Abstract
The presence of SARS-CoV-2 in the feces of infected patients and wastewater has drawn attention, not only to the possibility of fecal-oral transmission but also to the use of wastewater as an epidemiological tool. The COVID-19 pandemic has highlighted problems in evaluating the epidemiological scope of the disease using classical surveillance approaches, due to a lack of diagnostic capacity, and their application to only a small proportion of the population. As in previous pandemics, statistics, particularly the proportion of the population infected, are believed to be widely underestimated. Furthermore, analysis of only clinical samples cannot predict outbreaks in a timely manner or easily capture asymptomatic carriers. Threfore, community-scale surveillance, including wastewater-based epidemiology, can bridge the broader community and the clinic, becoming a valuable indirect epidemiological prediction tool for SARS-CoV-2 and other pandemic viruses. This article summarizes current knowledge and discusses the critical factors for implementing wastewater-based epidemiology of COVID-19.
Keywords: Wastewater-based epidemiology, Environmental monitoring, Coronavirus, SARS-CoV-2, COVID-19, Sewage
Graphical abstract
1. Introduction
The early 2020s will be remembered for the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes viral pneumonia called Coronavirus Disease 2019 (COVID-19). This new coronavirus strain, first detected in Wuhan (Hubei province, China), has rapidly spread across the world, causing the most consequential infection disease since the 1918 influenza pandemic.
SARS-CoV-2 is a positive-sense single-stranded RNA enveloped virus belonging to the Betacoronavirus genus in the family Coronaviridae (Order Nidovirales). The infection caused by SARS-CoV-2 is typically characterized by respiratory symptoms, indicative of airborne and droplet transmission (Chan et al., 2020). However, a significant proportion of patients infected with COVID-19 also show gastrointestinal symptoms and/or viral shedding in feces outlasting those from the respiratory tract (Holshue et al., 2020; Xu et al., 2020; Xiao et al., 2020a; Wu et al., 2020a, Wu et al., 2020b). Furthermore, intracellular staining of viral nucleocapsid protein in gastric, duodenal, and rectal epithelia showed that the virus can infect glandular epithelial cells in these areas and that virions are secreted from gastrointestinal cells (Xiao et al., 2020a). However, very limited evidence of SARS-CoV-2 infectivity in fecal samples has been obtained (Xiao et al., 2020b).
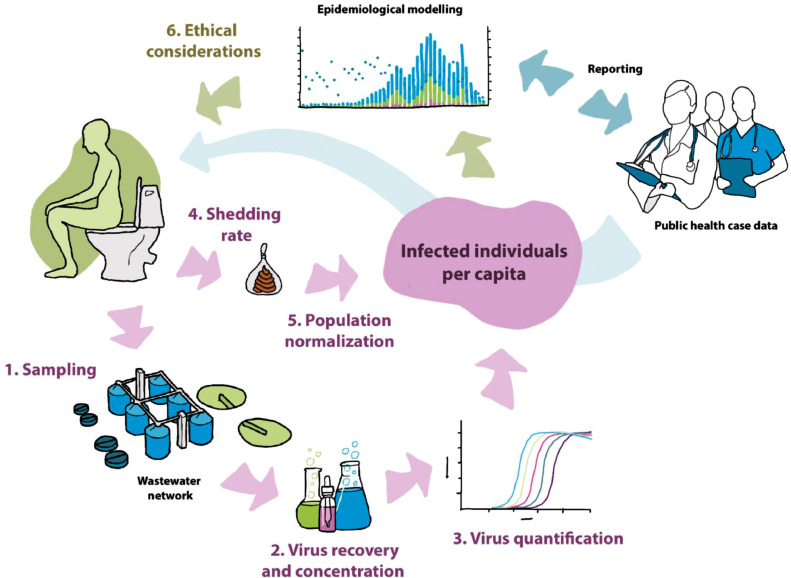
Whether SARS-CoV-2 virons in sewage are infective or not, the use of virus particles in wastewater as an epidemiological tool for community COVID-19 prevalence is possible. Individual wastewater samples represent a snapshot of infection within the population, whilst a structured longitudinal sampling strategy as part of an integrated wastewater-based epidemiology (WBE) program (see Fig. 1 ) can be an invaluable additional predictive tool for addressing the COVID-19 pandemic.
Fig. 1.
A structured approach to wastewater-based epidemiology (WBE) as used community COVID-19 surveillance. Highlighted factors flag the importance of how wastewater samples must be collected (1), processed, and concentrated (2) to provide exact enough data to combine with shedding data for population normalization. Infected individuals per capita are estimated based on SARS-CoV-2 RNA concentrations in wastewater (3), viral shedding rates per day per person (4), and the contributing population (5). These data are then merged with public health case data for epidemiological modelling with sufficient precisión to make community-scale predictions. Ethics (6) also must be considered to avoid stigmatism and media misinterpretation of wastewater monitoring data.
2. The concept
WBE was theorized in 2001 (Daughton and Jones-Lepp, 2001) and then implemented in 2005 to trace cocaine and other illicit drug use (Zuccato et al., 2005; Zuccato et al., 2008) and oseltamivir (Tamiflu) use during the 2009 influenza pandemic (Reddy, 2010; Singer et al., 2013). The approach relies on the assumption that any substance that is excreted by humans and is stable in wastewater can be used to back-calculate the original concentration excreted by the serviced population. This same concept can be translated to virus surveillance (Berchenko et al., 2017).
Contrary to other microorganisms, such as bacteria, viruses do not grow outside the host cells. Therefore, human viruses in wastewater can represent the concentrations excreted by the corresponding human population as long as they persist long enough (2–4 days) to be detected (Carducci et al., 2020; Kitajima et al., 2020). Therefore, monitoring temporal changes in viral concentrations and diversity in community wastewater samples can be used not only to determine the true extent of the infection in the population, but also the emergence of new viral strains and the early detection of new viral outbreaks (Adriaenssens et al., 2008; Ahmed et al., 2020; Daughton, 2020; Hart and Halden, 2020).
The utility and potential of a wastewater surveillance system have been previously demonstrated. During the global polio eradication program, it was utilized as a tool to assess polio circulation within populations and the evaluation of immunization efficacy against poliovirus (Hovi et al., 2012; Ndiaye et al., 2014; Roberts, 2013). It has also been used in the retrospective prediction of disease outbreaks of Hepatitis A and norovirus-associated gastroenteritis (Hellmér et al., 2014). However, WBE methods have not yet been applied to assess and predict viral disease outbreaks in a systematic way.
WBE could solve certain limitations in existing surveillance systems that have been highlighted during this COVID-19 pandemic or previous ones like the 2009 influenza A pandemic (Dawood et al., 2012; Simonsen et al., 2013). Specifically, the sensitivity and specificity of syndromic surveillance approaches greatly depend on the reporting and severity of clinical symptoms, and how much these signs overlap with existing diseases within the population (Mandi et al., 2004). In the case of SARS-CoV-2, a significant proportion of patients are either asymptomatic, presymptomatic or experience mild, non-specific symptoms and therefore go unreported, resulting in considerable underestimation of infection. In a range of studies, the rate of asymptomatic infection has been estimated at ca. 20–45% (Mizumoto et al., 2020; Oran and Topol, 2020; Wang et al., 2020). Whilst syndromic surveillance based on hospital admissions is likely to be more specific given the current prevalence of disease in the population, estimates of population infection based on hospital admissions represents a delay of days to weeks between infection and admission. In addition, a significant proportion of patients admitted to hospital will die.
In theory, individual sampling and testing represents the most accurate measure of active transmission and disease prevalence; however, the spatial and temporal scale of testing required to achieve adequate penetrance to gather granular information is impractical or economically challenging for most countries. Further, repeated testing would be required to ensure effective disease control. Novel rapid diagnostics may be useful in this respect, but they are not yet available and present their own challenges with respect to reliability and quality control. Therefore, there is a need for a more sensitive, specific and timely measure of infection in the population.
The biological information that wastewater contains can be used as an unbiased surveillance system and reflection of the community's health. Human viruses in wastewater are, by nature, biological markers of their own circulation in populations due to their DNA or RNA. Their detection in influents of wastewater treatment plants (WWTP) can suggest human sources and hence indicate what diseases are circulating within a population in almost real-time. WBE is scalable and cost-effective even in low-resource settings, provides rapid results, and can monitor a wide variety of emergent and re-emergent viral diseases and/or imported pathogens. In addition, the move towards centralized wastewater treatments plants in most urban centres (where SARS-CoV-2 is most prevalent) means that the viral load from 104 to 106 individuals can be captured in a single sample, facilitating the analysis for the whole community.
3. Enveloped viruses in wastewater: the paradigm shift
Historically, the study of viruses in wastewater has been focused on enteric non-enveloped viruses that replicate in the gastrointestinal tract and are readily transmitted via the fecal-oral route (e.g. Norovirus, Rotavirus). All viruses are susceptible to environmental degradation by factors such as temperature, UV light, and predation by the microbial community. Enteric viruses are highly resistant to heat, acids, and oxidants, and survive for long periods in the environment (Bosch et al., 2006). In contrast, enveloped viruses, such as coronaviruses, Ebola virus, or influenza viruses, are generally not associated with fecal-oral transmission in humans, and are considered more susceptible to inactivation because damage to the labile lipid envelope leads to loss of infectivity in aqueous environments.
Interestingly, not all enveloped viruses rapidly lose their infectivity. Studies in the last decade have expanded this view and revealed that some epidemic enveloped viruses like SARS-CoV, MERS-CoV, and now SARS-CoV-2 typically considered respiratory viruses can also be detected in the water cycle (Carducci et al., 2020; Kitajima et al., 2020; Singer and Wray, 2020; Wigginton et al., 2015). Temperature, pH, matrix composition or the presence of other microorganisms can influence survivability of enveloped viruses, but also, virus recovery and concentration methods historically optimized for non-enveloped viruses like enteric viruses can impact infectivity assay outcome and underestimate their infectivity rates (Aquino de Carvalho et al., 2017; Gundy et al., 2009; La Rosa et al., 2020; Ye et al., 2016).
Whether or not an enveloped infective virus persists long enough in wastewater to represent a threat to human health is still unclear and further research is needed. However, in the 2003 SARS-CoV outbreak, a clear link was made between localized SARS infections and the sewage network, albeit associated with fecal aerosolization (McKinney et al., 2006). In summary, the presence of viral nucleic acids in feces and wastewater indicate that the concept of WBE could be applied to a wide range of viruses beyond the enteric viruses, and that an enveloped virus such as SARS-CoV-2 is relevant to WBE (O'Brien and Xagoraraki, 2020; Sims and Kasprzyk-Hordern, 2020). Early data from the US suggest this may be possible (Peccia et al., 2020), but more work is needed to validate early results. Regardless, WBE offers a paradigm shift in infectious disease surveillance.
4. Critical factors in WBE implementation
4.1. Sampling
Sampling for WBE applications offers both spatial and temporal challenges that can compromise the degree to which the resulting data is “representative” of the study population (Fig. 1). Differences between urban and rural wastewater systems must be considered when the specific surveillance program is designed. Urban sewage systems can provide more representative samples of the community because wastewater ultimately is aggregated across the population through interceptors that can be used to partition the study population (O'Brien and Xagoraraki, 2020). Should viral concentrations be observed as higher in one interceptor than the rest, the corresponding serviced area would be of greater concern for a potential viral outbreak. Sampling in rural areas is more complex due to the lack of sewage collection systems and proximity to testing facilities. Therefore, watershed modeling and microbial source tracking are usually integral components of WBE, used to evaluate wastewater disposal and transport and to determine the best locations for effective sampling (O'Brien et al., 2017b).
The time of sampling is also crucial and should be based upon expected critical pathways, such as environmental reservoirs and locations where the virus is most easily transported and transmitted (O'Brien and Xagoraraki, 2020). Careful consideration needs to be given to the size of the catchment area and hence its susceptibility to diurnal changes in flow and/or viral detection rates (Cornman et al., 2018; Dong et al., 2015). For example, in some large urban areas there may be a delay of 24 h for the wastewater to go from the household to a centralized plant. Whilst autosamplers can be used to obtain composite samples over a representative time period, e.g. 24 h, refrigerated units necessary to prevent viral degradation are expensive. Hence, in many situations, grab sampling may represent the most practical and appropriate approach. The fate and decay rate of viruses may be different between systems that use enclosed underground sewer pipes, storm tanks, and systems that utilize septic tanks, catchments, and the open environment. Factors that lead to an increase in viral levels in wastewater influents include larger incidence rates in the community or cooler temperatures. On the contrary, dilution due to infiltration of precipitation or from surface water leads to lower levels. Detailed modeling of viral decay and wastewater flow rates through the system are therefore required to accurately relate viral concentration at the point of sampling to the presence of virus within the catchment population. In addition, sampling SARS-CoV-2 in wastewater from hospitals may be compromised by the wide range of disinfectants and detergents co-entering the sewage network.
4.2. Virus recovery and concentration
Virus detection in environmental samples likely requires viral concentration into a smaller volume to improve detection limits. There are a variety of available methods designed and optimized for non-enveloped viruses, which are based on combinations of filtrations, ultracentrifugation and polyethylene glycol (PEG) precipitation with a range of pH values, chemicals, filter types, centrifuge speeds and purification steps (La Rosa et al., 2020; Ye et al., 2016).
However, most methods are not suitable for enveloped viruses because the outer lipid layer renders these viruses more sensitive to temperature, pH and organic solvents like chloroform or cesium chloride solutions. Two general types of charged filters are commonly available. Electronegative and electropositive charged filters. Electropositive filtration is commonly used for non-enveloped viruses like enteric viruses with good recoveries and, by extension, they are also being employed for enveloped viruses like SARS-CoV (Wang et al., 2005). However, care must be taken since membrane filtration efficiencies will vary depending on the type of water sample (tap water, seawater, wastewater), the virus, the isoelectric point of the virus that influences the net charge of the viral particle, the pH, the presence in the sample of other contaminants with potential inhibitory activity for further steps in the process, or of organic material that could hamper the filtration (Armanious et al., 2016; Pepper and Gerba, 2015).
Virus culture and infectivity assays are not critical to implement WBE; however, they are crucial to evaluate the risk that a sample poses to human health or to potential animal reservoirs. This is not possible with PCR or sequencing techniques and therefore future infectivity studies are needed to fill this gap.
4.3. Virus quantification
Exact virus quantification is the principal goal of WBE because peaks in viral concentrations can indicate the potential onset of future disease outbreaks. SARS-CoV-2 primer/probe sets are already published targeting N, E, and RdRp genes (Corman et al., 2020). For RNA viruses, classical reverse transcription-PCR (RT-PCR) and RT-real time PCR (RT-qPCR) are still the gold standard methods to obtain qualitative and quantitative data, respectively. However, virus extraction concentrates from wastewater samples often contain diverse PCR inhibitors, including fats, proteins, and humic substances, which interfere with the PCR reaction (Gibson et al., 2012). They also contribute to bias in metagenomic analysis (Hall et al., 2014). Sometimes, these inhibitors can be minimized by dilution or by the addition of quenching agents.
However, future wastewater virus monitoring will need to depend on the development of better extraction and purification methods. New molecular techniques such as digital PCR (dPCR) can help with some problems. The partitioning effect of distributing the target nucleic acid into thousands of reaction wells has shown to be more sensitive and to decrease the effect of PCR inhibitory substances in complex matrices including wastewater (Polo et al., 2016; Varela et al., 2018).
Next-generation sequencing and metaviromics have the potential to revolutionize viral wastewater surveillance. However, standardized protocols for these technologies remain a challenge. The complexity of the matrix and the high abundance of genetic material in the samples often lead to more conservative results of viral detection as well as to poorer detection limits for a specific virus compared to qPCR (Fernandez-Cassi et al., 2018; Martínez-Puchol et al., 2020). Conversely, they can also detect new variants that are missed by conventional qPCR primer sets as de virus progressively mutates (Adriaenssens et al., 2018).
4.4. SARS-CoV-2 shedding rates and levels in wastewater
The shedding rate is the rate with which viruses are released from the body, and knowing rates are critical linking back wastewater data to human populations (Fig. 1). Many factors can impact the shedding rate of viruses in the feces, including viremia, the duration, severity and the stage of the disease, or age (Chen and Li, 2020). When data on viral loads in wastewater are absent, viral loads in human stool or urine samples may help to predict levels in wastewater during an outbreak event. It should be noted, however, that in contrast to fecal shedding, which occurs in ca. 50% of clinically diagnosed infections, shedding of SARS-CoV-2 in urine is far less common (<5% of confirmed infections)(Peng et al., 2020). SARS-CoV-2 has been detected by RT-qPCR in the feces at concentrations ranging from 103 to 10⁵ copies/mL (Pan et al., 2020; Zhang et al., 2020). The duration and shedding rate of the virus in asymptomatic cases, however, still remains extremely uncertain, but is expected to be much lower. In a pandemic scenario, concentrations in wastewater will therefore depend on the number of people infected in the community and the rate at which infected individuals shed the viruses. Regarding SARS-CoV-2, a few studies have assessed the levels in wastewater, ranging from 102 to 106 copies/L in untreated wastewater (Ahmed et al., 2020; Randazzo et al., 2020; Sherchan et al., 2020; Wu et al., 2020; Wurtzer et al., 2020). The establishment of correlations and comparisons between measured viral concentrations in wastewater and the reported clinical cases of disease or the extension of the outbreak in the population is the final goal of WBE. These correlations can serve as a validation for a prediction model that accounts for the factors discussed above, providing evidence for the notion that changes of viral concentrations in wastewater will indicate changes in disease cases in the human population.
4.5. Population normalization
An important step in the application of WBE is the estimation of the contributing population to wastewater samples, namely population normalization. For this purpose, both census and biomarker data can be used, which needs to be independently collected, but also integrated with common units (Fig. 1). Endogenous or exogenous human biomarkers can be used to estimate the serviced population in an area via statistical modeling (Table 1 ). Quantification of biomarkers would provide context to measured viral concentrations and ensure that differences in viral loads could not be attributed to changes in population. When observed viral concentrations are significantly high relative to the estimated population, a viral outbreak could be indicated (O'Brien and Xagoraraki, 2020; Sims and Kasprzyk-Hordern, 2020). Some substances that have been proposed as population biomarkers are creatinine, cholesterol, coprostanol, nicotine, cortisol, androstenedione, and the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA). However, all these biomarkers require capability in analytical chemistry, and there is still concern when applying these markers to different catchments due to differing consumption or disposal habits, stability, and sorption to particulate matter, which contribute to high uncertainties (Rico et al., 2017). Hence, human nucleic acid has a great potential to act as a population biomarker due to its limited affinity to other species in wastewater, stability, constant excretion by human, and the possibility of being quantifiable using the same pipelines and platforms as the viral nucleic acid of interest. Very promising viral indicators of human activity include crAssphage, pepper mild mosaic virus and adenovirus (Farkas et al., 2019). They are better than other human pathogenic viruses such as norovirus, which demonstrate strong seasonality in wastewater. Total nitrogen, phosphorus, biological oxygen demand and ammonium, have also been proposed as population biomarkers, but these better reflect human activity and industry footprint rather than populations (Choi et al., 2018; Daughton, 2018). Census information is also used in WBE. However, in some cases, this approach can underestimate the population compared to biomarkers (O'Brien et al., 2014).
Table 1.
Potential human biomarkers for population normalization in wastewater-based epidemiology at a community level.
| Biomarkers | Type | Description | Excreted in | References |
|---|---|---|---|---|
| 5-hydroxyindoleacetic acid | Endogenous | Metabolite of serotonin | Urine | Chen et al., 2014 |
| Ammonium | Endogenous | Form of ammonia | Urine | Been et al., 2014 |
| Androstenedione | Endogenous | Sex hormone precursor | Urine | Chen et al., 2014 |
| Cholesterol | Endogenous | Lipid molecule of cell membranes | Feces | Daughton 2012; Chen et al., 2014 |
| Coprostanol | Endogenous | Metabolite of cholesterol | Feces | Daughton 2012; Chen et al., 2014 |
| Cortisol | Endogenous | Steroid hormone | Feces | Chen et al., 2014 |
| Creatinine | Endogenous | Metabolite of creatine | Urine | Chen et al., 2014 |
| Homovanillic acid | Endogenous | Metabolite of catecholamine | Urine | Pandopulos et al., 2020 |
| Vanillylmandelic acid | Endogenous | Metabolite of catecholamine | Urine | Pandopulos et al., 2020 |
| Acesulfame | Exogenous | Artificial sweetener | Urine | O'Brien et al., 2017a |
| Atenolol | Exogenous | Hypertension beta blocker | Urine | O'Brien et al., 2017a; Rico et al., 2017 |
| Caffeine | Exogenous | Psychoactive drug | Urine | Chen et al., 2014; Rico et al., 2017 |
| Carbamazepine | Exogenous | Anticonvulsant medication | Urine/Feces | O'Brien et al., 2017a; Rico et al., 2017 |
| Codeine | Exogenous | Antiinflammatory drug | Urine | O'Brien et al., 2014; Rico et al., 2017 |
| Cotinine | Exogenous | Metabolite of nicotine | Urine | Chen et al., 2014 |
| Furosemide | Exogenous | Medication for Edema | Urine | O'Brien et al., 2014; Rico et al., 2017 |
| Gabapentin | Exogenous | Anticonvulsant medication | Urine | O'Brien et al., 2017a; Rico et al., 2017 |
| Hydrochlorothiazide | Exogenous | Diuretic medication | Urine | O'Brien et al., 2014; Rico et al., 2017 |
| Ibuprofen | Exogenous | Antiinflammatory drug | Urine | O'Brien et al., 2017a; Rico et al., 2017 |
| Iopromide | Exogenous | Radiographic contrast agent | Urine | O'Brien et al., 2014; Rico et al., 2017 |
| Naproxen | Exogenous | Anticonvulsant medication | Urine | O'Brien et al., 2014; Rico et al., 2017 |
| Nicotine | Exogenous | Stimulant found in tobacco | Urine | Chen et al., 2014; Rico et al., 2017 |
| Paracetamol | Exogenous | Pain and fever medication | Urine | O'Brien et al., 2014; Rico et al., 2017 |
| Salicylic-acid | Exogenous | Active metabolite of aspirin | Urine | O'Brien et al., 2014; Rico et al., 2017 |
| Venlafaxine | Exogenous | Antidepressant medication | Urine | O'Brien et al., 2014; Rico et al., 2017 |
Normalization of population is crucial to enable inter-city comparisons as well as to ensure that a significant increase in viral concentration in a wastewater sample does not correspond to an increase in population in the serviced area. In this sense, fluctuations in the population pose a challenge to WBE (e.g. due to tourism or commuter activity). Whilst these dynamics may have negligible impact on the levels of biomarkers in large populations, they might contribute to higher uncertainties in smaller populations (Chen et al., 2014; Ort et al., 2014).
4.6. Ethical considerations
WBE does not collect data on individuals so, a priori, the ethical risks are low. However, expanding WBE to include viral infectious diseases and outbreaks will pose new challenges with respect to ethical considerations. In many cases infectious diseases monitoring and control requires lockdowns and/or sampling of subgroups and small populations as it might provide faster interventions by public health authorities. However, these measures could also lead to stigmatizing behaviors. One of the best documented cases is the increased spread of Ebola in Western Africa due to fear-trigged behaviors. Stigmatism surrounding individuals infected with Ebola combined with a sense of distrust in health services and treatment centers resulted in efforts to hide cases, home treatment and increased chances of infecting family members and then other members of the community (Shultz et al., 2016). Similar ethical issues have also been observed in outbreaks such as SARS-CoV-1 and influenza, and social stigma associated with COVID-19 has already been reported. These earlier episodes lead to the publishing in 2017 of the first comprehensive international ethics guidelines on public health surveillance (WHO, 2017) highlighting that, ethical guidelines should be appropriately adapted to different social, economic and epidemiological circumstances. In this sense, care must be taken in disease reporting and to reduce media misinterpreting the publication's findings.
5. Conclusions
-
•
The presence of new highly pathogenic strains of enveloped viruses such as SARS-Co-2 in wastewater represents a new challenge and an opportunity to employ WBE for its surveillance.
-
•
There is a need for optimized recovery and concentration methods for enveloped viruses, as they have not previously been explored as part of a WBE approach.
-
•
The presence of viral RNA within wastewater, regardless of viral infectivity, constitutes an indirect population-level diagnostic tool.
-
•
Representative sampling, viral concentration in wastewater, population normalization, and ethical guidelines are crucial factors for reliable WBE approach.
-
•
A well validated WBE system is imperative for viral surveillance, particularly with respect to an early warning system for the next human pandemic
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Authors declare no conflict of interest.
Acknowledgements
This work was funded in part by Grant 2014-PG110 from the Xunta de Galicia (Spain), Grant EP/R511584/1 from the EPSRC IAA program (UK), and Grant NE/V004883/1 from the Natural Environment Research Council's (UK) COVID-19 Urgency programme. AC is supported by funding from the Centre of Expertise for Waters. We thank Eadington Graham for providing graphic visualizations for the manuscript.
References
- Adriaenssens E.M., Farkas K., Harrison C., Jones D.L., Allison H.E., McCarthy A.J. Viromic analysis of wastewater Input to a river catchment reveals a diverse assemblage of RNA viruses. mSystem. 2018;3:e00025-18. doi: 10.1128/mSystems.00025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino de Carvalho N., Stachler N.E., Cimabue N., Bibby K. Evaluation of Phi6 persistence and suitability as an enveloped virus surrogate. Environ. Sci. Technol. 2017;51(15):8692–8700. doi: 10.1021/acs.est.7b01296. [DOI] [PubMed] [Google Scholar]
- Armanious A., Aeppli M., Jacak R., Refardt D., Sigstam T., Kohn T., Sander M. Viruses at solid-water interfaces: a systematic assessment of interactions driving adsorption. Environ. Sci. Technol. 2016;50(2):732–743. doi: 10.1021/acs.est.5b04644. [DOI] [PubMed] [Google Scholar]
- Been F., Rossi L., Ort C., Rudaz S., Delémont O., Essevia P. Population normalization with ammonium in wastewater-based epidemiology: application to illicit drug monitoring. Environmental Science and Technology. 2014;48:8162–8169. doi: 10.1021/es5008388. [DOI] [PubMed] [Google Scholar]
- Berchenko Y., Manor Y., Freedman L.S., Kaliner E., Grotto I., Mendelson E., Huppert A. Estimation of polio infection prevalence from environmental surveillance data. Sci. Transl. Med. 2017;9(383) doi: 10.1126/scitranslmed.aaf6786. eaaf6786. [DOI] [PubMed] [Google Scholar]
- Bosch A., Pintó R.M., Abad F.X. Survival and transport of enteric viruses in the environment. Viruses Foods. 2006;1956:151–187. [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making Waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Kostakis C., Gerber J.P., Tscharke B.J., Irvine R.J., White J.M. Towards finding a population biomarker for wastewater epidemiology studies. Sci. Total Environ. 2014;487(1):621–628. doi: 10.1016/j.scitotenv.2013.11.075. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li L. SARS-CoV-2: virus dynamics and host response. Lancet Infect. Dis. 2020;20(5):515–516. doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P.M., Tscharke B.J., Donner E., O’Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O’Malley E., Crosbie N.D., Thomas K.V., Mueller J.F. Wastewater-based epidemiology biomarkers: past, present and future. TrAC - Trends Analyt. Chem. 2018;105:453–469. [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-.L., Ellis J., Zambon M., … Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveillance : Bulletin Europeen Sur Les Maladies Transmissibles = Eur. Commun. Dis. Bull. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornman R.S., Jr McKenna JE, Fike J., Oyler-McCance S.J., Johnson R. An experimental comparison of composite and grab sampling of stream water for metagenetic analysis of environmental DNA. PeerJ. 2018;6:e5871. doi: 10.7717/peerj.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Real-time estimation of small-area populations with human biomarkers in sewage. Sci. Total Environ. 2012;414:6–21. doi: 10.1016/j.scitotenv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. Monitoring wastewater for assessing community health: sewage Chemical-Information Mining (SCIM) Sci. Total Environ. 2018;619–620:748–764. doi: 10.1016/j.scitotenv.2017.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G., Jones-Lepp T.L. Pharmaceuticals and personal care products in the environment : scientific and regulatory issues LK- In ACS symposium series ; 791 TA - TT - Am. Chem. Soc. 2001 [Google Scholar]
- Dawood F.S., Iuliano A.D., Reed C., Meltzer M.I., Shay D.K., Cheng P.-.Y., Bandaranayake D., Breiman R.F., Brooks W.A., Buchy P., Feikin D.R., Fowler K.B., Gordon A., Hien N.T., Horby P., Huang Q.S., Katz M.A., Krishnan A., Lal R.…Widdowson M.-.A. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect. Dis. 2012;12(9):687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- Dong G. Wastewater sampling and characterization – Raw sewage monitoring and results analysis. Proceedings of the 9th Annual WIOA NSW Water Industry Operations Conference. Orange, PCYC. Water Industry Operator's Association of Australia. 2015:40–46. [Google Scholar]
- Fernandez-Cassi X., Timoneda N., Martínez-Puchol S., Rusiñol M., Rodriguez-Manzano J., Figuerola N., Bofill-Mas S., Abril J.F., Girones R. Metagenomics for the study of viruses in urban sewage as a tool for public health surveillance. Science of the Total Environment. 2018;618:870–880. doi: 10.1016/j.scitotenv.2017.08.249. [DOI] [PubMed] [Google Scholar]
- Gibson K.E., Schwab K.J., Spencer S.K., Borchardt M.A. Measuring and mitigating inhibition during quantitative real time PCR analysis of viral nucleic acid extracts from large-volume environmental water samples. Water Res. 2012;46(13):4281–4291. doi: 10.1016/j.watres.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1(1):10–14. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Hall R.J., Wang J., Todd A.K., Bissielo A.B., Yen S., Strydom H., Moore N.E., Ren X., Huang Q.S., Carter P.E., Peacey M. Evaluation of rapid and simple techniques for the enrichment of viruses prior to metagenomic virus discovery. J. Virol. Methods. 2014;195:194–204. doi: 10.1016/j.jviromet.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV 2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80(21):6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T., Shulman L.M., Van Der Avoort H., Deshpande J., Roivainen M., De Gourville E.M. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 2012;140(1):1–13. doi: 10.1017/S095026881000316X. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Bonadonna L, Lucentini L, Kenmoe S, Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at Amoy Gardens. J. Environ. Health. 2006;68(9):26–30. [PubMed] [Google Scholar]
- Martínez-Puchol S., Rusiñol M., Fernández-Cassi X., Timoneda N., Itarte M., Andrés C., Antón A., Abril J.F., Girones R., Bofill-Mas S. Characterisation of the sewage virome: comparison of NGS tools and occurrence of significant pathogens. Sci. Total Environ. 2020;713 doi: 10.1016/j.scitotenv.2020.136604. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship. 2020. Euro Surveill. 2020. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye A.K., Diop P.A.M., Diop O.M. Environmental surveillance of poliovirus and non-polio enterovirus in urban sewage in Dakar. Pan. Afr. Med. J. 2014;19:243. doi: 10.11604/pamj.2014.19.243.3538. 2007-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E.O., Xagoraraki I. A water-focused one-health approach for early detection and prevention of viral outbreaks. OneHealth. 2020;7 doi: 10.1016/j.onehlt.2019.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J.W., Banks A.P.W., Novic A.J., Mueller J.F., Jiang G., Ort C., Eaglesham G., Yuan Z., Thai P.K. Impact of in-Sewer Degradation of Pharmaceutical and Personal Care Products (PPCPs) Population Markers on a Population Model. Environ. Sci. Technol. 2017;51(7):3816–3823. doi: 10.1021/acs.est.6b02755. [DOI] [PubMed] [Google Scholar]
- O’Brien E., Nakyazze J., Wu H., Kiwanuka N., Cunningham W., Kaneene J.B., Xagoraraki I. Viral diversity and abundance in polluted waters in Kampala. Water Res. 2017;127:41–49. doi: 10.1016/j.watres.2017.09.063. [DOI] [PubMed] [Google Scholar]
- O’Brien J.W., Thai P.K., Eaglesham G., Ort C., Scheidegger A., Carter S., Lai F.Y., Mueller J.F. A Model to Estimate the Population Contributing to the Wastewater Using Samples Collected on Census Day. Environ. Sci. Technol. 2014;48(1):517–525. doi: 10.1021/es403251g. [DOI] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann. Intern. Med. 2020:M20–3012. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort C., Banta-Green C.J., Bijlsma L., Castiglioni S., Emke E., Gartner C., Kasprzyk-Hordern B., Reid M.J., Rieckermann J., Van Nuijs A.L.N. Sewage-based epidemiology requires a truly transdisciplinary approach. Gaia. 2014;23(3):266–268. doi: 10.14512/gaia.23.3.12. [DOI] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandopulos A.J., Gerber C., Tscharke B.J., O'Brien J., White J.M., Bade R. A sensitive analytical method for the measurement of neurotransmitter metabolites as potential population biomarkers in wastewater. J. Chromatogr. A. 2020;1612 doi: 10.1016/j.chroma.2019.460623. [DOI] [PubMed] [Google Scholar]
- Peccia, J., Zulli, A., Brackney, D.E., Grubaugh, N.D., Kaplan, E.H., Casanovas-Massana, A., Ko, A.I., Malik, A.A., Wang, D., Wang, M., Weinberger, D.M., & Omer, S.B. (2020). SARS-CoV-2 TNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. medRxiv doi: 10.1101/2020.05.19.20105999. [DOI]
- Peng L., Liu J., Xu W., Luo Q., Chen D., Lei Z., Huang Z., Li X., Deng K., Lin B., Gao Z. SARS‐CoV‐2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020:1–5. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper I.L., Gerba C.P. Environmental Microbiology: Third Edition. Elsevier Inc; 2015. Environmental Sample Collection and Processing. [DOI] [Google Scholar]
- Polo D., Schaeffer J., Fournet N., Le Saux J.-.C., Parnaudeau S., McLeod C., Le Guyader F.S. Digital PCR for quantifying norovirus in oysters implicated in outbreaks. Emerging Infect. Dis. 2016;22(12) doi: 10.3201/eid2212.160841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo, W., Truchado, P., Ferrando, E.C., Simon, P., Allende, A., & Sanchez, G. (2020). SARS-CoV-2 RNA titers in wastewater anticipated COVID-19 occurrence in a low prevalence area. medRxiv doi: 10.1101/2020.04.22.20075200. [DOI] [PMC free article] [PubMed]
- Reddy D. Responding to pandemic (H1N1) 2009 influenza: the role of oseltamivir. J. Antimicrob. Chemother. 2010;65 doi: 10.1093/jac/dkq014. suppl. 2ii35–ii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico M., Andrés-Costa M.J., Picó Y. Estimating population size in wastewater-based epidemiology. Valencia metropolitan area as a case study. J. Hazardous Mater. 2017;323(Pt A):156–165. doi: 10.1016/j.jhazmat.2016.05.079. [DOI] [PubMed] [Google Scholar]
- Roberts L. Israel’s Silent Polio Epidemic Breaks All the Rules. Science. 2013;342(6159) doi: 10.1126/science.342.6159.679. 679 LP – 680. [DOI] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M.K. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz J.M., Cooper J.L., Baingana F., Oquendo M.A., Espinel Z., Althouse B.M., Marcelin L.H., Towers S., Espinola M., McCoy C.B., Mazurik L., Wainberg M.L., Neria Y., Rechkemmer A. The Role of Fear-Related Behaviors in the 2013–2016 West Africa Ebola Virus Disease Outbreak. Curr. Psychiatry Rep. 2016;18(11) doi: 10.1007/s11920-016-0741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L., Spreeuwenberg P., Lustig R., Taylor R.J., Fleming D.M., Kroneman M., Van Kerkhove M.D., Mounts A.W., Paget W.J., Teams, the Gl C. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013;10(11) doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139(February) doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A.C., Wray R. Detection and survival of SARS-coronavirus in human stool, urine, wastewater and sludge. Preprints. 2020 doi: 10.20944/preprints202006.0216.v2. [DOI] [Google Scholar]
- Singer A.C., Järhult J.D., Grabic R., Khan G.A., Fedorova G., Fick J., Lindberg R.H., Bowes M.J., Olsen B., Söderström H. Compliance to oseltamivir among two populations in Oxfordshire, United Kingdom affected by influenza A (H1N1)pdm09, November 2009 – a waste water epidemiology study. PLoS ONE. 2013;8:e60221. doi: 10.1371/journal.pone.0060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M.F., Monteiro S., Rivadulla E., Santos R., Romalde J.L. Development of a novel digital RT-PCR method for detection of human sapovirus in different matrices. J. Virol. Methods. 2018;254(November 2017):21–24. doi: 10.1016/j.jviromet.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Wang X.W., Li J., Guo T., Zhen B., Kong Q., Yi B., Li Z., Song N., Jin M., Xiao W., Zhu X., Gu C., Yin J., Wei W., Yao W., Liu C., Li J., Ou G., Wang M., Fang T., Wang G., Qiu Y., Wu H., Chao F., Li J. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th Hospital of the Chinese People's Liberation Army. Water Sci. Technol. 2005;52(8):213–221. doi: 10.2166/wst.2005.0266. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kang H., Liu X., Tong Z. Asymptomatic cases with SARS‐CoV‐2 infection. J. Med. Virol. 2020:1–3. doi: 10.1002/jmv.25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2017. WHO Guidelines On Ethical Issues in Public Health Surveillance (1395) Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci.: Water Res. Technol. 2015;1(6):735–746. doi: 10.1039/c5ew00125k. [DOI] [Google Scholar]
- Wu, F., Xiao, A., Zhang. J., Gu, X., Lee, W.L., Kauffman, K., Hanage, W., Matus, M., Ghaeli, N., Endo, N., Duvallet, C., Moniz, K., Erickson, T., Chai, P., Thompson, J., & Alm, E. (2020). SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv doi: 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020:1–3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Zhao J., Huan J., Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerging Infect. Dis. 2020 doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nature Med. 2020 doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Zhang, N., Gong, Y., Meng, F., Bi, Y., Yang, P., & Wang, F. (2020). Virus shedding patterns in nasopharyngeal and fecal specimens of COVID-19 patients. MedRxiv 2020.03.28.20043059; doi: 10.1101/2020.03.28.20043059. [DOI] [PMC free article] [PubMed]
- Zuccato E., Chiabrando C., Castiglioni S., Bagnati R., Fanelli R. Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 2008;116(8):1027–1032. doi: 10.1289/ehp.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato E., Chiabrando C., Castiglioni S., Calamari D., Bagnati R., Schiarea S., Fanelli R. Cocaine in surface waters: a new evidence-based tool to monitor community drug abuse. Environ. Health : Global Access Sci. Source. 2005;4:14. doi: 10.1186/1476-069X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]