In nature, bacteria live in multicellular and multispecies communities. Microbial species can sense the density and composition of their community through chemical cues using a process called quorum sensing (QS). The marine pathogen Vibrio cholerae is found in communities on the chitinous shells of crustaceans in its aquatic reservoir. V. cholerae interactions with chitin are critical for the survival, evolution, and waterborne transmission of this pathogen. Here, we show that V. cholerae uses QS to regulate the expression of one locus required for V. cholerae-chitin interactions.
KEYWORDS: quorum sensing, cholera, protease
ABSTRACT
The marine facultative pathogen Vibrio cholerae forms complex multicellular communities on the chitinous shells of crustacean zooplankton in its aquatic reservoir. V. cholerae-chitin interactions are critical for the growth, evolution, and waterborne transmission of cholera. This is due, in part, to chitin-induced changes in gene expression in this pathogen. Here, we sought to identify factors that influence chitin-induced expression of one locus, the chitobiose utilization operon (chb), which is required for the uptake and catabolism of the chitin disaccharide. Through a series of genetic screens, we identified that the master regulator of quorum sensing, HapR, is a direct repressor of the chb operon. We also found that the levels of HapR in V. cholerae are regulated by the ClpAP protease. Furthermore, we show that the canonical quorum sensing cascade in V. cholerae regulates chb expression in an HapR-dependent manner. Through this analysis, we found that signaling via the species-specific autoinducer CAI-1, but not the interspecies autoinducer AI-2, influences chb expression. This phenomenon of species-specific regulation may enhance the fitness of this pathogen in its environmental niche.
IMPORTANCE In nature, bacteria live in multicellular and multispecies communities. Microbial species can sense the density and composition of their community through chemical cues using a process called quorum sensing (QS). The marine pathogen Vibrio cholerae is found in communities on the chitinous shells of crustaceans in its aquatic reservoir. V. cholerae interactions with chitin are critical for the survival, evolution, and waterborne transmission of this pathogen. Here, we show that V. cholerae uses QS to regulate the expression of one locus required for V. cholerae-chitin interactions.
INTRODUCTION
The facultative bacterial pathogen Vibrio cholerae, the causative agent of the diarrheal disease cholera, natively resides in the aquatic environment. In this niche, V. cholerae forms multicellular communities on biotic and abiotic chitinous surfaces, like the shells of crustaceans or marine snow (1, 2). Chitin is a polysaccharide made up of β-1,4-linked N-acetylglucosamine (GlcNAc) and serves as a major nutrient source for V. cholerae in the marine environment (1, 3, 4). The ability of V. cholerae to form chitin biofilms is critical for the waterborne transmission of cholera (5, 6). As chitin is the most abundant biopolymer in the ocean, the ability of Vibrio species to break down and utilize this highly insoluble polysaccharide also serves an important role in global nitrogen and carbon recycling (1, 4).
When V. cholerae is associated with a chitinous surface, chitin induces the expression of a subset of genes in V. cholerae. The genes induced by chitin include those required for chitin degradation, uptake, and catabolism (termed the chitin utilization program), as well as the genes required for natural transformation (7, 8). Transcriptional responses resulting from Vibrio-chitin interactions are highly regulated. One major chitin-responsive regulator is the orphan hybrid sensor kinase ChiS (7, 9). ChiS senses chitin indirectly through the periplasmic chitin binding protein (CBP) (9, 10). In the absence of chitin, CBP represses ChiS through interactions with its periplasmic domain (9, 10). In the presence of chitin, the CBP-chitin complex stimulates ChiS activity (9, 10). Thus, in the presence of chitin, ChiS is active and can facilitate expression of the chitin utilization program. Alternatively, ChiS can be genetically activated in the absence of chitin by deleting cbp (10, 11).
In the marine environment, V. cholerae not only senses chitin to modulate gene expression but also the presence of other bacteria through a process termed “quorum sensing” (QS) (12). This is a process by which bacteria indirectly sense other microbes in their community via small diffusible molecules called autoinducers (AIs). AIs are sensed by cognate sensor proteins. V. cholerae has four AI sensors, although the autoinducer molecules that serve as inducing cues are only known for two of them (13). AI sensing allows for cell-density-specific gene expression programs, which regulate “group” or “individual” behaviors (14). V. cholerae senses both chitin and AIs to regulate natural transformation on chitinous surfaces (15). Though a link between chitin utilization and quorum sensing has previously been suggested, it has not been directly studied (16).
To investigate regulation of the chitin utilization program in V. cholerae, most studies employ the chitobiose utilization operon (chb) (10, 11, 17, 18). The chb operon encodes the genes required for uptake and catabolism of the chitin disaccharide chitobiose and is highly induced in the presence of chitin oligosaccharides (7). Several mechanisms of chb regulation have already been identified. ChiS is the master regulator of the chitin utilization program in V. cholerae, and we have recently shown that this protein is a direct transcriptional activator required for induction of the chb locus (9, 10). Previous work has shown that carbon catabolite repression (CCR) can also play a role in regulating chitin responsive phenotypes, including ChiS-dependent induction of chb and natural transformation (18, 19). In addition, our group has previously found that the cell division licensing factor SlmA plays an essential role in activating chb expression (11). Tight regulation via these diverse signaling systems may act to ensure that the chitin utilization program is only expressed under conditions in which it will provide a competitive advantage.
Here, we sought to identify additional regulators of chb. Through a number of genetic screens and complementary molecular methods, we show that quorum sensing is an additional regulatory system that tunes expression of a chitin utilization locus in V. cholerae.
RESULTS
ClpA is identified in an unbiased screen for activators of Pchb.
To identify additional genes required for activation of the chb locus, we conducted a transposon mutant screen. This was carried out in a strain containing a chromosomally integrated Pchb-lacZ transcriptional reporter. As shown previously, induction of Pchb is dependent on the activity of the master regulator ChiS (7, 10, 11). In the absence of chitin, ChiS activity is repressed by CBP. In the presence of chitin, CBP repression of ChiS is relieved, which allows for ChiS-dependent activation of Pchb. In addition to being induced by chitin, ChiS can be activated genetically in the absence of chitin by deleting cbp (10, 11). As chitin oligomers are prohibitively expensive, a Δcbp mutation was used to induce ChiS-dependent Pchb-lacZ expression in our genetic screen exactly as previously described (11). So, the starting genotype for our screen was a strain containing Pchb-lacZ and a Δcbp mutation. This strain formed blue colonies on 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)-containing plates, and we screened for white colonies to identify putative activators that contribute to Pchb induction.
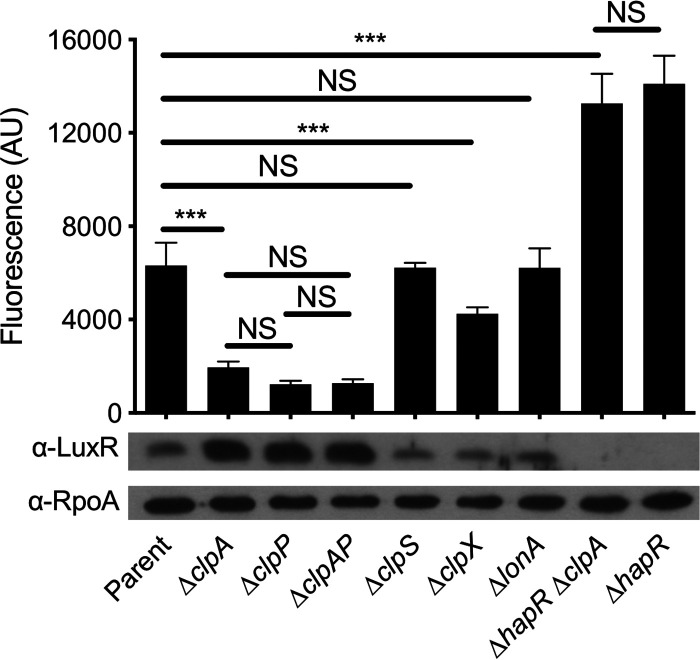
Of approximately 60,000 transposon mutants visually screened for loss of Pchb-lacZ expression, one gene identified was clpA (2 unique transposon insertions). Other hits identified in this screen are listed in Table S1 in the supplemental material. To study the effect of clpA on Pchb activity moving forward, we utilized a previously described chromosomally integrated Pchb-green fluorescent protein (GFP) reporter (10, 11). Using this reporter, we found that a ΔclpA mutation resulted in an ∼3-fold decrease in Pchb expression relative to the parent (Fig. 1). Importantly, complementation of this strain with an ectopic copy of clpA in trans restored Pchb expression to parent levels (see Fig. S1 in the supplemental material). ClpA is a AAA+ unfoldase that recognizes protein substrates, unfolds them, and feeds them into the ClpP protease where they are degraded (20). If ClpA was exhibiting its effect on Pchb expression as a part of the ClpAP machine, we hypothesized that a ΔclpP mutation should phenocopy a ΔclpA mutation. Indeed, ΔclpP and ΔclpP ΔclpA strains phenocopied a ΔclpA mutant for Pchb expression (Fig. 1). These results suggest that loss of the ClpAP protease decreases Pchb expression. As ClpAP degrades proteins, we hypothesized that ClpAP may indirectly promote activation of Pchb by degrading a repressor of the chb locus.
FIG 1.
HapR is a repressor of Pchb that is degraded by the ClpAP protease. Expression of a Pchb-GFP reporter and HapR protein levels were determined in the indicated mutant strains. The parent strain contained a Pchb-GFP reporter and a Δcbp mutation. A representative Western blot is shown below bars to indicate the protein levels for HapR and RpoA (a loading control) in the corresponding strains. An antibody against LuxR, which has 72% identity and 86% similarity to HapR, is cross-reactive with HapR and so was used to detect HapR protein levels. Fluorescence of cultures was determined on a plate reader from at least six independent biological replicates and is shown as the mean ± standard deviation (SD). Statistical comparisons were made by one-way ANOVA with Tukey’s posttest. NS, not significant. ***, P < 0.001.
HapR is a repressor of Pchb that is degraded by ClpAP.
To identify a putative repressor of Pchb that is targeted by ClpAP for degradation, we conducted a counterscreen using the Pchb-lacZ reporter. For the ΔclpA strain counterscreen, the parent strain had both Δcbp and ΔclpA mutations. This mutant is white on X-Gal plates because Pchb-lacZ is poorly expressed; inactivation of the putative repressor in this strain should result in restoration of Pchb-lacZ expression and yield a blue colony phenotype. In the ΔclpA strain counterscreen, we visually screened approximately 30,000 transposon mutants for reactivation of Pchb-lacZ expression and identified hapR (9 unique transposon insertions). Other hits identified in this screen are listed in Table S1. A ΔhapR mutation restored Pchb expression in the ΔclpA mutant background (Fig. 1). In fact, a ΔhapR mutation allowed for higher Pchb expression than that of the parent strain. This suggests that HapR represses Pchb expression when ClpAP is intact and that ClpAP does not degrade the entire pool of HapR in the cell (Fig. 1). Importantly, the level of Pchb expression observed in the ΔhapR ΔclpA mutant phenocopied the ΔhapR strain (Fig. 1). This epistasis between clpA and hapR suggests that they are involved in the same pathway for regulating Pchb expression. In addition, complementation of the ΔhapR strain with an ectopic copy of hapR in trans decreased Pchb expression almost to the level in the parent (see Fig. S1). Further, complementation of the ΔhapR ΔclpA strain with hapR in trans brought Pchb expression down to the level observed in the ΔclpA parent (Fig. S1).
We hypothesized that the reason Pchb expression was decreased in ΔclpAP mutants was due to increased HapR protein levels. Western blotting in these backgrounds revealed that HapR levels were, indeed, increased in strains containing mutations to clpA and/or clpP (Fig. 1). ClpAP-dependent degradation of HapR is not unique to V. cholerae but has previously been observed in Vibrio vulnificus where ClpAP degrades the HapR homolog SmcR (21). In addition to the ClpAP machine, it was shown that the Lon protease also plays a role in SmcR degradation. So, we next sought to investigate the role of other protease machines on induction of Pchb and HapR protein levels. Mutations to other Clp components (the ClpS adaptor protein or the ClpX unfoldase) did not have a marked impact on HapR protein levels. Also, a ΔclpS mutation did not affect Pchb expression levels, while a ΔclpX mutation slightly decreased Pchb expression (Fig. 1). Because HapR expression was not affected by the ΔclpX mutation, the observed decrease in Pchb expression may be attributed to a pleiotropic effect (i.e., an effect of clpX that is independent of HapR-dependent Pchb repression). In contrast to the effect of the Lon protease on SmcR levels in V. vulnificus, we did not observe an impact of the ΔlonA mutation on HapR levels in V. cholerae; the ΔlonA mutation, correspondingly, did not affect Pchb expression (Fig. 1). Together, these results establish that HapR is a repressor of Pchb and that HapR levels are controlled specifically by the ClpAP protease in V. cholerae.
HapR-mediated repression of Pchb occurs on chitinous surfaces.
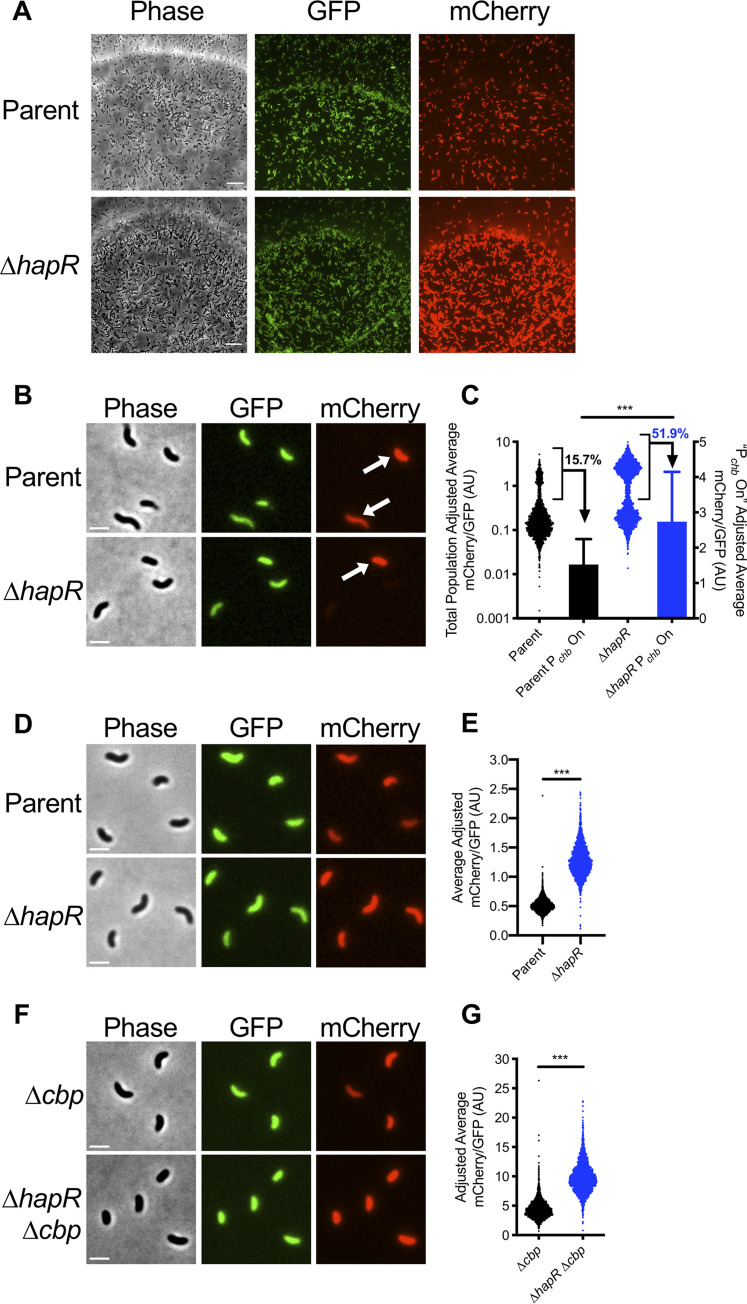
Thus far, we have studied Pchb regulation using a Δcbp mutation to induce ChiS activity. Natural induction of this locus, however, occurs in the presence of chitin oligosaccharides. So, we next wanted to test whether the repression of Pchb by HapR was observed in a more physiologically relevant setting. To test this, we cultured V. cholerae strains with CBP intact, a Pchb-mCherry reporter, and a construct that constitutively expresses GFP (Pconst2-GFP) on chitin beads (Fig. 2A). The Pconst2-GFP construct (derived from the insulated proD promoter [22]) served as an internal control for noise in gene expression and was used to normalize Pchb-mCherry expression in single cells (see Materials and Methods for details).
FIG 2.
HapR-mediated repression of Pchb occurs on chitinous surfaces. (A) Representative images of the indicated V. cholerae strains grown on chitin beads. The parent strain background contains both a Pchb-mCherry reporter and a Pconst2-GFP construct, which exhibits constitutive GFP expression. Scale bar = 10 μm. (B and C) Pchb expression in the indicated V. cholerae cbp+ strains grown on chitin beads. (B) Representative images of cells that were vortexed off chitin beads. Arrows demarcate cells where Pchb expression is induced (“Pchb on” cells). Scale bar = 2 μm. (C) Scatterplot and bar graph showing the relative expression of the Pchb-mCherry reporter in cells cultured on chitin beads. Scatterplots (left y axis) represent the entire population, whereas the bars (right y axis) represent only the Pchb on cells (bracketed in black on scatterplots). The percentage of cells in the Pchb on population is indicated. n = 2,120 for parent; n = 2,191 for ΔhapR strain; n = 333 for parent Pchb on; n = 1,139 for ΔhapR strain Pchb on. Data shown are from two independent biological replicates. (D and E) Pchb expression of the indicated V. cholerae strains where Pchb expression is induced by chitin oligosaccharides. (D) Representative images of cells grown with chitin oligosaccharides. Scale bar = 2 μm. (E) Scatterplot showing the relative expression of a Pchb-mCherry reporter in the indicated V. cholerae strains. n = 2,735 for parent; n = 2,384 for ΔhapR strain. Data shown are from two independent biological replicates. (F and G) Pchb expression of the indicated V. cholerae strains where Pchb expression is induced via deletion of cbp. (F) Representative images of cells grown in the absence of chitin. Scale bar = 2 μm. (G) Scatterplot showing the relative expression of a Pchb-mCherry reporter in the indicated V. cholerae strains. n = 2,407 for Δcbp strain; n = 2,313 for ΔhapR Δcbp strain. Data shown are from two independent biological replicates. Statistical comparisons in panels C, E, and G were made using Student's t test. ***, P < 0.001.
When cells were cultured on chitin beads, both the parent strain and the ΔhapR strain exhibited a bimodal distribution of Pchb expression (Fig. 2A to C). There were at least two possible explanations for the observed bimodality in Pchb gene expression in this experiment as follows: (i) the signaling pathway that leads to activation of chb has switch-like behavior that results in a population that exhibits bimodality in chb expression, or (ii) the environment during growth on chitin beads is heterogeneous and only some cells within the population have access to the chitin oligosaccharides necessary for activation of chb. Those cells that do not have access to chitin do not activate Pchb expression (Pchb off), while those that do have access to chitin do induce Pchb expression (Pchb on). To differentiate between these two possibilities, we induced expression of Pchb using the native inducer, chitin oligosaccharides, or genetically by deleting cbp. If the signaling circuit responsible for Pchb activation has switch-like behavior, we would predict that bimodality in gene expression would be maintained in both of these conditions; however, if bimodality is the result of heterogeneous access to chitin oligosaccharides when cells are cultured on chitin beads, we would expect bimodality to be lost. When Pchb was induced by chitin oligosaccharides or via deletion of cbp, all cells in the population are uniformly “Pchb on” (Fig. 2D and F), and the population becomes unimodal (Fig. 2E and G). Thus, these results showed that only the “Pchb on” cells have access to chitin oligosaccharides when grown on chitin beads. These results are consistent with chitin induction of natural transformation on chitin beads, where cells display bimodality in gene expression that is also likely due to heterogeneous access to inducing chitin oligosaccharides (23).
Next, we tested whether HapR influenced activation of Pchb on chitin beads by just assessing the expression level among “Pchb on” cells. As observed in bulk populations, the ΔhapR strain exhibited an ∼1.7-fold increase in Pchb expression when cultured on chitin beads (Fig. 2C) and an ∼2.5-fold increase when cultured with chitin oligosaccharides (Fig. 2E). These values were consistent with the ∼2-fold increase in Pchb expression observed in single cells when the population was induced via deletion of cbp (Fig. 2G). Finally, native chb transcripts (measured via reverse transcription-quantitative PCR [qRT-PCR]) were also induced ∼2-fold higher in a ΔhapR mutant compared to that of the parent when strains were induced with chitin oligosaccharides (see Fig. S2 in the supplemental material). Together, these results indicate that HapR is a bona fide repressor of Pchb under physiologically relevant inducing conditions.
HapR repression of Pchb is conserved in other V. cholerae El Tor isolates.
Previously, it was suggested that HapR was an activator of Pchb expression in the V. cholerae El Tor isolate A1552 (16). Specifically, the authors showed that deletion of hapR resulted in a decrease in Pchb expression when cells were cultured on chitin flakes. In the present study, we use the V. cholerae El Tor isolate E7946 to study Pchb regulation. To assess if HapR exhibits different effects on Pchb expression depending on the strain background, we assessed expression of a Pchb-mCherry reporter in hapR+ and ΔhapR derivatives of both E7946 and A1552. Consistent with our previous results, we observed that Pchb expression is elevated ∼2-fold in the ΔhapR derivative of both strain backgrounds when induced with chitin oligosaccharides or via deletion of cbp, which is consistent with HapR acting as a repressor of this locus (see Fig. S3 in the supplemental material). It is unclear what explains the discrepancy between our findings and those that were previously reported; however, these data suggest that they cannot be attributed to differences between strain backgrounds.
Deletion of HapR does not confer a growth advantage during growth on chitobiose.
Our data indicate that in the absence of HapR, Pchb expression is elevated. The chb locus encodes the genes required for uptake and degradation of the chitin disaccharide, chitobiose. Thus, we wanted to assess if the increased expression of Pchb confers a fitness advantage to ΔhapR mutant cells during growth on chitobiose. To test this, we conducted competitive growth assays with a 1:1 mixture of a parent and a ΔhapR mutant strain on minimal medium with chitobiose as the sole carbon source. We hypothesized that if the ΔhapR mutant had a competitive growth advantage due to increased expression of chb, then it should outcompete the parent strain in this assay. Even after ∼48 generations of growth on chitobiose, however, we did not observe a competitive advantage for the ΔhapR mutant (see Fig. S4 in the supplemental material). HapR is a global regulator that controls the expression of dozens of genes (24). Thus, even though a ΔhapR mutant has increased Pchb expression, there may be negative pleiotropic effects associated with the ΔhapR mutation that masks any competitive advantage of derepression of Pchb in this mutant during growth on chitobiose.
HapR binds Pchb in vitro and in vivo.
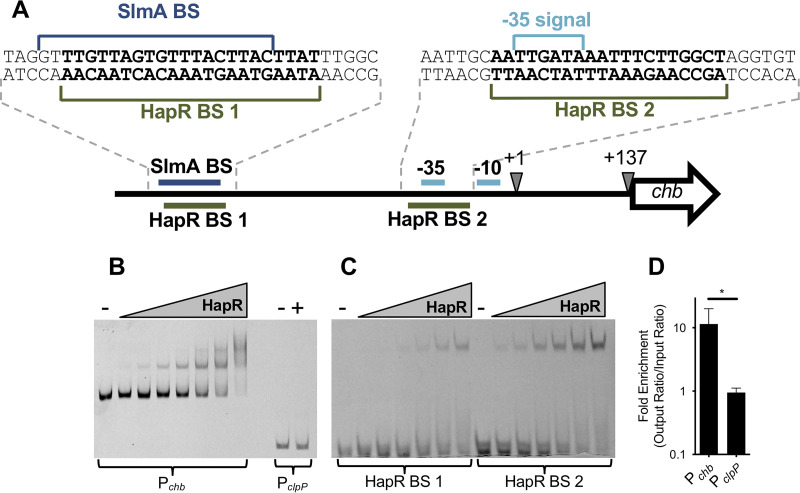
Thus far, our data suggest that HapR is a repressor of Pchb, but it does not distinguish whether HapR is a direct or indirect regulator of this locus. To assess if HapR could be regulating Pchb directly, we used an in silico approach to identify putative HapR binding sites (BSs) in Pchb based on consensus binding sequences generated for the HapR homologs LuxR (from Vibrio harveyi) and SmcR (from V. vulnificus) via chromatin immunoprecipitation sequencing (ChIP-seq) (25, 26). Using the Motif Alignment and Search Tool (MAST) in the Multiple Em for Motif Elicitation (MEME) suite (27), we identified two potential HapR binding sites in Pchb (Fig. 3A). Interestingly, these binding sites overlap with other elements in the chb promoter required for transcriptional activation. HapR BS 1 overlaps with the SlmA binding site, which is a critical activator of Pchb expression (11). HapR BS 2 overlaps with the −35 signal, which is required for RNA polymerase to bind to the promoter and initiate transcription. This sequence analysis suggests that the repressive effect of HapR may be due to HapR binding directly antagonizing SlmA and/or RNA polymerase (RNAP) at this locus.
FIG 3.
HapR binds Pchb in vitro and in vivo. (A) Promoter map of Pchb highlighting putative HapR binding sites (BSs). Other sites required for Pchb activation (the SlmA BS and the −35 and −10 signals) are highlighted. The exact sequences of the region containing the HapR BSs (bolded), the SlmA BS, and the −35 signal are shown. The transcriptional start site (+1) and the translational start site (+137) are also shown. (B) A representative EMSA using HapR and Cy5-labeled DNA probes of the indicated promoters. The Pchb probe was incubated with (from left to right) 0 nM (−), 12.5 nM, 25 nM, 50 nM, 100 nM, 200 nM, 400 nM, or 800 nM purified HapR. The PclpP probe was incubated with 0 nM (−) or 800 nM HapR (+). (C) A representative EMSA using HapR and 32-bp Cy5-labeled probes encompassing each of the putative HapR binding sites within the chb promoter (exact probe sequences are shown in panel A). The 32-bp probes were incubated with (from left to right) 0 nM (−), 100 nM, 200 nM, 400 nM, 800 nM, 1.6 μM, or 3.2 μM purified HapR. (D) ChIP-qPCR assays showing enrichment of the indicated promoters relative to rpoB, a reference locus that HapR does not bind to. Data are from five independent biological replicates and are shown as the mean ± SD. Statistical comparisons were made by Student's t test. *, P = 0.0240.
We next sought to determine whether HapR could directly bind to Pchb using both in vitro and in vivo approaches. First, we tested whether HapR could bind to Pchb in vitro using electrophoretic mobility shift assays (EMSAs). Consistent with the presence of two HapR binding sites in Pchb, we observed two shifts by EMSA when using a DNA probe of the chb promoter (Fig. 3B). HapR does not regulate the ClpP promoter and thus did not bind PclpP (Fig. 3B), which is consistent with previous studies (28). EMSAs were also done using 32-bp probes that encompassed each putative HapR BS (probe sequences can be found in Fig. 3A). We observed that HapR was able to bind to both probes, suggesting that HapR binds to both HapR BS 1 and HapR BS 2 (Fig. 3C). Next, we wanted to assess if HapR bound to Pchb in vivo via ChIP assays under physiologically relevant conditions. To that end, we first generated a FLAG-HapR strain that was functional for regulating Pchb expression (see Fig. S5 in the supplemental material). Using this strain in ChIP-quantitative PCR (qPCR) assays, we found that Pchb was, indeed, bound by HapR in vivo, while the negative control PclpP locus was not bound by HapR (Fig. 3D). Together, these data demonstrate that HapR binds to Pchb, which suggests that it is a direct repressor of this locus.
Quorum sensing regulates expression of Pchb through the cholera-specific autoinducer CAI-1.
HapR is the master regulator of quorum sensing (QS) in V. cholerae and is highly expressed at high cell density (HCD) (29). Thus far, we have established that HapR is a repressor of Pchb expression. Next, we wanted to probe the role of QS in regulating Pchb. To that end, we sought to test the effect of mutations in genes upstream of HapR in the V. cholerae QS cascade on Pchb induction and HapR protein levels.
QS in Vibrio species is controlled by autoinducer-responsive sensor proteins that indirectly modulate phosphorylation of the response regulator LuxO, which in turn indirectly regulates production of HapR (14). When autoinducer concentrations are low (i.e., at low cell density [LCD]), multiple histidine kinase sensors act as kinases (30–32). This results in high levels of phosphorylated LuxO, which prevents HapR production (see Fig. S6A in the supplemental material) (33). By contrast, at high autoinducer concentrations (i.e., at HCD), the sensors act as phosphatases, which ultimately leads to dephosphorylation of LuxO and allows for HapR production (Fig. S6B) (14, 34).
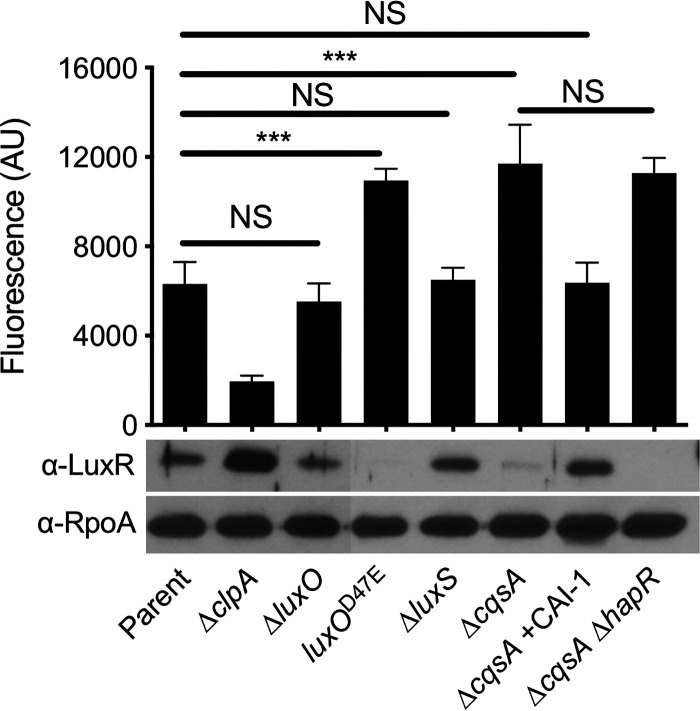
First, we assessed the impact of HCD on Pchb induction and HapR levels by deleting luxO, which genetically locks cells in an HCD state. Induction of the parent strain is tested under HCD conditions; thus, as expected, HapR levels were similar between the parent and the ΔluxO mutant; accordingly, expression of Pchb was also similar between the parent and ΔluxO mutant (Fig. 4). Next, we tested the effect of LCD on Pchb and HapR expression by generating a luxOD47E (D-to-E change at position 47 encoded by luxO) mutant, which mimics phosphorylated LuxO and genetically locks cells in an LCD state. In this strain, we saw that Pchb expression increased and was correlated with a decrease in HapR protein levels (Fig. 4). Together, these data suggest that HapR repression of Pchb is mediated through the canonical QS circuit.
FIG 4.
Quorum sensing regulates expression of Pchb through the cholera-specific autoinducer CAI-1. Expression of a Pchb-GFP reporter and HapR protein levels were determined in the indicated strains. The parent strain contains a Pchb-GFP reporter and a Δcbp mutation. A representative Western blot is shown below the bars to indicate the protein levels for HapR and RpoA (a loading control) in the corresponding strains. An antibody against LuxR, which has 72% identity and 86% similarity to HapR, is cross-reactive with HapR and so was used to detect HapR protein levels. Statistical comparisons were made by one-way ANOVA with Tukey’s posttest. NS, not significant. ***, P < 0.001. The Pchb-GFP fluorescence data for the “parent” and “ΔclpA” strains are identical to the data presented in Fig. 1 and were included here to ease comparisons.
Next, we wanted to move further upstream in the QS regulatory cascade to address whether distinct autoinducers differentially affected expression of Pchb and HapR. There are four parallel histidine kinase sensors that coordinate QS-dependent control of HapR expression (13); however, the autoinducer signal is only known for two of these systems. The sensor LuxPQ is responsive to the interspecies autoinducer AI-2, and the sensor CqsS is responsive to the V. cholerae-specific autoinducer CAI-1 (Fig. S6) (35). To assess the role of each autoinducer in regulation of Pchb, we made mutations to the synthase genes responsible for production of each autoinducer. LuxS makes AI-2 (36) and CqsA makes CAI-1 (35) (Fig. S6). In a strain that no longer produces AI-2 (ΔluxS strain), expression of Pchb was similar to that observed in the parent, and HapR levels remained similar in these two strains (Fig. 4). By contrast, a strain that is unable to produce CAI-1 (ΔcqsA strain) had increased expression of Pchb, likely due to the low level of HapR produced (Fig. 4). The observed decrease in Pchb expression in the ΔcqsA strain background was, indeed, due to a lack of CAI-1 production, as exogenously adding back synthetic CAI-1 restored HapR levels and repression of Pchb to the parent level (Fig. 4). In addition, a strain that does not make CAI-1 induced Pchb to the same level as that of a strain that does not produce both CAI-1 or HapR (Fig. 4). This epistasis indicates that CAI-1 production and HapR are involved in the same regulatory pathway for modulating expression of Pchb. These data support previous results, which indicate that CqsS signaling plays a dominant role in regulating HapR (23, 35, 37, 38). Together, these results indicate that Pchb expression is strongly influenced by QS signaling mediated by the V. cholerae-specific autoinducer CAI-1 and less via the interspecies autoinducer AI-2.
DISCUSSION
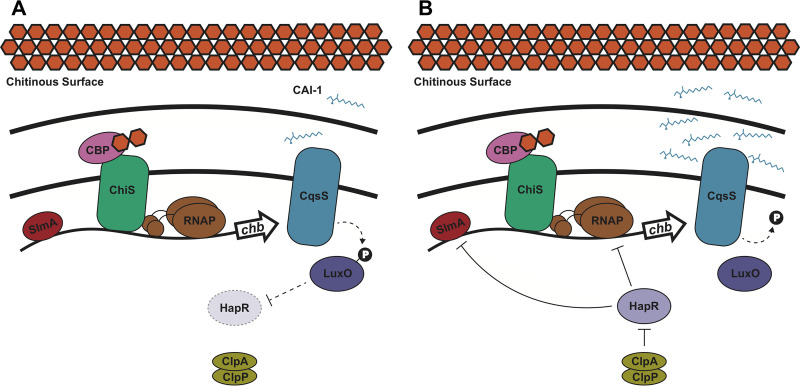
Here, we show that HapR acts as a repressor of chitobiose utilization genes in V. cholerae. When this organism forms communities on a chitinous surface, chitin induces expression of Pchb through activation of the chitin sensor ChiS (Fig. 5). V. cholerae then modulates Pchb expression depending on the presence of other V. cholerae cells, which it senses via the V. cholerae-specific autoinducer CAI-1. At low CAI-1 concentrations, Pchb expression is high because the repressor HapR is produced at a low level (Fig. 5A). At high CAI-1 concentrations, HapR is produced at higher levels and can repress Pchb expression (Fig. 5B). As the HapR binding sites in Pchb overlap with the SlmA binding site and the −35 signal, it is possible that HapR competes with these activators for binding at Pchb. Thus, HapR-mediated repression may occur through antagonism of the binding activity of SlmA and/or RNAP at Pchb.
FIG 5.
Model of quorum sensing regulation of chitobiose utilization genes in V. cholerae. When V. cholerae grows on a chitinous surface, the periplasmic chitin binding protein (CBP) binds to chitin oligosaccharides. This derepresses ChiS, which can then activate expression of the chitobiose utilization operon (chb) through recruitment of RNA polymerase (RNAP) (10). Binding of SlmA to Pchb is also required for transcriptional activation (11). (A) At low CAI-1 concentrations, the CqsS sensor indirectly (dashed line) promotes LuxO phosphorylation, which indirectly blocks HapR expression. In this state, Pchb is maximally expressed. (B) At high CAI-1 concentrations, the CqsS sensor indirectly dephosphorylates LuxO, which results in indirect activation of HapR expression. HapR then exerts a repressive effect on Pchb by decreasing expression of this locus ∼2-fold in the presence of the ClpAP protease machine. If HapR is not degraded by ClpAP, its repressive effect can result in an ∼7-fold decrease in Pchb expression. The mechanism of HapR repression may be through occluding binding of SlmA or RNAP to Pchb.
When HapR is produced, it gets proteolyzed by the ClpAP machine (Fig. 5B). While the dynamic range of HapR-dependent Pchb repression is only ∼2-fold when ClpAP is intact, the dynamic range of repression increases to ∼7-fold in the absence of ClpAP. Thus, it is tempting to speculate that regulation of ClpAP may modulate Pchb expression under some conditions. Currently, however, little is known about the regulation of ClpAP in V. cholerae. ClpAP is induced during heat shock in V. vulnificus (21). Also, in Escherichia coli, ClpP expression is induced during heat shock (39–41). Thus, it is believed that the ClpP protease plays a role in the heat shock response. In Bacillus subtilis, ClpP is upregulated under various stress conditions, suggesting that this protease may also be induced by a general stress response (42). Thus, one possibility is that stress-dependent regulation of ClpAP indirectly regulates Pchb. It has also been hypothesized that V. cholerae makes use of proteolysis machines to rapidly respond to changes in their environment; namely, the transition from the human gut to the aquatic environment after infection (43). ClpAP acting as an activator of Pchb may be a way for cells to rapidly induce expression of a locus that is not important in the human gut but is useful for survival in its aquatic reservoir. Consistent with this idea, chb is induced late in infection, suggesting that this is a critical locus for preparing to reenter the aquatic environment after infection of a host (44).
The mechanisms underlying distinct responses to AI-2 or CAI-1 remain a topic of interest in QS. It has been shown that the CAI-1 sensor CqsS plays a dominant role over LuxPQ in modulating HapR levels. Thus, it remains possible that the presence of CAI-1 allows for more robust regulation of HapR-regulated genes. CAI-1 has been shown to be critical for expression of the virulence factor hapA (38), natural transformation (15), and for repressing chitobiose utilization as shown in this study. HapA is a protease that has been implicated in mediating V. cholerae detachment from host epithelial cells, thereby promoting dissemination of cells back into the aquatic environment (45, 46); natural transformation and chitin utilization aid in V. cholerae fitness in the marine environment. By contrast, expression of tcpA, a protein that contributes to V. cholerae pathogenesis in the human gut, was shown to be primarily regulated by AI-2 (37). It is tempting to speculate that CAI-1 controls V. cholerae behaviors important for marine survival, whereas AI-2 controls behaviors involved in infection. It has been hypothesized that sensing both of these autoinducers plays a critical role in biofilm dispersal and that only when both are sensed are V. cholerae cells induced to leave a surface (i.e., QS works as a coincidence detector for both signals) (37). Thus, signaling via distinct autoinducers may allow V. cholerae to modulate responses depending on the context of the environment that they inhabit.
The data that we present here suggests that at HCD, V. cholerae dampens its expression of a chitin utilization locus. Below, we speculate on a few reasons why this regulation may be advantageous. Chitin polymers in the shells of crustacean zooplankton are long-chain polysaccharides in a crystalline insoluble lattice. In order to be used as a nutrient source, the long-chain chitin must be broken down into smaller, soluble chitin oligosaccharides. V. cholerae secretes chitinases that enzymatically degrade insoluble chitin into soluble chitin oligosaccharides for uptake and catabolism. The production and secretion of chitinases is an energetically costly process; thus, liberated chitin is a valuable “public good” in the context of a chitin biofilm (47). So, it is possible that QS regulation of chitin utilization allows V. cholerae to modulate chitin uptake based on the composition of its microbial community. When the level of V. cholerae cells (and the corresponding concentration of cholera-specific autoinducer CAI-1) in the community is high, chitin uptake may decrease among individual cells within the population in an effort to “share” liberated chitin oligosaccharides. By contrast, when the level of V. cholerae in the community is low, sensing of only the interspecies signal AI-2 (which is produced by many bacterial species) does not suppress chitin uptake and utilization, thus, allowing the V. cholerae within this population to maximally compete for liberated chitin oligosaccharides.
Another possibility is that this regulation allows V. cholerae to control its production of toxic metabolites. In a previous study, it was shown that cells in an HCD state alter their metabolic flux to produce neutral by-products as opposed to organic acids when grown in LB supplemented with a fermentable sugar like glucose (48). This regulation allows for a more stable community; by contrast, cells locked in an LCD state will excrete harmful metabolic by-products, which leads to the demise of the community (48). It has been shown that V. cholerae excretes ammonium as a potentially toxic by-product when grown on chitin (16). Also, because chitin oligosaccharides likely feed into glycolysis, it is possible that catabolism of chitin results in the production of potentially toxic organic acids. Thus, repressing chitin uptake and utilization may also help slow the rate of metabolism to prevent the accumulation of toxic intermediates in a dense community setting.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
V. cholerae strains were routinely grown at 30°C in LB medium and on LB agar supplemented when necessary with carbenicillin (20 μg/ml or 50 μg/ml), kanamycin (50 μg/ml), spectinomycin (200 μg/ml), trimethoprim (10 μg/ml), and/or chloramphenicol (2 μg/ml). Strains were grown in defined artificial salt water (DASW) medium for microscopy (see below for details), Instant Ocean for generating mutant strains (see below for details), or M9 minimal medium for competition experiments (see below for details).
Transposon mutagenesis.
Transposon mutant libraries were generated with a Carbr mini-Tn10 transposon exactly as previously described (49). Briefly, the transposon mutagenesis plasmid pDL1086 was first mated into parent strains containing chromosomally integrated Pchb-lacZ and a Δcbp mutation (activator screen) or ΔclpA Δcbp mutations (ΔclpA strain counterscreen). The parent strains also carried a chromosomally integrated Pchb-mCherry reporter at an ectopic site to ensure that candidate transposon mutants affected expression of Pchb and did not simply disrupt the Pchb-lacZ reporter. The activator screen parent also carried an additional copy of ChiS at an ectopic site, which prevented transposon hits in this known regulator of Pchb. Transposition was induced by plating cultures on LB agar supplemented with 50 μg/ml carbenicillin (Carb) at 42°C. To screen colonies, plates also contained 40 μg/ml X-Gal and 5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). IPTG was added to competitively inhibit the basal activity of the Pchb-lacZ reporter.
The sequences of transposon-genomic junctions in transposon mutants were determined by inverse PCR followed by Sanger sequencing. Briefly, genomic DNA was purified from mutants of interest and digested with the FatI restriction enzyme per the manufacturer’s instructions (NEB). Digested genomic DNAs were then incubated with T4 DNA ligase per manufacturer’s instructions (NEB) to generate self-ligated circles. The transposon-genomic junction was then amplified by PCR using the primers specified in Table 1 and subsequently Sanger sequenced (Eurofins Genomics).
TABLE 1.
Primers used in this study
| Primer | Sequencea | Description |
|---|---|---|
| Used for inverse PCR | ||
| BBC 244 | CCCGGGATCCTGTGTGAAATTGTTATCCGC | Tn10-specific primer for inverse PCR |
| BBC 434 | GTGTGGGCACTCGACATATGACAAG | Tn10-specific primer for inverse PCR |
| Used for reporter constructs | ||
| ABD 332 | GGCTGAACGTGGTTGTCGAAAATGAC | ΔlacZ F1 (up arm) |
| BBC 219 | GTTTATTTTTGTCGACTGTACAGCGTTTAAATAGAGGTCGATATTGACCC | ΔlacZ R1 (up arm) |
| ABD 342 | ATTTTTCAGTTGGCCTACAATGCTTTCC | ΔVC1807 F1 (up arm) |
| BBC 719 | CACCATACCCACGCCGAAACAAGGATTTTGAATTAAACGTTTCATTAGTC | ΔVC1807 R1 (up arm) |
| BBC 218 | CGCTGTACAGTCGACAAAAATAAAC | Kanr F (middle arm) |
| BBC 262 | TACCGAGGACGCGAAGCTG | Kanr R (middle arm) |
| BBC 266 | CAGCTTCGCGTCCTCGGTAGAATAAAGCAATCCGCAAGCG | Pchb F (middle arm) |
| BBC 267 | CCCGGGATCCTGTGTGAAATTGAGTTGCTTTCATTTCACTAATGG | Pchb R (middle arm) |
| BBC 732 | ctcaagccgaggagtaaagaagAGTTGCTTTCATTTCACTAATGG | Pchb fuse to lacZ R (middle arm) |
| BBC 252 | CAATTTCACACAGGATCCCGGGAGGAGGTAACGTAATGCGTAAAGGAGAAGAAC | GFP F (middle arm) |
| BBC 254 | tgtaggctggagctgcttcTTAGTTGTATAGTTCATCCATGCC | GFP R (middle arm) |
| BBC 1356 | TTGTTTCGGCGTGGGTATGGTGCGCTGTACAGTCGACAAAAATAAAC | mCherry F (middle arm) |
| BBC 206 | tgtaggctggagctgcttcttacttgtacagctcgtccatg | mCherry R (middle arm) |
| ABD 785 | CTTCTTTACTCCTCGGCTTGAG | Pchb stitch to lacZ F (down arm) |
| ABD 255 | gaagcagctccagcctacaCCACAATAAGCCAGAGAGCCTTAAG | ΔlacZ F2 (down arm) |
| ABD 256 | CCCAAATACGGCAACTTGGCG | ΔlacZ R2 (down arm) |
| ABD 341 | gaagcagctccagcctacaTAGTCGAAAATAAAAAAAAGAGGCTCGCCTC | ΔVC1807 F2 (down arm) |
| ABD 345 | CTTGCTAACCGTTGGTGTTACCAGC | ΔVC1807 R2 (down arm) |
| BBC 3083 | tggataactttacgggcatgcataaggctcgtataatatattcagggagaccacaacggtttccctctacaaataattttgtttaactttCAATTTCACACAGGATCCCGGG | Pconst2 F |
| BBC 3082 | ttatacgagccttatgcatgcccgtaaagttatccagcaaccactcatagacctagggcagcagatagggacgacgtggtgttagctgtgCTCATTAGGCACCCCAGGC | Pconst2 R |
| Used for SOE deletion, FLAG fusions, and point mutants | ||
| BBC 742 | attccggggatccgtcgacCTGCAGTTCagaagcagctccagcctaca | MiniFRT F |
| BBC 743 | tgtaggctggagctgcttctGAACTGCAGgtcgacggatccccggaat | MiniFRT R |
| ABD 123 | ATTCCGGGGATCCGTCGAC | Kanr, Specr, Carbr, Cmr, or Tmr cassette F |
| ABD 124 | TGTAGGCTGGAGCTGCTTC | Kanr, Specr, Carbr, Cmr, or Tmr cassette R |
| ABD 796 | TTAGAATCTGCGCCAGAAGCG | Δcbp F1 (up arm) |
| ABD 797 | gtcgacggatccccggaatCATAGCTGTTCCTTACTAGTTGC | Δcbp R1 (up arm) |
| BBC 920 | gtcgacggatccccggaatGCTCATCAGGTCGTCAGCC | Δ3′ cbp R1 (up arm) |
| ABD 798 | gaagcagctccagcctacaGTACTGGATCTGAAACCAGTTAAG | Δcbp F2 (down arm) |
| ABD 799 | GTATTGCGGAATGACCAGCATG | Δcbp R2 (down arm) |
| BBC 2755 | TTACCCCTAAGTCGGCGAGC | ΔVC0995 F1 (up arm) |
| BBC 2756 | gtcgacggatccccggaatAATATTCACCTTAAGTTCCCCC | ΔVC0995 R1 (up arm) |
| BBC 2757 | gaagcagctccagcctacaCTGCCTTAATCGAGTTTAAACCC | ΔVC0995 F2 (down arm) |
| BBC 2758 | GCACCACGATAGCAATAAGC | ΔVC0995 R2 (down arm) |
| CAK 407 | GCTACGCACTGCCAAATTACC | ΔclpA or ΔclpS F1 (up arm) |
| CAK 408 | gtcgacggatccccggaatAAGCATAAGGCCTCCTTAAGGAAC | ΔclpA R1 (up arm) |
| CAK 409 | gaagcagctccagcctacaCACTAGACATCTCACAATACGC | ΔclpA F2 (down arm) |
| CAK 410 | CCGCTAACATCTCAGGACTG | ΔclpA or ΔclpS R2 (down arm) |
| CKP 449 | TGCTCGGTTTTGATCCGTTC | ΔclpP or ΔclpX F1 (up arm) |
| CKP 450 | gtcgacggatccccggaatGGGCGACATTGCATTTTTTTC | ΔclpP R1 (up arm) |
| CKP 451 | gaagcagctccagcctacaGGCGAGTAAGCTCGTAATTG | ΔclpP F2 (down arm) |
| CKP 454 | GTCAAACTAGAAACCAGCGC | ΔclpP or ΔclpX R2 (down arm) |
| CKP 447 | gtcgacggatccccggaatTTTACTCATGACACTTCAATATTTG | ΔclpS R1 (up arm) |
| CKP 448 | gaagcagctccagcctacaCAAGCTTAAACTTGCCGGGC | ΔclpS F2 (down arm) |
| CKP 452 | gtcgacggatccccggaatGTCTGTCATTCGCTAACCTC | ΔclpX R1 (up arm) |
| CKP 453 | gaagcagctccagcctacaGCGGAGTAATCTCAAGCAAC | ΔclpX F2 (down arm) |
| CKP 489 | TTGACGCTCTGAAAGCAGAAG | ΔlonA F1 (up arm) |
| CKP 490 | gtcgacggatccccggaatCAAGTTCATATTTTTCTCTCTTCCG | ΔlonA R1 (up arm) |
| CKP 491 | gaagcagctccagcctacaGATGCATAGCAAAAATAAGTAAATC | ΔlonA F2 (down arm) |
| CKP 492 | CGAAGAATTATAAGTGCAAAGGC | ΔlonA R2 (down arm) |
| BBC 422 | ACGTTCAATAACCAGAATGTTGG | ΔhapR F1 (up arm) |
| BBC 423 | gtcgacggatccccggaatTTTTCGATTGATGCGTCCATAG | ΔhapR R1 (up arm) |
| CKP 511 | GTCtttgtcatcgtcatccttataatcCATAGGGGTATATCCTTGCC | 1× FLAG-hapR R1 (up arm) |
| BBC 408 | gaagcagctccagcctacaAACTAGTTTCTTGGGCAGCACAA | ΔhapR F2 (down arm) |
| CKP 510 | ATGgattataaggatgacgatgacaaaGACGCATCAATCGAAAAACG | 1× FLAG-hapR F2 (down arm) |
| BBC 409 | GTTCATAATGATTTCCTTGGTGCC | ΔhapR R2 (down arm) |
| BBC 374 | TGGCAAAAAGCGAGAGAAGAAG | ΔluxO F1 (up arm) |
| BBC 375 | gtcgacggatccccggaatCATGAGGACATATTTTGTTTTCTGC | ΔluxO R1 (up arm) |
| CKP 519 | TCATATCTGGCAAACGTAAcTCCAGCAGGATTAAGTCAGG | luxOD47E R1 (up arm) |
| BBC 376 | gaagcagctccagcctacaTAAGCGATGAGAGAATGGATCAAC | ΔluxO F2 (down arm) |
| CKP 518 | TGACTTAATCCTGCTGGAgTTACGTTTGCCAGATATGACG | luxOD47E F2 (down arm) |
| BBC 377 | TCACACCCGAATTTCCATCATGC | ΔluxO R2 (down arm) |
| CKP 554 | GTCTCTTAGCCGAGGTACTG | ΔcqsA F1 (up arm) |
| CKP 555 | gtcgacggatccccggaatCTTGTTCATCGCAATATATCCTAG | ΔcqsA R1 (up arm) |
| CKP 556 | gaagcagctccagcctacaTTTCGTTAAATGCATAAATAACAAAAAC | ΔcqsA F2 (down arm) |
| CKP 557 | AGTTGGAACCACTTCTTGTC | ΔcqsA R2 (down arm) |
| CKP 549 | TACAACTGCTTGGCACGC | ΔluxS F1 (up arm) |
| CKP 550 | gtcgacggatccccggaatTGGCATTTCCTTTCTCCC | ΔluxS R1 (up arm) |
| CKP 551 | gaagcagctccagcctacaCACTAAGTCGGTTCTGTAAACG | ΔluxS F2 (down arm) |
| CKP 552 | TTCTTAGCGTGATCAATTGC | ΔluxS R2 (down arm) |
| ABD 334 | AGTGCTCCGGACTCTTTGCTCTG | ΔlacZ F1 (up arm) |
| ABD 254 | gtcgacggatccccggaatCATCCCTCAAGCCGAGGAGTAAAG | ΔlacZ R1 (up arm) |
| ABD 255 | gaagcagctccagcctacaCCACAATAAGCCAGAGAGCCTTAAG | ΔlacZ F2 (down arm) |
| ABD 256 | CCCAAATACGGCAACTTGGCG | ΔlacZ R2 (down arm) |
| ABD 725 | GAAGCAGCTCCAGCCTACA | Detect F for all deletions |
| BBC 082 | gtcgacggatccccggaatCATAACTTACACCTTACTCACCCAG | Δcbp detect R |
| CAK 411 | TTGTTTGGTGCGATTATTGG | ΔclpA detect R |
| CKP 464 | TTGGCAGAACATCTTTGATC | ΔclpP detect R |
| CKP 463 | AAAGTATCCAGCTCACGGCG | ΔclpS detect R |
| CKP 465 | CCATGTGTGGATAGACAACC | ΔclpX detect R |
| CKP 493 | CAGTGCCACTTGGTCACCTG | ΔlonA detect R |
| BBC 410 | TAAATGGGGCTTGGAGAATTTAG | ΔhapR detect R |
| BBC 1911 | CGTAATCAAACTGCGAAAGTG | ΔluxO detect R |
| CKP 558 | AATTCGTAACTCTGAGCATG | ΔcqsA detect R |
| CKP 553 | TGGACCACGAACCTTAAACG | ΔluxS detect R |
| BBC 2759 | TTGTCAGAAAGCGTTTCTGC | ΔVC0995 detect R |
| ABD 399 | AACTGATGGCAGAAAAAGCCACTCAG | ΔlacZ detect R |
| BBC 993 | ttgattataaggatgacgatgac | 1× FLAG detect F |
| ABD 846 | CATAAACATGTTTCTGATCAGCAG | 1× FLAG-hapR detect R |
| BBC 380 | GCCAATAGAATGAGTCTATTGGCTG | luxOD47E detect F |
| CKP 520 | TCATATCTGGCAAACGTAgc | luxOD47E detect R |
| Used for EMSA probes | ||
| BBC 744 | cagcttcgcgtcctcggtaCGCAAATATAACTCAGGCAAAG | Pchb F |
| CKP 072 | cccgggatcctgtgtgaaattgCTTTGGCAGGAGTAAGAAAACACCTAG | Pchb R |
| CKP 865 | TAAGCAAACTGTAGCGTAGAAG | PclpP F |
| CKP 866 | TGCATTTTTTTCTTGGTAGC | PclpP R |
| CKP 504 | GCCAAATAAGTAAGTAAACACTAACAAACCTA | HapR BS 1 F |
| CKP 505 | TAGGTTTGTTAGTGTTTACTTACTTATTTGGC | HapR BS 1 R |
| CKP 506 | AATTGCAATTGATAAATTTCTTGGCTAGGTGT | HapR BS 2 F |
| CKP 507 | ACACCTAGCCAAGAAATTTATCAATTGCAATT | HapR BS 2 R |
| Used for qPCR and qRT-PCR | ||
| BBC 989 | GCATCTAGGTTTTGACGTTTTTAACG | Pchb amplify F |
| BBC 990 | AACACTCTCCAAGACCTACCTC | Pchb amplify R |
| ABD 132 | CTGTCTCAAGCCGGTTACAA | rpoB amplify F |
| ABD 133 | TTTCTACCAGTGCAGAGATGC | rpoB amplify R |
| CKP 865 | TAAGCAAACTGTAGCGTAGAAG | PclpP amplify F |
| CKP 867 | TCGTATGAACGCTCACCACG | PclpP amplify R |
| Used for purification vector | ||
| JDN 92 | agcagcggcctggtgccgcgcggcagcCatatggacgcatcaatcgaaaaa | Cloning 6×His-hapR into pET28b vector via IDAb, F |
| JDN 93 | tcagtggtggtggtggtggtgCTCGAGctagttcttatagatacacagcatattgagg | Cloning 6×His-hapR into pET28b vector via IDA, R |
| JDN 22 | CTCGAGcaccaccaccacca | Amplify pET28b vector backbone for IDA, F |
| JDN 23 | atGgctgccgcgcggcacca | Amplify pET28b vector backbone for IDA, R |
| Used for complementation constructs | ||
| BBC 832 | GCTTTTTGCTACAACGACCG | ΔVCA0692 F1 for up arm |
| BBC 828 | CACCATACCCACGCCGAAACAACAGTGATGTAGCGAATCGGAC | ΔVCA0692 R1 for up arm |
| BBC 243 | TTGTTTCGGCGTGGGTATGGTG | Tmr or Zeor F for middle arm |
| BBC 647 | tttttctatttctgaatcgattcatacgaCTCATTAGGCACCCCAGGC | Tmr F or Zeor for middle arm |
| CKP 948 | tcgtatgaatcgattcagaaatagaaaaaACCATTCTCGTTGTGTTGGG | PhapR-hapR F for middle arm |
| BBC 729 | tgtaggctggagctgcttcCTAGTTCTTATAGATACACAGCATATTG | PhapR-hapR R for middle arm |
| CKP 949 | tcgtatgaatcgattcagaaatagaaaaaCCCTCATGCATTTTATAACTG | PclpSA-clpSA F for middle arm |
| CKP 950 | tgtaggctggagctgcttcCTAGTGGACCACCTCTTCGC | PclpSA-clpSA R for middle arm |
| BBC 830 | gaagcagctccagcctacaGTTGAGTTGGATGCAGCACC | ΔVCA0692 F2 for down arm |
| BBC 834 | CACAATTTCTCGCTTAAAATGTCC | ΔVCA0692 R2 for down arm |
| CKP 509 | gcaggtggagcaggtggaGACGCATCAATCGAAAAACG | ΔVCA0692::PhapR-hapR detect F |
| BBC 1101 | CAGACGTACTATTAACAGGACTGAC | ΔVCA0692::PhapR-hapR detect R |
| CKP 235 | gtcgacggatccccggaatCAAATATATCCTCCTCACTATTTTGATTAG | ΔVCA0692::PclpSA-clpSA detect F |
| CKP 447 | gtcgacggatccccggaatTTTACTCATGACACTTCAATATTTG | ΔVCA0692::PclpSA-clpSA detect R |
Lowercase letters in primers indicate the sequence of overlap sequences needed for SOE PCR.
IDA, isothermal DNA assembly.
Generating mutant strains.
V. cholerae E7946 served as the parent for all strains generated in this study, except for those used in Fig. S3 in the supplemental material where we compare E7946 to A1552 (50, 51). Mutant constructs were generated by splicing by overlap extension PCR (SOE PCR) exactly as previously described with the primers indicated in Table 1 (52). PCRs were performed to generate up (F1/R1), middle, and down (F2/R2) arms. Up and down arms were designed to have 3-kb arms of homology to the genome at the site targeted for mutagenesis. All three arms were then mixed and used as a template for SOE PCRs with the F1 and R2 primers to generate the full-length mutant construct SOE product. Mutant constructs were introduced into V. cholerae cells by chitin-induced natural transformation and/or cotransformation exactly as previously described (53, 54) or by chitin-independent transformations using a plasmid that ectopically expresses tfoX and qstR as previously described (55). For chitin-dependent natural transformation, V. cholerae was grown to mid-log in LB medium, washed with Instant Ocean medium (7 g/liter; Aquarium Systems), and incubated with chitin flakes (Alfa Aesar) at a final optical density at 600 nm (OD600) of 0.1 overnight at 30°C. The next day, SOE PCR products were added to chitin-induced cells, incubated at 30°C for 5 h, and then outgrown and plated on selective media. For chitin-independent transformation, cells harboring pMMB67EH-tfoX-qstR were grown overnight in LB medium supplemented with 100 μM IPTG and 1 μg/ml chloramphenicol. The next day, 7 μl of the overnight culture was diluted into 350 μl of Instant Ocean medium. Then, SOE products were added as described above for chitin-dependent transformations. Mutant strains were confirmed by colony PCR, mismatch amplification mutation assay (MAMA) PCR (56), and/or sequencing. Complete lists of strains and primers used in this study are outlined in Table 2 and Table 1, respectively.
TABLE 2.
Strains used in this study
| Strain designation | Reference(s) in this report | Genotype | Reference |
|---|---|---|---|
| SAD 030 | V. cholerae E7946 WT; parent for all other E7946 strains; Fig. S2 parent | V. cholerae E7946 O1 El Tor | 50 |
| SAD 2825 | Activator screen strain | V. cholerae E7946 ΔVC1807::Pchb-mCherry, Kanr; Pchb-lacZ, Specr; ΔVCA0692::chiS, Tmr; Δ3′ cbp, pDL1086 Cmr Carbr | This study |
| SAD 2826 | ΔclpA strain counterscreen | V. cholerae E7946 ΔVC1807::Pchb-mCherry, Kanr; Pchb-lacZ, Specr; ΔclpA; Δcbp::Tmr; pDL1086, Cmr Carbr | This study |
| SAD 1309 | Fig. 1 and 4 parent; Fig. S5 HapR WT | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; Δcbp::Specr | 11 |
| SAD 2827 | Fig. 1 and 4 ΔclpA strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔclpA::Carbr; Δcbp::Specr | This study |
| SAD 2828 | Fig. 1 ΔclpP strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔclpP::Carbr; Δcbp::Tmr | This study |
| SAD 2829 | Fig. 1 ΔclpAP strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔclpA::Carbr; ΔclpP::Tmr; Δcbp::Specr | This study |
| SAD 2830 | Fig. 1 ΔclpS strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔclpS::Carbr; Δcbp::Tmr | This study |
| SAD 2831 | Fig. 1 ΔclpX strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔclpX::Carbr; Δcbp::Tmr | This study |
| SAD 2832 | Fig. 1 ΔlonA strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔlonA::Tmr; Δcbp::Carbr | This study |
| SAD 2833 | Fig. 1 ΔhapR ΔclpA strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔhapR::Specr; ΔclpA::Carbr; Δcbp::Tmr | This study |
| SAD 2834 | Fig. 1 ΔhapR strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔhapR::Specr; Δcbp::Carbr | This study |
| SAD 2835 | Fig. 2 parent | V. cholerae E7946 ΔlacZ::Pchb-mCherry, Kanr; ΔVCA0692::Pconst2-GFP, Specr | This study |
| SAD 2836 | Fig. 2 ΔhapR strain | V. cholerae E7946 ΔlacZ::Pchb-mCherry, Kanr; ΔVCA0692::Pconst2-GFP, Specr; ΔhapR::Cmr | This study |
| SAD 2837 | Fig. 2 Δcbp strain and Fig. S3 E7946 parent | V. cholerae E7946 ΔlacZ::Pchb-mCherry, Kanr; ΔVCA0692::Pconst2-GFP, Specr; Δcbp::Tmr | This study |
| SAD 2838 | Fig. 2 ΔhapR Δcbp strain and Fig. S3 E7946 ΔhapR strain | V. cholerae E7946 ΔlacZ::Pchb-mCherry, Kanr; ΔVCA0692::Pconst2-GFP, Specr; ΔhapR::Cmr; Δcbp::Tmr | This study |
| SAD 2839 | Fig. 3 ChIP strain and Fig. S5 1× FLAG-HapR | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; 1× FLAG-hapR; Δcbp::Tmr | This study |
| SAD 2840 | Fig. 4 ΔluxO strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔluxO::Specr; Δcbp::Carbr | This study |
| SAD 2841 | Fig. 4 luxOD47E strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; luxOD47E; Δcbp::Specr | This study |
| SAD 2842 | Fig. 4 ΔluxS strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔluxS::Cmr; Δcbp::Specr | This study |
| SAD 2843 | Fig. 4 ΔcqsA strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔcqsA::Tmr; Δcbp::Specr | This study |
| SAD 2844 | Fig. 4 ΔcqsA ΔhapR strain | V. cholerae E7946 ΔlacZ::Pchb-GFP, Kanr; ΔcqsA::Tmr; ΔhapR::Cmr; Δcbp::Specr | This study |
| SAD 306 | V. cholerae A1552 WT; parent for all other A1552 strains | V. cholerae A1552 O1 El Tor | 51 |
| SAD 2908 | E. coli strain used to mate pMMB tfoX-qstR into complementation strains used in Fig. S1 | E. coli S17 harboring pMMB67EH tfoX qstR, Cmr | This study |
| SAD 2909 | Fig. S1 ΔhapR strain PhapR-hapR | V. cholerae E7946 ΔlacZ::Pchb-GFP Kanr; ΔhapR::Specr; Δcbp::Carbr; ΔVCA0692::PhapR-hapR, Tmr | This study |
| SAD 2910 | Fig. S1 ΔhapR ΔclpA strain PhapR-hapR | V. cholerae E7946 ΔlacZ::Pchb-GFP Kanr; ΔhapR::Specr; ΔclpA::Carbr; Δcbp::Tmr; ΔVCA0692::PhapR-hapR, Zeor | This study |
| SAD 2911 | Fig. S1 ΔclpA strain PclpSA-clpSA | V. cholerae E7946 ΔlacZ::Pchb-GFP Kanr; ΔclpA::Carbr; Δcbp::Specr; ΔVCA0692::PclpSA-clpSA, Tmr | This study |
| SAD 2912 | Fig. S2 ΔhapR strain | V. cholerae E7946 ΔhapR::Cmr | This study |
| SAD 2845 | Fig. S3 A1552 parent | V. cholerae A1552 ΔVCA0692::Pconst2-GFP, Specr; ΔlacZ::Pchb-mCherry, Kanr; Δcbp::Tmr | This study |
| SAD 2846 | Fig. S3 A1552 ΔhapR strain | V. cholerae A1552 ΔVCA0692::Pconst2-GFP, Specr; ΔlacZ::Pchb-mCherry, Kanr; ΔhapR::Cmr; Δcbp::Tmr | This study |
| SAD 2847 | Fig. S4 in WT lacZ+ strain | V. cholerae E7946 ΔVC1807::Cmr; ΔVC0995 | This study |
| SAD 2848 | Fig. S4 WT ΔlacZ strain | V. cholerae E7946 ΔlacZ::Specr; ΔVC1807::Cmr; ΔVC0995 | This study |
| SAD 2849 | Fig. S4 ΔhapR ΔlacZ strain | V. cholerae E7946 ΔlacZ::Specr; ΔVC1807::Cmr; ΔVC0995; ΔhapR::Tmr | This study |
| JDN 71 | Fig. 3B and C purified HapR | E. coli BL21(DE3) harboring pET28b-6×His-hapR, Kanr | This study |
Measuring GFP and mCherry fluorescence in bulk populations.
GFP and mCherry fluorescence in reporter strains were determined exactly as previously described (57). Briefly, single colonies were grown in LB overnight at 30°C. Where indicated, medium was supplemented with 10 μM CAI-1. CAI-1 was synthesized exactly as previously described (58). The next day, cells were washed and resuspended to an OD600 of 1.0 in Instant Ocean medium (7 g/liter; Aquarium Systems). Fluorescence was determined on a BioTek H1M plate reader. For GFP measurements, excitation was set to 500 nm and emission was set to 540 nm; for mCherry measurements, excitation was set to 580 nm and emission was set to 610 nm.
Antibody generation.
Purified Vibrio harveyi LuxR protein (300 μg; purified as previously described [59]) was sent to Cocalico Biologicals, Inc. for serial injection into a rabbit host for antibody generation. Serum obtained from the third bleed has been used for Western analyses.
Western blotting.
From overnight cultures, cells were concentrated to an OD600 of 20 in Instant Ocean medium. Cells were lysed on a FastPrep-24 classic instrument at 4°C, and then lysates were clarified by centrifugation. Lysates were then boiled with an equal volume of 2× SDS-PAGE sample buffer (220 mM Tris, pH 6.8, 25% glycerol, 1.2% SDS, 0.02% bromophenol blue, and 5% β-mercaptoethanol). Proteins were separated on a 15% SDS polyacrylamide gel by SDS electrophoresis, electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane, and probed with rabbit polyclonal α-LuxR serum or mouse monoclonal α-RpoA (BioLegend) primary antibodies. LuxR is the HapR homolog in Vibrio harveyi and has 72% identity and 86% similarity to V. cholerae HapR. The LuxR antibody was empirically found to be cross-reactive with V. cholerae HapR, and so it was used to detect HapR protein levels. Blots were then incubated with α-rabbit or α-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies, developed using Pierce ECL Western blotting substrate (Thermo Fisher), and exposed to film.
Chitin bead culturing.
Chitin beads (200 μl of a 50% slurry; NEB) and overnight cultures of the indicated strains were washed with defined artificial salt water (DASW) medium, which was prepared exactly as previously described (8). Chitin beads were inoculated with V. cholerae cells to an OD600 of 0.1 in a final volume of 5 ml in a Costar 6-well plate (Corning). Chitin mixtures were incubated statically at 30°C for 7 days before imaging.
Microscopy data collection and analysis.
To image chitin beads, cultured beads were gently transferred to a coverslip using wide-bore pipette tips. To image chitin-grown cells, chitin bead reaction mixtures were vortexed and then centrifuged at 250 × g for 1 min. Cells found in the supernatant were transferred to a coverslip. To image Δcbp strains, overnight cultures grown in LB were washed and resuspended to an OD600 of 0.2 in DASW and then transferred to a coverslip. Samples on the coverslip were placed under a 0.2% Gelzan pad and imaged on an inverted Nikon Ti2 microscope with a Plan Apo 60× objective, yellow fluorescent protein (YFP) and mCherry filter cubes, a Hamamatsu ORCA-Flash 4.0 camera, and Nikon NIS-Elements imaging software.
The strains used to examine Pchb expression across a population contained (i) a Pchb-mCherry reporter and (ii) a reporter that drove constitutive expression of GFP, Pconst2-GFP. By using these reporters, the expression of the Pchb-mCherry could be normalized to GFP expression in each cell. Images were analyzed on Fiji using the MicrobeJ plugin (60) to determine mean intensity of cells in the YFP and mCherry channels. GFP was assessed using a YFP filter set to avoid background fluorescence from chitin beads, which was stronger in the GFP channel. Background fluorescence was subtracted from each channel, and the mCherry/GFP fluorescence was determined for each individual cell.
HapR protein purification.
A plasmid expressing a hexahistidine-tagged hapR wild-type (WT) allele was generated using Gibson assembly. The hapR insert was amplified from V. cholerae E7946 and inserted into a pET28b vector (Novagen) using the primers listed in Table 1. The plasmid was then electroporated into E. coli BL21(DE3) for protein overexpression. This strain was grown overnight in LB medium with kanamycin, back-diluted 1:100 into 1 liter of LB medium with kanamycin, and grown to an OD600 of 0.4 to 0.6 at 30°C. Expression of HapR was induced by IPTG to a final concentration of 1 mM, and cultures were grown for 4 h shaking at 30°C. The cells were pelleted and frozen at −80°C. The pellet was resuspended in 25 ml buffer A (25 mM Tris, pH 8, 500 mM NaCl), and an Avestin EmulsiFlex-C3 emulsifier was used to lyse cells. The soluble lysate was applied to a HisTrap HP nickel nitrilotriacetic acid (Ni-NTA) column using an Äkta Pure fast protein liquid chromatography (FPLC) system in buffer A and eluted from the column with a gradient of buffer B (25 mM Tris, pH 8, 500 mM NaCl, 1 M imidazole). The purified protein was concentrated to approximately 5 ml using Sartorius Vivaspin turbo 10,000 molecular weight cutoff (MWCO) centrifugal concentrators. The sample was manually injected into the Äkta Pure and separated via size exclusion chromatography on a HiLoad 16/600 Superdex 75-pg column equilibrated with gel filtration buffer (25 mM Tris, pH 7.5, 200 mM NaCl). Eluted fractions were analyzed by SDS-PAGE (15% gel), pooled, and concentrated using the same centrifugal concentrators previously mentioned. The samples were then immediately frozen in liquid nitrogen with a final concentration of 10% glycerol and stored at −80°C.
Electrophoretic mobility shift assay.
Binding reactions contained 10 mM Tris HCl, pH 7.5, 1 mM EDTA, 10 mM KCl, 1 mM dithiothreitol (DTT), 50 μg/ml bovine serum albumin (BSA), 0.1 mg/ml salmon sperm DNA, 5% glycerol, 1 nM Cy5-labeled DNA probe, and HapR at the indicated concentrations (diluted in 10 mM Tris, pH 7.5, 10 mM KCl, 1 mM DTT, and 5% glycerol). Reaction mixtures were incubated at room temperature for 20 min in the dark and then electrophoretically separated on polyacrylamide gels in 0.5× Tris-borate-EDTA (TBE) buffer. Gels were imaged for Cy5 fluorescence on a Typhoon 9210 instrument. Short DNA probes (30 bp) were made by end-labeling one primer of a complementary pair (primers listed in Table 1) using 20 μM Cy5-dCTP and terminal deoxynucleotidyl transferase (TdT; Promega). Complementary primers (one labeled with Cy5 and the other unlabeled) were annealed by slow cooling at equimolar concentrations in annealing buffer (10 mM Tris, pH 7.5, and 50 mM NaCl). Pchb and PclpP probes were made by Phusion PCR, where Cy5-dCTP was included in the reaction at a level that would result in incorporation of 1 to 2 Cy5-labeled nucleotides in the final probe as previously described.
ChIP-qPCR assays.
Assays were carried out exactly as previously described (10). Briefly, overnight cultures were diluted to an OD600 of 0.08 and then grown for 6 h at 30°C. Cultures were cross-linked using 1% paraformaldehyde and then quenched with a 1.2 molar excess of Tris. Cells were washed with phosphate-buffered saline (PBS) and stored at −80°C overnight. The next day, cells were resuspended in lysis buffer (1× FastBreak cell lysis reagent [Promega], 50 μg/ml lysozyme, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 1× protease inhibitor cocktail; 100× inhibitor cocktail contained the following: 0.07 mg/ml phosphoramidon [Santa Cruz], 0.006 mg/ml bestatin [MP Biomedicals/Fisher Scientific], 1.67 mg/ml AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride; DOT Scientific], 0.07 mg/ml pepstatin A [GoldBio], and 0.07 mg/ml E64 [Gold Bio]) and then lysed by sonication, resulting in a DNA shear size of ∼500 bp. Lysates were incubated with anti-FLAG M2 magnetic beads (Sigma), washed to remove unbound proteins, and then bound protein-DNA complexes were eluted off with SDS. Samples were digested with proteinase K, then cross-links were reversed. DNA samples were cleaned up and used as a template for quantitative PCR (qPCR) using iTaq Universal SYBR green supermix (Bio-Rad) and primers specific for the genes indicated (primers are listed in Table 1) on a StepOne qPCR system. Standard curves of genomic DNA were included in each experiment and were used to determine the abundance of each amplicon in the input (derived from the lysate prior to ChIP) and output (derived from the samples after ChIP). Primers to amplify rpoB served as a baseline control in this assay because HapR does not bind this locus. Data are reported as “fold enrichment,” which is defined as the ratio of the test promoter (Pchb or PclpP)/rpoB found in the output divided by the same ratio found in the input.
Chitobiose competition.
Overnight cultures grown in LB were washed with M9 medium and mixed 1:1. For each growth reaction mixture, 102 cells of this mixture was added to M9 medium supplemented with 0.2% chitobiose and 10 μM synthetic CAI-1 and grown shaking at 30°C for 24 h. After 24 h, ∼102 cells from this mixture was used to inoculate fresh growth reactions to achieve additional generations of growth on chitobiose. This was repeated a third time after another 24 h. CAI-1 was supplemented throughout these experiments to ensure consistently high levels of HapR expression throughout transfer steps. After each 24-h growth period, the CFU per milliliter was determined for each strain in the mixture by dilution plating on LB agar supplemented with X-Gal. Competing strains were discerned by blue/white screening as one was a lacZ+ strain and the other was a ΔlacZ strain. A lacZ+ strain was competed against a ΔlacZ strain (parent-parent competition) or against a ΔlacZ ΔhapR strain (ΔhapR strain-parent competition). V. cholerae can grow on chitobiose through the activity of the following two sugar transporters: the GlcNAc phosphotransferase system (PTS) transporter (VC0995) or the chitobiose ABC transporter (VC0616-0620). Because we wanted to study the regulation of the transporter encoded within the chb locus, all strains for this assay had a deletion in VC0995 that renders growth on chitobiose dependent on the chb locus as previously described (11). Competitive indices were calculated as the CFU ratio of the ΔlacZ strain/lacZ+ strain after growth for the indicated number of generations divided by the CFU ratio of the ΔlacZ strain/lacZ+ strain in the initial inoculum.
Statistics.
Statistical comparisons were determined using Student's t test or one-way analysis of variance (ANOVA) with Tukey’s posttest using GraphPad Prism software.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ryan Chaparian for offering experimental advice, David Grainger for helpful discussions, and Wai-Leung Ng for providing synthetic CAI-1.
This work was supported by grant R35GM128674 from the National Institutes of Health to A.B.D. and grant R35GM124698 from the National Institutes of Health to J.C.V.K.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Pruzzo C, Vezzulli L, Colwell RR. 2008. Global impact of Vibrio cholerae interactions with chitin. Environ Microbiol 10:1400–1410. doi: 10.1111/j.1462-2920.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 2.Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283. doi: 10.1128/AEM.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahar S, Sultana M, Naser MN, Nair GB, Watanabe H, Ohnishi M, Yamamoto S, Endtz H, Cravioto A, Sack RB, Hasan NA, Sadique A, Huq A, Colwell RR, Alam M. 2011. Role of shrimp chitin in the ecology of toxigenic Vibrio cholerae and cholera transmission. Front Microbiol 2:260. doi: 10.3389/fmicb.2011.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt DE, Gevers D, Vahora NM, Polz MF. 2008. Conservation of the chitin utilization pathway in the Vibrionaceae. Appl Environ Microbiol 74:44–51. doi: 10.1128/AEM.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colwell RR, Huq A, Islam MS, Aziz KM, Yunus M, Khan NH, Mahmud A, Sack RB, Nair GB, Chakraborty J, Sack DA, Russek-Cohen E. 2003. Reduction of cholera in Bangladeshi villages by simple filtration. Proc Natl Acad Sci U S A 100:1051–1055. doi: 10.1073/pnas.0237386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huq A, Yunus M, Sohel SS, Bhuiya A, Emch M, Luby SP, Russek-Cohen E, Nair GB, Sack RB, Colwell RR. 2010. Simple sari cloth filtration of water is sustainable and continues to protect villagers from cholera in Matlab, Bangladesh. mBio 1:e00034-10. doi: 10.1128/mBio.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meibom KL, Li XB, Nielsen AT, Wu CY, Roseman S, Schoolnik GK. 2004. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci U S A 101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Roseman S. 2004. The chitinolytic cascade in Vibrios is regulated by chitin oligosaccharides and a two-component chitin catabolic sensor/kinase. Proc Natl Acad Sci U S A 101:627–631. doi: 10.1073/pnas.0307645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klancher CA, Yamamoto S, Dalia TN, Dalia AB. 2020. ChiS is a noncanonical DNA-binding hybrid sensor kinase that directly regulates the chitin utilization program in Vibrio cholerae. bioRxiv doi: 10.1101/2020.01.10.902320. [DOI] [PMC free article] [PubMed]
- 11.Klancher CA, Hayes CA, Dalia AB. 2017. The nucleoid occlusion protein SlmA is a direct transcriptional activator of chitobiose utilization in Vibrio cholerae. PLoS Genet 13:e1006877. doi: 10.1371/journal.pgen.1006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung SA, Chapman CA, Ng WL. 2015. Quadruple quorum-sensing inputs control Vibrio cholerae virulence and maintain system robustness. PLoS Pathog 11:e1004837. doi: 10.1371/journal.ppat.1004837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball AS, Chaparian RR, van Kessel JC. 2017. Quorum sensing gene regulation by LuxR/HapR master regulators in vibrios. J Bacteriol 199:e00105-17. doi: 10.1128/JB.00105-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suckow G, Seitz P, Blokesch M. 2011. Quorum sensing contributes to natural transformation of Vibrio cholerae in a species-specific manner. J Bacteriol 193:4914–4924. doi: 10.1128/JB.05396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun S, Tay QX, Kjelleberg S, Rice SA, McDougald D. 2015. Quorum sensing-regulated chitin metabolism provides grazing resistance to Vibrio cholerae biofilms. ISME J 9:1812–1820. doi: 10.1038/ismej.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto S, Mitobe J, Ishikawa T, Wai SN, Ohnishi M, Watanabe H, Izumiya H. 2014. Regulation of natural competence by the orphan two-component system sensor kinase ChiS involves a non-canonical transmembrane regulator in Vibrio cholerae. Mol Microbiol 91:326–347. doi: 10.1111/mmi.12462. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S, Ohnishi M. 2017. Glucose-specific enzyme IIA of the phosphoenolpyruvate:carbohydrate phosphotransferase system modulates chitin signaling pathways in Vibrio cholerae. J Bacteriol 199:e00127-17. doi: 10.1128/JB.00127-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blokesch M. 2012. Chitin colonization, chitin degradation and chitin-induced natural competence of Vibrio cholerae are subject to catabolite repression. Environ Microbiol 14:1898–1912. doi: 10.1111/j.1462-2920.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 20.Sauer RT, Baker TA. 2011. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem 80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- 21.Lee KJ, Jung YC, Park SJ, Lee KH. 2018. Role of heat shock proteases in quorum-sensing-mediated regulation of biofilm formation by Vibrio species. mBio 9:e02086-17. doi: 10.1128/mBio.02086-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis JH, Rubin AJ, Sauer RT. 2011. Design, construction and characterization of a set of insulated bacterial promoters. Nucleic Acids Res 39:1131–1141. doi: 10.1093/nar/gkq810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo Scrudato M, Blokesch M. 2012. The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet 8:e1002778. doi: 10.1371/journal.pgen.1002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Mekalanos JJ. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell 5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 25.van Kessel JC, Ulrich LE, Zhulin IB, Bassler BL. 2013. Analysis of activator and repressor functions reveals the requirements for transcriptional control by LuxR, the master regulator of quorum sensing in Vibrio harveyi. mBio 4:e00378-13. doi: 10.1128/mBio.00378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DH, Jeong HS, Jeong HG, Kim KM, Kim H, Choi SH. 2008. A consensus sequence for binding of SmcR, a Vibrio vulnificus LuxR homologue, and genome-wide identification of the SmcR regulon. J Biol Chem 283:23610–23618. doi: 10.1074/jbc.M801480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME suite: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaparian RR, Tran MLN, Miller Conrad LC, Rusch DB, van Kessel JC. 2020. Global H-NS counter-silencing by LuxR activates quorum sensing gene expression. Nucleic Acids Res 48:171–183. doi: 10.1093/nar/gkz1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jobling MG, Holmes RK. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol 26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 30.Freeman JA, Lilley BN, Bassler BL. 2000. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol 35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- 31.Freeman JA, Bassler BL. 1999. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J Bacteriol 181:899–906. doi: 10.1128/JB.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. 2005. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell 18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Tu KC, Bassler BL. 2007. Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev 21:221–233. doi: 10.1101/gad.1502407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman JA, Bassler BL. 1999. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol 31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 35.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 36.Surette MG, Miller MB, Bassler BL. 1999. Quorum sensing in Escherichia coli, Salmonella Typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A 96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bridges AA, Bassler BL. 2019. The intragenus and interspecies quorum-sensing autoinducers exert distinct control over Vibrio cholerae biofilm formation and dispersal. PLoS Biol 17:e3000429. doi: 10.1371/journal.pbio.3000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurley A, Bassler BL. 2017. Asymmetric regulation of quorum-sensing receptors drives autoinducer-specific gene expression programs in Vibrio cholerae. PLoS Genet 13:e1006826. doi: 10.1371/journal.pgen.1006826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroh HE, Simon LD. 1990. The ClpP component of Clp protease is the sigma 32-dependent heat shock protein F21.5. J Bacteriol 172:6026–6034. doi: 10.1128/jb.172.10.6026-6034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grossman AD, Erickson JW, Gross CA. 1984. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell 38:383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- 41.Katayama Y, Gottesman S, Pumphrey J, Rudikoff S, Clark WP, Maurizi MR. 1988. The two-component, ATP-dependent Clp protease of Escherichia coli. Purification, cloning, and mutational analysis of the ATP-binding component. J Biol Chem 263:15226–15236. [PubMed] [Google Scholar]
- 42.Gerth U, Kruger E, Derre I, Msadek T, Hecker M. 1998. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol Microbiol 28:787–802. doi: 10.1046/j.1365-2958.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 43.Pennetzdorfer N, Lembke M, Pressler K, Matson JS, Reidl J, Schild S. 2019. Regulated proteolysis in Vibrio cholerae allowing rapid adaptation to stress conditions. Front Cell Infect Microbiol 9:214. doi: 10.3389/fcimb.2019.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schild S, Tamayo R, Nelson EJ, Qadri F, Calderwood SB, Camilli A. 2007. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe 2:264–277. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benitez JA, Spelbrink RG, Silva A, Phillips TE, Stanley CM, Boesman-Finkelstein M, Finkelstein RA. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect Immun 65:3474–3477. doi: 10.1128/IAI.65.8.3474-3477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benitez JA, Silva AJ, Finkelstein RA. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect Immun 69:6549–6553. doi: 10.1128/IAI.69.10.6549-6553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. 2014. Solutions to the public goods dilemma in bacterial biofilms. Curr Biol 24:50–55. doi: 10.1016/j.cub.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawver LA, Giulietti JM, Baleja JD, Ng WL. 2016. Quorum sensing coordinates cooperative expression of pyruvate metabolism genes to maintain a sustainable environment for population stability. mBio 7:e01863-16. doi: 10.1128/mBio.01863-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDonough E, Kamp H, Camilli A. 2016. Vibrio cholerae phosphatases required for the utilization of nucleotides and extracellular DNA as phosphate sources. Mol Microbiol 99:453–469. doi: 10.1111/mmi.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller VL, DiRita VJ, Mekalanos JJ. 1989. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J Bacteriol 171:1288–1293. doi: 10.1128/jb.171.3.1288-1293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yildiz FH, Liu XS, Heydorn A, Schoolnik GK. 2004. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol Microbiol 53:497–515. doi: 10.1111/j.1365-2958.2004.04154.x. [DOI] [PubMed] [Google Scholar]
- 52.Dalia AB, Lazinski DW, Camilli A. 2013. Characterization of undermethylated sites in Vibrio cholerae. J Bacteriol 195:2389–2399. doi: 10.1128/JB.02112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalia AB, McDonough E, Camilli A. 2014. Multiplex genome editing by natural transformation. Proc Natl Acad Sci U S A 111:8937–8942. doi: 10.1073/pnas.1406478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalia AB. 2018. Natural cotransformation and multiplex genome editing by natural transformation (MuGENT) of Vibrio cholerae. Methods Mol Biol 1839:53–64. doi: 10.1007/978-1-4939-8685-9_6. [DOI] [PubMed] [Google Scholar]
- 55.Simpson CA, Podicheti R, Rusch DB, Dalia AB, van Kessel JC. 2019. Diversity in natural transformation frequencies and regulation across Vibrio species. mBio 10:e02788-19. doi: 10.1128/mBio.02788-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cha RS, Zarbl H, Keohavong P, Thilly WG. 1992. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl 2:14–20. doi: 10.1101/gr.2.1.14. [DOI] [PubMed] [Google Scholar]
- 57.Dalia AB. 2016. RpoS is required for natural transformation of Vibrio cholerae through regulation of chitinases. Environ Microbiol 18:3758–3767. doi: 10.1111/1462-2920.13302. [DOI] [PubMed] [Google Scholar]
- 58.Ng WL, Perez LJ, Wei Y, Kraml C, Semmelhack MF, Bassler BL. 2011. Signal production and detection specificity in Vibrio CqsA/CqsS quorum-sensing systems. Mol Microbiol 79:1407–1417. doi: 10.1111/j.1365-2958.2011.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaparian RR, Olney SG, Hustmyer CM, Rowe-Magnus DA, van Kessel JC. 2016. Integration host factor and LuxR synergistically bind DNA to coactivate quorum-sensing genes in Vibrio harveyi. Mol Microbiol 101:823–840. doi: 10.1111/mmi.13425. [DOI] [PubMed] [Google Scholar]
- 60.Ducret A, Quardokus EM, Brun YV. 2016. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1:16077. doi: 10.1038/nmicrobiol.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.