ABSTRACT
The aim of this work was to report the detection of a putative novel Ehrlichia strain associated with the tick Amblyomma triste. Free-living adult ticks determined as A. triste were collected by drag-sampling in Argentina and Uruguay. Molecular detection of Ehrlichia agents was performed targeting three different loci: 16S rRNA gene, dsb gene and a fragment of groESL heat shock operon. In total, 164 adults of A. triste (38 from INTA E.E.A Delta del Paraná in Argentina and 126 from Toledo Chico in Uruguay) were analyzed. One tick (0.6%) collected in INTA E.E.A. Delta del Paraná (Argentina) was positive. The phylogenetic analyses show that the Ehrlichia strain found in this study (named Ehrlichia sp. strain Delta) represents an independent lineage within the genus Ehrlichia, close to E. chaffeensis and E. muris. This is also the first report of an Ehrlichia agent infecting the tick A. triste. The medical and veterinary significance of Ehrlichia sp. strain Delta remains to be demonstrated. However, it is important to mention that adults of A. triste are aggressive to humans and domestic mammals. Therefore, the potential role of A. triste in the transmission of Ehrlichia agents to humans or domestic animals across its distributional range should be highlighted, even more considering that Ehrlichia sp. strain Delta is phylogenetically related to the zoonotic E. chaffeensis, which is recognized as pathogenic to both humans and animals.
KEYWORDS: Ehrlichia, ticks, Amblyomma, South America
Introduction
Bacteria of the genus Ehrlichia (Rickettsiales: Anaplasmataceae) are alpha-proteobacterial, tick-transmitted, obligate intracellular parasites with medical and veterinary importance that can infect monocytes, neutrophils, endothelial cells or neutrophils [1,2]. Formally there are six recognized species: Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia ruminantium, Ehrlichia muris and Ehrlichia minasensis, but different strains of putative novel species of Ehrlichia have been molecularly detected in the last 20 years [1–14, among others].
Amblyomma triste is part of the Amblyomma maculatum group, which also includes to Amblyomma maculatum sensu stricto and Amblyomma tigrinum [15]. This complex is widely distributed throughout the Neotropical and Nearctic regions, from southern U.S.A. to Argentina and Uruguay [1,15,16]. These three species have medical and veterinary relevance since their adult stages are aggressive to humans and domestic mammals, and because they transmit the human pathogen Rickettsia parkeri [16]. In the Southern Cone of America, specifically in Argentina and Uruguay, A. triste adults are prone to infest humans, dogs and cattle, and it is the principal vector of R. parkeri [16–19].
In Argentina, the records of Ehrlichia spp. correspond to E. canis detected in blood samples of dogs and in ticks from the Rhipicephalus sanguineus group [20–24], Ehrlichia sp. strain San Luis (closely related to E. chaffeensis) infecting A. tigrinum and Amblyomma parvum ticks [11,25], Ehrlichia sp. strain La Dormida associated to Amblyomma neumanni [14], others Ehrlichia spp. infecting A. tigrinum [11,12], and two reports of Ehrlichia cf. E. chaffeensis infecting A. parvum [26] and the marsh deer Blastocerus dichotomus [27]. Furthermore, Ripoll et al. [28] presented serology-based evidence of human infection with E. chaffeensis (or an antigenically related species) in Jujuy Province, and Halac [29] reported a case of human disease in Cordoba Province attributable to infection with Ehrlichia, although the evidence presented by this author is not enough to confirm it. No Ehrlichia species have been detected in Uruguay so far.
Due to the relevance of A. triste in terms of public health and veterinary issues, the knowledge of the potential role that this tick play in the transmission of tick-borne pathogens constitutes a relevant trait. Therefore, the aim of this work was to report the detection of a putative novel Ehrlichia strain associated with the tick A. triste and to describe its phylogenetic relationship with other species and strains of the genus Ehrlichia.
Materials & methods
Questing adults ticks (unfed) were collected by drag-sampling of the vegetation using a 1.50 m white cloth flag between September, October and November of 2017 at the Estación Experimental Agropecuaria Delta del Paraná, Instituto Nacional de Tecnología Agropecuaria (INTA E.E.A. Delta del Paraná), Campana (34º11´S, 58º50´W), Buenos Aires Province, Argentina, and Toledo Chico (34º44´S, 56º06´W), Canelones Department, Uruguay. Both localities belong to the Pampa Biogeographic Province as described in Morrone et al. [30]. Ticks were determined following Nava et al. [16].
DNA extraction of each tick was carried out by using the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) following the manufacturer’s instructions. Molecular diagnosis by PCR was performed according to methods previously described by the authors cited in Table 1. Initial screening for Anaplasmataceae was performed with a PCR-amplified fragment of the 16S rRNA gene following conditions described in Parola et al. [31] and Anaplasma centrale was used as a positive control. Samples showed to be positive to Ehrlichia were further used to amplify a ca. 350-bp fragment of the dsb gene following the methods described in Aguiar et al. [32] and Almeida et al. [6], and a ca. 1100-bp fragment of groESL heat shock operon of Ehrlichia according to Liz et al. [33], with E. canis as a positive control. Nuclease-free water was used as a negative control for all PCRs.
Table 1.
Used primers for the detection of Ehrlichia.
| Target | Name | Primer sequences (5´-3´) | Reference |
|---|---|---|---|
| 16S rRNA | EHR16SD | GGTACCYACAGAAGAAGTCC | [30] |
| EHR16SR | TAGCACTCATCGTTTACAGC | ||
| dsb | dsb-330 | GATGATGTCTGAAGATATGAAACAAAT | [6,31] |
| dsb-380 | ATTTTTAGRGATTTTCCAATACTTGG | ||
| dsb-728 | CTGCTCGTCTATTTTACTTCTTAAAGT | ||
| groESL | HS1a | AITGGGCTGGTAITGAAAT | [32] |
| HS6a | CCICCIGGIACIAIACCTTC | ||
| HS43 | ATWGCWAARGAAGCATAGTC | ||
| HSVR | CTCAACAGCAGCTCTAGTAGC |
PCR-products were purified using the Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, USA) and sequenced with a 3500 Genetic Analyzer sequencer (Applied Biosystems, Foster City, USA). Sequences were edited using BioEdit Sequence Alignment Editor [34] with manual edition whenever it was necessary, aligned with the program Clustal W [35] and compared with those sequences of Ehrlichia and Anaplasma deposited in GenBank by using BLAST (www.ncbi.nlm.nih.gov/blast). Phylogenetic analyses were performed with Maximum-likelihood (ML) and best-fitting substitution models were determined with the Akaike Information Criterion using the ML model test implemented in MEGA 5.0 [36]. Support for the topologies was tested by bootstrapping over 1000 replications and gaps were excluded from the comparisons. Sequences of Anaplasma marginale (dsb) and Neorickettsia risticii (groESL) were included as outgroup.
Results
In total, 218 adults of A. triste (38 from INTA E.E.A. Delta del Paraná and 180 from Toledo Chico) were analyzed for Ehrlichia and Anaplasma infection. One tick (0.45%) was positive for the initial PCR of the Anaplasmataceae family. The positive tick was a female collected in INTA E.E.A Delta del Paraná. The sequence obtained from a fragment of the 16S rRNA gene (GenBank accession number: MT672744) matched with a high similarity with different 16S sequences belonging to species and strains of the genus Ehrlichia.
The positive sample of the 16S rRNA fragment was also positive for the heminested dsb and nested groESL PCRs. Both amplicons were sequenced (GenBank accession number of dsb sequence: MT681331; GenBank accession number of groESL sequence: MT681330) and phylogenetically analyzed because they have enough polymorphism to differentiate Ehrlichia spp. at the species level. The ML phylogenetic trees for the dsb fragment (Figure 1) show that the Ehrlichia strain found in this study, named as Ehrlichia sp. strain Delta, represent an independent lineage within the genus Ehrlichia, close to E. chaffeensis and E. muris. They form a clade with 70% bootstrap support. The phylogenetic analysis of groESL sequences (Figure 2) also indicates that Ehrlichia sp. strain Delta is genetically different from the remaining Ehrlichia spp. and that it also constitutes a clade (89% bootstrap support) with E. chaffeensis and E. muris, and other strains not yet formally described. The dsb sequence of Ehrlichia sp. strain Delta differed by more than 10% with the remaining sequences of Ehrlichia spp. available in GenBank (an alignment of 231 bp was analyzed), and that of groESL by more than 4.75% (an alignment of 980 bp was analyzed).
Figure 1.
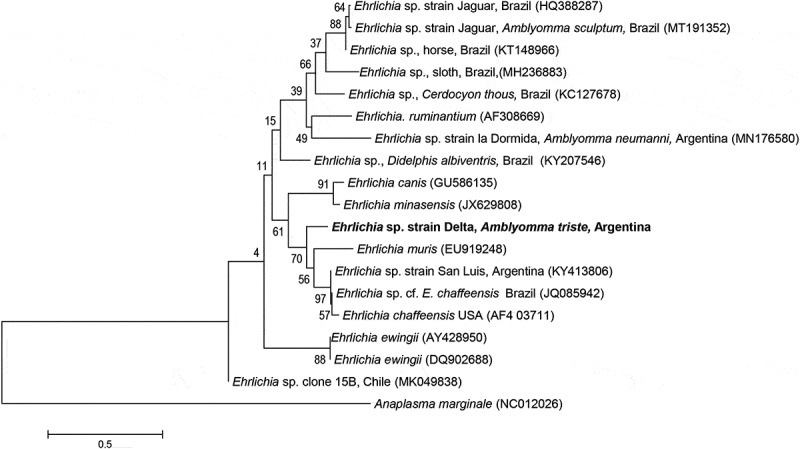
Maximum-likelihood tree constructed from dsb sequences of Ehrlichia spp. (substitution model: Tamura 3 parameter + G). Numbers represent bootstrap support generated from 1,000 replications. GenBank accession numbers are in brackets.
Figure 2.
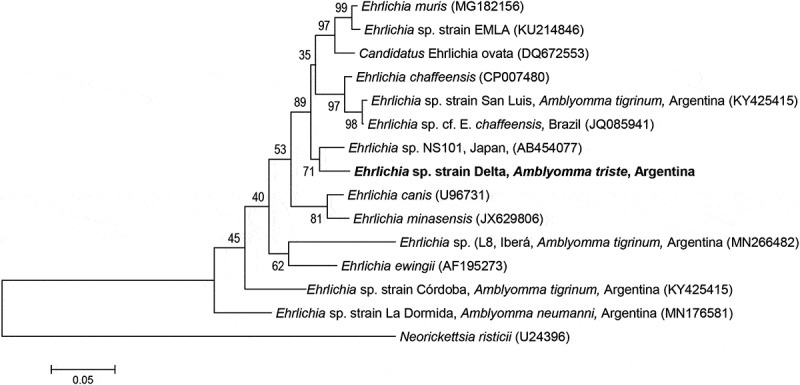
Maximum-likelihood tree constructed from groESL sequences of Ehrlichia spp. (substitution model: GTR + G). Numbers represent bootstrap support generated from 1,000 replications. GenBank accession numbers are in brackets.
Discussion
This is the first report of the genus Ehrlichia in the tick A. triste. In the current work, DNA sequences from three different loci were studied to infer the phylogenetic relationships of the Ehrlichia sp. detected in A. triste. Specifically, dsb and groESL molecular markers have enough polymorphism to characterize the erhlichial agents at lower taxonomic levels. Phylogenetic analyses showed that Ehrlichia sp. strain Delta is closely related to E. chaffeensis and E. muris (Figures 1 and 2).
In Argentina, the previous reports of ehrlichial agents close to E. chaffeensis were in A. tigrinum and A. parvum ticks from the north-central area of the country [11,25], where A. triste is not distributed. Interestingly, Guillemi et al. [27] report Ehrlichia cf. E. chaffeensis infecting the marsh deer B. dichotomus from Paraná Delta Area and Iberá Wetland from Argentina, but no sequences of dsb and groESL genes were obtained from that Ehrlichia detected in B. dichotomous. Therefore, a comparison between both Ehrlichia strains is currently not feasible. Previously, Venzal et al. [37] found no positive ticks for Ehrlichia in an analysis of 51 specimens of A. triste from Uruguay. The A. triste ticks from Uruguay analyzed during this work were also negative for Ehrlichia infection.
Amblyomma triste, A. maculatum and A. tigrinum are phylogenetically very close [15]. Amblyomma triste sensu stricto is found in Argentina, Paraguay, Uruguay and southern Brazil, ecologically associated with wetlands and flood areas [15,16]. In the Southern Cone of America, this tick is distributed in the sub-basins of the Paraná and Uruguay rivers, and in the Samborombón Bay in the province of Buenos Aires, including Buenos Aires city [18,38,39]. The principal hosts for the adults of A. triste are the marsh deer B. dichotomus, wild and domestic carnivorous (dogs, Panthera onca, Puma concolor, Herpailurus yagouaroundi, Chrysocyon brachyurus, Lycalopex vetulus), cattle and Hydrochoerus hydrochaeris [16,18]. Adults of this tick are also aggressive to humans (16). Small rodents of the families Cricetidae (subfamily Sigmodontidae) and Caviidae are the principal hosts for immature stages [16,18,40].
Vertical transmission of bacteria of the genus Ehrlichia appears to be exclusively transstadial because the transovarial transmission has not been demonstrated for this genus [1]. Thus, infection with Ehrlichia is acquired by a tick during feeding of larvae or nymphs, and then the infection pass to adults ticks by transtadial transmission. Sigmondontid or caviid rodents, principal hosts for immature stages of A. triste, are candidate to be reservoir hosts responsible for maintenance of the enzootic cycle of Ehrlichia sp. strain Delta detected in this work, as it was also hypothesized for the Ehrlichia sp. strain San Luis detected in A. tigrinum, which is very closely related to E. chaffeensis [11]. In this sense, it is important to highlights that E. chaffeensis, E. muris and other closely related ehrlichial agents have been detected in rodents [41,42].
The medical and veterinary significance of Ehrlichia sp. strain Delta remains to be demonstrated. However, it is important to mention that A. triste adults are aggressive to humans and domestic mammals as cattle and dogs [16]. Therefore, the potential role of A. triste in the transmission of Ehrlichia agents to humans or domestic animals across its distributional range should be highlighted, even more considering that Ehrlichia sp. strain Delta is phylogenetically related to the zoonotic E. chaffeensis, which is recognized as pathogenic to both humans and animals [40]. The finding of this work plus those data obtained in the last years in South America regarding the circulation of Ehrlichia spp. other than E. canis (i.e. E. minasensis, Ehrlichia sp.strain Córdoba, Ehrlichia sp.strain San Luis, Ehrlichia sp. strain L8 (Iberá), Ehrlichia sp. strain La Dormida, Ehrlichia sp. cf. E. chaffeensis, Ehrlichia sp. clone 15B, Ehrlichia sp. from Didelphis albiventris, Ehrlichia sp. from Cerdocyon thous, Ehrlichia sp. from sloth, Ehrlichia sp. from horse, Ehrlichia sp. strain Jaguar and Ehrlichia sp. strain Delta [5,6,8,11–14,26,27,43,44, this work]), clearly highlight the need to consider as targets these microorganisms when serological, molecular and clinical studies on tick-borne pathogens in humans and domestic and wild mammals are conducted in countries of this continent.
Acknowledgments
We are grateful to INTA and Asociación Cooperadora INTA Rafaela for the financial support to SN.
Funding Statement
This work was supported by INTA (PEI109) and Asociación Cooperadora INTA Rafaela.
Disclosure statement
The authors of the current study declare no conflict of interest.
References
- [1].Brouqui P, Matsumoto K.. Bacteriology and phylogeny of Anaplasmataceae. In: Raoult D, Parola P, editors. Rickettsial diseases. New York (NY): Informa; 2007. p. 179–198. [Google Scholar]
- [2].Dumler JS, Barbet F, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combi. Int J Syst Evol Microbiol. 2001;51:2145–2165. [DOI] [PubMed] [Google Scholar]
- [3].Shibata SI, Kawahara M, Rikihisa Y, et al. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J Clin Microbiol. 2000;38:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Loftis AD, Reeves WK, Spurlock JP, et al. Infection of a goat with a tick-transmitted Ehrlichia from Georgia, U.S.A., that is closely related to Ehrlichia ruminantium. J Vector Ecol. 2006;31:213–223. [DOI] [PubMed] [Google Scholar]
- [5].Widmer CE, Azevedo FCC, Almeida AP, et al. Tick-borne bacteria in free-living jaguars (Panthera onca) in Pantanal, Brazil. Vector Borne Zoonotic Dis. 2011;11:1001–1005. [DOI] [PubMed] [Google Scholar]
- [6].Almeida PA, Marcili A, Labruna MB.. Novel Ehrlichia and Hepatozoon agents infecting the crab-eating fox (Cerdocyon thous) in Southeastern Brazil. J Med Entomol. 2013;50:640–646. [DOI] [PubMed] [Google Scholar]
- [7].Gofton E, Doggett S, Ratchford A, et al. Bacterial profiling reveals novel “Ca. Neoehrlichia”, Ehrlichia, and Anaplasma species in Australian human-biting ticks. PLoS One. 2015;10:0145449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cabezas Cruz A, Zweygarth E, Vancová M, et al. Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus. Int J Syst Evol Microbiol. 2016;66:1423–1430. [DOI] [PubMed] [Google Scholar]
- [9].Laroche M, Marie J, Mediannikov O, et al. A novel ehrlichial agent detected in tick in French Polynesia. Ticks Tick Borne Dis. 2016;7:1203–1208. [DOI] [PubMed] [Google Scholar]
- [10].Luo L, Sun J, Yan J, et al. Detection of a novel Ehrlichia species in Haemaphysalis longicornis tick from China. Vector Borne Zoonotic Dis. 2016;16:363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cicuttin GL, De Salvo MN, Nava S. Two novel Ehrlichia strains detected in Amblyomma tigrinum ticks associated to dogs in peri-urban areas of Argentina. Comp Immunol Microbiol Infect Dis. 2017;53:40–44. [DOI] [PubMed] [Google Scholar]
- [12].Eberhardt AT, Fernandez C, Fargnoli L, et al. A putative novel strain of Ehrlichia infecting Amblyomma tigrinum associated with Pampas fox (Lycalopex gymnocercus) in Esteros del Ibera ecoregion, Argentina. Ticks Tick Borne Dis. 2019;11:101318. [DOI] [PubMed] [Google Scholar]
- [13].Muñoz-Leal S, Clemes YS, Lopes MG, et al. Novel Ehrlichia sp. detected in Magellanic penguins (Sphenicus magellanicus) and in the seabird tick Ixodes uriae from Magdalena Island, southern Chile. Ticks Tick Borne Dis. 2019;10:101256. [DOI] [PubMed] [Google Scholar]
- [14].Fargnoli L, Fernadez C, Monje LD. Novel Ehrlichia strain infecting cattle tick Amblyomma neumanni, Argentina, 2018. Emerging Infect Dis. 2020;26:1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lado P, Nava S, Mendoza-Uribe L, et al. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: phenotypic plasticity or incipient speciation? Parasit Vectors. 2018;11:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nava S, Venzal JM, Gonzalez-Acuña DA, et al. Ticks of the Southern Cone of America: diagnosis, distribution and hosts with taxonomy, ecology and sanitary importance. London: Elsevier, Academic Press; 2017. [Google Scholar]
- [17].Venzal JM, Estrada-Peña A, Castro O, et al. Amblyomma triste Koch, 1844 (Acari: Ixodidae): hosts and seasonality of the vector of Rickettsia parkeri in Uruguay. Vet Parasitol. 2008;155:104–109. [DOI] [PubMed] [Google Scholar]
- [18].Nava S, Mangold AJ, Mastropaolo M, et al. Seasonal dynamics and hosts of Amblyomma triste (Acari: Ixodidae) in Argentina. Vet Parasitol. 2011;181:301–308. [DOI] [PubMed] [Google Scholar]
- [19].Romer Y, Borras P, Govedic F, et al. Clinical and epidemiological comparison of Rickettsia parkeri rickettsiosis, related to Amblyomma triste and Amblyomma tigrinum, in Argentina. Ticks Tick Borne Dis. 2020;11:101436. [DOI] [PubMed] [Google Scholar]
- [20].Eiras DF, Craviotto MB, Vezzani D, et al. First description of natural Ehrlichia canis and Anaplasma platys infections in dogs from Argentina. Comp Immunol Microbiol Infect Dis. 2013;36:169–173. [DOI] [PubMed] [Google Scholar]
- [21].Cicuttin GL, Tarragona EL, De Salvo MN, et al. Infection with Ehrlichia canis and Anaplasma platys (Rickettsiales: Anaplasmataceae) in two lineages of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) from Argentina. Ticks Tick Borne Dis. 2015;6:724–729. [DOI] [PubMed] [Google Scholar]
- [22].Cicuttin GL, De Salvo MN, Gury Dohmen FE. Molecular characterization of Ehrlichia canis infecting dogs, Buenos Aires. Ticks Tick-borne Dis. 2016;7:954–957. [DOI] [PubMed] [Google Scholar]
- [23].Cicuttin GL, De Salvo MN, Silva D, et al. Ehrlichia canis (Rickettsiales: Anaplasmataceae) en garrapatas Rhipicephalus sanguineus sensu lato del linaje templado (Acari : Ixodidae), provincia de Buenos Aires, Argentina. Rev FAVE (Sección Ciencias Veterinarias). 2017;16:93–96. [Google Scholar]
- [24].Tarragona ET, Flores FS, Herrera CL, et al. Primer reporte de un caso de ehrlichiosis monocítica canina en la provincia de Santa Fe, Argentina. Rev FAVE (Sección Ciencias Veterinarias). 2019;18:49–54. [Google Scholar]
- [25].Monje LD, Fernandez C, Percara A. Detection of Ehrlichia sp. strain San Luis and Candidatus Rickettsia andeanae in Amblyomma parvum ticks. Ticks Tick Borne Dis. 2018;10:111–114. [DOI] [PubMed] [Google Scholar]
- [26].Tomassone L, Nuñez P, Gurtler R, et al. Molecular detection of Ehrlichia chaffeensis in Amblyomma parvum ticks, Argentina. Emerg Infect Dis. 2008;14:1953–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guillemi EC, Orozco MM, Argibay HD, et al. Evidence of Ehrlichia chaffeensis in Argentina through molecular detection in marsh deer (Blastocerus dichotomus). Int J Parasitol Parasites Wildl. 2019;8:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ripoll CM, Remondegui CE, Ordoñez G, et al. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg. 1999;61:350–354. [DOI] [PubMed] [Google Scholar]
- [29].Halac E. Infección por Ehrlichia en un niño: características clínicas y revisión de la bibliografía. Arch Argent Pediatr. 2016;114:199–200. [DOI] [PubMed] [Google Scholar]
- [30].Morrone JJ. Biogeographic areas and transition zones of Latin American and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol. 2006;51:467–494. [DOI] [PubMed] [Google Scholar]
- [31].Parola P, Roux V, Camicas JL, et al. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans R Soc Trop Med Hyg. 2000;94:707–708. [DOI] [PubMed] [Google Scholar]
- [32].Aguiar DM, Cavalcante GT, Pinter A, et al. Prevalence of Ehrlichia canis (Rickettsiales: Anaplasmataceae) in dogs and Rhipicephalus sanguineus (Acari: Ixodidae) ticks from Brazil. J Med Entomol. 2007;44:126–132.30. [DOI] [PubMed] [Google Scholar]
- [33].Liz JS, Anderes J, Sumner JW, et al. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J Clin Microbiol. 2000;38:1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hall TA. BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- [35].Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. [DOI] [PubMed] [Google Scholar]
- [36].Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Venzal JM, Estrada-Peña A, Portillo A, et al. Detection of Alpha and Gamma-Proteobacteria in Amblyomma triste (Acari: Ixodidae) from Uruguay. Exp Appl Acarol. 2008;44:49–56. [DOI] [PubMed] [Google Scholar]
- [38].Cicuttin GL, Sassaroli JC, Ardiles MI, et al. Presencia de dos especies de garrapatas (Acari: Ixodidae) con importancia médica en la Ciudad de Buenos Aires. Medicina (B Aires) (Buenos Aires). 2013;73:389–390. [PubMed] [Google Scholar]
- [39].Guglielmone AA, Nava S, Mastropaolo M, et al. Distribution and genetic variation of Amblyomma triste (Acari: Ixodidae) in Argentina. Ticks Tick Borne Dis. 2013;4:386–390. [DOI] [PubMed] [Google Scholar]
- [40].Wolf RW, Aragona M, Muñoz-Leal S, et al. Novel Babesia and Hepatozoon agents in the Brazilian Pantanal, with the first record of the tick Ornithodoros guaporensis in Brazil. Ticks Tick-borne Dis. 2016;7:449–456. [DOI] [PubMed] [Google Scholar]
- [41].Yabsley MJ. Natural History of Ehrlichia chaffeensis: vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet Parasitol. 2010;167:136–148. [DOI] [PubMed] [Google Scholar]
- [42].Pritt BS, Allerdice MEJ, Sloan LM, et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int J Syst Evol Microbiol. 2017;67:2121–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Soares HS, Marcili A, Barbieri AM, et al. Novel Anaplasma and Ehrlichial organisms infecting the wildlife of two regions of the Brazilian Amazon. Acta Trop. 2017;174:82–87. [DOI] [PubMed] [Google Scholar]
- [44].Lopes MG, Muñoz-Leal S, Lima JYR, et al. Ticks, rickettsial and ehrlichial infection in small mammals from Atlantic forest remnants in northeastern Brazil. Int J Parasitol Parasites Wildl. 2018;7:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]


