Abstract
Low- and middle-income countries (LMICs) face many challenges in controlling COVID-19. Healthcare resources are limited and so are ICU beds. RT-PCR testing is conducted on a limited scale and treatment options are few. There is no vaccine. Therefore, what low-cost solutions remain for the prevention, diagnosis, and treatment of SARS-CoV-2? How should these essential health services be delivered in order to reach the most vulnerable in our societies? In this editorial we discuss several important strategies for controlling COVID-19 including: vaccination, molecular and serological diagnostics, hygiene and WaSH interventions, and low-cost therapeutics. We also discuss the delivery of such services in order to reach the most in need. The proposed integrated control strategy requires immediate action and political will in order to reduce the widening health inequalities caused by the pandemic.
Keywords: SARS-CoV-2, control, vaccination, diagnostics, hygiene, therapeutics
The search for a SARS-CoV-2 vaccine
There is an intense global race to find a COVID-19 vaccine. As of August 29, 2020, 143 vaccine candidates were in preclinical evaluation and 46 candidate vaccines were being tested in human clinical trials (Phases I–III) (Anon, 2020a, Anon, 2020b). Nine vaccine candidates had entered Phase III trials in several countries, utilizing thousands of volunteers. The Phase III candidates were developed by: Sinovac Life Sciences Co., Ltd, China (NCT04456595); the University of Oxford, UK (ISRCTN89951424); CanSino Biologics, China (NCT04526990); Wuhan Institute of Biological Products, China (ChiCTR2000034780); Beijing Institute of Biological Products, China (ChiCTR2000034780); ModernaTX Inc., USA (NCT04470427); Gamaleya Research Institute, Russia (NCT04530396); BioNTech/Fosun Pharma/Pfizer (NCT04368728); and Janssen Pharmaceutical Companies (NCT04505722) (Anon, 2020a).
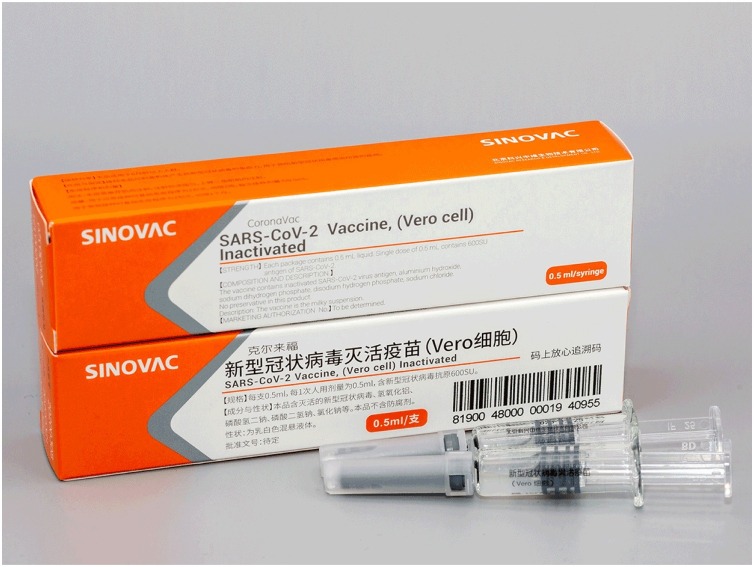
To develop these vaccines, various new and old techniques have been used to produce an immune response. These include: genetic vaccines, where one or more of the coronavirus’s genes are used; viral vector vaccines, where a virus (unable to replicate) is used to deliver coronavirus genes into cells to make viral proteins; protein-based vaccines, using coronavirus spike protein or its fragment; and whole-virus vaccines, using weakened or inactivated viruses (Anon, 2020b). Sinovac Biotech, Co. Ltd Beijing has developed a promising inactivated SARS-CoV-2 Vaccine (Vero Cell) and tested it successfully in Phase I/II trials among 744 healthy Chinese participants aged 18–59 years (NCT04352608) (Figure 1 ). The results from Phase I/II trials showed that a two-dose schedule of the vaccine was well tolerated, safe (without any serious adverse reactions), and immunogenic (producing high titers of antibodies). Sinovac is presently conducting Phase III trials in Brazil, Saudi Arabia, Turkey, Chile, and Indonesia, and soon in Bangladesh. According to many vaccinologists, inactivated vaccines may be the best choice against COVID-19 because there is no risk of reversion to a virulent form. Inactivated vaccines have been extremely effective over the past century in inducing protection against many deadly viral pathogens, such as polio, rabies, HAV, and influenza.
Figure 1.
Sinovac’s SARS-CoV 2 (Vero Cell) inactivated vaccine – one of China’s leading vaccine candidates.
Another promising vaccine developed by the University of Oxford is a chimpanzee adenoviral vectored coronavirus vaccine, which was developed within 100 days after deciphering the genetic sequence of the virus. The results published in the Lancet showed that a single dose of vaccine was safe and immunogenic in Phase I/II trials (Folegatti et al., 2020). Oxford’s commercial and manufacturing partner AstraZeneca has already received advance orders for billions of doses worldwide. Combined Phase II/III trials and a separate Phase III study to test the safety and efficacy of the vaccine are being conducted among tens of thousands of participants in the UK, Brazil, and South Africa (Mahase, 2020). These two vaccine candidates have created hope that a vaccine will be available for early 2021.
There are still questions on whether these vaccines will be available for LMICs or if vaccine production facilities will be adequate to assure a reliable supply within a suitable timeline frame to meet global demand. More specifically, there must be a transparent global allocation system to prioritize access to the vaccines at low cost for frontline healthcare workers and to people living in poorer countries with a higher risk of severe illness and death. As COVID-19 is highly contagious (R0 2.5), we will need to vaccinate approximately 80% of the population with a vaccine with 80% proven efficacy. To ensure equity of access and international deployment we must support global randomized controlled trials of several leading vaccine candidates through the ‘The WHO Solidarity Vaccines Trials’ (Krause et al., 2020). To provide the vaccines free of cost to resource-poor nations, we will need a global fund supported by the World Bank, the Gates Foundation, the Wellcome Trust, and the G8 nations (Moon et al., 2011). Even if successful, it is unlikely that vaccination will be a standalone strategy for controlling SARS-CoV-2 (Ross et al., 2020).
RT-qPCR diagnosis of COVID-19
WHO has recommended nucleic acid amplification tests using RT-qPCR for the routine diagnosis of COVID-19 infection (WHO, 2020). This is the gold standard for diagnosing COVID-19 and is practiced all over the world, including resource-poor countries. However, most LMICs are struggling to test samples and track the true infection rate due to a lack of laboratory facilities, trained manpower and regular supply of RT-qPCR kits. Therefore, the infection rates these countries are forecasting may actually represent only the tip of the iceberg. At the beginning of the COVID-19 pandemic, Bangladesh had only one RT-qPCR laboratory – at the Institute of Epidemiology and Disease Control Research (IEDCR) – designated for diagnosis of COVID-19 infection for the whole country (170 million), but now there are 77 RT-PCR labs (Figure 2 ) conducting approximately 20 000 tests daily (Directorate General of Health Services (DGHS), 2020). A lack of trained staff capable of performing the molecular biology experiments required for testing for COVID-19 (e.g. viral RNA extraction and qPCR) and able to interpret the results is a major limitation in resource-poor countries. Recently, several publications have reported the successful use of loop-mediated isothermal amplification (LAMP)-based protocols to test for COVID-19 in urine, saliva, as well as oropharyngeal and nasopharyngeal swabs, both with or without the requirement for viral RNA extraction (Nagura-Ikeda et al., 2020). Therefore, alternative testing protocols such as LAMP, which utilize rapid antigen detection with limited resources and available manpower, will be extremely useful.
Figure 2.
The icddr,b virology laboratory in Bangladesh, where approximately 500 RT-qPCR COVID-19 tests are performed daily for the government by 10 staff members.
Serological testing of COVID-19
Serological tests are comparatively easier to perform, and require less technical expertise and equipment compared with nucleic acid-based detection (Zainol Rashid et al., 2020). Serological tests can complement RT-PCR in the diagnosis of acute infection, sick or hospitalized patients with severe symptoms who have tested negative with RT-PCR, or for determining the antibody status of healthcare professionals (and other workers) who are ready to return to work after being ill with COVID-19. Serological testing could also be used for investigating the attack rate of an ongoing outbreak in the community, detecting the prevalence of asymptomatic carriers, and for the selection of donors of convalescent sera for treatment purposes (Bai et al., 2020). At the national level, expanding testing capacity through antibody testing will enable large-scale screening at the population level, generating crucial intelligence on estimates of disease spread and mortality attributable to COVID-19 and ensuring timely implementation of containment measures.
Due to the unprecedented demand for rapid diagnostic testing to enable the efficient treatment and mitigation of COVID-19, the US FDA has allowed more widespread access to serology testing by issuing emergency use authorization for serological tests (Anon, 2020c). One of the lab-based automated testing platforms for serological testing is Elecsys® Anti-SARS-CoV-2, by Roche Diagnostics, which is a qualitative total antibody test (IgM and IgG) that detects antibodies against SARS-CoV-2 in patients using the nucleocapsid protein. The Elecsys assay has high clinical sensitivity (99.5%; ≥14 days’ post-PCR confirmation) and overall specificity (99.81%), resulting in highly reliable and accurate results. Moreover, it is a quick test, providing results within 18 minutes. Combining such immunoassays with molecular diagnostics is deemed the best approach for Bangladesh and other LMICs, and is presently being conducted in 21 states of India (Anon, 2020d). The Directorate General of Drug Administration of Bangladesh has not yet approved any serological tests for the country except for research purposes.
Low-cost therapeutics
In the absence of a vaccine to tackle COVID-19, many repurposed drugs have been identified in observational series, or are being used according to anecdotal, in vitro, or extrapolated evidence. The repositioning of old drugs for use as antiviral treatments for COVID-19 patients is an intuitive strategy during the pandemic because the safety profiles, side-effects, posology, and drug interactions of these drugs are already established. These drugs include remdesivir, chloroquine, favipiravir, danoprevir, ritonavir, bromhexine hydrochloride, hydrochloroquine, and convalescent plasma. In a resource-limited country such as Bangladesh, we feel strongly that if such drugs are scientifically proven to be safe and effective, then they should be made available to the general population and free for the indigent population. The icddr,b laboratory is at present conducting a randomized, double-blind, placebo-controlled trial in three COVID-19 dedicated hospitals in Dhaka city, comparing ivermectin and doxycycline in combination or ivermectin alone for the treatment of adult Bangladeshi patients.
The pharmaceutical industry in Bangladesh continues to produce 98% of the medicines used domestically, and exports high-quality drugs to over 150 countries, including Europe and North America. This outstanding growth of the drug industry occurred following the Bangladesh government’s 1982 Drug Act, promoting local pharmaceutical enterprizes. This resulted in local institutions being able to produce inexpensive, high-quality, generic drugs that do not require extensive and costly human trials. Bangladesh, as an LMIC, is exempt from patent restrictions until 2032, and is free to copy any drug on the market or in the pipeline, while more developed countries can only copy out-of-patent drugs. As a result, soon after Abbott Pharmaceutical announced that one of its antiviral drugs (remdesivir) showed good safety and efficacy against severe COVID-19 in the USA, Bangladeshi Pharma were the first to make generic copies of the drug and export them globally.
WASH preventive measures
The rapid monitoring of COVID-19 transmission pathways is required for prevention, intervention, and control. Studies have shown that COVID-19 viral RNA can be persistently shed in the feces for a maximum of 33 days after the patient has tested negative for respiratory viral RNA (Gupta et al., 2020), although it is yet to be confirmed that fecal–oral transmission is indeed possible (Quilliam et al., 2020). Safely managing fecal waste from infected, recovering, and recovered patients poses a significant challenge in developing countries and in urban slums. Despite several uncertainties, new horizons are opening up, as shown by recent reviews on testing for SARS-CoV-2 in wastewater, aimed at early detection and monitoring of outbreaks. Thus far, researchers have found traces of the SARS-CoV-2 in sewage in the Netherlands, Australia, China, India, the USA, and Sweden. Evidence from studies on hand hygiene and influenza potentially provides a useful comparison for COVID-19. A systematic review by Saunders-Hastings et al. (2017) showed frequent handwashing to have a significant protective effect against pandemic influenza (Figure 3 ). Aiello et al. (2008) found that handwashing reduces the rate of respiratory infections by removing respiratory pathogens from the hands, thus preventing them from entering the body or passing on to other people. Further evidence suggests that washing hands with soap after defecation and before eating can cut the respiratory infection rate by up to 25% (Curtis and Cairncross, 2003). Convenient and accessible handwashing techniques are therefore needed on entering or leaving households and in public places, especially after coughing or sneezing.
Figure 3.
A public foot-operated hand-washing station designed to halt transmission.
Delivery of essential health services
A potential outbreak response program for COVID-19 prevention can be deployed at three levels: a mass strategy, a district/ward strategy, and a household strategy. A mass strategy can be deployed within a city or town, where residents will be informed of the ‘COVID prevention program’ in their respective district or ward via SMS messages, local health centers, EPI centers, pharmacies, and community notice boards. Health education would focus on the risk factors for COVID-19 and better cough hygiene practices. Mass media on COVID-19 could showcase a Protect your family from COVID-19 video, to illustrate effective WASH practices aimed at lowering risk. As part of a district/ward strategy, an early warning surveillance system could be deployed using: rapid diagnostic testing (e.g. the Roche antibody test) for suspected COVID cases, at health facilities and local hospitals; periodic testing of municipality sewage water for the presence of SARS-CoV-2; Android-based phone reporting of real-time test results; GIS risk mapping of patients’ addresses. Finally, a family emergency WASH kit could be utilized as a household strategy for families with a newly diagnosed family member. The kit for COVID-19 could comprise: a prevention poster for the family on how to minimize the risk of acquiring and transmitting COVID-19; ‘soapy water’ package (soap and three dispensers) – enough for a family of five for 30 days; daily household disinfectant with bleach (3–6% sodium hypochlorite); and masks for wearing at home.
Conclusions
Vaccination alone will not halt the COVID-19 pandemic. Low-cost, evidence-based, integrated control strategies will be required. We must ensure access to reliable diagnostics in order to determine the true burden of disease in the community. To support the existing health system, data on the safety and effectiveness of locally available, affordable, and cost-effective therapeutic drugs need to be generated in order to treat COVID-19 patients. A combination of effective vaccination, treatment, and WASH will ensure enhanced protection against COVID-19. Collaboration at the international, national, regional, and local level is parmount if we are to halt the spread of infection and end the pandemic.
Conflicts of interest
We declare no conflicts of interest.
Funding
No funding was required.
Ethical approval
Ethical approval sought.
References
- Aiello A.E., Coulborn R.M., Perez V., Larson E.L. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health. 2008;98(8):1372–1381. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO’s Draft landscape of COVID-19 candidate vaccines. Available at https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines[Accessed 29 August 2020].
- Coronavirus Vaccine Tracker. Available at https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.[Accessed 29 August 2020].
- https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance.
- https://indianexpress.com/article/explained/coronavirus-numbers-tracker-serological-survey-delhi-mumbai-6518055/.
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323(February (14)):1406–1407. doi: 10.1001/jama.2020.2565. Epub ahead of print. PMID: 32083643; PMCID: PMC7042844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis V., Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3(5):275–281. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- Directorate General of Health Services (DGHS), Government of Bangladesh. Available from: https://dghs.gov.bd/images/docs/Notice/rt_pcr_lab.pdf [Accessed 25 July 2020].
- Folegatti P.M., Ewer K.J., Aley P.K. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial [published online ahead of print, 2020 Jul 20] Lancet. 2020 doi: 10.1016/S0140-6736(20)31604-4. S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Parker J., Smits S., Underwood J., Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces – a rapid review. Colorectal Dis. 2020;22(6):611–620. doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P., Fleming T.R., Longini I., Henao-Restrepo A.M., Peto R., World Health Organization Solidarity Vaccines Trial Expert Group COVID-19 vaccine trials should seek worthwhile efficacy [published online ahead of print, 2020 Aug 27] Lancet. 2020 doi: 10.1016/S0140-6736(20)31821-3. S0140-6736(20)31821-31823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: Oxford team begins vaccine trials in Brazil and South Africa to determine efficacy. BMJ. 2020;369:m2612. doi: 10.1136/bmj.m2612. Published 2020 June 29. [DOI] [PubMed] [Google Scholar]
- Moon S., Jambert E., Childs M., von Schoen-Angerer T. A win-win solution? A critical analysis of tiered pricing to improve access to medicines in developing countries. Global Health. 2011;7:39. doi: 10.1186/1744-8603-7-39. Published 2011 October 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura-Ikeda M., Imai K., Tabata S., Miyoshi K., Murahara N., Mizuno T., Horiuchi M., Kato K., Imoto Y., Iwata M., Mimura S., Ito T., Tamura K., Kato Y. Clinical evaluation of self-collected saliva by RT-qPCR, direct RT-qPCR, RT-LAMP, and a rapid antigen test to diagnose COVID-19. Clin Microbiol. 2020;(July) doi: 10.1128/JCM.01438-20. JCM.01438-20, Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam R.S., Weidmann M., Moresco V., Purshouse H., O’Hara Z., Oliver D.M. COVID-19: The environmental implications of shedding SARS-CoV-2 in human faeces. Environ Int. 2020;140(July):105790. doi: 10.1016/j.envint.2020.105790. Epub 2020 May 6. PMID: 32388248; PMCID: PMC7200326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A.G., Rahman M., Alam M., Zaman K., Qadri F. Can we’ WaSH’ infectious diseases out of slums? Int J Infect Dis. 2020;92:130–132. doi: 10.1016/j.ijid.2020.01.014. [DOI] [PubMed] [Google Scholar]
- Saunders-Hastings P., Crispo J.A.G., Sikora L., Krewski D. Effectiveness of personal protective measures in reducing pandemic influenza transmission: a systematic review and meta-analysis. Epidemics. 2017;20:1–20. doi: 10.1016/j.epidem.2017.04.003. [DOI] [PubMed] [Google Scholar]
- WHO . 2020. Critical Preparedness, Readiness and Response Actions for COVID-19: Interim Guidance. https://apps.who.int/iris/bitstream/handle/10665/331511/Critical preparedness,readiness and response actions COVID-102020-03-22_FINAL-eng.pdf?sequence=1&isAllowed=y, [Accessed 3 April 2020] [Google Scholar]
- Zainol Rashid Z., Othman S.N., Abdul Samat M.N., Ali U.K., Wong K.K. Diagnostic performance of COVID-19 serology assays. Malays J Pathol. 2020;42(April (1)):13–21. PMID: 32342927. [PubMed] [Google Scholar]