Abstract
Background
The impact of primary tumor location on overall survival (OS), recurrence-free survival (RFS), and long-term outcomes has not been well established in patients undergoing potentially curative resection of colorectal liver metastases (CRLM).
Methods
A single-institution database was queried for initial resections for CRLM 1992–2004. Primary tumor location determined by chart review (Right=cecum to transverse; Left=splenic flexure to sigmoid). Rectal cancer (distal 16cm), multiple primaries, and unknown location were excluded. Kaplan Meier and Cox regression methods were used. Cure was defined as actual 10-year survival with either no recurrence or resected recurrence with at least 3 years of disease-free followup.
Results
907 patients were included with a median followup of 11 years. 578 patients (64%) had left-sided and 329 (36%) right-sided primaries. Median OS for patients with a left-sided primary was 5.2 years (95% CI: 4.6–6.0) versus 3.6 years (95% CI: 3.2–4.2) for right-sided (p=0.004). On multivariable analysis, the hazard ratio (HR) for right-sided tumors was 1.22 (95% CI: 1.02–1.45, p=0.028) after adjusting for common clinicopathologic factors. Median RFS was marginally different stratified by primary location (1.3 vs 1.7 years; p=0.065). On multivariable analysis, location of primary was not significantly associated with RFS (p=0.105). Observed cure rates were 22% for left and 20% for right-sided tumors.
Conclusions
Among patients undergoing resection of CRLM, left-sided primary tumors were associated with improved median OS. However, long-term and recurrence-free survival were not significantly different stratified by primary location. Patients with left-sided primary tumors display a prolonged clinical course suggestive of more indolent biology.
Introduction
Colorectal cancer is the third most common cancer in the United States with approximately 140,000 new cases diagnosed annually.1 Large population-based studies have demonstrated that survival following surgery for colon cancer differs by tumor location.2–4 In these reports, tumor location is classified as proximal (right) or distal (left) depending on the relationship to the splenic flexure of the colon. This distinction is based on embryology because the right colon develops from the midgut and the left colon from the hindgut. Tumors within these unique regions are known to differ in regard to clinicopathologic factors, such as microsatellite instability (MSI) and BRAF mutation rates.5–7 The prognostic implication of primary tumor location has also been expanded to patients with metastatic disease.8 In recent prospective trials of metastatic and unresectable colorectal cancer, improved progression-free survival (PFS) and overall survival (OS) were observed in patients with left-sided primary tumors.9–11 However, unlike those with widespread metastatic disease, patients with limited colorectal liver metastases (CRLM) are candidates for surgery with the potential for long-term survival and cure.12–14 The prognostic implication of primary tumor location has not been well examined in patients with resectable CRLM and long-term follow-up.
Among patients selected for hepatectomy, preoperative factors such as the size and number of hepatic metastases reflect the extent of liver disease and are associated with outcomes.15–17 Prognostic variables related to the primary tumor also impact survival. Controlling for the size and number of hepatic metastases, lymph node involvement and lymphovascular invasion (LVI) have also been associated with differences in survival after hepatic resection.18 However, these previous studies and current clinical risk scores did not examine the significance of right versus left-sided primary tumors following hepatic resection of CRLM. Two recent studies have analyzed primary tumor location in patients undergoing hepatectomy for CRLM.19,20 Left-sided primary tumors were associated with an improved median OS in both. These studies, however, were limited by a short follow-up. Thus, the ultimate clinical implication of primary tumor location in patients undergoing hepatectomy for CRLM requires further study.
The aim of this study was to determine the impact of primary tumor location on recurrence-free (RFS), OS, and actual 10-year survival for patients undergoing hepatic resection for metastatic colon cancer.
Methods
Study Design and Patients
All patients referred to a surgeon at Memorial Sloan Kettering Cancer Center are recorded in a prospectively-maintained departmental database. A waiver of Health Insurance and Portability and Accountability Act authorization was obtained and patients were queried from the database that had initial hepatic resection for CRLM from 1992–2004 without macroscopic (R2) residual disease. All patients in the selected time interval had sufficient follow-up to assess for 10-year survival outcomes. Patients with postoperative death or less than 90 day follow-up were excluded. Consistent with previous publications, right-sided colon primary was defined as tumors in the cecum, ascending colon, hepatic flexure, or transverse colon.2,3 Left-sided primary tumors were defined as those in the splenic flexure, descending colon, and sigmoid. Patients with rectal cancer (distal 16cm), multiple primaries, or unknown location of primary were excluded. Rectal cancer was excluded as it represents a subset of patients with unique therapeutic strategies and distinct outcomes.
Demographic and clinicopathologic variables were supplemented with review of the medical record. Clinical risk score (CRS) has been previously reported and was dichotomized into low (0–2) and high (3–5) risk groups.15 Disease-free interval (DFI) less than 12 months is a variable in the CRS, and also includes patients with synchronous CRLM. Positive margin was defined as malignant cells at the inked surface of the transected liver.24 Extrahepatic disease included patients with additional metastatic lesions known at the time of hepatectomy and resected at the same time or within the next 6 months.25 Perioperative chemotherapy was defined as any chemotherapy administered within 3 months of surgery. Cure was defined as actual 10-year survival with either no recurrence or resected recurrence with at least 3 years of disease-free follow-up from the time of last resection.
Data Analysis
Categorical variables were reported with frequency and percentage. Continuous variables were reported as median and range. Differences between groups were assessed with the chi-square test or Fisher’s exact test. RFS and OS were calculated from the date of hepatic resection until the time of first recurrence (for RFS) or until the time of death (for OS), whichever came first. Patients alive by the end of the study were censored. OS after recurrence was calculated among the subset that had a recurrence. RFS, OS and OS among recurrence patients were estimated using Kaplan-Meier methods and compared using log-rank test. A Cox proportional hazards model was used to evaluate the independent association between location of primary tumors and outcomes and adjusted for known clinical confounders. Variables with a higher percentage of unknown, such as LVI and perineural invasion (PNI) of the primary, were not included in the multivariable OS and RFS models. Instead, subset analyses were carried out to examine the association between tumor location and OS after controlling for LVI or PNI on patients with complete data. A P value <0.05 was considered statistically significant and 95% confidence intervals were used where appropriate. All analyses were done in SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
Patient Characteristics
Overall, 1316 patients underwent initial, complete hepatic resection for CRLM between 1992 and 2004. From this group, patients with postoperative death (n=35) or no follow-up beyond 90 days (n=70) were excluded. Patients with rectal primaries (n=292), multiple primaries (n=8) and unknown primary location (n=4) were excluded. The remaining 907 patients formed the study population with 329 (36.3%) right-sided and 578 (63.7%) left-sided primary tumors. Perioperative chemotherapy was administered to >90% of patients (n=837), this included patients that received chemotherapy preoperatively only (n=42, 4.6%), post-operatively only (n=384, 42.3%), and both (n=411, 45.3%). Perioperative hepatic artery infusion (HAI) pump was utilized for 29.5% of patients (n=268, neoadjuvant=21, adjuvant=247). Detailed clinicopathologic factors of the primary tumor and extent of disease are listed in Table 1. Left-sided primary was associated with younger median age (62.6 vs 65.4 years, p=0.001) and less frequently a DFI less than 12 months (50.5 vs 61.4%, p=0.002).
Table 1.
Patient Demographics and Clinicopathologic Characteristics
| Patient Characteristics | Right-Sided Primary (Total=329) | Left-Sided Primary (Total=578) | |
|---|---|---|---|
| N (%) | N (%) | p | |
| Age at Surgery, median (range) | 65.4 (26–86) | 62.6 (23–89) | <0.001 |
| Gender | 0.553 | ||
| Male | 180 (54.7) | 328 (56.7) | |
| Female | 149 (45.3) | 250 (43.3) | |
| T stage for primary tumor | 0.885 | ||
| 1 | 9 (2.7) | 13 (2.2) | |
| 2 | 38 (11.6) | 69 (11.9) | |
| 3 | 241 (73.3) | 412 (71.3) | |
| 4 | 25 (7.6) | 48 (8.3) | |
| Unknown | 16 (4.9) | 36 (6.2) | |
| N stage for primary tumor | 0.144 | ||
| N0 | 126 (38.3) | 223 (38.6) | |
| N1 | 113 (34.3) | 231 (40.0) | |
| N2 | 88 (26.7) | 119 (20.6) | |
| Unknown | 2 (0.6) | 5 (0.9) | |
| LVI for primary tumor | 0.665 | ||
| No | 108 (32.8) | 183 (31.7) | |
| Yes | 82 (24.9) | 160 (27.7) | |
| Unknown/Missing | 139 (42.2) | 235 (40.7) | |
| PNI for primary tumor | 0.318 | ||
| No | 122 (37.1) | 186 (32.1) | |
| Yes | 35 (10.6) | 69 (11.9) | |
| Unknown/Missing | 172 (52.3) | 323 (55.9) | |
| Tumor grade | 0.157 | ||
| Well | 4 (1.2) | 16 (2.8) | |
| Moderate | 255 (77.5) | 451 (78.0) | |
| Poor | 34 (10.3) | 41 7.1) | |
| Unknown/Missing | 36 (10.9) | 70 (12.1) | |
| CEA | 0.155 | ||
| <200 | 255 (77.5) | 447 (77.3) | |
| ≥200 | 38 (11.6) | 48 (8.3) | |
| Unknown/Missing | 36 (10.9) | 83 (14.3) | |
| DFI | 0.002 | ||
| <12 month | 202 (61.4) | 292 (50.5) | |
| ≥12 month | 127 (38.6) | 286 (49.5) | |
| Size of largest liver tumor | 0.858 | ||
| <5 | 222 (67.5) | 387 (67.0) | |
| ≥5 | 107 (32.5) | 191 (33.0) | |
| Multiple liver tumor | 0.809 | ||
| 1 | 172 (52.3) | 307 (53.1) | |
| >1 | 157 (47.7) | 271 (46.9) | |
| Clinical Risk Score | 0.664 | ||
| 0–2 | 172 (52.3) | 283 (49.0) | |
| 3–5 | 119 (36.2) | 209 (36.2) | |
| Unknown | 38 (11.6) | 86 (14.9) | |
| Extrahepatic Disease | 0.263 | ||
| No | 304 (92.4) | 545 (94.3) | |
| Yes | 25 (7.6) | 33 (5.7) | |
| Margin | 0.191 | ||
| Negative | 308 (93.6) | 527 (91.2) | |
| Positive | 21 (6.4) | 51 (8.8) | |
| Perioperative Chemotherapy | 0.919 | ||
| Yes | 304 (92.4) | 533 (92.2) | |
| No | 25 (7.6) | 45 (7.8) | |
| Hepatic Artery Infusion Pump | 0.381 | ||
| Yes | 103 (31.3) | 165 (28.5) | |
| No | 226 (68.7) | 413(71.4) |
Recurrence-Free Survival
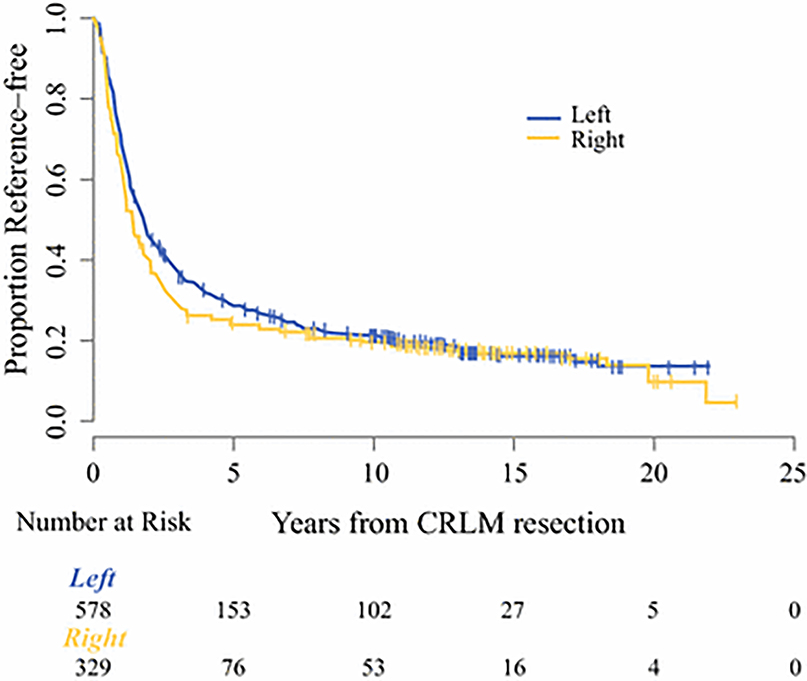
Recurrence following hepatic resection was identified in 70.0% (635/907) of patients. For the entire study cohort, Kaplan-Meier estimates of RFS at 3 years was 34% (95% CI: 31–37) and at 5 years was 27% (95% CI: 24–30). Figure 1 demonstrates a marginal difference in RFS with regard to primary location that approaches but does not reach statistical significance (log rank p=0.065). The median RFS for patients with a right-sided primary tumor was 1.3 years (95% CI: 1.1–1.6) as compared to 1.7 years (95% CI: 1.5–2.0) for those with a left-sided primary tumor. Three and 5-year RFS was 29.0% (95% CI: 24.2–34.0) and 24.3% (95% CI: 19.8–29.1) respectively for right-sided tumors. For left-sided primary tumors, 3 and 5-year RFS was 37.0% (95% CI: 33.0–41.0) and 28.6% (95% CI: 24.9–32.4) respectively. On multivariable analysis, location of primary tumor was not associated with a significant difference in RFS (HR 1.14; 95% CI: 0.97–1.35, p=0.105) Table 2.
Figure 1.
Kaplan-Meier curve of recurrence-free survival (RFS) stratified by primary location. Median RFS for patients with a right-sided primary tumor was 1.3 years (95% CI: 1.1–1.6) versus 1.7 years (95% CI: 1.5–2.0) for those with a left-sided primary tumor (p=0.065).
Table 2.
Univariate and multivariable analysis of factors associated with RFS following hepatic resection for CRLM.
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%) | p-value | |
| Location | ||||
| Right Colon | 1.15 (0.99,1.33) | 0.066 | 1.14 (0.97,1.35) | 0.105 |
| Left Colon | Ref | Ref | ||
| Age at Surgery* | 0.97 (0.91,1.04) | 0.472 | 0.98 (0.91,1.06) | 0.626 |
| CEA (>=200 vs <200) | 1.83 (1.44,2.31) | <0.001 | 1.34 (1.04,1.74) | 0.025 |
| DFI (<12 vs > 12 months) | 1.23 (1.07,1.45) | 0.004 | 1.18 (0.99,1.39) | 0.052 |
| Largest liver tumor (>=5 vs <5) | 1.48 (1.27,1.73) | <0.001 | 1.41 (1.18,1.70) | <0.001 |
| N stage of primary tumor | <0.001 | <0.001 | ||
| N0 | Ref | Ref | ||
| N1 | 1.33 (1.12,1.57) | 1.44 (1.20,1.74) | ||
| N2 | 1.62 (1.33,1.95) | 1.63 (1.32,2.01) | ||
| Number of liver lesions | <0.001 | 0.004 | ||
| One | Ref | Ref | ||
| More than one | 1.34 (1.15,1.55) | 1.27 (1.01,1.49) | ||
| Margin Status | <0.001 | <0.001 | ||
| Negative | Ref | Ref | ||
| Positive | 2.02 (1.58,2.59) | 2.2 (1.67,2.89) | ||
| Extrahepatic disease | <0.001 | <0.001 | ||
| No | Ref | Ref | ||
| Yes | 2.48 (1.89,3.25) | 2.3 (1.73,3.27) | ||
Note: HR estimated every 10 yrs increased in age at surgery
Overall Survival
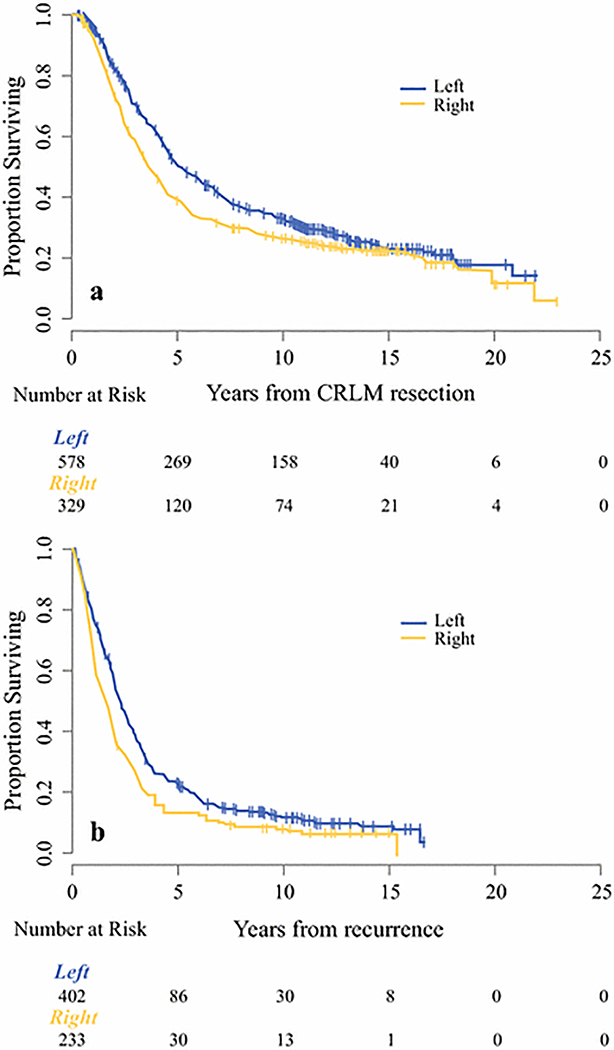
Median follow-up among survivors was 11.4 years. Five-year OS for the whole cohort was 46% (95% CI: 42–49). Figure 2a demonstrates that OS following hepatectomy was significantly different stratified by primary location, with a median OS of 3.6 years (95% CI: 3.2–4.2) for patients with a right-sided primary tumor as compared to 5.2 years (95% CI: 4.6–6.0) for those with a left-sided primary tumor (log-rank=0.004). Five-year OS for right-sided primary tumors was 38.5% (95% CI: 33.3–43.9) compared to 50.4% (95% CI: 46.1–54.5) for left-sided primary tumors. The difference in OS by primary location was adjusted for known prognostic clinicopathologic variables and the results of the multivariable model are in Table 3. Controlling for these factors, the HR for patients with a right-sided primary was 1.22 (95% CI: 1.02–1.45, p=0.028) as compared to the left-sided primary. Among patients with complete data on LVI status (n=533), location of primary tumor remained significantly associated with OS (HR 1.25, 95% CI: 1.01–1.54, p=0.039, data not shown). However, among the subset with known PNI status (n=412), location of primary was not associated with OS (HR 1.17, 95% CI: 0.92–1.48, p=0.1774, data not shown).
Figure 2.
a. Kaplan-Meier curve of overall survival (OS) stratified by primary location. Median OS of 3.6 years (95% CI: 3.2–4.2) for patients with a right-sided primary tumor versus 5.2 years (95% CI: 4.6–6.0) for those with left-sided primary tumors (p= 0.004).
b. OS as measured from the time of recurrence (n=635) stratified by primary location. Patients with a right-sided primary had a median OS after recurrence of 1.5 years (95% CI: 1.2–1.8) versus 2.2 years (95% CI: 2.0–2.5) for patients with left-sided primary (p<0.001).
Table 3.
Univariate and multivariable analysis of factors associated with OS following hepatic resection for CRLM.
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%) | p-value | |
| Location | ||||
| Right Colon | 1.26 (1.08,1.48) | 0.003 | 1.22 (1.02,1.45) | 0.028 |
| Left Colon | Ref | |||
| Age at Surgery* | 1.06 (0.99,1.14) | 0.084 | 1.11 (1.03,1.20) | 0.010 |
| CEA (>=200 vs <200) | 1.88 (1.47,2.39) | <0.001 | 1.36 (1.04,1.78) | 0.025 |
| DFI (<12 vs > 12 months) | 1.17 (1.00,1.37) | 0.044 | 1.08 (0.89,1.28) | 0.427 |
| Largest liver tumor (>=5 vs <5) | 1.44 (1.22,1.69) | <0.001 | 1.44 (1.19,1.75) | <0.001 |
| N stage of primary tumor | <0.001 | <0.001 | ||
| N0 | Ref | Ref | ||
| N1 | 1.36 (1.13,1.63) | 1.47 (1.21,1.79) | ||
| N2 | 1.74 (1.42,2.14) | 1.92 (1.53,2.41) | ||
| Number of liver lesions | <0.001 | 0.005 | ||
| One | Ref | Ref | ||
| More than one | 1.32 (1.12,1.53) | 1.28 (1.08,1.52) | ||
| Margin Status | <0.001 | <0.001 | ||
| Negative | Ref | Ref | ||
| Positive | 1.84 (1.42,2.38) | 2.0 (1.47,2.60) | ||
| Extra Hepatic disease | <0.001 | <0.001 | ||
| No | Ref | Ref | ||
| Yes | 2.0 (1.51,2.66) | 2.1 (1.51,2.97) | ||
Note: HR estimated every 10 yrs increased in age at surgery
Among 635 patients with recurrence, patients with a right-sided primary had a median OS after recurrence of 1.5 years (95% CI: 1.2–1.8) as compared to 2.2 years (95% CI: 2.0–2.5) for patients with a left-sided primary. Five-year estimated OS after recurrence was 13.5% (95% CI: 9.0–18.3) for patients with a right-sided primary tumor and 22.7% (95% CI: 18.6–27.3) for those with a left-sided primary tumor (Figure 2b, p<0.001).
Utilizing our definition of cure based on actual 10-year survival, cure was observed in 20.3% (67/329) of patients with a right-sided primary compared to 22.0% (127/578) of patients with a left-sided primary tumor (p=0.57).
Discussion
Location of the primary tumor has become an increasingly recognized prognostic factor in metastatic colorectal cancer (mCRC).8–11 Analysis of recent prospective trials in patients with mCRC treated with palliative chemotherapy have demonstrated prolonged PFS and OS associated with left-sided primary tumors. The overall impact, however, of primary tumor location in patients undergoing potentially curative resection of CRLM requires further evaluation. Two recent studies have looked at primary tumor location and early outcomes following hepatic resection19,20 However, our study is the first to investigate the effect of primary tumor location in a cohort of patients with hepatectomy for CRLM and long-term follow-up. Patients with left-sided primary tumors had a significantly improved median OS as compared to right-sided tumors, and improved OS was also observed among the subgroup of patients that had recurred. However, with long-term follow-up, there was no difference in RFS stratified by primary tumor location or the rates of observed cure. Overall, patients with left-sided primary tumors displayed a more prolonged and indolent clinical course without any difference in the ultimate rates of death.
In our series, patients with liver metastases from left-sided primary tumors were younger and more often presented with longer DFI. The latter finding supports the idea of a more protracted course for patients with left-sided primary tumors. However, other variables related to the primary tumor (T-stage, nodal status, LVI, PNI, grade), liver metastases (number and size), extrahepatic disease, and resection (margin status) were not different between patients stratified by primary tumor location. Thus, the difference in outcomes following hepatic resection supports the hypothesis that left-sided primary tumors harbor more indolent tumor biology not fully characterized by clinicopathologic factors in current risk scores. The aim of this study was not to re-evaluate all potential variables related to survival, although known perioperative factors remained associated with OS (age, CEA >200, size and number of liver tumors, margin, extrahepatic disease, and lymph node stage of primary). Among factors that comprise the CRS, DFI <12 months was the only variable that did not reach statistical significance on multivariate analysis and further highlights the limitations of clinical prognostic factors. Rather, this study was designed to control for known clinicopathologic variables related to survival and determine the impact of primary location in a large, consecutive series of patients.
Patients with left-sided primary tumors had significantly different median OS. These results correspond with what has been previously reported regarding the impact of primary location on OS in patients with metastatic colon cancer treated with palliative chemotherapy.9–11 The patient cohort that undergoes resection of CRLM is highly-selected. However, it appears that left-sided primary location is a consistent factor that correlates with a prolonged clinical course. Patients with left-sided primary tumors also displayed a longer interval between recurrence and death. This finding suggests that recurrences after hepatic resection from right-sided tumors may be less amenable to resection, less responsive to therapy, or simply more rapidly progressive. Recent evidence indicates that primary location impacts the efficacy of chemotherapy in RAS wt patients.9,10 In these studies, the authors suggest that further molecular and genetic factors related to the primary tumor (MSI, methylation, BRAF mutations) may provide a better explanation for this difference. Although patients with BRAF mutations infrequently present with resectable CRLM, the frequency of such mutations is higher in right-sided primary tumors.26 The patients in this study are from a time period before molecular data was collected. Thus, we are not able to elucidate the associations between primary tumor location and these mutations in our dataset. Nonetheless, primary location, as a potential surrogate for higher-risk disease characteristics, adds a component to our current understanding of metastatic colon cancer.
The impact of primary location after resection of CRLM appears to diminish with regard to overall rates of cure. While primary tumor location impacts median OS, the observed cure rates were not different. Prolonged follow-up demonstrated that approximately 20% of patients were cured regardless of primary location. Therefore, primary tumor location, by itself, has limited utility to improve current methods of patient selection for hepatic resection of CRLM. Our findings suggest the primary location, at present, should not change surgical decision-making. However, this study lays the foundation for further investigations regarding primary tumor location, molecular data, and specific treatments such as systemic or hepatic artery infusion chemotherapy.
This is the first study to examine the impact of primary tumor location on resected CRLM patients with long-term survival. Two recent publications have also reported an improved median OS after hepatic resection in patients with left-sided tumors.19,20 However, these studies differ with regard to RFS and duration of follow-up. Sasaki et al recently published a report of primary location on RFS and OS following liver resection for CRLM.19 In a cohort of 475 patients, these authors found improved RFS in patients with right-sided primary tumors. However, patients with right-sided tumors were more likely to recur with advanced disease and had worse OS after recurrence. These results do not correspond with our larger dataset. On the contrary, RFS in our cohort demonstrated a marginally worse RFS for patients with a right-sided primary though it did not reach statistical significance. In a study set of 725 patients, Yamashita et al recently reported a significantly worse RFS and OS for patients with right-sided primary tumors.20 Both studies had less then 3 years median follow-up among survivors. On the contrary, with a median follow-up among survivors of 11 years, our study has the potential to more thoroughly address the impact of primary location on long-term survival outcomes. In our study, after adjusting for known clinical confounders, patients with right-sided primary tumors were at increased risk of all cause mortality than left-sided tumors.
Our study has several limitations. As a retrospective analysis, it is subject to inherent bias regarding, selection, follow-up, and missing data. Furthermore, many of the current prognostic variables regarding the primary colon tumor were not routinely collected for patients undergoing hepatectomy, including MSI status and KRAS and BRAF mutations, due to the years and scope of the project.27–29 In the same way, LVI and PNI status, though potentially important, were missing for many patients and thus not included in the full model. Hepatic artery infusion has been shown to impact HDFS, but we did not include it in the multivariate model because the rates were similar between groups and it was a clinical decision as opposed to prognostic factor.23 Further investigations, that minimize missing data and include molecular characteristics of the primary, will help further explain the reason behind these observed differences in outcomes. Nonetheless, this is a large series with prolonged follow-up that reveals the impact of primary location on survival and cure following hepatic resection of metastatic colon cancer.
Conclusion
Among patients selected for hepatic resection of CRLM, left-sided primary tumors were independently associated with an improved median OS. However, primary tumor location was not associated with differences in RFS or long-term survival. Patients with left-sided primary tumors display a prolonged clinical course suggestive of more indolent tumor biology.
Synopsis.
After hepatic resection of CRLM, patients with left-sided primary tumors had an associated improved median OS but not long-term survival or RFS. Patients with left-sided primary tumors display a prolonged clinical course suggestive of more indolent tumor biology.
Acknowledgments
Support: This work was supported in part by the Biostatistics Core and NIH/NCI P30 CA008748 Cancer Center Support Grant.
Footnotes
Disclosures and Conflicts of Interest: none
References
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin 65:5–29, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Benedix F, Kube R, Meyer F, et al. : Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 53:57–64, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Weiss JM, Pfau PR, O’Connor ES, et al. : Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol 29:4401–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yahagi M, Okabayashi K, Hasegawa H, et al. : The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: a Systematic Review and Meta-analysis. J Gastrointest Surg 20:648–55, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Gervaz P, Bucher P, Morel P: Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol 88:261–6, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Benedix F, Meyer F, Kube R, et al. : Influence of anatomical subsite on the incidence of microsatellite instability, and KRAS and BRAF mutation rates in patients with colon carcinoma. Pathol Res Pract 208:592–7, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bufill JA: Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 113:779–88, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Price TJ, Beeke C, Ullah S, et al. : Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 121:830–5, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Tejpar S, Stintzing S, Ciardiello F, et al. : Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venook A, Niedzwiecki D, Innocenti F, et al. : Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 34:abstr 3504, 2016 [Google Scholar]
- 11.Holch JW, Ricard I, Stintzing S, et al. : The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 70:87–98, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. : Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25:4575–80, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Pulitano C, Castillo F, Aldrighetti L, et al. : What defines ‘cure’ after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB (Oxford) 12:244–9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigano L, Ferrero A, Lo Tesoriere R, et al. : Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol 15:2458–64, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Fong Y, Fortner J, Sun RL, et al. : Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–18; discussion 318–21, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakaria S, Donohue JH, Que FG, et al. : Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg 246:183–91, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordlinger B, Guiguet M, Vaillant JC, et al. : Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 77:1254–62, 1996 [PubMed] [Google Scholar]
- 18.Cardona K, Mastrodomenico P, D’Amico F, et al. : Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol 20:148–54, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Sasaki K, Andreatos N, Margonis GA, et al. : The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol 114:803–809, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Yamashita S, Brudvik KW, Kopetz SE, et al. : Embryonic Origin of Primary Colon Cancer Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colon Cancer Liver Metastases. Ann Surg, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly JM, Kemeny N, Oderman P, et al. : Long-term hepatic arterial infusion chemotherapy. Anatomic considerations, operative technique, and treatment morbidity. Arch Surg 119:936–41, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Allen PJ, Nissan A, Picon AI, et al. : Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg 201:57–65, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kemeny N, Huang Y, Cohen AM, et al. : Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 341:2039–48, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Sadot E, Groot Koerkamp B, Leal JN, et al. : Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg 262:476–85; discussion 483–5, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpizo DR, Are C, Jarnagin W, et al. : Liver Resection for Metastatic Colorectal Cancer in Patients with Concurrent Extrahepatic Disease: Results in 127 Patients Treated at a Single Center. Annals of Surgical Oncology 16:2138–2146, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Missiaglia E, Jacobs B, D’Ario G, et al. : Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 25:1995–2001, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Schirripa M, Bergamo F, Cremolini C, et al. : BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer 112:1921–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karagkounis G, Torbenson MS, Daniel HD, et al. : Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 119:4137–44, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nash GM, Gimbel M, Cohen AM, et al. : KRAS mutation and microsatellite instability: two genetic markers of early tumor development that influence the prognosis of colorectal cancer. Ann Surg Oncol 17:416–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]