Abstract
Purpose
To assess the prognostic value of pneumonia severity score (PSS), pectoralis muscle area (PMA), and index (PMI) on chest computed tomography (CT) in adult coronavirus disease 2019 (COVID-19) patients.
Method
The chest CT images of COVID-19 patients were evaluated for the PSS as the ratio of the volume of involved lung parenchyma to the total lung volume. The cross-sectional areas of the pectoralis muscles (PMA, cm2) were also measured automatically on axial CT images, and PMI was calculated as the following formula: PMI = PMA / patient’s height square (m2). The relationship between clinical variables, PSS, PMA, sex-specific PMI values, and patient outcomes (intubation, prolonged hospital stay, and death) were investigated using multivariable logistic regression analysis. All patients were followed for more than a month.
Results
One-hundred thirty patients (76 males, 58.46 %) were included in the study. Fifteen patients (11.54 %) were intubated, 24 patients (18.46 %) had prolonged hospital stay, and eight patients (6.15 %) died during follow-up. Patients with comorbidity had a higher mean of PSS (6.3 + 4.5 vs 3.9 + 3.8; p = 0.001). After adjusting the confounders, PSS was an independent predictor of intubation (adjusted Odds Ratio [OR]: 1.73, 95 % CI 1.31−2.28, p < 0.001), prolonged hospital stay (OR: 1.20, 95 % CI 1.09−1.33, p < 0.001), and death (OR: 2.13, 95 % CI 1.1−4.13, p = 0.026. PMI value was a predictor of prolonged hospital stay (OR: 0.83, 95 % CI 0.72−0.96, p = 0.038) and death (OR: 0.53, 95 % CI 0.29−0.96, p = 0.036). Incrementally increasing PMA value was a predictor of prolonged hospital stay (OR: 0.93, 95 % CI 0.89−0.98, p = 0.01) and intubation (OR: 0.98, 95 % CI 0.96−1, p = 0.036).
Conclusion
PSS, PMA, and PMI values have prognostic value in adult COVID-19 patients and can be easily assessed on chest CT images.
Keywords: Computed tomography, COVID-19, Pneumonia, Sarcopenia, Prognosis
1. Introduction
In December 2019, a novel type of coronavirus (SARS-CoV-2) had been identified, and the World Health Organization named the infection of SARS-CoV-2 as "coronavirus disease 2019 (COVID-19)" [1]. By June 3, 2020, there were more than 6.2 million approved cases with COVID-19 and more than 370,000 COVID-19 related deaths worldwide [2]. Although the detection of viral RNA in nasopharyngeal swabs or tracheal aspirates by reverse transcription-polymerase chain reaction (RT-PCR) is the gold standard for diagnosis, chest computed tomography (CT) can provide valuable information about the diagnosis, disease severity and patients prognosis [[3], [4], [5]]. Although some of the COVID-19 patients are asymptomatic or mildly symptomatic, the mortality rate has been shown to be high in elderly patients and patients with comorbidity such as diabetes [6].
The decrease of skeletal muscle function or strength and muscle mass by aging has been defined as sarcopenia, which is an essential factor in the development of frailty [7]. Many studies have shown that the area of the pectoralis, psoas, and paravertebral muscles on cross-sectional CT images is associated with lean muscle mass, handgrip strength, sarcopenia, and health [[7], [8], [9], [10]]. Although low pectoralis muscle area and index have been shown as critical prognostic factors in many benign and malignant disorders [[11], [12], [13], [14], [15]], its prognostic effect in patients with COVID-19 is unknown. Herein, our aim was to evaluate the effect of pneumonia severity on chest CT, gender-specific pectoral muscle area (PMA) values, and patient's height-adjusted PMA values (PMI, pectoralis muscle index) on chest CT in the prognosis and outcomes of adult COVID-19 patients.
2. Materials and methods
This retrospective study was approved by Pamukkale University Clinical Research Ethics Committee and written informed consent was waived.
2.1. Study population
We investigated consecutive adult patients diagnosed with COVID-19 by RT-PCR test between 20 March 2020 and 30 April 2020. Among these patients, those who underwent unenhanced chest CT examination were included in the study. Patients younger than 18 years old, major motion artifacts on CT, and patients whose pectoral muscles were not included in the field of view on chest CT images were excluded from the study.
The chest CT indications were determined according to the Republic of Turkey Ministry of Health’s COVID-19 Patient Management Algorithm [16]. The chest CT indications were one of the following findings is present at the time of initial examination in adult patients who have suspicious clinical findings of COVID-19 pneumonia: respiratory distress, tachypnea (> 22 / min), loss of consciousness with tachycardia (> 125 beats/min), or low oxygen saturation level in room air (SpO2 <93 %). Moreover, in the absence of these clinical findings, patients with > 50 years of age and comorbidity or patients with worsening clinical conditions were an indication for CT [16].
2.2. Chest computed tomography image acquisition
Chest CT images were obtained using a multi-detector CT scanner (Brilliance 16, Philips Medical Systems) at deep inspiration in the supine position. The CT scanner was dedicated only to patients with suspicion of COVID-19. The CT room and CT scanner were sanitized using standard cleaning procedures and approved disinfectants after each procedure. The chest CT parameters were 16 × 0.75 mm slice collimation, 0.75 s rotation time, 3 mm slice thickness and 1 mm slice reconstructions, 250–300 mm field of view, 90−120 kV tube voltage, 50–90 mA s effective tube current-time product, and 512 × 512 matrix.
2.3. Visual CT assessment
First, the suitability of CT images was evaluated by a single board-certified chest radiologist (F.U.) with five years of experience in thoracic imaging. Patients whose CT images were not suitable for the study were excluded. Then, all chest CT images were independently evaluated by two observers with five (F.U.) and two years (M.D.) of experience in thoracic imaging, who were unaware of the patient’s laboratory and clinical findings. In the CT image evaluation, lung window settings (with a window center of -500 HU and a window width of 1500 HU) were used, and visual (semiquantitative) pneumonia severity score (PSS) was evaluated in each patient. The PSS was assessed by a simple CT scoring method, which was described by Chung et al. [17] and verified by Li et al. [18]. The involvement severity was evaluated separately for each lobe, and the scores found for each lobe were summed for obtaining the PSS. The percentage of each lobe involvement was calculated as follows: no pneumonia (0%) = 0 points, minimal involvement (1–25 % volume of the lung lobe) = 1 point, mild involvement (26–50 %) = 2 points, moderate involvement (%51–75) = 3 points, severe involvement (76–100 %) = 4 points [17,18]. In the presence of disagreement between the two observers for the PSS score, these CT images were reevaluated by a third observer with 22 years of experience in cardiothoracic imaging, and an agreement was reached.
2.4. CT image analysis
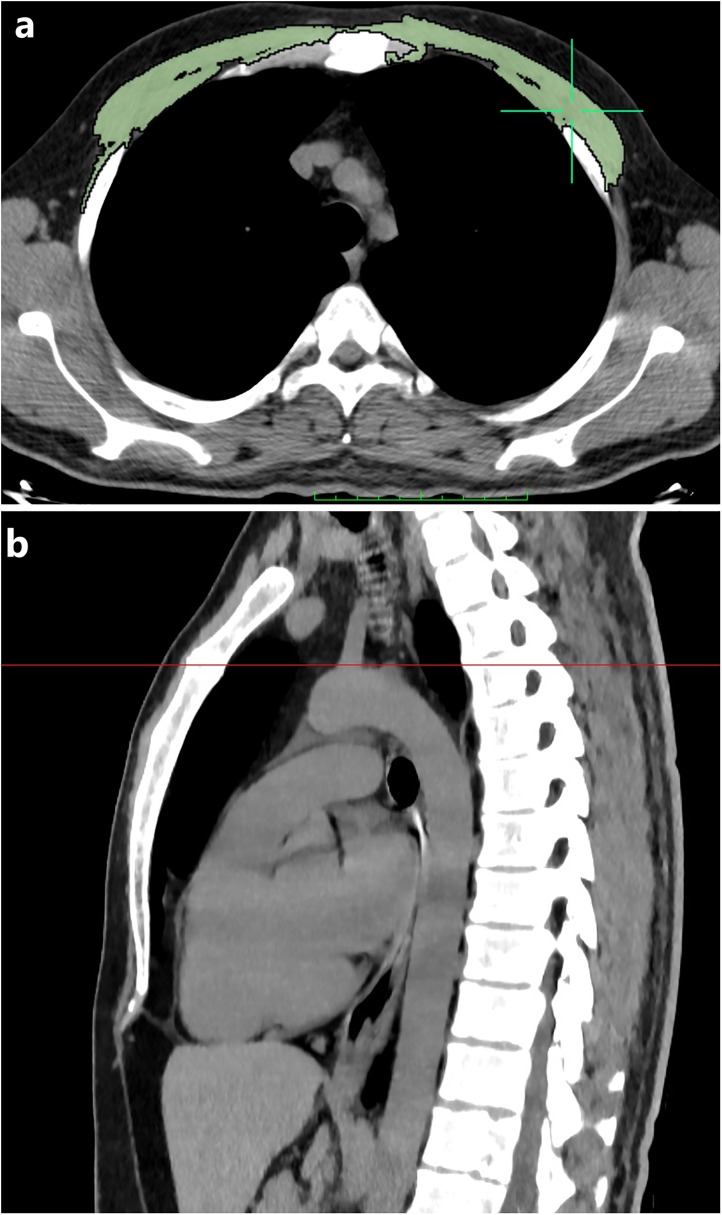
All CT images were anonymized prior to quantitative evaluation, and all patients were randomly numbered. On a single axial chest CT image just above the aortic arch was selected in each patient, and Pectoralis muscle area (PMA) was measured by a trained radiologist (F.U.) using a free DICOM viewer (Horos software Version 3.3.3; Available at https://horosproject.org/). Both of the pectoralis major and minor muscles were manually shaded using a predefined attenuation range of –50 and 90 Hounsfield unit (HU), and muscle areas were automatically measured between those CT attenuation values, on an axial CT slice just above the aortic arch [19]. PMA value was obtained by summing bilateral pectoralis major and minor muscle areas (Fig. 1 ). To investigate the interobserver agreement, PMA measurements were repeated by a second observer (E.S.) from 60 consecutive patients among the whole population, using the same method and blindly to the first measurement results. In the event of insufficient skeletal muscle segmentation, the user replaced the skeletal muscle contours with a manual tool. The axial CT image just above the aortic arch has been selected because it can be easily identified, reproduced in a large population of cases, and has been previously described as successful for estimating skeletal muscle mass [19]. Besides, PMA values were normalized by the height of the patient, and the gender specific pectoralis muscle index (cm2/m2, PMI) was calculated by the PMA (cm2) value divided by the square of the patient height (m2).
Fig. 1.
The pectoralis muscle area (PMA) measurement on chest computed tomography (CT) image in a 41-year-old male patient with COVID-19. Axial chest CT image just above the arcus aorta level used for PMA measurement. (a) PMA measurement was performed on axial CT image using a predefined attenuation values of -50 and 90 Hounsfield units. Bilateral pectoralis muscles are colored green (pectoralis major and minor muscles). (b) Sagittal reformatted chest CT image shows the level of just above the aortic arch.
2.5. Clinical examination
The presence of comorbidities such as diabetes, congestive heart failure, malignancy, hypertension, chronic renal failure, hepatic cirrhosis, and chronic lung disease (asthma and chronic obstructive pulmonary disease) was noted. Besides, smoking status and amount of tobacco use (packs/year) were recorded in all patients. Patients were clinically divided into four groups [20]: (a) Mild disease: mild or minimal clinical symptoms without pneumonia on chest CT; (b) common disease: fever, respiratory symptoms without respiratory distress, no need for supplemental oxygen and pneumonia on chest CT; (c) severe disease: fever or suspected respiratory infection and severe respiratory distress and/or increased respiratory rate ≥ 30 breaths/min and/or decreased oxygen saturation (SpO2) on room air with ≤ 93 % and/or PaO2/FiO2 ≤ 300MMHG; (d) critical disease: respiratory failure requiring mechanical ventilation, septic shock and other organ failure requiring intensive care unit (ICU) monitoring and treatment.
All patients were followed through 31 May 2020, and the days between the symptom onset and chest CT examination, the presence of intubation, length of hospital stay, and death was examined. Length of hospital stay was categorized as prolonged if more than ten days. The outcomes were assessed longitudinally over the entire study period. The most severe outcome was assigned in patients with multiple hospital admission due to COVID-19. For example, patients who were initially admitted to the hospital and followed at home and then readmitted requiring a hospital stay and intubation were assigned to the intubated group.
2.6. Statistical analysis
While categorical data were represented as a proportion (%), continuous data represented as mean with standard deviation (SD) or median with interquartile range (IQR). To evaluate the data normality Shapiro-Wilk W test was used. Categorical and continuous data were compared using a chi-squared or Fisher's exact test and Student t-test or the Mann-Whitney U test, respectively. The correlation between the continuous variables was calculated using the Pearson correlation coefficient (r). Interobserver agreement was evaluated by the intraclass correlation coefficient (ICC) score, and an ICC score < 0.4 accepted as poor agreement, 0.4–0.75 as moderate, 0.75−0.9 as good agreement, and > 0.9 as excellent agreement.
All patients were divided into gender-specific pectoralis muscle index (PMI) tertiles. Low PMI was categorically defined as the smallest tertile of this height square-adjusted and gender-stratified pectoralis muscle area (PMA) values. We used the PMI tertiles to estimate the muscle mass of patients regardless of the gender and physical structure.
The outcomes of interest for this study the presence of intubation, length of hospital stay, and death. To estimate the relative effect of variables by calculating unadjusted odds ratios (ORs) for categorical outcomes, Logistic regression analysis was used. In the univariate analysis, statistically significant variables (p < 0.05) were used for multivariate modeling. Statistically, a p-value of < 0.05 was considered significant. The statistics were performed using SPSS (version 24.0, IBM, Armonk, NY, USA) and MedCalc (MedCalc Software Ltd, Ostend, Belgium) statistical software.
3. Results
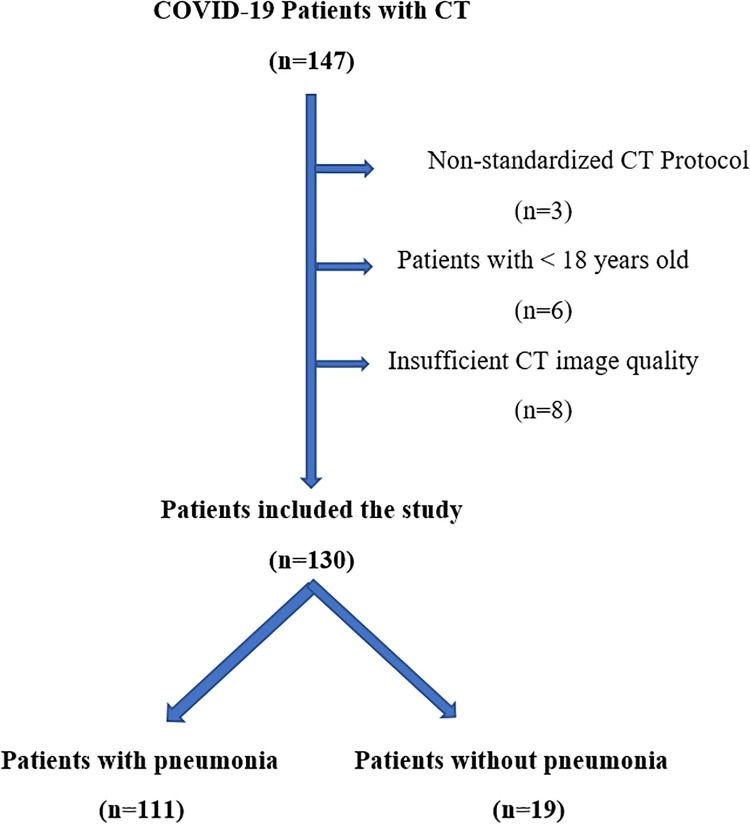
One hundred and thirty patients with COVID-19 were included in the study according to the exclusion and inclusion criteria (Fig. 2 ). The mean follow-up time of all patients was 40.6 + 6.5 days. There were 76 males (58.5 %) and 54 females (41.5 %) with a median age of 48 years (IQR; 36–64). Fifty-six patients (43.08 %) had at least one comorbidity, and the most common comorbidities were hypertension in 25 patients (19.23 %), diabetes mellitus in 23 patients (17.69 %), and asthma in 17 patients (13.08 %). Fifty-four patients (41.54 %) had a high fever (forehead temperature of > 37.3 °C) at the time of admission. The median body temperature at the time of admission was 36.8 degrees Celsius (°C) (IQR, 36.4–37.8 °C). Thirty-six (27.69 %) patients were smokers (26 patients current, ten patients’ ex-smokers), and the median smoking pack-years were 20 (range; 1–80). The median number of days from symptom onset to the CT examination was four days [IQR; 2–7 days]. (Table 1 ).
Fig. 2.
Patient selection and inclusion flow diagram of patients with novel coronavirus 19 disease (COVID-19).
Table 1.
The descriptive characteristics of the study population.
| Characteristics | Total Population (n = 130) | Females (n = 54) | Males (n = 76) | p-value |
|---|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | ||
| Age (years) | 48 (18–86) | 47 (18–84) | 48 (20–86) | 0.765 |
| PSS Observer-1 | 4 (0–17) | 4 (0–17) | 4 (0–14) | 0.061 |
| PSS Observer-2 | 4 (0–16) | 4 (0–15) | 4 (0–16) | 0.056 |
| PSS Consensus | 4 (0–17) | 3 (0–14) | 5 (0–17) | 0.058 |
| Weight (kg) | 72 (39−99) | 70 (39−92) | 78 (56−99) | < 0.001 |
| Height (cm) | 164 (148−191) | 161 (148−179) | 170 (149−191) | < 0.001 |
| Body mass index (BMI) | 26.9 (17.1−36.5) | 26.1 (17.1−32.6) | 27.3 (21−36.5) | 0.31 |
| PMA (cm2) | 33.7 (17.2−63) | 27.1 (17.2−39.3) | 41.6 (21.1−63) | < 0.001 |
| PMI (cm2/m2) | 12.4 (5.3−21.6) | 10.2 (5.3−15.7) | 14.1 (6.9−21.6) | < 0.001 |
| Onset to admission time | 4 (1−12) | 3 (1−11) | 5 (1−12) | 0.016 |
| Forehead temperature (oC) | 36.8 (36.2−39.7) | 36.7 (36.2−39.6) | 37.3 (36.5−39.7) | 0.042 |
PSS; pneumonia severity score, °C; Celsius degree.
The median PMA and PMI values in males and females were 42.1 cm2 (IQR, 31.3–47.2 cm2), 14 cm2/m2 (IQR, 14.5–16 cm2/m2), and 27.1 cm2 (IQR, 23.5–32.2 cm2), 10.2 cm2/m2 (8.7–12 cm2/m2) respectively (Table 1). There was an excellent agreement between observers for pectoralis muscle area (PMA) measurements with an ICC of 0.919 (95 % CI, 0.868−0.951). The tertile cut-off values of PMI in males were < 12.73 cm2/m2 for first, 12.73-15.13 cm2/m2 for second, and > 15.13 cm2/m2 for third tertile, respectively. The tertile cut-off values of PMI in females was < 9 cm2/m2, 9-10.9 cm2/m2, > 10.9 cm2/m2, respectively. A PMI value of in the first tertile in both genders accepted as low PMI (Table 2 ).
Table 2.
Comparison of the frequencies of COVID-19 patients according to pneumonia severity score and pectoralis muscle index values.
| Variables | All patients (n = 130) | Low PSS (< 4) n = 74 | High PSS (> 4) n = 56 | P-value | Low PMI n = 44 | Normal PMI n = 86 | P-value | |
|---|---|---|---|---|---|---|---|---|
| 0.516 | ||||||||
| Male | 76 (58.46 %) | 38 (51.35 %) | 38 (67.86 %) | 25 (56.82 %) | 51 (59.3 %) | |||
| Female | 54 (41.54 %) | 36 (48.65 %) | 18 (32.14 %) | 19 (43.18 %) | 35 (40.7 %) | |||
| Age median (years) [IQR] | 0.001 | 0.0001 | ||||||
| Male | 48 [36, 65] | 44 [34, 70] | 48 [38, 60] | 69 [57, 77] | 44 [34, 50] | |||
| Female | 48 [36, 64] | 42 [27, 52] | 66 [52, 76] | 50 [41, 66] | 47 [33, 61] | |||
| Onset to admission time (days) [IQR] | Median | 4 [2,7] | 3 [2,7] | 5 [3,7] | 0.017 | 4 [2,5] | 5 [3,7] | 0.081 |
| Smoking History (%) | Smoking History (%) | 0.770 | 0.530 | |||||
| Never | 94 (72.31 %) | 56 (75.68 %) | 38 (67.86 %) | 30 (68.18 %) | 64 (74.442 %) | |||
| Former | 10 (7.69 %) | 7 (9.46 %) | 3 (5.36 %) | 5 (11.36 %) | 5 (5.81 %) | |||
| Current | 26 (20 %) | 11 (14.86 %) | 15 (26.79 %) | 9 (20.45 %) | 17 (19.7 %) | |||
| Comorbidity | 0.037 | 0.092 | ||||||
| Hypertension | 25 (19.23 %) | 5 (6.76 %) | 20 (35.71 %) | 12 (27.27 %) | 13 (15.12 %) | |||
| Diabetes | 23 (17.69 %) | 9 (12.16 %) | 14 (25 %) | 7 (15.91) | 16 (18.60 %) | |||
| Asthma / COPD | 17 (13.08 %) | 10 (13.51 %) | 7 (12.5 %) | 5 (11.36 %) | 12 (13.95 %) | |||
| Immunosuppression | 12 (9.23 %) | 7 (9.46 %) | 5 (8.93 %) | 6 (13.64 %) | 6 (6.98 %) | |||
| Malignancy | 10 (7.69 %) | 8 (10.81 %) | 2 (3.57 %) | 5 (11.36 %) | 5 (5.81 %) | |||
| Hearth failure | 9 (6.92 %) | 5 (6.76 %) | 4 (7.14 %) | 4 (9.09 %) | 5 (5.81 %) | |||
| CVD | 6 (4.62 %) | 4 (5.41 %) | 2 (3.57 %) | 3 (6.82 %) | 3 (3.49 %) | |||
| Chronic renal failure | 1 (0.77 %) | 0 | 1 (1.79 %) | 1 (2.27 %) | 0 | |||
| Febrile at initial presentation (%) | 54 (41.54 %) | 26 (35.14 %) | 28 (50 %) | 0.062 | 18 (40.91) | 36 (41.86 %) | 0.532 | |
| Clinical severity score (%) | Clinical severity score (%) | 0.0001 | 0.031 | |||||
| Mild | 19 (14.62 %) | 19 (25.68 %) | 0 | 6 (13.64 %) | 9 (10.47 %) | |||
| Common | 86 (66.15 %) | 51 (68.92 %) | 35 (62.5 %) | 25 (56.82 %) | 64 (74.42 %) | |||
| Severe | 12 (9.23 %) | 4 (5.41 %) | 8 (14.29 %) | 4 (9.09 %) | 9 (10.47 %) | |||
| Critical | 13 (10 %) | 0 | 13 (23.21 %) | 9 (20.45 %) | 4 (4.65 %) | |||
PSS; pneumonia severity score, PMI; pectoralis muscle index, IQR; interquartile range.
Pneumonia was not present on CT in 19 patients (14.6 %). There was an excellent agreement between observers for pneumonia severity score (PSS) with an ICC of 0.971 (95 % CI, 0.957−0.981). The median PSS was 4 (IQR, 1–7) and patients with a PSS < 4 was accepted as low PSS (Table 2). Patients with a comorbidity had a higher mean PSS (mean + SD PSS values were 6.3 + 4.5 vs 3.9 + 3.8; p = 0.001). Besides, patients with longer time for symptom onset to the CT examination (more than 5 days from symptom onset) had a higher PSS (4.8 + 4.3 vs 0.34 + 0.48, p < 0.001). In the study population, patients with the critical, severe, common and mild disease frequency were 13 (10 %), 12 (9.2 %), 86 (66.2 %), and 19 (14.6 %), respectively. The PSS showed a significant correlation with clinical classification (p < 0.001, r = 0.609).
The median value of hospital stay length was seven days (IQR, 6–10 days), and 24 patients (18.46 %) had prolonged hospital stay (> 10 days). At the time of writing, three patients (2.31 %) were still stay in hospital. Eight patients (6.15 %) died during follow-up (median age; 69, IQR; 64–82). Patients who died were significantly older (68.6 + 13.2 years’ vs 48.3 + 16.6 years, p = 0.001) and found to have higher mean PSS (12.9 + 4.9 vs 4.3 + 3.6, p < 0.001) and lower mean PMI (7.9 + 2.1 cm2/m2 vs 12.5 + 3.5 cm2/m2, p < 0.001) values.
After adjusting the confounders (age, sex, comorbidities and symptom onset to admission time), we found that PSS, symptom onset to admission time (days), late admission (> 5 days), febrile at initial presentation, PMI, and PMA values were found to be significantly associated with several poor outcomes (Table 3, Table 4 ). Incrementally increasing pneumonia severity score (PSS) was found to be an independent predictor of intubation (adjusted Odds Ratio [OR]: 1.73, 95 % CI 1.31−2.28, p < 0.001), prolonged hospital stay (OR: 1.20, 95 % CI 1.09−1.33, p < 0.001), and death (OR: 2.13, 95 % CI 1.1−4.13, p = 0.026). Incrementally increasing PMI value was a predictor of prolonged hospital stay (OR: 0.83, 95 % CI 0.72−0.96, p = 0.038) and death (OR: 0.53, 95 % CI 0.29−0.96, p = 0.036). Incrementally increasing PMA value was a predictor of prolonged hospital stay (OR: 0.93, 95 % CI 0.89−0.98, p = 0.01) and intubation (OR: 0.98, 95 % CI 0.96−1, p = 0.036). Moreover, the presence of low PMI was found to be a predictor of intubation (OR: 3.9, 95 % CI 0.8−18.8, p = 0.025) and death (OR: 11.6, 95 % CI 1.5−89.9, p = 0.019) (Table 3, Table 4).
Table 3.
Univariate and multivariate analysis of variables for intubation and prolonged hospital stay (> 10 days).
| Intubation (n = 15) |
Prolonged Hospital Stay (n = 24) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis |
Multivariate Analysis |
Univariate Analysis |
Multivariate Analysis |
|||||
| Variable | Unadjusted OR | p-value | Adjusted OR | p-value | Unadjusted OR | p-value | Adjusted OR | p-value |
| Age median (years) | 1.07 (1.03–1.12) | < 0.001 | 1.08 (0.96−1.23) | 0.187 | 1.02 (0.99–1.05) | 0.089 | – | |
| Age > 65 years | 5.12 (1.67−15.67) | 0.005 | 76.2 (3.5−1653) | 0.006 | 1.62 (0.65−4.05) | 0.305 | 1.81 (0.6−5.42) | 0.29 |
| Male gender | 2.19 (0.66−7.29) | 0.201 | – | 2.12 (0.9−5.1) | 0.09 | – | ||
| Onset to admission time (days) | 1.26 (1.05−1.51) | 0.012 | 1.52 (1.16−2) | 0.002 | 1.11 (0.96−1.27) | 0.009 | 1.02 (0.87−1.19) | 0.164 |
| Late admission (> 5 days) | 4.76 (1.52−14.98) | 0.008 | 14.36 (2.98−69.24) | < 0.001 | 1.1 (0.47−2.56) | 0.825 | 0.62 (0.22−1.68) | 0.738 |
| Smoking History | ||||||||
| Never | reference | reference | reference | reference | ||||
| Former or current | 1.36 (0.39−4.67) | 0.909 | 0.53 (0.06−4.6) | 0.191 | 1.6 (0.68−3.81) | 0.283 | 1.35 (0.54−3.4) | 0.835 |
| BMI median (kg/m2) | 1.01 (0.96−1.06) | 0.142 | – | 1.04 (1−1.08) | 0.342 | – | ||
| Comorbidity | 4.28 (1.28−14.26) | 0.018 | 4.68 (0.37−59.9) | 0.236 | 1.33 (0.59−2.98) | 0.495 | – | |
| Hypertension | 2.35 (0.72−7.63) | 0.155 | – | 1.3 (0.48−3.47) | 0.606 | – | ||
| Diabetes | 3.8 (1.2−12.1) | 0.023 | 0.62 (0.2−1.97) | 0.414 | – | |||
| COPD/Asthma | 9.14 (2.43−34.4) | 0.994 | – | 1.65 (0.72−3.75) | 0.219 | – | ||
| Immunosuppression | 0.67 (0.8−5.6) | 0.710 | – | 0.61 (0.13−2.9) | 0.534 | – | ||
| Malignancy | 1.2 (0.14−10.2) | 0.867 | – | 3 (0.37−24.9) | 0.302 | – | ||
| Hearth failure | 6.52 (2.1−20.3) | 0.001 | 1.44 (0.1−22.1) | 0.792 | 4.13 (1.6−10.4) | 0.003 | 4.62 (0.96−22.2) | 0.056 |
| CVD | 2.39 (0.64−8.94) | 0.995 | – | 1.79 (0.73−4.4) | 0.588 | – | ||
| Febrile at initial presentation | 0.9 (0.3−2.7) | 0.855 | 0.22 (0.04−1.27) | 0.66 | 3.76 (1.58−8.9) | 0.003 | 3.46 (1.34−8.9) | 0.009 |
| Low PSS (PSS < 4) | 0.09 (0.02−0.43) | 0.002 | 0.11 (0.02−0.56) | 0.082 | 0.17 (0.07−0.43) | < 0.001 | 0.21 (0.08−0.53) | 0.001 |
| PSS | 1.6 (1.30−1.97) | < 0.001 | 1.73 (1.31−2.28) | < 0.001 | 1.21 (1.1−1.34) | < 0.001 | 1.2 (1.09−1.33) | 0.007 |
| Low PMI | 4.76 (1.51−14.99) | 0.008 | 3.9 (0.8−18.8) | 0.025 | 1.1 (0.47−2.56) | 0.035 | 1.84 (0.35−9.76) | 0.057 |
| PMI | 0.95 (0.81−1.11) | 0.544 | 0.86 (0.71−1.05) | 0.33 | 0.91 (0.81−1.03) | 0.038 | 0.83 (0.72−0.96) | 0.038 |
| PMA | 1 (0.99−1.01) | 0.699 | 0.98 (0.96−1) | 0.04 | 0.99 (0.97−1) | 0.782 | 0.93 (0.89−0.98) | 0.01 |
ICU; intensive care unit, OR; odds ratio, COPD; chronic obstructive pulmonary disease, CVD; cardiovascular disease, PSS; pneumonia severity score, PMI; pectoralis muscle index, PMA; pectoralis muscle area.
Table 4.
Univariate and multivariate analysis of variables for death.
|
Exitus (n = 8) |
||||
|---|---|---|---|---|
| Univariate Analysis |
Multivariate Analysis |
|||
| Variable | Unadjusted OR | p-value | Adjusted OR for PSS | p-value |
| Age median (years) | 1.12 (1.04−1.2) | 0.003 | 1.08 (0.99−1.18) | 0.088 |
| Age > 65 years | 6.8 (1.52−30.5) | 0.012 | 34.8 (0.34−356.1) | 0.133 |
| Male gender | 1.24 (0.28−5.41) | 0.777 | – | |
| Onset to admission time (days) | 1.11 (0.88−1.41) | 0.391 | 0.98 (0.42−2.28) | 0.084 |
| Late admission (> 5 days) | 3.54 (0.81−15.6) | 0.093 | 14.7 (1.61−135.4) | 0.017 |
| Smoking History | ||||
| Never | reference | reference | ||
| Former or current | 2.85 (0.34−2.4) | 0.336 | 3.41 (0.8−7.41) | 0.122 |
| BMI median (kg/m2) | 1.06 (0.98−1.1) | 0.052 | – | |
| Comorbidity | – | – | ||
| Hypertension | 2.7 (0.6−12.1) | 0.196 | – | |
| Diabetes | 9.54 (2.09−43.45) | 0.004 | 20.1 (1.58−256) | 0.021 |
| COPD/Asthma | – | – | ||
| Immunosuppression | 1.43 (0.16−12.7) | 0.749 | – | |
| Malignancy | 1.77 (0.19−16.08) | 0.609 | – | |
| Hearth failure | 8.41 (1.86−38.09) | 0.006 | – | |
| CVD | – | – | ||
| Febrile at initial presentation | 0.44 (0.08−2.25) | 0.321 | 0.39 (0.05−3.29) | 0.391 |
| Low PSS (PSS < 4) | 0.1 (0.01−0.8) | 0.031 | 0.13 (0.01−1.277) | 0.08 |
| PSS | 1.59 (1.25−2.02) | < 0.001 | 2.13 (1.1−4.13) | 0.026 |
| Low PMI | 31.1 (5.97−162) | < 0.001 | 11.6 (1.5−89.9) | 0.019 |
| PMI | 0.48 (0.30−0.77) | 0.002 | 0.53 (0.29−0.96) | 0.036 |
| PMA | 0.85 (0.75−0.97) | 0.015 | 0.76 (0.5−1.15) | 0.071 |
ICU; intensive care unit, OR; odds ratio, COPD; chronic obstructive pulmonary disease, CVD; cardiovascular disease, PSS; pneumonia severity score, PMI; pectoralis muscle index, PMA; pectoralis muscle area.
4. Discussion
In the present study, our results revealed that pneumonia severity score (PSS), pectoralis muscle area (PMA), and index (PMI) values on chest CT are significantly associated with several adverse outcomes, such as intubation, prolonged hospital stay, and death. Furthermore, symptoms onset to admission time (days), late admission (> 5 days), diabetes mellitus, and high fever (> 37.3 °C) at initial presentation are independent clinical predictors for several adverse outcomes. Age and heart failure were significantly predicted poor outcomes in univariate analysis. However, in multivariate analysis, when factors such as age, pneumonia severity score, symptoms onset to admission time (days), low PMI, and other comorbidities were included, lost its significance. To our knowledge, this is the first study to assess the role of PSS, PMA, and PMI on chest CT in the outcomes of adult COVID-19 patients rather than on the basis of laboratory and/or clinical findings.
Chung et al. [17] examined 21 adult COVID-19 patients, and they showed that patients with high pneumonia severity score (PSS) had a more advanced clinical disease score. Furthermore, Li and colleagues [18] demonstrated an excellent interobserver agreement with ICC of 0.976 (95 % CI; 0.962–0.985) for the PSS assessment on CT. Similarly, we found an excellent agreement between observers with an ICC value of 0.971 (95 % CI, 0.957−0.981), and PSS on chest CT images was significantly correlated with clinical disease score (p < 0.001, r = 0.609).
Du and colleagues [21] investigated 179 patients with COVID-19, and they reported that older age (> 65 years) and underlying cerebrovascular or cardiovascular diseases are strong and independent predictors for mortality. Moreover, Petrilli et al. [22] investigated 5279 COVID-19 patients, and they reported that older age (> 75 years), heart failure, male gender, and malignancy were independent factors for mortality. In the present study, older age (> 65 years) is predicted death in univariate analysis, but when the confounders were included, older age (> 65 years) lost significance in multivariate analysis. However, diabetes mellitus, PSS, and PMI were significant and independent predictors of mortality in the present study.
Guan et al. [23] investigated 1099 COVID-19 patients, and they reported the prevalence of intubation as 2.3 % (n = 25) and mortality as 1.4 % (n = 15). Petrilli and colleagues [22] reported the prevalence of mortality as 7.41 % (n = 391/5279) in COVID-19 patients who admitted to the hospital. The differences between these studies may be due to the different disease prevalence, the differences between hospital and ICU capacities, differences in patients' comorbidity, age, and gender differences between patient populations. The mortality rate of COVID-19 was 6.15 % (n = 8/130) in the present study. However, in the present study, unlike these previous studies, only patients who underwent chest CT were included and analyzed. Therefore, patients with mild disease and no chest CT imaging indications were not investigated in this study. Petrilli et al. [22] reported that chronic obstructive pulmonary disease and asthma were not risk factors for severe disease or mortality in COVID-19 patients. Similarly, our results showed that chronic obstructive lung disease and asthma are not risk factors for adverse outcomes or mortality. Although some previous studies estimate that high smoking rates explain some of the morbidity in those patients [24,25], Petrilli and colleagues [22] have shown that tobacco use is not associated with a hospital stay or an increased risk of critical disease. While the number of patients in the present study was small, we also did not found any relationship between smoking history and morbidity or mortality.
Pectoralis muscle area (PMA) and sex-specified PMA have been shown to be strong predictors of sarcopenia [11]. Moreover, it has been shown that reduced PMA and PMI are associated with poor prognosis of chronic obstructive pulmonary disease [12], non-small cell lung cancer [13], and idiopathic pulmonary fibrosis [14]. In addition, the reduced PMA and PMI are associated with mortality in patients with ventricular assist device implantation [15]. However, the effects of low PMA and PMI values on chest CT in COVID-19 patients were previously unknown, which are predictors of sarcopenia. The present study shows a significant association of PSS, PMA, and PMI with intubation, prolonged hospital stay, and mortality among COVID-19 patients who underwent chest CT at the admission.
In a large meta-analysis including 33 studies and 16,003 patients by Kumar et al. [26], the presence of diabetes in COVID-19 patients has been shown to significantly increase disease severity and mortality (p < 0.01 for both). Similarly, we found that the presence of diabetes in COVID-19 patients is an independent risk factor for mortality (p = 0.021). However, we found the confidence interval of the odds ratio in this relationship quite wide. We suggest that this is because our study population is small, and the number of patients with diabetes is low. Few studies have investigated the prognostic impact of the time between the onset of symptoms and hospital admission in COVID-19 patients. Chen and colleagues [27] showed that patients with longer symptoms were significantly more likely to be transferred to intensive care units (p < 0.001). Sung et al. (28) showed that COVID-19 patients with longer time from disease onset to hospital admission had more severe disease than those with shorter ones. Similarly, we found that patients with high PSS admitted to the hospital significantly later (p = 0.017) and late admission was independently associated with intubation and mortality. We suggest that this may be because patients postpone their admission to hospitals due to the pandemic and try to stay home until serious symptoms develop.
This study had several limitations. First, the study has been retrospectively performed in a single tertiary university hospital, and the sample size was relatively small. However, to our knowledge, this is the first study that is investigating the CT-derived prognostic factors in adult COVID-19 patients. Second, the number of patients between clinical disease severity groups were not homogenous; patients with common disease severity was intense. However, these groups are not homogenous in the normal course of the COVID-19, and most of the patients have been shown to have a mild or asymptomatic disease [17,18,22]. Third, computed tomography of the chest was not performed to all COVID-19 patients. Only patients with CT indication, according to the Ministry of the Health guideline, underwent chest CT, and those were included in our study [16]. Fourth, all of the COVID-19 patients in our tertiary medical center who underwent chest CT have not included in the study due to the exclusion criteria (e.g., major motion artifacts on chest CT). Fifth, the presence of sarcopenia was not evaluated by other quantitative (e.g. grip strength, Dual-energy X-ray absorptiometry) and qualitative tests (e.g. Chair stand test, 400-m walk test). However, it is very difficult to apply these tests during a pandemic. Lastly, the study population was treated within a single medical center, and all patients were from a single geographic region. Therefore, factors associated with adverse outcomes might differ in different populations and regions.
In conclusion, pneumonia severity score (PSS), pectoralis muscle area (PMA), and pectoralis muscle index (PMI) are significantly associated with several adverse outcomes in adult COVID-19 patients. These parameters can be easily assessed on chest CT images of COVID-19 patients, and we suggest that these parameters can be useful in routine clinical practice since those have prognostic value and do not require additional examination. Furthermore, symptoms onset to admission time (days), late admission (> 5 days), age > 65 years, diabetes mellitus, and high fever (> 37.3 °C) at initial presentation are independent predictors for several adverse outcomes.
CRediT authorship contribution statement
Furkan Ufuk: Conceptualization, Data curation, Formal analysis, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Mahmut Demirci: Data curation, Formal analysis, Investigation, Visualization, Writing - original draft. Ergin Sagtas: Data curation, Formal analysis, Investigation, Writing - original draft. Ismail Hakkı Akbudak: Methodology, Resources, Writing - review & editing. Erhan Ugurlu: Resources, Writing - review & editing. Tugba Sari: Formal analysis, Methodology, Resources.
Declaration of Competing Interest
All of the authors declare that they have all participated in the design, execution, and analysis of the paper, and that they have approved the final version.
Funding
The authors received no specific funding for this work.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva: 2020. Coronavirus Disease 2019 (COVID-19) Situation Report–87.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200603-covid-19-sitrep-135.pdf?sfvrsn=39972feb_2 Available at:Accessed June 4, 2020. [Google Scholar]
- 3.Xu Z., Shi L., Wang Y., Zhang J., Huang L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin Gd, Ryerson Cj, Haramati Lb, Sverzellati N., Kanne Jp, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Radiology. 2020;296(1):172–180. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ufuk F., Savaş R. Chest CT features of the novel coronavirus disease (COVID-19) Turk. J. Med. Sci. 2020;50(4):664–678. doi: 10.3906/sag-2004-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura J.M., Ansari A.Z., D’Souza D.M., Moffatt-Bruce S.D., Merritt R.E., Kneuertz P.J. Computed tomography-assessed skeletal muscle mass as a predictor of outcomes in lung Cancer surgery. Ann. Thorac. Surg. 2019;108:1555–1564. doi: 10.1016/j.athoracsur.2019.04.090. [DOI] [PubMed] [Google Scholar]
- 9.Kim G., Kang S.H., Kim M.Y., Baik S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. 2017;12(10):e0186990. doi: 10.1371/journal.pone.0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamidi M., Ho C., Zeeshan M., O’Keeffe T., Hamza A., et al. Can Sarcopenia Quantified by Computed Tomography Scan Predict Adverse Outcomes in Emergency General Surgery? J. Surg. Res. 2019;235:141–147. doi: 10.1016/j.jss.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.S., Kim E.Y., Kang S.M., Ahn H.K., Kim H.S. Single cross-sectional area of pectoralis muscle by computed tomography - correlation with bioelectrical impedance based skeletal muscle mass in healthy subjects. Clin. Physiol. Funct. Imaging. 2017;37:507–511. doi: 10.1111/cpf.12333. [DOI] [PubMed] [Google Scholar]
- 12.Bak S.H., Kwon S.O., Han S.S., Kim W.J. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: a case control study. Respir. Res. 2019;20:226. doi: 10.1186/s12931-019-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cury S.S., de Moraes D., Freire P.P., de Oliveira G., Marques D.V.P., et al. Tumor transcriptome reveals high expression of IL-8 in non-small cell lung Cancer patients with low pectoralis muscle area and reduced survival. Cancers (Basel). 2019;11:1251. doi: 10.3390/cancers11091251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon S.W., Choi J.S., Lee S.H., Jung K.S., Jung J.Y., et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir. Res. 2019;20 doi: 10.1186/s12931-019-1001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teigen L.M., John R., Kuchnia A.J., Nagel E.M., Earthman C.P., et al. Preoperative pectoralis muscle quantity and attenuation by computed tomography are novel and powerful predictors of mortality after left ventricular assist device implantation. Circ. Heart Fail. 2017;10:e004069. doi: 10.1161/CIRCHEARTFAILURE.117.004069. [DOI] [PubMed] [Google Scholar]
- 16.2020. The Republic of Turkey Ministry of Health’s COVID-19 Patient Management Algorithm.https://hsgm.saglik.gov.tr/tr/covid-19-i-ngilizce-dokumanlar/covid-19-i-ngilizce-algoritmalar.html Accessed June 4. [Google Scholar]
- 17.Chung M., Bernheim A., Mei X., Zhang N., Huang M., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K., Fang Y., Li W., Pan C., Qin P., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur. Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz A.A., Martinez C.H., Harmouche R., Young T.P., McDonald M.L., et al. Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir. Res. 2018;19:62. doi: 10.1186/s12931-018-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.China National Health Commission . 2020. Diagnosis and Treatment of Pneumonitis Caused by New Coronavirus (trial Version 7)http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml Available at:Accessed May 30. [Google Scholar]
- 21.Du RH Liang L.R., Yang C.Q., Wang W., Cao T.Z., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakladar J., Shende N., Li W.T., Rajasekaran M., Chang E.Y., Ongkeko W.M. Smoking-Mediated Upregulation of the Androgen Pathway Leads to Increased SARS-CoV-2 Susceptibiliy. Int. J. Mol. Sci. 2020;21:E3627. doi: 10.3390/ijms21103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattaruzza M.S., Zagà V., Gallus S., D’Argenio P., Gorini G. Tobacco smoking and COVID-19 pandemic: old and new issues. A summary of the evidence from the scientific literature. Acta Biomed. 2020;91:106–112. doi: 10.23750/abm.v91i2.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A., Arora A., Sharma P., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung H.K., Kim J.Y., Heo J., et al. Clinical Course and Outcomes of 3,060 Patients with Coronavirus Disease 2019 in Korea, January-May 2020. J. Korean Med. Sci. 2020;35:e280. doi: 10.3346/jkms.2020.35.e280. [DOI] [PMC free article] [PubMed] [Google Scholar]