Abstract
BACKGROUND
In 2015 and 2016, Colombia had a widespread outbreak of Zika virus. Data from two national population-based surveillance systems for symptomatic Zika virus disease (ZVD) and birth defects provided complementary information on the effect of the Zika virus outbreak on pregnancies and infant outcomes.
METHODS
We collected national surveillance data regarding cases of pregnant women with ZVD that were reported during the period from June 2015 through July 2016. The presence of Zika virus RNA was identified in a subgroup of these women on real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) assay. Brain or eye defects in infants and fetuses and other adverse pregnancy outcomes were identified among the women who had laboratory-confirmed ZVD and for whom data were available regarding pregnancy outcomes. We compared the nationwide prevalence of brain and eye defects during the outbreak with the prevalence both before and after the outbreak period.
RESULTS
Of 18,117 pregnant women with ZVD, the presence of Zika virus was confirmed in 5926 (33%) on rRT-PCR. Of the 5673 pregnancies with laboratory-confirmed ZVD for which outcomes had been reported, 93 infants or fetuses (2%) had brain or eye defects. The incidence of brain or eye defects was higher among pregnancies in which the mother had an onset of ZVD symptoms in the first trimester than in those with an onset during the second or third trimester (3% vs. 1%). A total of 172 of 5673 pregnancies (3%) resulted in pregnancy loss; after the exclusion of pregnancies affected by birth defects, 409 of 5426 (8%) resulted in preterm birth and 333 of 5426 (6%) in low birth weight. The prevalence of brain or eye defects during the outbreak was 13 per 10,000 live births, as compared with a prevalence of 8 per 10,000 live births before the outbreak and 11 per 10,000 live births after the outbreak.
CONCLUSIONS
In pregnant women with laboratory-confirmed ZVD, brain or eye defects in infants or fetuses were more common during the Zika virus outbreak than during the periods immediately before and after the outbreak. The frequency of such defects was increased among women with a symptom onset early in pregnancy. (Funded by the Colombian Instituto Nacional de Salud and the Centers for Disease Control and Prevention.)
Zika virus is a flavivirus that is transmitted to humans primarily by the bite of an infected mosquito of the aedes genus.1 Sexual transmission and transmission of Zika virus from mother to fetus have also been reported.2–4 Congenital Zika virus infection can cause serious defects of the brain and eye5 and has been associated with neurodevelopmental abnormalities, such as seizures, joint contractures, swallowing difficulties, vision impairments, and hearing loss in infants.6–11
Preliminary monitoring of Zika virus disease (ZVD) began in Colombia after the recognition of the Zika outbreak in Brazil in May 2015. In August 2015, the Colombian Instituto Nacional de Salud (INS) began national surveillance of ZVD.12 In early October 2015, the first cluster of laboratory-confirmed cases (9 patients from northern Colombia) was identified, and an outbreak of ZVD was declared in the country. On July 25, 2016, Colombia declared an end to the Zika epidemic, with reports of clinical ZVD symptoms in more than 100,000 persons nationwide.13 Colombia had the second highest number of reported cases among the 50 countries with autochthonous transmission during the outbreak in the Americas.14
In 2016, we described a cohort of pregnant women with symptomatic ZVD for whom data had been reported to the Colombian national surveillance system, but the majority of women who had been infected during the first or second trimester were still pregnant.15 Using national passive surveillance data regarding birth defects, we identified an increase by a factor of four in the number of microcephaly cases from the previous year, although we did not have data regarding Zika testing in these cases.16 The data showed a peak number of microcephaly cases approximately 24 weeks after the peak of the outbreak, which suggested that the greatest risk for adverse outcomes was likely among women who were infected in early pregnancy. Colombia had two national surveillance systems in place before the epidemic — one to capture symptomatic ZVD and the other to capture birth defects — which allowed for the monitoring of the effect of the Zika virus outbreak at the population level.
To document the extent of the 2015–2016 Zika virus outbreak in Colombia, we evaluated the incidence and geographic distribution of symptomatic ZVD among all pregnant women who were reported to the national surveillance system. Here, we report the number of brain or eye defects that could have been caused by Zika virus infection and other adverse pregnancy outcomes according to the trimester of symptom onset among pregnant women with laboratory-confirmed ZVD. Since the majority of Zika virus infections are mild or have no symptoms, we describe the prevalence of brain and eye defects reported nationally, regardless of known Zika virus infection during pregnancy, along with assessing the geographic distribution and comparing the prevalence before and after the outbreak.
Methods
Public Health Surveillance System
The Colombian national public health surveillance system for notifiable conditions monitors dengue, chikungunya, and Zika virus, along with maternal morbidity, acute flaccid paralysis (in children ≤15 years of age), and birth defects, including disorders of the central nervous system. Information that is collected by health care centers is compiled and transmitted weekly to the national surveillance system.
We report on data from 18,117 pregnant women with clinical symptoms of ZVD that were recorded by the national surveillance system from June 15, 2015, to July 31, 2016.13 ZVD cases included those reported to the INS, regardless of laboratory confirmation. Laboratory-confirmed ZVD was defined as the presence of clinical symptoms of ZVD and a serum sample positive for Zika virus RNA on real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) assay. In October 2015, the INS mandated immediate reporting of all ZVD cases and implemented enhanced surveillance of microcephaly and other defects of the central nervous system.12 The initial case definition for ZVD included fever and at least one of the following symptoms: nonpurulent conjunctivitis, headache, rash, pruritus, or arthralgia, with no known alternative cause. On December 24, 2015, the symptom criteria were revised to include fever and rash and at least one of the following symptoms: nonpurulent conjunctivitis, headache, pruritus, arthralgia, myalgia, or malaise. Health care providers documented whether patients met the symptom criteria, but specific symptoms were not captured.
Laboratory Testing for Zika Virus
The INS arboviral reference laboratory prioritized rRT-PCR testing for Zika virus RNA in a subgroup of ZVD cases from four populations: infants, pregnant women, adults 65 years of age or older, and persons with coexisting illnesses.12 Molecular testing was performed with the use of two methods: a single-target rRT-PCR assay, which uses a published method with reflex testing for dengue and chikungunya for those with negative results for Zika virus,17 and the Trioplex rRT-PCR assay, which detects RNA from all three viruses simultaneously.18,19 Since both of these tests are most sensitive within 7 days after symptom onset, we analyzed rRT-PCR results in pregnant women that were stratified according to the number of days from symptom onset to sample collection.
Brain or Eye Defects and Other Adverse Pregnancy Outcomes
We collected data regarding brain and eye defects and other adverse pregnancy outcomes for pregnant women with ZVD for whom data regarding the pregnancy outcome and the gestational age at the time of symptom onset were available. Data from ZVD surveillance were linked to data from surveillance of birth defects and vital statistics, which included information on live births and fetal, neonatal, and infant deaths. Zika virus–associated birth defects were defined as brain or eye defects that were identified on the basis of a standard case definition informed by published studies and clinical and epidemiologic subject-matter experts.20 We used clinical terms from the International Classification of Diseases, 10th Revision, Clinical Modification for the selected defects. (Data regarding the clinical terms are provided in note F1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.)
We calculated the occurrence of these defects among live births and counted pregnancy losses at any gestational age. Among live births excluding multiple births, we collected data regarding the status of infants as preterm (<37 weeks) and low birth weight (<2500 g); we report incidence of infant death before the age of 1 year. Outcomes are reported overall and according to the trimester of symptom onset, which was calculated on the basis of the date of the woman’s last menstrual period and symptom onset (first trimester, 0 to 90 days of gestation; second and third trimester, >90 days of gestation). Our primary analysis focused on adverse pregnancy and infant outcomes among women with laboratory-confirmed ZVD, since a clinical diagnosis on the basis of symptoms may be difficult in regions in which dengue and other infections are endemic.
In addition, we collected data regarding the nationwide prevalence of brain or eye defects, regardless of ZVD status, from September 2015 through April 2017, a time period that accounts for all pregnancies throughout the epidemic period. We calculated the prevalence of brain or eye defects as the number of infants or fetuses reported with these defects per 10,000 live births, and we compared the prevalence for the same defects before the outbreak in 2014 and after the outbreak in 2018. Confidence intervals were calculated with the use of exact Poisson regression.21
Geographic Distribution of ZVD and Brain or Eye Defects
We assessed the geographic distribution of laboratory-confirmed ZVD among pregnant women and cases of brain or eye defects in infants or fetuses by mapping these data according to reporting area (32 departments and 4 districts). (Data regarding the reporting areas are provided in note F2 in the Supplementary Appendix.) The cumulative incidence of laboratory-confirmed ZVD among pregnant women was calculated as the number of cases per 100,000 women of childbearing age on the basis of census data.22
Results
From June 15, 2015, to July 31, 2016, a total of 18,117 pregnant women with clinical symptoms of ZVD were reported. The number of ZVD cases steadily increased from September 2015 through January 2016, with the largest number of cases reported during the week of January 24, 2016 (epidemiologic week 3) (Fig. 1). During the period from January 31 through March 27, 2016 (epidemiologic weeks 4 to 12), the number of reported cases decreased sharply; a gradual but consistent decline in cases occurred through August 7, 2016 (epidemiologic week 31). A similar pattern according to epidemiologic week was seen for laboratory-confirmed cases. Of the 18,117 pregnant women with ZVD, 8215 (45%) underwent serum testing for Zika virus on rRTPCR; 5926 (33%) of the women who were tested had positive results (Fig. S1 in the Supplementary Appendix).
Figure 1. Pregnant Women with Symptoms of Zika Virus Disease (ZVD) in Colombia, According to the Date of Symptom Onset (June 15, 2015–July 31, 2016).
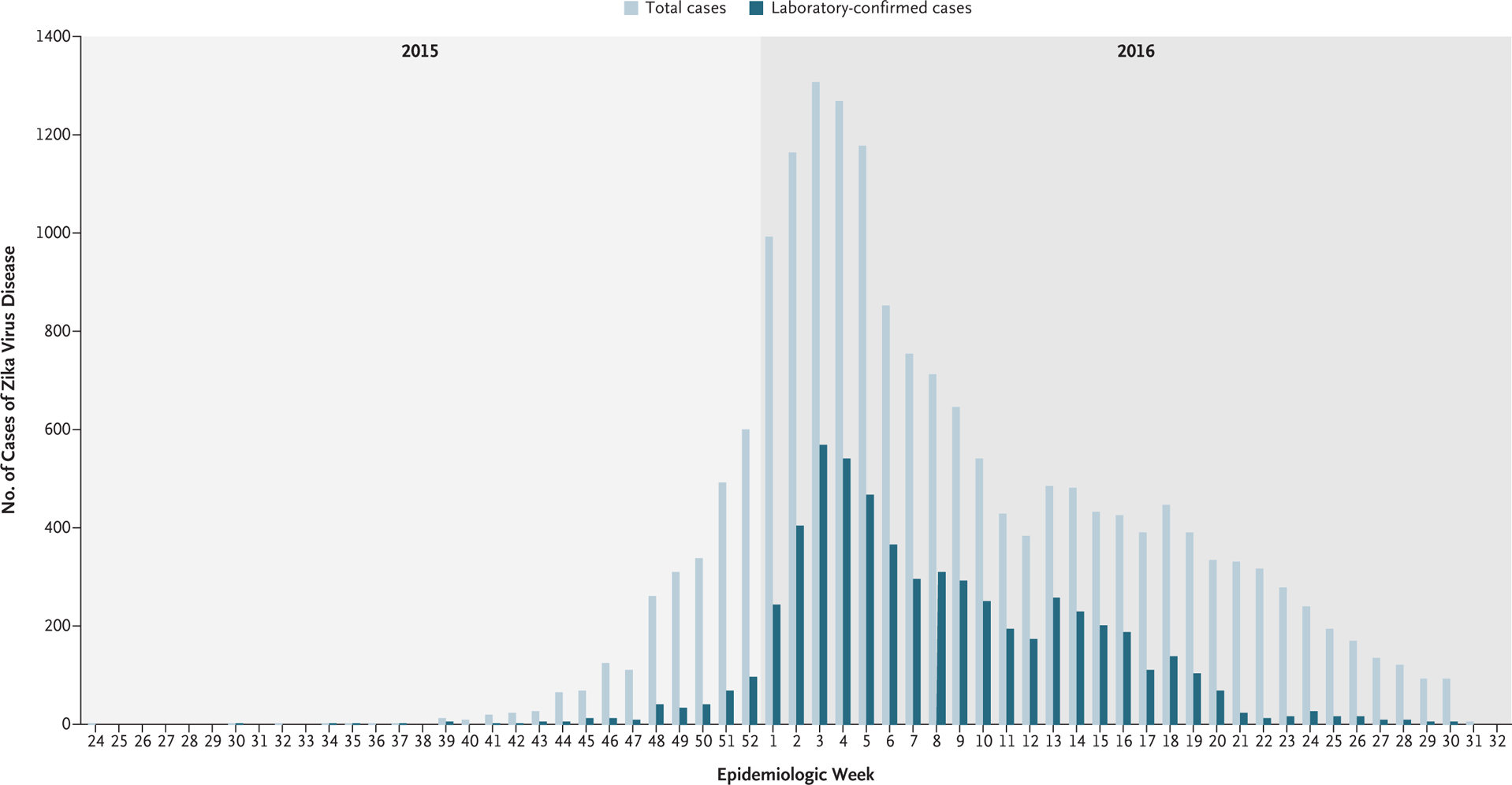
Shown is the distribution of symptom onset among 18,117 pregnant women who were reported to the Colombian national surveillance system as having ZVD during the most recent outbreak. The numbers of total cases and laboratory-confirmed cases are provided. Laboratory confirmation was determined by the presence of Zika virus RNA on real-time reverse transcriptase–polymerase-chain-reaction (rRT-PCR) assay.
The gestational age at the time of symptom onset and pregnancy outcome were reported for 5673 of 5926 infants or fetuses (96%) carried by women with laboratory-confirmed Zika virus infection (including one set of twins) (Fig. 2). Of the 5673 pregnancies, 790 (14%) had at least one adverse outcome (Table 1). Brain or eye defects were reported in 93 infants or fetuses (2%), with 75 (81%) among live births and 18 (19%) among pregnancy losses. The percentage of brain and eye defects was higher among the women with symptom onset during the first trimester than among those with onset during the second or third trimester (3% [40 of 1192] vs. 1% [53 of 4481]). Among pregnancy losses, a lower percentage of birth defects was seen among the women with symptom onset during the first trimester than among those with onset during the second or third trimester; however, the mean gestational week at the time of pregnancy loss was 14 weeks and 27 weeks in the two subgroups, respectively, which may account for some of the difference in the frequency of known defects.
Figure 2. Analysis of Surveillance Data and Pregnancy Outcomes.
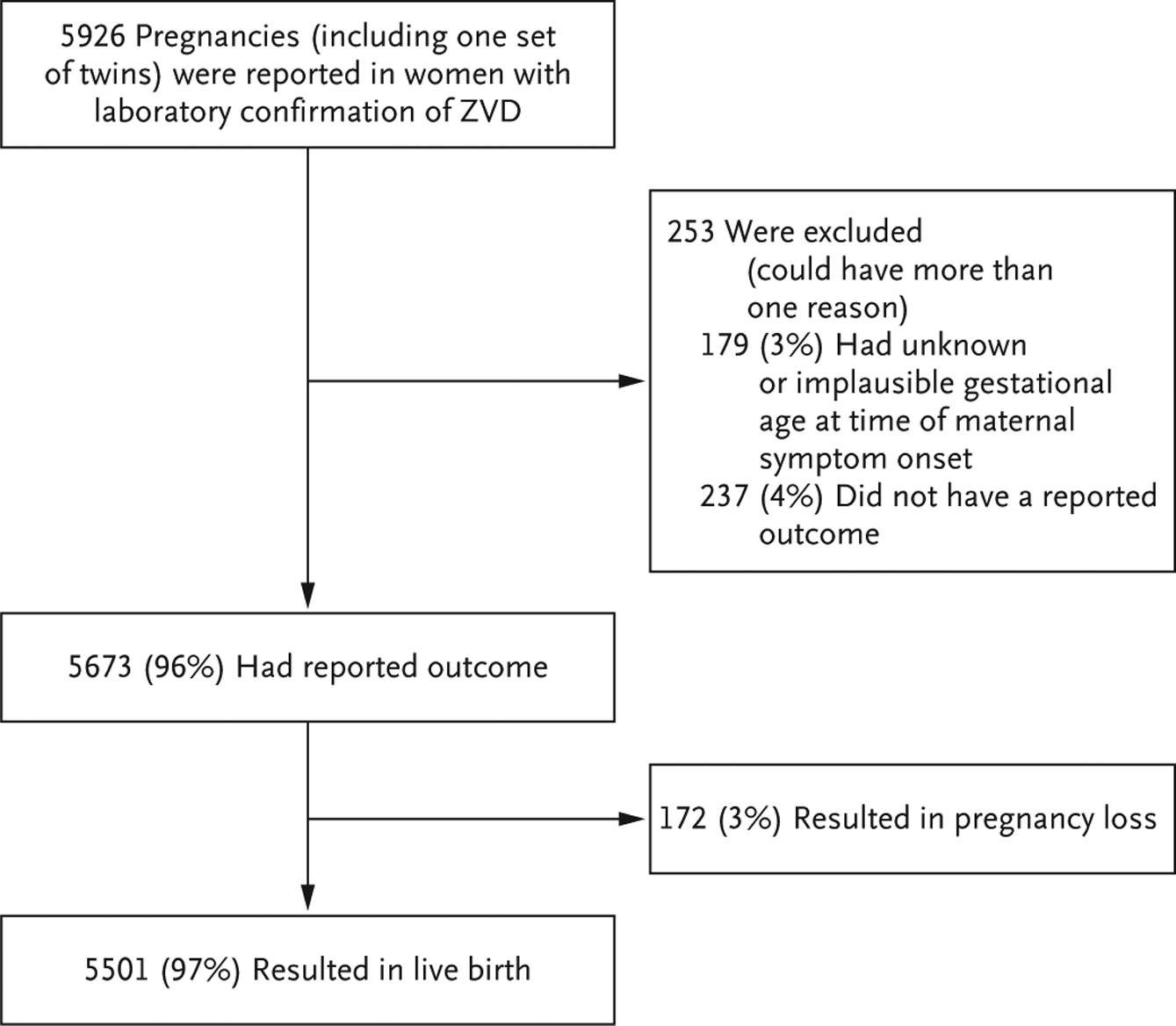
To clarify the outcomes of pregnancies (including one set of twins), data are provided for infants or fetuses rather than pregnant women. The presence of Zika virus infection in infants or fetuses was confirmed on rRT-PCR assay.
Table 1.
Characteristics of Pregnant Women with Laboratory-Confirmed ZVD and Data Regarding Pregnancy Outcome, According to Trimester of ZVD Symptom Onset (June 15, 2015–July 31, 2016).*
| Characteristic | Timing of Symptom Onset of Laboratory-Confirmed ZVD | ||
|---|---|---|---|
| First Trimester (N = 1192) | Second or Third Trimester (N = 4481) | All Trimesters (N = 5673) | |
| no. of infants or fetuses/total no (%) | |||
| Maternal demographic data | |||
| Age | |||
| <18 yr | 79/1191 (7) | 356/4479 (8) | 435/5670 (8) |
| 18–24 yr | 438/1191 (37) | 1701/4479 (38) | 2139/5670 (38) |
| 25–34 yr | 545/1191(46) | 1962/4479 (44) | 2507/5670 (44) |
| ≥35 yr | 129/1191 (11) | 460/4479 (10) | 589/5670 (10) |
| Race or ethnic group† | |||
| Mestizo or White | 1034/1060 (98) | 3886/3998 (97) | 4920/5058 (97) |
| Other | 26/1060 (2) | 112/3998 (3) | 138/5058 (3) |
| Residence | |||
| Urban | 935/1060 (88) | 3495/3998 (87) | 4430/5058 (88) |
| Other | 125/1060 (12) | 503/3998 (13) | 628/5058 (12) |
| Insurance type | |||
| Employer-based | 586/1060 (55) | 2043/3998 (51) | 2629/5058 (52) |
| Subsidized | 407/1060 (38) | 1699/3998 (42) | 2106/5058 (42) |
| Other | 67/1060 (6) | 256/3998 (6) | 323/5058 (6) |
| Adverse pregnancy and infant outcomes | |||
| Any adverse outcome‡ | 255/1192 (21) | 535/4481 (12) | 790/5673 (14) |
| Pregnancy loss | 97/1192 (8) | 75/4481 (2) | 172/5673 (3) |
| Brain or eye defect§ | 40/1192 (3) | 53/4481 (1) | 93/5673 (2) |
| Preterm birth¶ | 104/1061 (10) | 305/4365 (7) | 409/5426 (8) |
| Low birth weight¶ | 71/1061 (7) | 262/4365 (6) | 333/5426 (6) |
| Infant death | 8/1095 (1) | 40/4406 (1) | 48/5501 (1) |
Listed are data for infants or fetuses carried by pregnant women who had laboratory confirmation of Zika virus disease (ZVD) and for whom a pregnancy outcome was available. Percentages may not total 100 because of rounding.
Race or ethnic group was reported by the women.
Data may be listed for more than one category of adverse outcome.
This category includes both pregnancy losses and live births.
Included in this category are live births of infants without brain or eye defects with the exclusion of multiple births.
Among the 5426 live births without a report of birth defects, 8% of the infants were born preterm, and 6% had a low birth weight. Women with an onset of ZVD symptoms during the first trimester were more likely to have pregnancy loss or a preterm birth than those with an onset of symptoms later in pregnancy (8% vs. 2% for pregnancy loss and 10% vs. 7% for preterm birth). The deaths of 48 infants (1%) were reported, with no difference in incidence according to the trimester of symptom onset. Findings regarding adverse pregnancy and infant outcomes in the total cohort of pregnant women with ZVD were consistent with the results among women with laboratory-confirmed ZVD (Table S1).
Laboratory confirmation of ZVD in pregnant women was widely distributed across Colombia (Fig. 3A), with 33 of 36 reporting areas identifying at least 1 laboratory-confirmed case. Fifteen reporting areas reported an aggregate of 5290 cases of laboratory-confirmed ZVD (89% of the total cases). From September 2015 through April 2017, a total of 1451 infants or fetuses with a brain or eye defect were reported to the national surveillance system regardless of the mother’s ZVD status during pregnancy, for a prevalence of 13 cases (95% confidence interval [CI], 13 to 14) per 10,000 live births. All reporting areas had cases of brain and eye defects (Fig. 3B), with a prevalence that ranged from 5 to 81 per 10,000 live births in each reporting area. The prevalence of brain and eye defects combined was 8 (95% CI, 7 to 9) per 10,000 live births in 2014 and 11 (95% CI, 10 to 12) per 10,000 live births in 2018.
Figure 3. Laboratory-Confirmed ZVD among Pregnant Women and Reported Cases of Brain or Eye Defects among Infants or Fetuses.
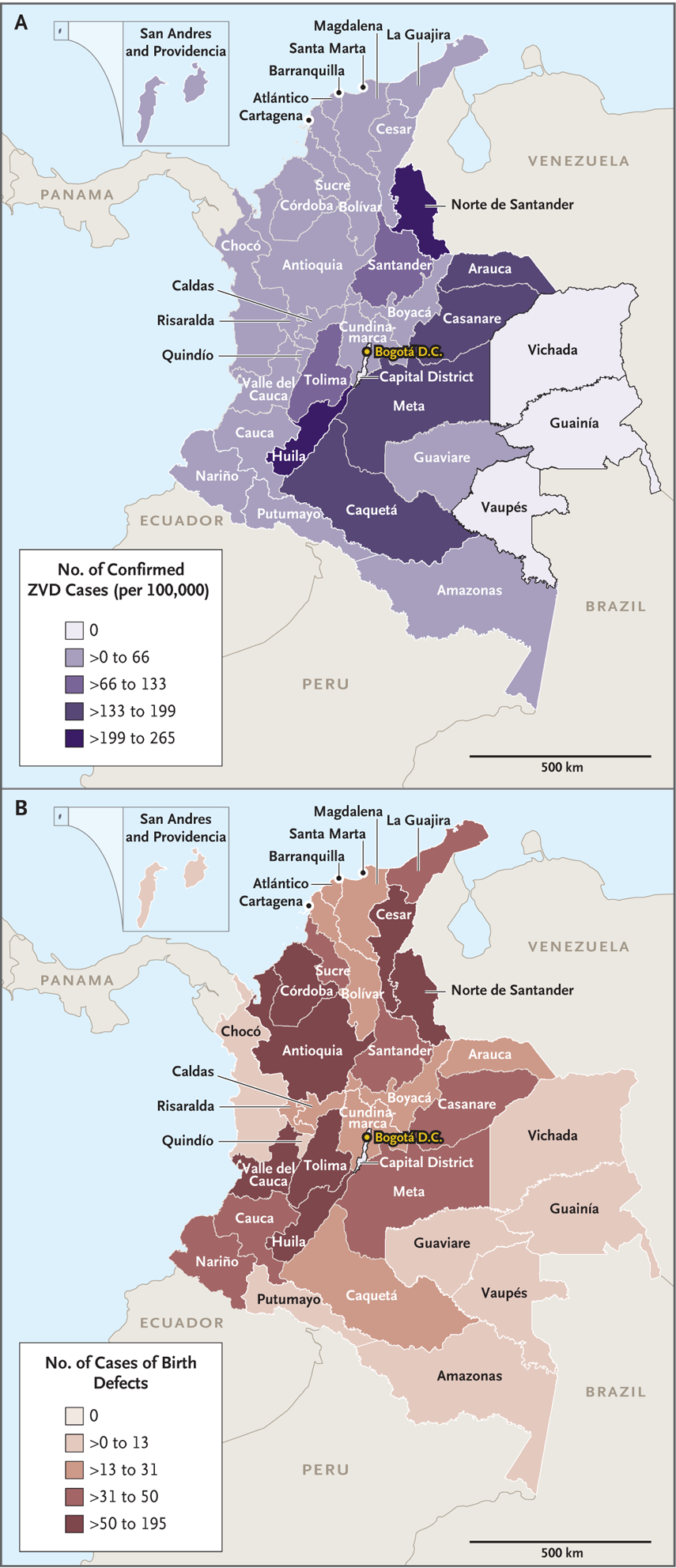
Panel A shows the number of pregnant women with laboratory-confirmed ZVD per 100,000 women of childbearing age in Colombia from June 15, 2015, to July 31, 2016, during the most recent outbreak of Zika virus infection. Panel B shows the number of reported cases of brain or eye defects among infants or fetuses in Colombia from September 2015 through April 2017.
Discussion
During the Zika virus outbreak in Colombia from June 2015 through July 2016, the presence of ZVD in more than 18,000 pregnant women was reported to the national surveillance system. Among the women in this group who had laboratory-confirmed ZVD, 2% of their infants or fetuses had a brain or eye defect. Such defects were more common among infants carried by women who had an onset of ZVD symptoms during the first trimester of pregnancy than among those with a later symptom onset (3% vs. 1%). After the exclusion of infants or fetuses with brain or eye defects, 8% were born preterm and 6% had a low birth weight. However, these frequencies of preterm delivery and low birth weight were lower than population estimates in Colombia during 2014 (19% and 9%, respectively).23
Pregnant women were prioritized for Zika virus testing during the epidemic because of the risk of serious fetal harm; approximately one third of these women had positive test results. A major barrier to testing was the short window for sample collection. Although prolonged viremia has been observed among women with Zika virus infection in pregnancy,24–26 acute infection is most likely to be detectable in samples obtained within 1 week after symptom onset.27 Samples may not have been obtained from women who were reported to the national surveillance program more than 1 week after the onset of ZVD symptoms, and the range of intervals from symptom onset to sample collection was wide for the entire cohort. The manifestation of ZVD is often mild, which may limit the ability to pinpoint the date of symptom onset. Thus, inaccurate estimates of the time from symptom onset to sample collection may have occurred, a factor that complicates the interpretation of laboratory results.
Our results show that laboratory-confirmed ZVD cases among pregnant women and, subsequently, brain or eye defects in their infants or fetuses were geographically widespread in Colombia during the outbreak period. The 93 cases of brain or eye defects that were reported in women with laboratory-confirmed ZVD represents only 6% of the overall 1451 infants or fetuses with brain or eye defects that were reported nationally (prevalence, 13 per 10,000 live births). These nationwide numbers are consistent with a high percentage of undetected and asymptomatic Zika virus infections occurring during pregnancy. Alternatively, the remaining 1358 infants or fetuses with brain or eye defects that were not directly linked to maternal ZVD may be the result of other causes. However, the nationwide prevalence of brain or eye defects during the outbreak was significantly higher than that in the years either before or after the outbreak. Since the risk of such birth defects is similar regardless of whether a woman has known ZVD symptoms, surveillance that is limited to women with clinical symptoms of ZVD will not identify all brain or eye defects that are related to Zika virus infection.28–30 These findings highlight the importance of surveillance for birth defects, which can complement the surveillance of ZVD in order to characterize the full effect of a Zika virus outbreak.
The incidence of brain or eye defects among the infants or fetuses of women with laboratory-confirmed ZVD (2%) was lower than that reported in other countries on the basis of a similar definition for the defects. In territories of the United States (including American Samoa, Federated States of Micronesia, Marshall Islands, Puerto Rico, and the U.S. Virgin Islands), 5% of pregnancies in which the mother had laboratory-confirmed or possible Zika virus infection resulted in a ZVD-associated birth defect; among the women who had confirmed infection during the first trimester, ZVD-associated birth defects occurred in 8%.30 Underreporting of brain or eye defects among pregnancy losses and live births is likely in Colombia because surveillance for birth defects relies on passive reporting from providers. Furthermore, many brain defects, such as intracranial calcifications, can be diagnosed only with neuroimaging. Although national guidelines recommend neuroimaging, a comprehensive eye examination, and standard evaluation for infants born to women with Zika virus exposure,12 it is unclear how commonly such evaluations were performed during the outbreak period, even among infants with known congenital exposure. On the basis of active surveillance data in the U.S. territories, it is possible that as many as 40% of infants born to women with laboratory evidence of possible Zika virus infection do not undergo postnatal neuroimaging.11 In our study, the highest risk of brain or eye defects occurred when ZVD symptoms were reported during the first trimester of pregnancy, a finding that is consistent with reports of outcomes in other populations.11,28,31 However, 80% of our cohort reported symptom onset in the second or third trimester, which could be one reason for the relatively low percentage of defects reported in the overall cohort.
Much attention has been given to the disparity in the number of confirmed cases of congenital syndrome associated with Zika virus infection reported to the Pan American Health Organization (PAHO) in Brazil (2952 cases) as compared with the cases in Colombia (248) as of January 2018.14 Reasons for the differences may include population size and the percentage of the population in Colombia residing above 2200 m, an altitude at which aedes mosquitoes do not circulate. But the main factor may be the difference in case definitions. Brazil reported all cases meeting either the confirmed or probable PAHO case definition; the probable case definition did not require laboratory confirmation. In contrast, Colombia reported only cases consistent with the PAHO case definition for a confirmed case, including requiring the detection of Zika virus in a specimen obtained from the infant. However, we found the expected increase in the incidence of brain or eye defects that were potentially associated with Zika virus infection overall in our cohort of pregnant women with laboratory-confirmed ZVD (2%) and among those with symptom onset in the first trimester (3%).
Variation in the case definition for birth defects associated with Zika virus infection during pregnancy has made it challenging to compare the findings from published studies. In a cohort of women in Rio de Janeiro who had undergone postnatal neuroimaging (e.g., transfontanel ultra-sonography, computed tomography, and magnetic resonance imaging [MRI]), 58 of 125 women with Zika virus infection (46%) had an adverse pregnancy outcome. However, among the adverse outcomes that were identified, only 3% involved the presence of microcephaly. In the other cases, adverse outcomes were identified only on neuroimaging, including findings of unclear clinical significance (e.g., excessive hypersignaling in white matter on T2-weighted MRI).7,32 Other reports that have relied on a narrower case definition for ZVD-associated birth defects have used active methods for ascertaining outcomes. In these studies, structural brain or eye defects were reported in 5 to 10% of infants or fetuses carried by women with Zika virus infection. Of the surviving infants, approximately 9% were found to have a neurodevelopmental abnormality that was potentially linked to Zika virus infection by 1 year of age.11
Early in 2016, the Zika virus outbreak was deemed to be a public health emergency of international concern because of the threat posed to pregnant women and their developing infants.33 A rapid clinical and public health response was essential to identify the causal link between the virus and serious birth defects, and efforts are ongoing to understand the neurodevelopmental consequences of Zika virus infection during pregnancy.5,11 In a separate cohort of 70 Colombian infants with documented maternal Zika virus infection during pregnancy but no clinical signs of congenital Zika syndrome and normal prenatal neuroimaging, an assessment of neurodevelopment by standardized tools in infants between 4 and 18 months of age showed developmental scores falling further below the mean as the children aged.34 This finding emphasizes the importance of long-term follow-up of children with Zika virus exposure.35 In Colombia, as in every country that has had a Zika virus outbreak, affected children will need to be followed to identify all disabilities associated with this infection and to ensure that families are linked to needed services.
Supplementary Material
Acknowledgments
Supported by the Colombian Instituto Nacional de Salud (INS) and the CDC under an interagency agreement with the Office of Infectious Disease, Bureau for Global Health, U.S. Agency for International Development.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the following persons for their contributions to this project: Pablo Chaparro, M.D., Esther Cuevas, M.S., Claudia Hugett, M.S., and Natalia Tolosa, M.D., of the INS; Andres Espinosa-Bode, M.D., Rita Helfand, M.D., Christina A. Nelson, M.D., Ann M. Powers, Ph.D., Jennita Reefhuis, Ph.D., Jessica Ricaldi Camahuali, M.D., Carol Y. Rao, Sc.D., and Sherry Farr, Ph.D., of the CDC and other staff members who worked on the Colombia Zika virus response; Christopher Carr, M.P.H., of the Oak Ridge Institute for Science and Education; Alexandra Caycedo, D.D.S., M.A., and Andrea Rodriguez, D.D.S., M.P.H., of Vynsova Partners for supporting surveillance by collecting medical records and samples; the Sivigila group for data collection and reporting of the national surveillance data at the INS; the staff members at the Ministry of Health; and the Secretaries of Health in all departments, districts, and municipalities in Colombia.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the U.S. Agency for International Development.
References
- 1.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med 2016; 374: 1552–63. [DOI] [PubMed] [Google Scholar]
- 2.Calvet G, Aguiar RS, Melo ASO, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 2016; 16: 653–60. [DOI] [PubMed] [Google Scholar]
- 3.Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17: 880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission — continental United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 215–6. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects — reviewing the evidence for causality. N Engl J Med 2016; 374: 1981–7. [DOI] [PubMed] [Google Scholar]
- 6.Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 2016; 387: 2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016; 375: 2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 2016; 388: 891–7. [DOI] [PubMed] [Google Scholar]
- 9.Leal MC, Muniz LF, Ferreira TSA, et al. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection — Brazil, November 2015–May 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 917–9. [DOI] [PubMed] [Google Scholar]
- 10.Melo AS, Aguiar RS, Amorim MM, et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol 2016; 73: 1407–16. [DOI] [PubMed] [Google Scholar]
- 11.Rice ME, Galang RR, Roth NM, et al. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection — U.S. territories and freely associated states, 2018. MMWR Morb Mortal Wkly Rep 2018; 67: 858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Instituto Nacional de Salud. Circular externa 0043 de 2015. October 14, 2015. (https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/circular-conjunta-externa-0043.pdf).
- 13.Instituto Nacional de Salud. Epidemiological week 30. 2016. (https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2016%20Boletin%20epidemiologico%20semana%2030.pdf).
- 14.Pan American Health Organization. Zika cumulative cases 2018. (https://www.paho.org/hq/index.php?option=com_content&view=article&id=12390:zika-cumulative-cases&Itemid=42090&lang=en).
- 15.Pacheco O, Beltrán M, Nelson CA, et al. Zika virus disease in Colombia — preliminary report. N Engl J Med 2020;383:e44. [DOI] [PubMed] [Google Scholar]
- 16.Cuevas EL, Tong VT, Rozo N, et al. Preliminary report of microcephaly potentially associated with Zika virus infection during pregnancy — Colombia, January–November 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1409–13. [DOI] [PubMed] [Google Scholar]
- 17.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14: 1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Trioplex real-time RT-PCR assay: instructions for use. 2017. (https://www.cdc.gov/zika/pdfs/trioplex-real-time-rt-pcr-assay-instructions-for-use.pdf).
- 19.Santiago GA, Vázquez J, Courtney S, et al. Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat Commun 2018; 9: 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson SM, Delaney A, Jones AM, et al. Updated baseline prevalence of birth defects potentially related to Zika virus infection. Birth Defects Res 2019; 111: 938–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly L Simple SAS macros for the calculation of exact binomial and Poisson confidence limits. Comput Biol Med 1992; 22: 351–61. [DOI] [PubMed] [Google Scholar]
- 22.Departamento Administrativo Nacional de Estadística. Series de población 1985–2020 (https://www.dane.gov.co/index.php/en/statistics-by-topic-1/population-and-demography/population-series-1985-2020).
- 23.Departamento Administrativo Nacional de Estadística. Nacimientos por área de ocurrencia y sexo, según grupos de edad de la madre. 2014. (https://www.dane.gov.co/index.php/estadisticas-por-tema/salud/nacimientos-y-defunciones/nacimientos/nacimientos-2014).
- 24.Driggers RW, Ho C-Y, Korhonen EM, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 2016; 374: 2142–51. [DOI] [PubMed] [Google Scholar]
- 25.Lozier MJ, Rosenberg ES, Doyle K, et al. Prolonged detection of Zika virus nucleic acid among symptomatic pregnant women: a cohort study. Clin Infect Dis 2018; 67: 624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meaney-Delman D, Oduyebo T, Polen KN, et al. Prolonged detection of Zika virus RNA in pregnant women. Obstet Gynecol 2016; 128: 724–30. [DOI] [PubMed] [Google Scholar]
- 27.Sharp TM, Fischer M, Muñoz-Jordán JL, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep 2019; 68(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honein MA, Dawson AL, Petersen EE, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017; 317: 59–68. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds MR, Jones AM, Petersen EE, et al. Vital signs: update on Zika virus– associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure — U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep 2017; 66: 366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro-Mendoza CK, Rice ME, Galang RR, et al. Pregnancy outcomes after maternal Zika virus infection during pregnancy — U.S. Territories, January 1, 2016–April 25, 2017. MMWR Morb Mortal Wkly Rep 2017; 66: 615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med 2018; 378: 985–94. [DOI] [PubMed] [Google Scholar]
- 32.Honein MA, Jamieson DJ. Monitoring and preventing congenital Zika syndrome. N Engl J Med 2016; 375: 2393–4. [DOI] [PubMed] [Google Scholar]
- 33.Gulland A Zika virus is a global public health emergency, declares WHO. BMJ 2016; 352: i657. [DOI] [PubMed] [Google Scholar]
- 34.Mulkey SB, Arroyave-Wessel M, Peyton C, et al. Neurodevelopmental abnormalities in children with in utero Zika virus exposure without congenital Zika syndrome. JAMA Pediatr 2020; 174: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honein MA, Woodworth KR, Gregory CJ. Neurodevelopmental abnormalities associated with in utero Zika virus infection in infants and children — the unfolding story. JAMA Pediatr 2020; 174: 237–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


