Abstract
Clinical manifestations of COVID-19 affect many organs, including the heart. Cardiovascular disease is a dominant comorbidity and prognostic factors predicting risk for critical courses are highly needed. Moreover, immunomechanisms underlying COVID-induced myocardial damage are poorly understood.
Objective
To elucidate prognostic markers to identify patients at risk.
Results
Only patients with pericardial effusion (PE) developed a severe disease course, and those who died could be identified by a high CD8/Treg/monocyte ratio. Ten out of 19 COVID-19 patients presented with PE, 7 (78%) of these had elevated APACHE-II mortality risk-score, requiring mechanical ventilation. At admission, PE patients showed signs of systemic and cardiac inflammation in NMR and impaired cardiac function as detected by transthoracic echocardiography (TTE), whereas parameters of myocardial injury e.g. high sensitive troponin-t (hs-TnT) were not yet increased. During the course of disease, hs-TnT rose in 8 of the PE-patients above 16 ng/l, 7 had to undergo ventilatory therapy and 4 of them died. FACS at admission showed in PE patients elevated frequencies of CD3+CD8+ T cells among all CD3+ T-cells, and lower frequencies of Tregs and CD14+HLA−DR+-monocytes. A high CD8/Treg/monocyte ratio predicted a severe disease course in PE patients, and was associated with high serum levels of antiviral cytokines. By contrast, patients without PE and PE patients with a low CD8/Treg/monocyte ratio neither had to be intubated, nor died.
Conclusions
PE predicts cardiac injury in COVID-19 patients. Therefore, TTE should be performed at admission. Immunological parameters for dysfunctional antiviral immunity, such as the CD8/Treg/monocyte ratio used here, supports risk assessment by predicting poor prognosis.
Keywords: COVID-19, SARS-CoV-2, Pericardial effusion, CD8, Treg, Monocyte
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has quickly progressed to a global pandemic health emergency, affecting various organs, and recent case reports associated myocardial involvement with poor prognosis [[1], [2], [3], [4]]. However, it has not been elucidated whether cardiac dysfunction and potential myocarditis is a primary result of SARS-CoV-2 induced cardiomyocyte damage or secondary to COVID-19 induced inflammation and the heart is the primary target or secondary bystander of disease [5]. Even though, autopsy studies have found evidence of direct myocyte assault by the virus, this was not associated with an influx of inflammatory cells [6]. Furthermore, studies investigating this potential link and identifying prognostic factors for cardiac injury and survival remain to be defined. Here, we correlated cardiac and immunologic parameters with clinical courses of COVID-19 patients to investigate potential mechanisms involved in the development of SARS-CoV-2 induced cardiac injury.
2. Material and methods
After approval form the Ethics Committee at the University Hospital Bonn, Germany (No. 107/20) and in accordance with the Declaration of Helsinki and §15 of the Medical Association Nordrheins' professional code of conduct, we enrolled 19 patients administered to the University Medical Center Bonn with symptoms typical for COVID-19. Infection was confirmed by smear test for SARS-CoV-2 RNA according to WHO standards.
Primary evaluation included transthoracic echocardiography (TTE), blood test and calculation of the APACHE II score (Table 2). Data were recorded by our patient data management system. Routine serum diagnostics were measured. All patients were followed up until discharge or death.
Table 2.
Clinical data for APACHE II score.
The mortality risk score APACHE II and respective clinical parameters upon admission in COVID-19 patients with pericardial effusion (PE), compared to patients without PE. Data were analyzed using Mann-Whitney-U test. Mean and SD are shown.
| Data | No PE, n = 9 | No PE, n = 10 | p-Value |
|---|---|---|---|
| Age (years) | 59.6 ± 13.8 | 69.9 ± 14.0 | 0.1109 |
| Temperature | 37.2 ± 1.3 | 37.3 ± 0.7 | 0.4373 |
| MAD (mmHg) | 90.8 ± 11.7 | 90.3 ± 12.1 | 0.8376 |
| pH | 7.485 ± 0.081 | 7.413 ± 0.033 | 0.0509 |
| Heart rate (bpm) | 87.4 ± 20.9 | 87.6 ± 16.4 | 0.9672 |
| Respiratory rate (breaths/min) | 14.9 ± 2.3 | 18.6 ± 5.3 | 0.0691 |
| Sodium (mmol/l) | 134.7 ± 6.1 | 134.3 ± 5.2 | 0.7123 |
| Potassium (mmol/l) | 3.7 ± 0.6 | 4.1 ± 0.4 | 0.3042 |
| Creatinine (mg/dl) | 0.81 ± 0.25 | 2.10 ± 2.68 | 0.4875 |
| Acute renal failure | 1 (11) | 2 (20) | 0.5957 |
| Hematocrit (%) | 37.8 ± 5.7 | 34.8 ± 6.1 | 0.2868 |
| Leukocytes (G/l) | 5.82 ± 2.57 | 6.29 ± 2.30 | 0.8065 |
| Glasgow coma scale (pts) | 15.0 ± 0.0 | 11.4 ± 5.8 | 0.0814 |
| FiO2 (%) | 34.9 ± 25.3 | 51.0 ± 23.2 | 0.0193 |
2.1. Cardiac structure and function analyses
TTE was performed using a Philips Affiniti with a 15 MHz probe (Philips, Germany). Left ventricular ejection fraction (EF) was calculated using the biplane method.
2.2. Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging was performed using a clinical whole-body magnetic resonance imaging system (Ingenia 1.5 T; Philips Healthcare, Netherlands). A 32-channel torso coil with digital inter-face was used for signal reception. Functional cine, T2-weighted edema and late gadolinium enhancement imaging in standard heart sections was performed. For myocardial T1-mapping, a 3(3)3(3)5 modified Look-Locker inversion recovery mapping sequence was applied. For myocardial T2-mapping, a six-echo gradient spin-echo sequence was used. Image analysis was conducted according to the updated Lake Louise criteria.
2.3. Serum analyses
Quantification of cytokines including sE-Selectin; GM-CSF; ICAM-1/CD54; IFN alpha; IFN gamma; IL-1 alpha; IL-1 beta; IL-4; IL-6; IL-8; IL-10; IL-12p70; IL-13; IL-17A/CTLA-8; IP-10/CXCL10; MCP-1/CCL2; MIP-1alpha/CCL3; MIP-1 beta/CCL4; sP-Selectin; TNF alpha was performed using the Inflammation 20-plex Human ProcartaPlex-Panel from thermo-fisher according to the manufacturer's instruction. High sensitive troponin-t (hs-TnT) was analyzed in our routine laboratory using Elecsys® electrochemiluminescence immunoassay (ECLIA) Roche, Germany.
2.4. Flow cytometry analysis
Blood was collected in EDTA-tubes and analyzed within 24 h. Some patients could not be analyzed at all time points due to logistical reasons. 100 μl whole blood were incubated with dried custom-designed format reagents (DuraClone tubes, Beckman Coulter) containing the antibodies CD3-AA700, CD4-APC, CD8-KrOr, CD14-AA750, CD16-FITC, CD25-PE, CD56-ECD, CD127-PC7, HLA-DR-PB. Fix- and Lyse Solution were used according to manufacturer's protocol. The CD8+ T cells/Treg/monocyte quotient was calculated by dividing percentages of CD3+CD8+ T cells by CD3+CD4+CD25+CD127− Tregs by CD14+HLA−DR+ monocytes of all circulating cells.
2.5. Statistical analysis
Data are presented as scatter plot with median in figures and as Mean ± SD in tables. Statistics were calculated with PRISM (V8.4.2; GraphPad, USA). Categorial variables were compared by Chi2 test. Continuous variables between two groups were tested using Mann-Whitney U test. Survival and intubation probability were visualized as Kaplan-Meier curves and results were validated using Log-rank (Mantel-Cox) test. p < 0.05 was considered statistically significant (*). **, p < 0.01; ***, p < 0.001. Statistics were approved by the Bonn Institute of Medical Biometry and Informatics.
3. Results
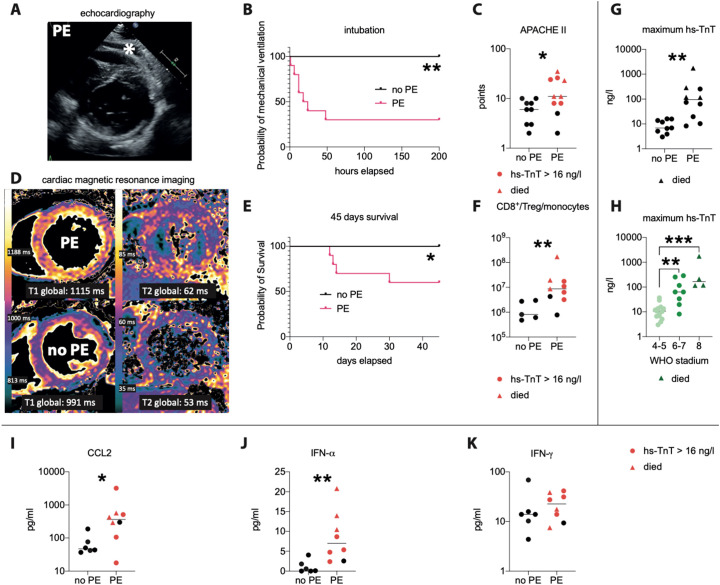
At admission, high-sensitive Troponin T (hs-TnT) was not markedly elevated in any COVID-19 patient (12.5 ± 9.3 ng/l, mean ± SD), but TTE revealed pericardial effusions (PE) in 10 of 19 patients (Fig. 1A). Seven of these PE patients subsequently developed respiratory failure and required invasive ventilatory support within 48 h (Fig. 1B). PE patients had higher APACHE II-scores (Fig. 1C) and poorer survival (Fig. 1E). Baseline characteristics, as well as pre-existing medical conditions did not differ between the groups (Table 1 ). In correlation with APACHE-scores, PE patients exhibited impaired left-ventricular EF (Table 1). Cardiac magnetic resonance imaging revealed a diffuse inflammatory pattern with myocardial edema in PE patients, but no evidence for direct viral damage such as necrosis (Fig. 1D). Cardiac inflammation was not detected in patients lacking PE. Myocardial damage marker such as hs-TnT increased only in PE patients during the course of disease (Fig. 1G).
Fig. 1.
PE, hs-TnT and the CD8/Treg/monocyte quotient predict heart injury and clinical course of COVID-19 patients.
(A): representative transthoracic echocardiography (TTE) image, asterisk indicates pericardial effusion. (B): necessity for intubation, (C) APACHE II-score. (D): Representative cardiac magnetic resonance images from a patient with (upper row) and without PE (lower row), showing a diffuse inflammatory pattern with increased markers of edema (T1 and T2 relaxation times) only in PE patients. (E) Survival of COVID-19 patients with or without pericardial effusion (PE) at admission, showing that a PE predicts poorer prognosis. (F): ratio between CD8+ T cells to Tregs and CD14+ HLA-DR+ monocytes, as a parameter for disease severity, which was higher in patients with PE, especially in those who subsequently developed a hs-TnT rise (red symbols) and those who died (red triangles). (G): hs-TnT correlates with PE and with disease severity indicated by (H) the new WHO COVID-19 classification. (I–K): Serum concentrations of the antiviral cytokines CCL2 (I), IFN-α (J) and IFN-γ (K) in patients exhibiting a pericardial effusion (PE) or not (no PE). Subsequent hs-TnT rise (red symbols), deceased (red triangle). Data is presented as scatter plot with median (line). p < 0.05 was considered statistically significant (*). **, p < 0.01; ***, p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Patients baseline characteristics and preexisting conditions.
Baseline characteristics, symptoms, underlying health conditions, and coagulation parameter in COVID-19 patients being admitted with pericardial effusion (PE), compared to patients without PE. For metric data, Mann-Whitney-U test was used, for categorial variables, Chi2 test was performed. Mean and SD are shown.
| Pre-existing diseases and conditions at admission | No PE, n = 9 | PE, n = 10 | p-Value |
|---|---|---|---|
| Female gender, n (%) | 5 (56) | 5 (50) | 0.8087 |
| BMI (kg/m2) | 24.2 ± 6.3 | 29.1 ± 4.9 | 0.0724 |
| Dyspnea, n (%) | 6 (67) | 9 (90) | 0.2129 |
| Cough, n (%) | 8 (89) | 6 (60) | 0.1533 |
| Syncope, n (%) | 0 (0) | 2 (20) | 0.1561 |
| Nausea, n (%) | 0 (0) | 1 (10) | 0.3297 |
| Fatigue, n (%) | 5 (56) | 8 (80) | 0.2524 |
| Fever, n (%) | 6 (67) | 7 (70) | 0.8759 |
| COPD, n (%) | 0 (0) | 3 (30) | 0.0734 |
| Previous pneumonia, n (%) | 1 (11) | 2 (20) | 0.5957 |
| Smoking, n (%) | 2 (22) | 2 (20) | 0.9056 |
| Arterial hypertension, n (%) | 4 (11) | 8 (80) | 0.1087 |
| Previous stroke, n (%) | 0 (0) | 2 (20) | 0.1561 |
| Diabetes mellitus, n (%) | 2 (22) | 3 (30) | 0.7007 |
| Hyperlipidemia, n (%) | 2 (22) | 6 (60) | 0.0959 |
| Coronary artery disease, n (%) | 0 (0) | 3 (30) | 0.0734 |
| Previous myocardial infarction, n (%) | 0 (0) | 3 (30) | 0.0734 |
| Heart valve disease, n (%) | 0 (0) | 1 (10) | 0.3297 |
| Atrial fibrillation, n (%) | 1 (11) | 2 (20) | 0.5957 |
| Previous cardiac surgery, n (%) | 0 (0) | 1 (10) | 0.3297 |
| Chronic renal failure, n (%) | 1 (11) | 2 (20) | 0.5957 |
| Liver disease, n (%) | 1 (11) | 0 (0) | 0.2788 |
| Chronic venous disease, n (%) | 0 (0) | 1 (10) | 0.3297 |
| Peripheral artery disease, n (%) | 1 (11) | 1 (10) | 0.9372 |
| Hypothyroidism, n (%) | 3 (33) | 1 (20) | 0.2129 |
| Leukocytes (G/l) | 5.82 ± 2.57 | 6.29 ± 2.30 | 0.8065 |
| Neutrophils (%) | 71.4 ± 7.65 | 77.0 ± 10.6 | 0.3154 |
| Lymphocytes (%) | 16.8 ± 6.62 | 14.5 ± 5.46 | 0.3675 |
| Platelet count (cells) | 206.1 ± 100.1 | 173.6 ± 61.6 | 0.3072 |
| INR | 1.10 ± 0.16 | 1.31 ± 0.84 | 0.8569 |
| Quick | 84.4 ± 16.6 | 84.6 ± 25.7 | 0.7123 |
| pTT | 27.8 ± 6.2 | 32.2 ± 9.4 | 0.2857 |
| hs-TnT at admission (ng/l) | 8.12 ± 5.66 | 17.5 ± 10.5 | 0.0719 |
| Left-ventricular ejection fraction at admission (%) | 73.1 ± 4.71 | 63.4 ± 7.61 | 0.0122 |
| Correlation between EF and APACHE II at admission | r: −0.5826 | R2: 0.3394 | 0.02 |
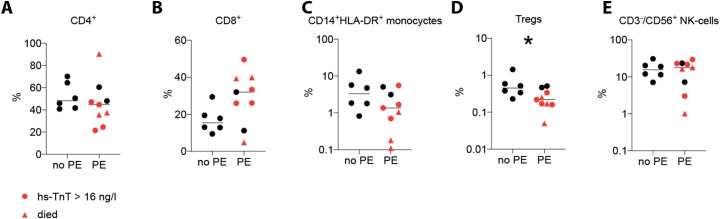
Next, we used flow-cytometry to identify inflammatory parameters that can predict at admission which of the PE patients would develop a severe disease course. This technique revealed normal frequencies of total leukocytes, lymphocytes and neutrophil blood counts in PE patients at admission (Table 1). However, among the T cells, the frequencies of CD8+ T cells, important effectors of early antiviral immunity [7], were increased, whereas regulatory T cells (Tregs), which control immune responses including CD8+ T cells [[8], [9], [10]], and monocytes, which have been reported to accumulate at sites of Covid-19 infection and simultaneously disappear from the circulation [11], were less frequent in PE patients (Fig. 2 ). We combined these 3 parameters altered in severely affected COVID-19 patients by calculating the CD8/Treg/monocyte ratio as a parameter for a dysfunctional antiviral immune response. Indeed, this ratio was low in all patients without PE and in three PE patients, and none of those developed a severe disease course (Fig. 1F). The other PE patients showed a high CD8/Treg/monocyte ratio and developed an hs-TnT increase (Fig. 1G), which correlated with the WHO COVID-19 classification (Fig. 1H). These patients also showed high serum levels of pro-inflammatory cytokines (Fig. 1I–K), consistent with the notion of a cytokine storm. Notably, an increase was seen also for the chemokine CCL2 (Fig. 1I), which mobilizes blood monocytes, possibly to counteract monopenia. Four of the patients with a high CD8/Treg/monocyte ratio eventually died.
Fig. 2.
Immune cell blood frequencies in COVID-19 patients.
Percentages of CD4+ of CD3+ T cells (A), CD8+ of CD3+ T cells (B), CD14+ HLA-DR+ monocytes (C), Tregs (D) and NK cells (E) upon admission in patients exhibiting a pericardial effusion (PE) versus patients without PE (no PE).
4. Discussion
The present study addressed cardiac involvement in COVID-19 and provides clinical and molecular information on underlying immunomechanisms, thus supporting an association between (hyper)inflammation and myocardial damage. Myocardial involvement was evident in the form of a PE, long before serum markers of cardiomyocyte damage became significantly elevated. The PE was associated with a severe disease course, including respiratory failure and fatal outcome. Consistent with previous reports of cardiac replication with SARS-CoV-2 in autopsy cases [6], cardiac MRI revealed myocarditis in COVID19 patients with PE [12], which was followed by elevated serum parameters cardiac damage, especially hs-TnT [2]. Further, we detected a reduced EF and increased markers of edema (T1 and T2 relaxation times) only in PE-patients. A recent report showed that recovered COVID-19 patients frequently showed signs of myocardial dysfunction in cardiac NMR [13]. Our findings are consistent with that report and demonstrate that inflammatory alterations of the heart are detectable already early in ongoing infection.
It has been speculated that an excessive immune response against COVID-19 might worsen cardiac dysfunction [14]. We here introduce the CD8/Treg/monocyte ratio to quantify an inadequate immune response that can target the heart. That ratio was low in all patients without PE and in those PE patients who survived, but high in patients who developed respiratory failure or died. Thus, a high CD8/Treg/monocyte ratio might serve as an early indicator for a severe cardiac involvement outcome in COVID-19, comparable to proteinuria recently shown to predict severe kidney involvement in this infection [15].
5. Limitations
Our results should be substantiated in a larger patient cohort to increase predictive power. Furthermore, our patients were enrolled upon admission and therefore disease state may be different. Nevertheless, the severity of their symptoms necessitated, justifying the admission date as a reasonable the start-point for our study. Also, patients with cardiac involvement are likely to feature affection of other organs as well, which may also affect disease-progression. Finally, future studies are warranted to clarify whether and how much the antiviral response against SARS-CoV-19 contributes to myocardial injury.
6. Conclusions
In conclusion, our findings indicate that a PE predicts imminent cardiac injury in COVID-19 patients. Thus, echocardiography should be performed at hospital admission as it identifies patients at risk, as already propagated [16]. Immunological parameters for disease severity, such as the CD8/Treg/monocyte ratio used here, supports risk assessment by predicting poor prognosis. Notably, myocardial inflammation preceded heart injury in our patients, supporting the notion that antiviral immunity contributed to myocardial damage [17]. Finally, the presence of a PE, together with the CD8/Treg/monocyte ratio and hs-TnT may also serve as progression parameters.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgments
We thank Chrystel Flores for technical support.
Footnotes
Supported by grants from the German Heart Foundation F/27/20 and F/28/29 (to Drs. Dürr, Heine, Luetkens and Velten), from the Deutsche Forschungsgemeinschaft (DFG EXC2151 – 390873048 to Drs. Heine and Kurts, DFG CRCTR259 to Drs. Zimmer, Nickenig and Kurts, and a DFG Gottfried-Wilhelm Leibniz Price to Dr. Kurts).
References
- 1.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F., Cao S., Liu X., Xiang Y., Zhao Q., Huang H., Yang B., Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox S.E., Li G., Akmatbekov A., Harbert J.L., Lameira F.S., Brown J.Q., Vander Heide R.S. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 4.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. The heart in COVID19: primary target or secondary bystander? JACC Basic Transl. Sci. 2020;5(5):537–542. doi: 10.1016/j.jacbts.2020.04.001. eCollection 2020 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindner D., Fitzek A., Brauninger H., Aleshcheva G., Edler C., Meissner K., Scherschel K., Kirchhof P., Escher F., Schultheiss H.P., Blankenberg S., Puschel K., Westermann D. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Maria A., Moretta L. HLA-class I-specific inhibitory receptors in HIV-1 infection. Hum. Immunol. 2000;61:74–81. doi: 10.1016/s0198-8859(99)00169-x. [DOI] [PubMed] [Google Scholar]
- 8.Ganji A., Farahani I., Khansarinejad B., Ghazavi A., Mosayebi G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells Mol. Dis. 2020;83 doi: 10.1016/j.bcmd.2020.102437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., Tong S.Y.C., Lewin S.R., Kedzierska K. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajivalili M., Hosseini M., Haji-Fatahaliha M. Gaining insights on immune responses to the novel coronavirus, COVID-19 and therapeutic challenges. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luetkens J.A., Isaak A., Zimmer S., Nattermann J., Sprinkart A.M., Boesecke C., Rieke G.J., Zachoval C., Heine A., Velten M., Duerr G.D. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ. Cardiovasc. Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.010897. [DOI] [PubMed] [Google Scholar]
- 13.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3557. Online ahead of print. PMID: 32730619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D’Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schroder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buonsenso D., Pata D., Chiaretti A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir. Med. 2020;8:e27. doi: 10.1016/S2213-2600(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]