Abstract
Background
COVID-19 is a pandemic disease that has paralyzed social life and the economy around the world since the end of 2019, and which has so far killed nearly 600,000 people. The rapidity of its spread and the lack of detailed research on the course and methods of transmission significantly impede both its eradication and prevention.
Scope and approach
Due to the high transmission rate and fatality resulting from COVID-19 disease, the paper focuses on analyzing the current state of knowledge about SARS-CoV-2 as well as its potential connection with food as a source of pathogen and infection.
Key findings and conclusions
There is currently no evidence (scientific publications, WHO, EFSA etc.) that COVID-19 disease can spread directly through food and the human digestive system. However, according to the hypothesis regarding the primary transmission of the virus, the source of which was food of animal origin (meat of wild animals), as well as the fact that food is a basic necessity for humans, it is worth emphasizing that food can, if not directly, be a carrier of the virus. Particular attention should be paid to this indirect pathway when considering the potential for the spread of an epidemic and the development of prevention principles.
Keywords: COVID-19, Food safety, Prevention, Virus transmission
1. The pandemic outbreak – introduction
Viral diseases have plagued Earth since before the dawn of civilization, but only in the last century have technological advances led to a characterization of the etiological agents and their epidemiology (Cook, 2013). Coronaviruses (CoVs) are one of the major pathogens that primarily target the human respiratory system. Previous outbreaks of coronavirus diseases include severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) (Rothan & Byrareddy, 2020). In 2002 SARS infection was confirmed in 8098 individuals, 774 patients died. The epicenter of the disease was Guangdong, China, from where it spread internationally to more than twelve countries (Arshad Ali, Baloch, Ahmed, Arshad Ali, & Iqbal, 2020). Two MERS outbreaks took place, the first epidemic in 2012 caused a total of 2494 cases with 858 deaths (Arshad Ali et al., 2020), while the second one in 2017 had 2037 laboratory-confirmed infection cases and 710 deaths (Zhou, Li, Zhao, Chu, Wang & Yan, 2017). MERS demonstrated clinical features similar to SARS with prominent gastrointestinal symptoms and acute kidney failure. It has been reported that up to 20% of MERS cases could be considered as primary infections due to contact with camels. Camels infected by MERS coronavirus (MERS-CoV), especially juvenile camels, were found to shed a large amount of the virus from the upper respiratory tract. Camel milk may also have played a role in the virus transmission; as MERS-CoV can survive in camel milk for a prolonged period, the consumption of unpasteurized camel milk was found to be a source of infection in some MERS patients (Zhou et al., 2017).
At the end of December 2019 patients with pneumonia of unknown etiology were admitted to Wuhan hospital, then reported to the Chinese Center for Disease Control and Prevention and to the World Health Organization (WHO) Country Office in China (Arshad Ali et al., 2020). As infection was caused by a coronavirus it was provisionally called the Wuhan virus, and then 2019 novel coronavirus (2019-nCoV). On 11th February 2020, the International Committee on Taxonomy of Viruses and WHO announced an official name, which was coronavirus disease 2019 (COVID-19), while the pathogen acquired the name severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
So far this disease has spread rapidly from Wuhan, first to other Chinese districts, and then to 216 countries worldwide (Coronavirus Disease (COVID-2019) Situation Report -173, 2020; Wu, Wu, Liu, & Yang, 2020). The chronology of COVID-19 infections is as follows. The first cases were reported in December 2019. From 18th December 2019 through 29th December 2019, five patients were hospitalized with acute respiratory distress syndrome and one of these patients died (Lu, 2020). By 2nd January 2020, 41 patients admitted to hospital had been identified as having laboratory-confirmed COVID-19 infection. As of 22nd January 2020, a total of 571 cases of the 2019 novel coronavirus were reported in 25 provinces in China (Lu, 2020). As of 30th January 2020, 7734 cases had been confirmed in China and 90 other cases had also been reported from other countries that included Taiwan, Thailand, Vietnam, Malaysia, Nepal, Sri Lanka, Cambodia, Japan, Singapore, the Republic of Korea, the United Arab Emirates, the United States, the Philippines, India, Australia, Canada, Finland, France and Germany. At this time, the case fatality rate was calculated to be 2.2% (170/7824) (Bassetti, Vena, & Giacobbe, 2020; Rothan & Byrareddy, 2020). The first case of COVID-19 infection confirmed in the United States of America occurred on 27th January 2020.
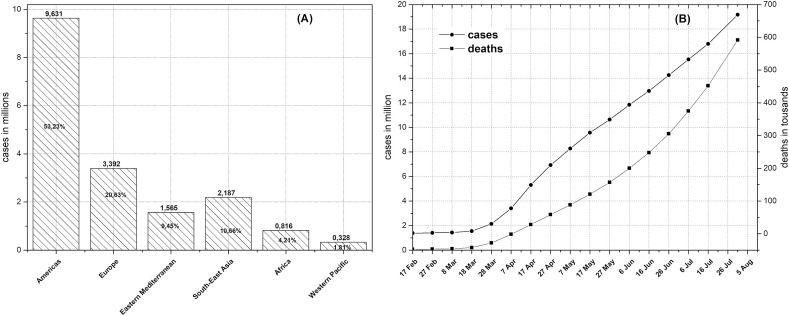
According to WHO data, the spread of the disease on all continents has led to over twelve million cases and nearly 600,000 deaths (Fig. 1 ). As of 3th August 2020, most cases were recorded in USA (4,582,276 cases, 153,757 deaths), Brazil (2,707,877 cases, 93,563 deaths), India (1,803,695 cases, 38,135 deaths) and Russia (856,264 cases, 14,207 deaths). In total, 55.53% of cases and 43.64% of deaths occurred in the four countries mentioned above (Coronavirus Disease (COVID-2019) Situation Report -173, 2020).
Fig. 1.
Spread of coronavirus around the world from February 2020. (A) Regional pread of disease and (B) Evolution of cases and deaths [based on (Coronavirus Disease (COVID-2019) Situation Report -173, 2020)].
2. Is COVID-19 the first respiratory transmitted outbreak which is foodborne?
The whole world is currently struggling with the coronavirus pandemic, but epidemiologic investigation has proven that many initial cases were associated with a ‘wet market’, the Huanan South Seafood Wholesale Market in Wuhan, China. This suggests that COVID-19 could be the first respiratory transmitted outbreak which is foodborne. Outbreaks of infectious diseases are common, and usually happen through one of several means of transmission: food, water, person-to-person or environmental. Regardless of the initial means of transmission, outbreaks are followed by spreading through direct human contacts (Jalava, Kauppinen, Al-Hello, & Räsänen, 2019). Initial human-to-human transmission was limited, and cases clustered around the Huanan market, mainly among elderly men. Taking these two points into account, and given that coronaviruses are derived from animals, this suggests the possibility of zoonotic transmission in the first instance.
Because most COVID-19 patients lived around or worked at the ‘wet market’ in Wuhan, where fruit, vegetables, seafood and live (often wild) animals were also on sale, it was suspected that the main mechanism of transmission was from animal to human (Chen, Zhou, et al., 2020). Consumption of exotic animal meat is common in China, because it is believed to have health promoting properties. Therefore, in Asian wet markets, alongside fresh vegetables, live animals can be bought. Such animals include: chicken, snakes, civets, rats, giant salamanders, dogs, wolves, pangolins, pheasants, bats, marmots, deer, rabbits, beavers, porcupines, hatchling crocodiles and other domestic and exotic fare. These animals are kept alive in cages, close to each other, and slaughtered for meat just before sale and consumption. Therefore, viruses can easily spread from an infected animal to another animal or human. They can also contaminate plant food. Taking into account the number of cases related to one location, the exposure may have been substantial, and possibly occurred within a limited time period. This would indicate the presence of either highly viremic animals, contaminated food or a seafood animal environment. This was why the Chinese government temporarily closed the Huanan market starting from 1st January 2020. It is worth remembering that the closing of the animal markets also played a key role in tempering the scope and duration of the SARS epidemic (Baric, 2008). Very little information has been released on animal or food exposure, and among the first cases, only a few human-to-human transmission events were reported, and these were household transmissions. This mode of transmission became more evident later (Wang, Hu, et al., 2020).
There is, of course, considerable controversy regarding the source of coronavirus in humans. Some recent studies have demonstrated that SARS-CoV-2 genes can be detected in samples collected before the outbreak in Wuhan. A study published in April 2020 suggests that the COVID-19 epidemic had already started in France in late December 2019 (Deslandes et al., 2020). The authors reported the case of 42-year-old patient hospitalized in the intensive care unit of a hospital near Paris. The patient was an unemployed male born in Algeria. He had lived in France for many years and his last foreign trip was to Algeria in August 2019. Another example is a study from the Italian National Institute of Health (Istituto Superiore di Sanità - ISS). They collected forty samples of sewage water during October 2019 to February 2020 from wastewater treatment plants located in northern Italy. They reported the presence of SARS-CoV-2 in sewage water from Milan and Turin on the 18th of December 2019 (CS N°39/2020 - Studio ISS Su Acque Di Scarico, a Milano e Torino Sars-Cov-2 Presente Già a Dicembre - ISS, 2020). Further studies also show that the virus was found in frozen sewage water collected in Barcelona (Spain) even in March 2019 (Chavarria-Miró et al., 2020). The above mentioned studies provide evidence that SARS-CoV-2 was present in the environment before it began to spread rapidly in China. Therefore, it is possible that the Wuhan ‘wet market’ was not the first source of SARS-CoV-2 (Mackenzie & Smith, 2020; Zhang, Yang, Zhang, & Lin, 2020).
New findings have been published by a UK research team (Forster, Forster, Renfrew, & Forster, 2020), which analyzed 160 complete SARS-CoV-2 genomes sampled from across the world between 24th December 2019 and 4th March 2020. The authors revealed three distinct 'variants' of SARS-CoV-2, consisting of clusters of closely related lineages, which they named ‘A’, ‘B’ and ‘C’. Variant A, most closely related to the virus found in both bats and pangolins, is described as ‘the root of the outbreak’. Type B of the virus derives from variant A, separated by two mutations, while type C has in turn formed from type B. Type A was present in Wuhan, but was not the city's predominant virus type. Wuhan largely contained type B, which was also the most common type found in East Asia. It is interesting that this variant didn't travel much beyond that region without further mutations, implying some kind of ‘resistance’ against this type of coronavirus outside East Asia. Type A was found in Chinese individuals and Americans reported to have lived in Wuhan, and mutated versions of type A were found in patients from America and Australia. On the other hand, type C is the major variant in Europe, it is absent in the mainland Chinese samples, but was found in Singapore, Hong Kong, Taiwan and South Korea.
All these studies suggest the virus may be months older than we thought, they further suggest that the virus did not originate in the animal markets of Wuhan, but probably from south of Hubei province. Nonetheless, bats and pangolins can still be considered its animal source.
3. General characteristics of coronaviruses
SARS-CoV-2 (2019-nCoV during the early stages of the pandemic) is a novel species from within the group of human coronaviruses (HCoVs) that causes COVID-19 disease. As with other human coronaviruses, SARS-CoV-2 belongs to the family Coronaviridae (subfamily: Orthocoronavirinae) in the order Nidovirales (Walker et al., 2019). Before the COVID-19 outbreak there were only six coronaviruses that were known to infect humans and cause respiratory diseases. Out of them, four (HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1) are endemic globally and cause only mild upper respiratory disease. In rare cases some of them can cause severe infection of infants and the elderly. The other two, SARS-CoV and MERS-CoV can infect the lower respiratory tract and cause severe acute respiratory syndrome in humans (Paules, Marston, & Fauci, 2020; Chen, Liu, & Guo, 2020).
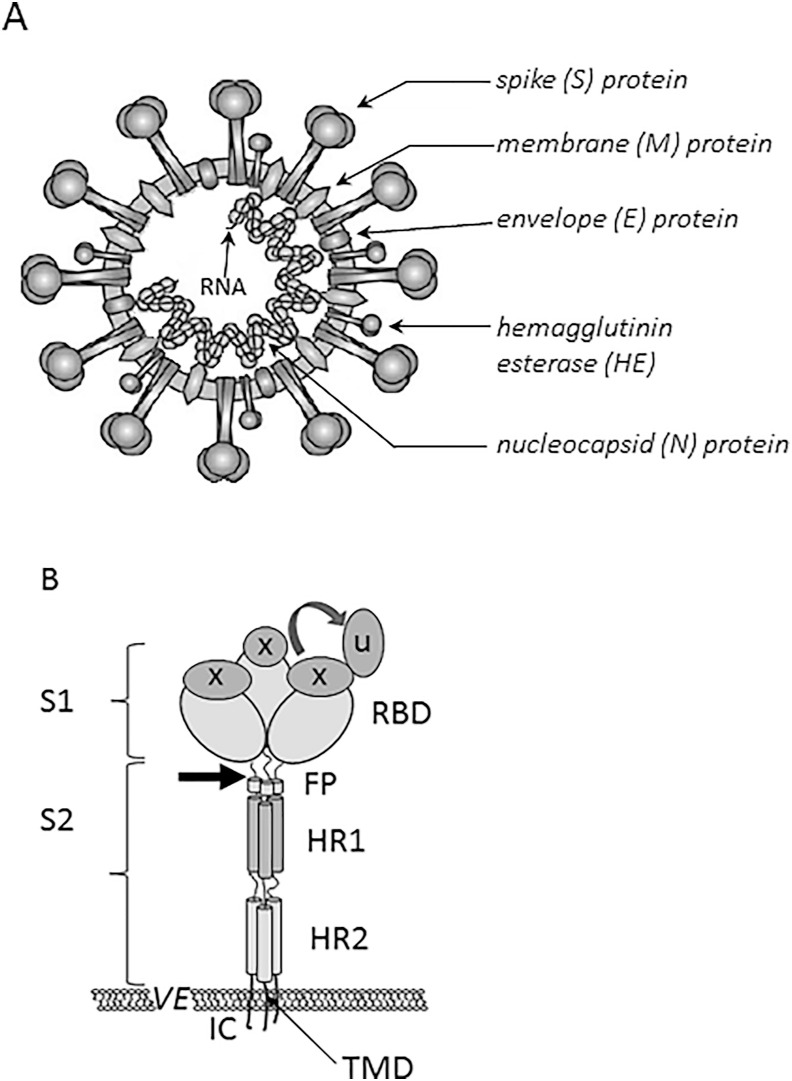
Coronavirus virions are enveloped and pleomorphic (usually of spherical shape) with a diameter of 80 nm–120 nm (Fung & Liu, 2019). They have large club-shaped projections (spikes) composed of a type I glycoprotein called spike (S) protein (Lai & Cavanagh, 1997). The S protein is trimeric, highly glycosylated and rises some 20 nm above the virion envelope, giving the virion the appearance of a crown (the origin of the name ‘coronavirus’) (Li, 2016; Masters, 2006). It has a major influence on viral tropism and pathogenic phenotype. Some coronaviruses (e.g. human betacoronaviruses) also contain an additional layer of short spikes, which consist of hemagglutinin-esterase (HE) protein, also a type I glycoprotein (Fig. 2 A). Both large and small spikes are anchored in the envelope, which is a lipid bilayer formed when virion is leaving the host cell. The envelope is also associated with a small integral membrane (M) protein and a small envelope (E) protein. Inside the envelope is a ribonucleoprotein (RNP) composed of a single nucleocapsid (N) protein bound with the genomic RNA in a beads-on-a-string fashion forming a helical nucleocapsid. Coronaviruses have nonsegmented, single-stranded positive-sense RNA (Lai & Cavanagh, 1997). With lengths ranging from 27.3 kb (HCoV-229E) to 31.3 kb (murine coronavirus MHV) coronavirus genomes are among the largest mature RNA molecules known to biology. Almost two thirds of the entire RNA (20 kb–22 kb) is occupied by the coronavirus polymerase gene comprising two overlapping open reading frames (ORF1a and ORFlb) (Fung & Liu, 2019; Hulswit, de Haan, & Bosch, 2016; Masters, 2006; Mousavizadeh & Ghasemi, 2020; Weiss & Leibowitz, 2011).
Fig. 2.
The structure of SARS-CoV-2 on the basis of (Mousavizadeh & Ghasemi, 2020; Shang et al., 2020; The Enemy Within, 2020.; Wang, Xia, et al., 2020). (A) The structure of a SARS-CoV-2 particle. (B) A scheme of the structure of coronavirus spike. S1, receptor-binding subunit; S2, membrane fusion subunit; TMD, transmembrane domain; IC, intracellular tail; VE, viral envelope. The receptor binding domain (RBD) is switching between a standing-up position (u) and a lying-down position (x). FP (fusion peptide), HR1 (heptad repeat 1), and HR2 (heptad repeat 2) are structural elements in a coronavirus S2 subunit. Arrow shows the protease cleavage site.
The coronavirus infection cycle includes the following steps: attachment, entry into the host cell, translation of the replicase-transcriptase, replication of the genome and transcription of mRNAs, and finally the assembly and budding of newly packaged virions. Virion attachment to the cell host depends on the binding of S protein to the host cell surface receptor(s).
The S protein can be functionally divided into two subunits, subunit S1 is responsible for host recognition and receptor binding, while the S2 subunit is involved in cell membrane fusion (Lu et al., 2020). S1 contains a signal peptide, followed by an N-terminal domain (NTD) and receptor-binding domain (RBD), whereas S2 contains conserved fusion peptide (FP) and heptad repeat regions (HR1 and HR2) (Wang, Xia, et al., 2020) (Fig. 2 B ). Some authors also include a transmembrane domain (TMD) and a cytoplasmic domain (CP) as components of the S2 subunit (Chan, Kok, et al., 2020). In the natural state, S protein on the surface of coronavirus is inactive and subunit S2 is hidden inside S1. It has been proven that receptor binding (the specific interaction between RBD in S1 and the cognate host cell receptor) needs proteolytical activation, which results in S1 dissociation. The dissociation of subunit S1 triggers drastic conformational changes leading to exposure of S2 and the fusion peptide, which is followed by interaction between HR1 and HR2 and fusion between the virus envelope and the cellular membrane (Shang et al., 2020; Wang, Xia, et al., 2020).
Receptor binding is the major determinant of host range and tissue tropism for a coronavirus (Table 1 ). Among known receptors recognized by HCoVs on cells are surface receptors such as angiotensin converting enzyme 2 (ACE2), dipeptidyl peptidase 4 (DPP4), aminopeptidase N (APN), and 9-O-acetylated sialic acid (Fung & Liu, 2019; Hulswit et al., 2016; Lim, Ng, Tam, & Liu, 2016; Masters, 2006). The S2 subunit of SARS-CoV-2 showed around 93% sequence identity with bat-SL-CoVZC45 and bat-SL-CoVZXC21, much higher than that of the S1 subunit, which had only around 68%–70% identity with these bat-derived viruses (Wang, Xia, et al., 2020). However, the core domain of RBD is highly conserved and phylogenetic analysis found that although SARS-CoV-2 was closer to bat-SL-CoVZC45 and bat-SL-CoVZXC21 at the whole-genome level, its receptor binding was closer to that of SARS-CoV (Chan, Kok, et al., 2020; Lu et al., 2020; Wang, Xia, et al., 2020). Also, the HR1 and HR2 domains shared a 92.6% and 100% identity, respectively, with those of SARS-CoV (Wang, Xia, et al., 2020).
Table 1.
Comparison of known human coronaviruses.
| Genus | Alphacoronavirus | Betacoronavirus | |||||
|---|---|---|---|---|---|---|---|
| Strain | HCoV-229E | HCoV-NL63 | HCoV-OC43 | HCoV-HKU1 | SARS-CoV | SARS-CoV-2 | MERS-CoV |
| Disease | mild upper respiratory tract infection |
mild upper respiratory tract infection |
mild upper respiratory tract infection |
pneumonia | Severe acute respiratory syndrome (SARS) | Coronavirus Disease 2019 (COVID-19) | Middle East respiratory syndrome (MERS) |
| Receptor on human cells | APN | ACE2 | ASA | ASA | ACE2 | ACE2 | DPP4 |
| Tissue/organ tropism | Monocytic and granulocytic lineage; synaptic membranes of the central nervous system; intestinal, lung and kidney epithelial cells | Arterial and venous endothelium; arterial smooth muscle; small intestine, respiratory tract epithelium; alveolar monocytes and macrophages | Sub-maxillary mucin | Airway epithelium, alveolar cells | Arterial and venous endothelium; arterial smooth muscle; small intestine, respiratory tract epithelium; alveolar monocytes and macrophages | Respiratory tract epithelium; kidney, small intestine; liver and prostate; activated leukocytes | |
| Natural host/reservoir/ | bats | bats | rodents | rodents | bats | bats | bats |
| Intermediate host | dromedary camels, alpacas | palm civets | cattle | mice | palm civets, raccoon dog | pangolin, bats | dromedary camels |
| Symptoms | Malaise, headache, nasal discharge, sneezing, sore throat, fever, cough | Cough, Rhinorrhea, tachypnea, fever, hypoxia, croup | Malaise, headache, nasal discharge, sneezing, sore throat, fever, cough | Fever, running nose, cough, dyspnea | Fever, myalgia, headache, malaise, dry cough, dyspnea, respiratory distress, diarrhea | Fever, dry cough, dyspnea, myalgia, headache, diarrhea, fatigue, shortness of breath, sore throat, nausea, vomiting, rhinorrhea, conjunctivitis | Fever, cough, chills, sore throat, myalgia, arthralgia, dyspnea, pneumonia, diarrhea and vomiting, acute renal impairment |
| Transmission | Respiratory droplets, fomites | Respiratory droplets, fomites | Respiratory droplets, fomites | Respiratory droplets, fomites | Respiratory droplets, fomites, fecal-oral | Respiratory droplets, fomites, fecal-oral | Respiratory droplets, fomites |
| Transmission by asymptomatic patient | not reported | not reported | not reported | not reported | very rare | often | no |
| References | (Belouzard et al., 2012, Corman et al., 2015, Corman, Muth, Niemeyer, & Drosten, 2018, Fung and Liu, 2019, Lim et al., 2016, Ye et al., 2020, Chen et al., 2020, Fan et al., 2019, Fung and Liu, 2019, Wang et al., 2006, Xu et al., 2009); (Chen et al., 2020, Fan et al., 2019, Fung and Liu, 2019, Lim et al., 2016, Wang et al., 2006, Xu et al., 2009, Ye et al., 2020) | (Fung & Liu, 2019; Lim et al., 2016; Ye et al., 2020) | (Belouzard et al., 2012; Fung & Liu, 2019; Lim et al., 2016; Ye et al., 2020) | (Dijkman et al., 2013, Fung and Liu, 2019, Hu et al., 2015, Lim et al., 2016, Ye et al., 2020) | (Chen et al., 2020, Fan et al., 2019, Fung and Liu, 2019, Lim et al., 2016, Wang et al., 2006, Xu et al., 2020, Ye et al., 2020) | (Chan et al., 2020, Fung and Liu, 2019, Gu et al., 2020, Hu et al., 2020, Lim et al., 2016, Liu et al., 2020, Lu et al., 2020, Meng, Huang, Zhou, Li, & Wu, 2020, Meselson, 2020, Wang & Du, 2020, Ye et al., 2020, Zheng, 2020) | (Al Hosani et al., 2018, Alagaili et al., 2014, Azhar et al., 2014, Fung and Liu, 2019, Lim et al., 2016, Ye et al., 2020) |
APN – aminopeptidase N, ACE2 – angiotensin-converting enzyme 2, DPP4 – dipeptidyl peptidase 4, ASA – 9-O-acetylated sialic a.
It is interesting that although the SARS-CoV-2 RBD has a significantly higher human ACE2 binding affinity than SARS-CoV RBD, the cryo-electron microscopy structure of a SARS-CoV-2 spike revealed that its RBD is mostly in the lying-down state, a state associated with ineffective receptor binding (Shang et al., 2020). It is possible that hidden RBD contributes to the immune evasion of SARS-CoV-2 as one of the conformational masking strategies.
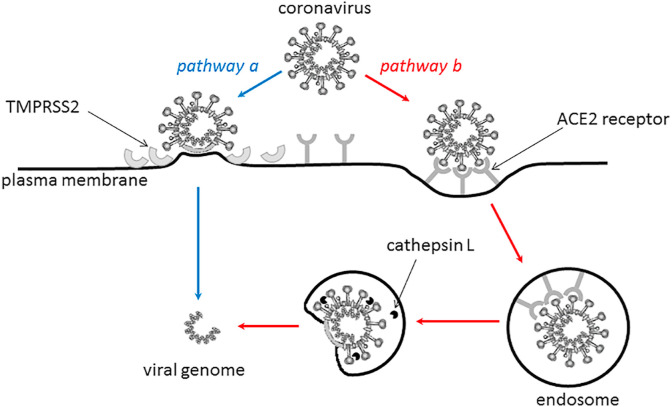
The coronavirus can enter into the target cell through two routes (Fig. 3 ), a direct fusion on the cellular surface (pathway ‘a') or via endocytosis (pathway ‘b'), the latter takes place in the endosomal compartment and is initiated by additional triggers. Some studies have shown that entry of SARS-CoV may be pH-dependent (Yang, Huang, Ganesh, Leung, Kong & Schwartz, 2004), and that the endosomal protease cathepsin L (Huang et al., 2006) might be involved, suggesting that pH level and cathepsin L may facilitate virus entry (Belouzard et al., 2012; Lim et al., 2016; Zhou et al., 2015). Several human proteases, such as transmembrane serine proteinase 2 (TMPRSS2) or furin, can cleave the spike protein and induce its activation. If these proteases are located near the spike-ACE2 binding interface, they will cleave the glycoprotein S to expose the S2 region and the fusion peptide region. Direct fusion on the cellular membrane is possible because the fusion peptide region contains a lot of hydrophobic amino acids, and therefore, easily inserts into the lipid-containing cell membrane, enabling fusion between viral membrane and host cell membrane with subsequent entry of the viral genome into the cell. As TMPRSS2 is expressed by human lung cells, it is thought to play an important part in SARS-CoV-2 virus entry into respiratory system cells (Wang, Xia, et al., 2020). It has also been shown that furin (distributed mainly in the Golgi apparatus) can proteolytically activate the MERS-CoV spike (S) protein after receptor binding, and then mediate fusion between the viral and cellular membranes (Millet & Whittaker, 2014). However, a virus cell entry assay and a cell-cell fusion assay conducted by Matsuyama et al. (Matsuyama et al., 2018) provided no evidence that the S protein was activated by exogenous furin.
Fig. 3.
The possible routes of SARS-CoV-2 entry into the target cell: pathway ‘a' – through a direct fusion on the cellular surface, pathway ‘b' – via endocytosis (on the basis of (The Enemy Within, 2020)).
After entry, the viral genomic RNA is released into the cytoplasm, and the uncoated RNA serves as a transcript to cap-dependent translation. It begins with the translation of ORF1a and ORF1b into polyproteins pp1a (4382 amino acids) and pp1ab (7073 amino acids), this is due to a ‘slippery’ sequence and an RNA pseudoknot structure near the end of ORF1a (Fung & Liu, 2019; Guo, Cao, et al., 2020; Masters, 2006). Then, with the assembly of the replicase-polymerase, the full-length positive strand of genomic RNA is transcribed to form a full-length negative-strand template for the synthesis of new genomic RNAs and overlapping subgenomic negative-strand templates. These subgenomic mRNAs are then transcribed and translated to produce the structural and accessory proteins (Hulswit et al., 2016). New genomic RNA binds with protein N to form helical nucleocapsid, which then interacts with other viral structural proteins (S, E and M proteins) to form the mature virion, which is released by budding through the host cell membrane.
4. Coronaviruses in animals are a potential source for epidemics in humans
Coronaviruses can be classified into four genera (Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus). It seems that bats and birds, the warm blooded flying vertebrates, are ideal hosts for the coronavirus gene source, bats mainly for alphacoronaviruses and betacoronaviruses, while birds for gammacoronaviruses and deltacoronaviruses (Woo, Lau, Lam, Lau, Tsang & Lau, 2012; Yin & Wunderink, 2018). However, coronaviruses or coronavirus-like infections have also been reported in swine, cattle, horses, cats, dogs, rats, ferrets, rabbits and various wildlife species, often being asymptomatic (Gong et al., 2017; Pratelli, 2008; Saif, 2004; Shi & Hu, 2008). The alphacoronaviruses are represented by the human pathogen HCoV-NL63 or HCoV-229E, whereas betacoronaviruses contain human pathogens like HCoV-OC43, all responsible for common cold or diarrhea, or causing asymptomatic infections (van der Hoek, 2007; Walsh, Shin, & Falsey, 2013; Ye et al., 2020; Zeng et al., 2018). However, betacoronaviruses also include dangerous SARS-CoV responsible for the severe acute respiratory syndrome epidemic 2002–2003 (Ksiazek et al., 2003) and MERS-CoV which caused Middle East respiratory syndrome in 2012 and 2017 (Table 1). HCoVs can activate apoptotic cascades also causing damage in various tissues and organs (Fung & Liu, 2019; Lim et al., 2016).
The novel SARS-CoV-2 has some similar characteristics when compared to SARS-CoV and other HCoVs (El Zowalaty & Järhult, 2020; McCloskey & Heymann, 2020; Xu et al., 2020; Yang et al., 2020), however, it is a novel, separate strain. The main human-to-human transmission pathway of SARS-CoV, MERS-CoV and SARS-CoV-2 is by droplets and aerosols (Chan, Yuan, et al., 2020; Meselson, 2020). However, there is one very important difference between these viruses. Asymptomatic and pre-symptomatic transmission of SARS-CoV-2 has been reported, this might contribute to its rapid spread around the world (Chan, Yuan, et al., 2020; Li, Ji, Wang, Wang, Hao & Dai, 2020), while transmission of SARS and MERS from an asymptomatic patient is extremely rare (Moon & Son, 2017; Yin & Wunderink, 2018). In contrast to SARS-CoV, SARS-CoV-2 and MERS-CoV, direct human-to-human transmission has not been reported for the other four HCoVs (Yin & Wunderink, 2018). This difference is highly significant in terms of how much potential a virus has to cause a pandemic. In 2012 MERS did not become a pandemic disease because the peak of virus shedding occurs after the onset of symptoms, which means that public health measures, such as isolating people with clinical symptoms, were sufficiently effective in limiting the spread of the virus (Anderson et al., 2004). Similarly with the SARS epidemic, rare cases of asymptomatic infections, a lower infectiousness and virus transmission typically occurring after disease onset, allowed public health care to introduce intervention strategies that controlled the outbreak (Baric, 2008). The SARS epidemic raised awareness of the importance of rapid reporting, infection control, and the tracing and quarantining of contacts in controlling new emerging diseases. Conversely, with influenza, the archetypal pandemic disease, the majority of virus transmission occurs before the host realizes that they are ill (Daly, 2017). The same applies to SARS-CoV-2.
Phylogenetic analyses have revealed that SARS-CoV-2 and SARS-CoV have high nucleotide sequence homology (79%–82%), and this was the highest sequence identity among the six other known pathogenic HCoVs (Zhou, Yang, Wang, Hu, Zhang & Zhang, 2020). However, the coronavirus strains isolated from animals seem to be more closely related to SARS-CoV-2. Genetically diverse SARS-like coronaviruses have been isolated from various species of horseshoe bats in China. Moreover, some strains are highly similar to SARS-CoV even in the spike protein, and are able to use the same receptor as SARS-CoV for cell entry (Hu, Ge, Wang, & Shi, 2015). Some coronavirus isolates obtained from human samples (Wuhan hospital patients) have an 86.9% nucleotide sequence identity to that of a bat SARS-like coronavirus isolate: bat-SL-CoVZC45 genome (MG772933.1) (Zhu et al., 2020). Wu et al. (Wu, Zhao, et al., 2020) revealed that bat-SL-CoVZC45, that had previously been sampled in China, had a nucleotide identity of 89.1% with virus isolated from bronchoalveolar lavage fluid from a patient with confirmed SARS-CoV-2 infection. In terms of the encoded genes (for proteins pp1ab, pp1a, envelope, matrix, accessory protein 7a, and nucleocapsid), phylogenetic analyses have shown that SARS-CoV-2 is more closely related to the SARS-like bat coronavirus than human SARS-CoV (Wu, Peng, et al., 2020). There are 380 amino acid substitutions between the amino acid sequences of SARS-CoV-2 and the corresponding consensus sequences of SARS and SARS-like viruses. Among them, 27 amino acid substitutions were found in the spike protein, including 6 mutations occurring in the receptor binding domain. These changes could affect the host tropism and transmission property of SARS-CoV-2 compared to SARS-CoV. Zhou et al. (Zhou et al., 2020) showed that SARS-CoV-2 had 96.2% overall genome sequence identity throughout the genome compared to BatCoV RaTG13 (a bat coronavirus detected in Rhinolophus affinis from Yunnan province) and more than 88.1% identity with a bat SARS-like CoV (bat-SL-CoVZC45, R. sinicus from Zhejiang province). Chan, Kok, et al., (2020) reported that the genome of SARS-CoV-2 isolated from a patient in Wuhan had 89% nucleotide identity with bat SARS-like-CoVZXC21 and 82% with that of human SARS-CoV BJ01 2003 (AY278488) and human SARS-CoV Tor2 (AY274119). The phylogenetic trees of their orf1a/b, spike, envelope, membrane and nucleoprotein also clustered closely with those of the bat, civet and human SARS coronaviruses. The S2 subunit of spike protein of SARS-CoV-2 strain from Wuhan was highly conserved and shared 99% identity with those of the two bat SARS-like CoVs (SL-CoV ZXC21 and ZC45) and human SARS-CoV (Chan, Kok, et al., 2020). Other research has also revealed the similarity of the SARS-CoV-2 genome to various coronavirus genomes from pangolins. Six strains of coronaviruses (GX/P2V, GX/P3B, GX/P4L, GX/P5E, GX/P1E and GX/P5L) isolated from Malayan pangolins (Manis javanica) from the Guangxi province of China, had genomes characterized by 85.5%–92.4% sequence similarity to SARS-CoV-2 (Lam et al., 2020).
Bats are the only mammals that can fly, therefore, they can easily spread viruses to other vertebrates even in remote regions (Calisher, Childs, Field, Holmes, & Schountz, 2006). They can migrate for a long distance, are able to survive hibernation and are characterized by extreme longevity. Among the viruses found in bats are rabies virus, Nipah virus, Hendra virus, Ebola virus, Marburg virus and various coronaviruses with SARS-like viruses among them (Calisher et al., 2006; Fan, Zhao, Shi, & Zhou, 2019; Han et al., 2015; Wang et al., 2006). Although SARS-CoV-2 was found in bats, its transmission route among hosts is not clear, but it appears that the virus is becoming more transmissible between humans. Moreover, a natural reservoir host tends to coevolve with its viruses, but does not usually display clinical signs of infection.
5. The spread of coronaviruses and transmission to humans
For zoonotic virus transmission to occur from a wildlife reservoir the following four events are required: inter-species contact, cross-species virus transmission (spillover), sustained transmission, and virus adaptation within the spillover species (Wang et al., 2006). The exact mechanism of transmission from reservoir host to intermediate host is often unknown. However, the fecal-oral route seems to be the predominant method of coronavirus transmission among animals, especially when the animal species concerned are neighbors. It follows that coronavirus transmission between animals in Chinese ‘wet markets’ was very plausible, especially so as the live animals kept in cages were stressed and therefore practicing poor hygiene standards. Animal-to-human transmission requires either direct contact or occurs through objects/food contaminated with animal feces, urine, blood or aerosols (Wang et al., 2006).
To infect a new host species, coronaviruses must adapt to the receptor of their new host. This can occur either by mutation or by recombination with a coronavirus infecting their new host. Bats harbor coronaviruses with great genetic diversity, and they are considered a main reservoir of mammalian coronaviruses (Fan et al., 2019; Hu, Ge, Wang & Shi, 2015). It is believed that most currently circulating alphacoronaviruses and betacoronaviruses in different mammals are linked by evolution to ancestral coronaviruses originating from bats. Coronaviruses have a strong history of host shifting, and many HCoVs are recognized as having an animal reservoir. Phylogenetic analysis has shown that bovine CoV (BCoV) was the origin for HCoV-OC43 following a relatively recent cross-species transmission event. The natural reservoir of MERS-CoV is presumed to be in dromedary camels, from which zoonotic transmissions repeatedly give rise to infections of the lower respiratory tract in humans (Hulswit et al., 2016). Some data suggests that HCoV-229E may actually be transferred from dromedary camels as is the case with the MERS coronavirus (Corman, Muth, Niemeyer, & Drosten, 2018). Four novel deltacoronaviruses were detected in fecal samples from eight birds of four different species (Lau et al., 2018), which indicates possible virus transmission by the fecal-oral route. Moreover, genome analysis has provided evidence of recent inter-species transmission between birds and their prey, possibly along the food chain, as well as avian-to-swine transmission. This suggests that somewhere in the course of evolution, the food (prey) has become the source of the pathogen for the predator. Also, HCoV-NL63, HCoV-229E, SARS-CoV and MERS-CoV were predicted to have originated from bats, while HCoV-OC43 and HCoV-HKU1 to have probably originated from rodents (Bolles, Donaldson, & Baric, 2011; Cui, Li, & Shi, 2019; Hu et al., 2015; Huynh et al., 2012; Ye et al., 2020).
In the case of SARS-CoV, the virus appeared in 2002 in live animal markets in China, closely related viruses were isolated from Himalayan palm civets, raccoon dogs and Chinese ferrets (Ye et al., 2020). Recent virology and genetic studies indicate that although horseshoe bats (Rhinolophus genus) are reservoir hosts of SARS-CoV and MERS-CoV, the virus was spread to humans from palm civets and dromedary camels respectively, which were intermediary hosts during the cross-species event (Zheng, 2020). In the case of SARS-CoV-2, the pangolin seems to be the intermediary host. In 2016–2017, swine acute diarrhea syndrome (SADS) was observed in pigs in Guangdong province, with a mortality rate of up to 90% for piglets 5 days or younger. A novel HKU2-related bat coronavirus (SADS-CoV) was identified as the agent causing the disease, and it was revealed that isolates were almost identical (95%) with Rhinolophus bat coronavirus HKU2 (Cui et al., 2019).
The high diversity of coronaviruses is attributable to their potentially high mutation rates associated with RNA replication, estimated as 10−3 to 10−5, offspring differ by 1–2 mutations each from their parent (Duffy, 2018). The recombination frequencies within the coronavirus family are very high (about 25%) during mixed infections, this is probably a result of discontinuous RNA transcription. Moreover, with the largest RNA genome among viruses, coronaviruses have an increased opportunity for mutations. These genomic characteristics create a high diversity of coronaviruses, and allow for rapid change and adaptation to novel hosts (mainly due to mutations in spike proteins), ecological niches, tissue tropism, and even generation of novel coronavirus species sometimes causing major zoonotic outbreaks with disastrous consequences (Bolles et al., 2011; Duffy, 2018; Lau et al., 2018; Woo, Lau, Huang, & Yuen, 2009).
Therefore, the genetic diversity of SARS-like coronaviruses in bats, and their high mutation rates, trigger changes that cause direct interspecies transmission to humans. Analysis of receptor binding and the pathogenesis of SARS suggests that SARS-CoV was most likely generated in bats through sequential recombination of bat SARS related coronaviruses, and that this recombination occurred in bats before coronavirus infected civets or other mammals (Lau et al., 2015). The introduced SARS-CoV underwent rapid mutations in S and ORF8 and successfully spread in market civets. After several independent spillovers to humans, some of the strains underwent further mutations in S protein and became epidemic during the SARS outbreak 2002–2003. The same situation applies to MERS. The natural reservoirs of MERS-CoV (or an ancestral MERS-like CoV) are bats. It is hypothesized that bat MERS-like CoV jumped to camels several decades ago, then the virus evolved and adapted with accumulating mutations in camels, which has then been transmitted to humans very recently (Hu et al., 2015). A study of Sabir et al. demonstrated that dromedary camels share three species of coronavirus with humans and therefore they serve as an important reservoir for the maintenance and diversification of the MERS-CoVs and are the source of human infections with this virus (Sabir et al., 2016).
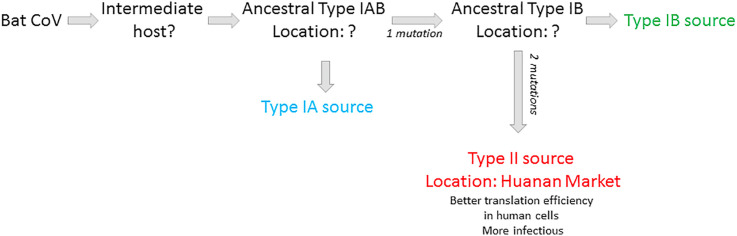
The outbreak of COVID-19 was first linked to Huanan Seafood Wholesale Market, where non-aquatic live animals, including several kinds of wild animal, were sold. After introduction to a human organism (probably some months before December 2019) a mutation in the virus took place, this enabled human-to-human transmission, which then became the main route of the virus spreading. However, based on current evidence, it is possible that the market was not the original source of SARS-CoV-2 transmission to humans (Mackenzie & Smith, 2020; Yu, Tang, Zhang, & Corlett, 2020). The results of Zhang et al. (Zhang, Yang, et al., 2020) suggest the outbreak of type II COVID-19 likely occurred in the Huanan market, while the initial transmission of the type I virus to humans probably occurred at a different location in Wuhan. Genomes of type I and II differ only in three sites: 8750, 28112 and 29063 (based on the genome coordinates MN938384.1), and the corresponding nucleotides are T, C and T/C in type I, and C, T and C in Type II, respectively. Type I can be further divided into type IA and IB based on the nucleotide at the site 29063. The authors demonstrated that the three sites in Type IA and two in Type IB are identical to those in BatCoV RaTG13, suggesting that Type I may be more closely related to the ancestral human-infecting strain than Type II. Type IA seems to be the earliest transmission source, but it did not occur in the Huanan Market [Fig. 4 ].
Fig. 4.
Possible routes of SARS-CoV-2 evolution [based on ( Zhang, Yang, et al., 2020)].
Genomic variations of SARS-CoV-2 analyzed by Yu et al. (Yu, Tang, et al., 2020) identified 58 haplotypes in 93 viral genomes. By comparing these to the bat-RaTG13-CoV genome as the outgroup, they found that haplotypes H13 and H38 might be considered as ancestral haplotypes, and that H1 was derived from the intermediate haplotype H3. It should be noted that all samples from the Huanan Market belonged to H1 or its derived haplotypes, but none of the available market samples encoded H3 haplotype. This suggests that H3 haplotype might have originated and spread outside the market before an early stage of population expansion. The non-synonymous mutation from H3 to H1 would probably have enhanced the infectiousness of SARS-CoV-2, infected humans would have transmitted the H1 haplotype of SARS-CoV-2 to workers or sellers in the market. The crowded market boosted rapid transmission of haplotype H1 to buyers at an early stage of human-to-human transmission so the virus had spread to the whole city in early December 2019. Such an explanation is supported by other findings of the authors (Yu et al., 2020), who reported 120 substitution sites associated with 119 codons, among which 79 were non-synonymous and 40 synonymous substitutions. Among the 79 non-synonymous substitutions there were 40 which changed the biochemical properties of the amino acid, and could therefore be associated with better virus adaptation. This is consistent with the study of Zhang et al. (Zhang, Yang, et al., 2020), which reported that mutation at 8750 and 29063 caused higher translational efficiencies for the Type II strains than for Type I. This enabled faster production of Type II virus particles resulting in easier spread and leading to its becoming the dominant strain, implying that Type II is more transmissible than Type I. This might explain why Type II strains were more prevalent in Wuhan. All these results suggest that unrecognized SARS-CoV-2 was circulating before December 2019, and that it was imported from an unknown location to the Wuhan market, where it quickly and efficiently amplified (Forster et al., 2020; Lu et al., 2020; Zhang, Yang, et al., 2020).
The means of SARS-CoV-2 entry into cells is similar to SARS-CoV. In both cases it requires the recognition of a host receptor, human angiotensin-converting enzyme 2 (ACE2) and its binding with the spike S protein, but in the case of SARS-CoV-2 the affinity of S protein to the receptor is 10-fold–20-fold higher (Chen, Guo, Pan & Zhao, 2020; Zheng, 2020). As well as for SARS-CoV-2, ACE2 is also the receptor for SARS-CoV and HCoVNL63 (Table 1) (Cui et al., 2019; Li, Moore, Vasilieva, Sui, Wong & Berne, 2003; Qiu et al., 2020). This surface molecule is localized on arterial and venous endothelial cells, arterial smooth muscle cells, epithelia of the small intestine and epithelia of the respiratory tract, however, the highest expression of ACE2 is in lung alveolar epithelial cells and enterocytes of the small intestine, causing these tissues and relevant organs to be the most sensitive to infection with SARS-CoV-2 (Perlot & Penninger, 2013; Yin & Wunderink, 2018). The presence of ACE2 was confirmed in pangolin, cat, cow, buffalo, goat, sheep and pigeon, which provided ample opportunity for the interspecies transmission of the SARS-CoV-2 from bats both to and among these animals (Qiu et al., 2020). Moreover, any of these animals might serve as an intermediate host for SARS-CoV-2. This should be taken into account in the control of COVID-19.
It has already been reported that a active SARS-like coronavirus isolated from bat fecal samples (bat SL-CoV-WIV1), which has typical coronavirus morphology, used ACE2 from humans, civets and Chinese horseshoe bats for cell entry (Ge et al., 2013). These results provide the strongest evidence that bats are natural reservoirs of SARS-CoV. In addition to the recent results on MERS-CoV in a Saudi Arabian bat, and of bat CoVs closely related to MERS-CoV in China, Africa, Europe and North America, these results also suggested that bat coronaviruses remain a substantial global threat to public health. Moreover, cell entry studies of Hu et al. demonstrated that three newly identified SARS-CoVs with different S protein sequences were able to use human ACE2 as the receptor. It means that they are close to becoming infectious to humans, so we should be prepared for future SARS-like disease outbreaks (Hu, Zeng, Yang, Ge, Zhang & Li, 2017).
There are several hypotheses on how coronaviruses are transmitted to humans (Han et al., 2015). The first holds that bats, which are the virus reservoir, contaminate fruit or plants with virus from their saliva, feces or urine, these fruit or plants are then consumed by intermediate hosts, such as pigs, horses and nonhuman primates; while humans are infected by direct contact with animals, or by consuming products of the intermediate animal hosts. Coronavirus particles were detected in, among others, the saliva, milk and feces of infected animals, which are often asymptomatic, and therefore can be transmitted to humans (Azhar et al., 2014; Guan et al., 2003; Killerby, Biggs, Midgley, Gerber, & Watson, 2020; Saif, 2004; Shi & Hu, 2008; van Doremalen, Bushmaker, Karesh, & Munster, 2014; Wang & Anderson, 2019). SARS-CoV-2 genomic material has been also found in sewage (Medema, Heijnen, Elsinga, Italiaander, & Brouwer, 2020) and river water (Guerrero-Latorre et al., 2020). Aerosol transmission of SARS-CoV-2 from bat feces to humans is also possible (Franklin & Bevins, 2020). Coronaviruses can also be transmitted to humans directly from bats via bites or scratches, or by eating bat meat (a practice still popular in China) which has been undercooked (Chan, To, Tse, Jin, & Yuen, 2013; Han et al., 2015; Lu, Wang, & Gao, 2015; Woo, Lau, & Yuen, 2006; Xu et al., 2020). Also in the case of SARS-CoV some evidence indicates that eating meals prepared from infected palm civets could be one of the ways that the virus jumps to humans (Bolles et al., 2011; Hilgenfeld & Peiris, 2013; Lam et al., 2020; Wang et al., 2006; Wang, Yan, Xu, Liang, Kan & Zheng, 2005). When the COVID-19 outbreak was analyzed, some papers indicated pangolin as a source of the virus, as pangolin are used not only for their meat but also for traditional Chinese medicine (Guan et al., 2003; Ye et al., 2020). Although the whole genome sequences of CoVs isolated from pangolin are less similar to SARS-CoV-2 than bat CoV (RaTG13), the receptor-binding domain (RBD) of this pangolin virus is identical to that of SARS-CoV-2 in all 6 of the key amino acids. It is highly probable that the bat virus jumped to humans several months before the outbreak was detected, but was not highly infectious. Some studies proved that substitution of one or more amino acids in S protein is enough for virus introduction into new host species (Belouzard et al., 2012; Thackray & Holmes, 2004). Another possibility is that pangolin CoV and bat CoV met in a human host, and thanks to a high recombination rate they interchanged genes and the new SARS-CoV-2 virus was formed with a genetic sequence that makes it highly infectious to humans (Lam et al., 2020; Zheng, 2020). It is well documented that bats, pangolins, civets and other animals were sold in the Wuhan ‘wet market’ where the COVID-19 pandemic started.
It is also worth considering the possibility of SARS-CoV-2 spillover from infected humans to domestic animals and native wildlife that could subsequently serve as new reservoir hosts for the virus and enable the virus spillback into the human population. The viral dissemination from human feces into the environment could have occurred via the wastewater treatment system. Although the impact on livestock and wildlife health is yet unknown, spillover events of zoonotic links are frequent in the Coronaviridae family (Franklin & Bevins, 2020). It is not known whether wildlife species can serve as reservoir hosts for SARS-CoV-2, but recent research on domestic animals indicates that ferrets and cats are susceptible to SARS-CoV-2, which then replicated efficiently and was transmissible to naïve cats via droplet transmission (Shi, Wen, Zhong, Yang, Wang & Huang, 2020). Another risk associated with the establishment of SARS-CoV-2 in a wildlife host population is the possibility of the formation of novel virus variants by mutation in novel hosts or by recombination of SARS-CoV-2 with other bat coronaviruses (Franklin & Bevins, 2020).
6. Is food a source of SARS-CoV-2 infection in humans?
To date, the detailed SARS-CoV-2 infection pathways are still unclear, but studies indicate the possibility of virus entry not only through the respiratory system but also the digestive tract. This is due to the fact that the SARS-CoV-2 entry receptor, ACE2, shows significant expression in lung AT2 cells, upper and layered epithelial cells, and epithelial enterocytes from the ileum and colon. This indicates that not only the respiratory system but also the digestive system are potential routes of infection (Zhang, Kang, et al., 2020; Zhou et al., 2017). It is also worth adding that the intestinal symptoms of SARS-CoV-2 may be associated with affected enterocytes expressing ACE2. The ACE2 receptor (for humans hACE2), besides being the receptor for some coronaviruses, is an enzyme attached to the outer surface (cell membranes) of cells in various organs of the body, it lowers blood pressure by catalyzing the cleavage of angiotensin II (a vasoconstrictor peptide) into angiotensin 1-7 (a vasodilator) (Letko, Marzi, & Munster, 2020). Studies carried out during previous epidemics of coronaviruses, mainly SARS-CoV, indicate that, on the one hand, the presence of the virus in the entire gastrointestinal tract (biopsy and feces results even in discharge patients) may be a factor responsible for gastrointestinal symptoms in patients and a potential relapse of the disease (Gu, Han, & Wang, 2020). On the other hand, it is possible to transfer and spread the virus through non-droplet transmission. This was also confirmed by cases of SARS-CoV-2 described in the United States (Holshue et al., 2020) and in Hubei (Zhang, Chen, et al., 2020). Detailed patient tests showed significant gastrointestinal discomfort and the presence of the virus in both the respiratory tract and feces (Holshue et al., 2020; Zang et al., 2020).
It is worth recalling that vomiting or flushing the toilet can generate the aerosols causing subsequent airborne transmission (Johnson, Mead, Lynch, & Hirst, 2013; Knowlton et al., 2018). Spreading the virus through aerosolization and vaporization of fecal waste water has been demonstrated for adenoviruses and torque teno virus as they have already been detected in aerosols surrounding toilets (Verani, Bigazzi, & Carducci, 2014). But some recent studies suggest that coronavirus can also be spread by feces aerosolization contaminating various surfaces (Guo, Wang, et al., 2020; McDermott, Alicic, Harden, Cox, & Scanlan, 2020). Furthermore, according to recent opinions, the monitoring of municipal wastewater for the presence of the virus may enable prediction of a new outbreak a few days before symptoms occur (Mao, Zhang, & Yang, 2020). This method is much cheaper than genetic tests of people. All this means that proper hygiene is essential to stop SARS-CoV-2 from spreading as virions can also contaminate the hands and clothes of people managing food.
Data collected in February 2020 by researchers in Hong Kong confirmed that approximately 18% of patients with COVID-19 in the study group showed gastrointestinal symptoms (Cheung et al., 2020). However, virus RNA was detected in the stool samples of over 48% of patients. Fecal RNA was detected even in cases with a negative result for respiratory samples. The relationship between infections/respiratory manifestations and the gastrointestinal tract is not fully understood, but it is known that patients with respiratory tract infections usually show bowel dysfunction or secondary bowel complications that are associated with a more severe clinical course of the disease, which clearly indicates a cross effect. We currently do not have clinical evidence which indicates that modulation of the intestinal microbiota plays a therapeutic role in the treatment of COVID-19. However, it is suggested that influencing intestinal microorganisms may be a therapeutic option at least to alleviate the disease (Gao, Chen, & Fang, 2020). Recommendations developed in February 2020 by the National Health Committee of China and the National Administration of Traditional Medicine in China directly suggest that probiotics could be used to treat patients with severe COVID-19 infection to maintain the balance of intestinal microbiota and to prevent secondary bacterial infection (Gao et al., 2020).
The possibility of oral coronavirus infection was also demonstrated by previous studies on twin viruses, especially MERS-CoV. In this case it was found, among other things, that the route of infection of nearly 1/5 of the MERS cases was direct contact with camels (Cauchemez et al., 2016). These animals shed a large amount of virus from the upper respiratory tract, but the possibility of infection also results from other factors. Authors mention camel milk among them (Memish et al., 2014). MERS-CoV was detected in 41.9% of camel milk samples (Reusken et al., 2014) with a long shelf life. Primary MERS-CoV infection can occur through breathing droplets and/or saliva during direct contact with camels or through the consumption of unpasteurized camel milk or undercooked camel meat (Durai, Batool, Shah, & Choi, 2015). Additionally, studies have also shown that the MERS coronavirus significantly loses its potential for infection in fasted-state gastric fluid (low pH). Instead, it is resistant to gastric fluid after a meal and intestinal fluid that contains digestive enzymes and bile salts (Zhou et al., 2017). Studies on the survival of the virus in milk also concerned other types of milk, including goat's and cow's milk. In all cases, the survival of MERS-CoV was found in unpasteurized milk after more than 48 h (van Doremalen et al., 2014). This situation can trigger the transmission mechanism of MERS coronavirus to humans and infection of the mouth and later the lower respiratory tract. Pasteurization of milk can prevent food-borne transmission of SARS-CoV (Rabenau et al., 2005). To inactivate this type of virus, it is sufficient to warm the milk to a temperature of about 60 °C for 30 min (Rabenau et al., 2005). It is also known that human coronaviruses HCoV-229E and HCoV-OC43 remain active in buffer solutions longer than in a dried state and are able to survive in suspension at room temperature for several days (Sizun, Yu, & Talbot, 2000).
There are three main routes for human-to-human COVID-19 transmission: direct or indirect contact transmission, short range transmission by droplets, and long range transmission by aerosol (Adhikari et al., 2020).
6.1. Direct transmission and food
From the beginning of the pandemic, direct transmission of the virus was indicated as the main route, and many studies confirmed person-to-person transmission. As was reported (Auwaerter, 2020) the mean incubation period of SARS-CoV-2 is 5 days–6 days (range 2 days–12 days). For people quarantined, two weeks' observation is recommended to exclude infection, but up to 24 days’ asymptomatic time from exposure was described. Moreover, viral shedding could occur following recovery, but it is unclear what role this plays in transmission. Some suggest that high viral load may equate with disease severity, but studies to date have not been standardized. McIntosh (McIntosh, 2020) suggested, on the basis of literature review, the possibility that patients might be more infectious in the earlier stage of infection, but additional data is needed to confirm this hypothesis. Transmission of SARS-CoV-2 from a person presenting symptoms as well as from presymptomatic and asymptomatic individuals has been previously documented (Chan, Yuan, et al., 2020; Hu et al., 2020; Rothe et al., 2020; Yu, Zhu, Zhang, & Han, 2020).
The possibility of the virus spreading by an infected person without symptoms is very dangerous because this makes isolation impossible. This means that any activity involving direct contact with another person (e.g. store, marketplace, restaurant, transport) is a potential threat. Results from a study of Kwok et al. (Kwok, Gralton, & McLaws, 2015) showed that people touch their face about 23 times per hour. Of all face touches, 44% involved contact with a mucous membrane, 36% involved the mouth, 31% involved the nose, 27% involved the eyes, and 6% were a combination of these regions (Kwok et al., 2015). This means that during normal daily activities and shaking hands with an asymptomatic infected person there is a considerable risk of transmission.
6.2. Indirect transmission and food
Transmission of coronaviruses from contaminated surfaces (indirect transmission) has been postulated, this includes self-inoculation of mucous membranes of the nose, eyes or mouth, emphasizing the importance of a detailed understanding of coronavirus persistence on inanimate surfaces (Kampf, Todt, Pfaender, & Steinmann, 2020). During normal daily activity all people touch many things besides their face. Thereby, an infected person (either presymptomatic or asymptomatic) can unknowingly contaminate various environmental surfaces with viruses which can then be transferred to the fingers of other individuals. The frequency and relative importance of this type of transmission remains unclear. It could be a more probable source of infection in settings where there is heavy viral contamination (McIntosh, 2020). It was confirmed by Winther et al. (Winther, McCue, Ashe, Rubino, & Hendley, 2007) that transfer of virus from sites contaminated with mucus involved fingertip contact, which is usual in normal daily activities such as flipping light switches, dialling phone numbers or holding a telephone receiver.
Food may become contaminated directly by the unsanitized hands of harvesters in farm fields, processors, and those who prepare and serve food in restaurants and at home (Cook, 2013). Similarly, when shopping, especially when buying food, people often carefully choose ripe fruit, undamaged vegetables, they analyze the ingredients of the meal on the packaging or look for data on allergens on the label. Each time they pick up a product, touch it and either put it in their shopping cart or return it to the shelf. Therefore, infected hands of sellers in the store as well as of clients could be a potential source of SARS-CoV-2 transmission.
Taking into account the stability of SARS-CoV-2 on various surfaces (next chapter) indirect transmission of the virus is therefore quite possible. Proper hand-washing was one of the first recommendations to prevent the spread of COVID-19. Thorough washing of the surfaces of products could also be a useful intervention to reduce virus levels, but some foods, such as raspberries, strawberries and crinkly lettuce, are difficult to thoroughly wash (Cook, 2013). According to EFSA, there is currently no evidence that food is a likely source or route of transmission for SARS-CoV-2 (Coronavirus, 2020). Regarding food safety, WHO has issued precautionary recommendations including advice on following good hygiene practices during food handling and preparation, such as washing hands, cooking meat thoroughly and avoiding potential cross-contamination between cooked and uncooked foods. It is emphasized everywhere that SARS-CoV-2 is an enveloped virus, so hand washing with basic soap for at least 30 s is enough to inactivate the virus.
The possibility of rapid transmission of viruses through contaminated hands is already known for other pathogens. Rhinoviruses are non-enveloped, positive-stranded RNA viruses belonging to the Enterovirus genus within the Picornaviridae family, and are the main causative agent of the common cold, the most frequent infection worldwide (L'Huillier et al., 2015). It was demonstrated that rhinoviruses on the fingers of volunteers have been efficiently transferred by contact to the nasal and conjunctival mucosa, where they routinely produced infection. Five out of 10 recipients (50%) developed infection after exposure to virus-contaminated coffee cup handles and 9 out of 16 (56%) became infected after exposure to contaminated plastic tiles (Gwaltney & Hendley, 1982). Human-to-human transmission of influenza is mediated mainly by the airborne route, but direct contact via contaminated hands followed by self-inoculation of the upper respiratory tract is probably equally important. Nevertheless, it remains difficult to establish whether large contaminated respiratory droplets could lead to finger contamination (Lowen, Steel, Mubareka, & Palese, 2008; Mubareka et al., 2009; Tellier, 2006, 2009). This may be dependent upon the virus type, inoculum size and external conditions (i.e. temperature, humidity). Thomas et al. (Thomas, Boquete-Suter, Koch, Pittet, & Kaiser, 2014) experimentally investigated the survival of influenza A (H3N2) and A (H1N1)pdm09 viruses on human fingers. Their findings could help to set up the promotion of hand hygiene to prevent influenza hand contamination. A similar conclusion could be taken in connection with SARS-CoV-2.
At present, no infection with SARS-CoV-2 via meat transmission has been detected. According to the German Federal Institute for Risk Assessment BfR (Can the New Type of Coronavirus Be Transmitted via Food and Objects?, 2020), livestock used for the production of meat cannot be infected with SARS-CoV-2, for that reason it is unable to transmit the virus to humans via this path. Moreover, meat and meat products are protected by packaging. To prevent not only COVID-19 but also other diseases, meat and poultry should be heated sufficiently before consumption (Coronavirus: No Evidence That Food Is a Source or Transmission Route, 2020).
Contamination of dairy products has been associated with transmission of bacteria and viruses. Results obtained by van Doremalen et al. (van Doremalen et al., 2014) show that MERS-CoV, when introduced into milk, can survive for prolonged periods. However, pasteurization of milk can prevent foodborne transmission. The authors concluded that heat treatment decreased MERS-CoV below the detection limit of the titration assay, and this might function as a relatively easy and cost-effective measure to prevent transmission. According to EFSA and BfR (Can the New Type of Coronavirus Be Transmitted via Food and Objects?, 2020; Coronavirus: No Evidence That Food Is a Source or Transmission Route, 2020) no infections with SARS-CoV-2 via milk transmission have been detected to date.
While COVID-19 can theoretically be transmitted by touching a contaminated surface and then touching the nose, mouth or eyes, this is not thought to be its primary mode of transmission (How COVID-19Spreads, 2020). However, proper hygiene and hand washing technique are also important due to the possibility of a fecal-oral route of virus transmission. An infective SARS-CoV-2 strain was isolated from a stool specimen of a laboratory-confirmed COVID-19 severe pneumonia case. This means that stool samples may contaminate hands, food and water, and may cause infection by invading the oral cavity, respiratory mucosa or conjunctiva (Zhang, Chen, et al., 2020).
6.3. Droplets and aerosol transmission
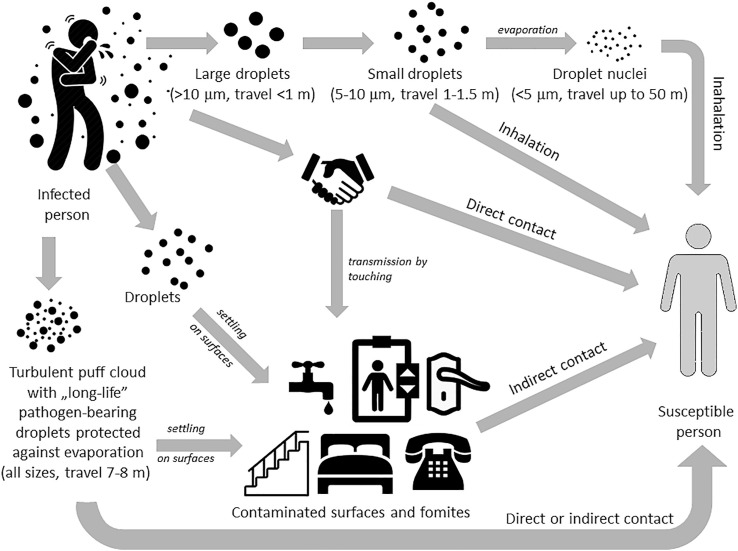
SARS-CoV-2 was detected in saliva (To et al., 2020) so people can spread the virus by coughing, speaking, breathing, sneezing or singing, as well as vomiting or during dental treatment. All these activities create an aerosol (an infectious aerosol when it contains pathogens), which is projected into the surrounding air Fig. 5 . It is suspected that exhaled air from both the nose and mouth is able to mix with air in the breathing zone of another person standing nearby (e.g. doctors at the bedside, customers in a store or restaurant, travelers on public transport) (Tang, Li, Eames, Chan, & Ridgway, 2006). So wearing a protective face mask was recommended in many countries as a preventive measure. But a major problem is the lack of clinical trials on the impact of masks on viral infection rates. Wearing a face mask probably does not prevent coronavirus infection, but can limit infectious aerosols spreading. Either way, an infected individual causes the virus to spread (Fig. 5), either directly (touching various objects) or by producing an infectious aerosol which settles on surfaces (fomites).
Fig. 5.
Transmission routes involving direct contact, indirect contact by contaminated surfaces and fomites, as well as by droplets (short range) and droplet nuclei (long range) spreading [on the basis of (Tang et al., 2006)].
An infected person when coughing emits about 3000 droplet nuclei, while one sneeze produces up to 40,000 droplets (Tang et al., 2006). During normal breathing, exhalation can project droplets up to 1 m in room air, which may be inhaled by another person nearby causing disease development. The infectivity of such aerosol depends on many factors, with the size of infectious particles being the main agent. The World Health Organization and other agencies, such as the Centers for Disease Control and Prevention, use a dichotomous classification between large vs small droplets and droplets vs aerosol. Accordingly, larger droplets (>10 μm) with more mass are more strongly influenced by gravity but less so by air flows, so they move more ‘ballistically’ and fall to the ground more quickly, usually within a distance < 1 m. Small infectious droplets (5 μm–10 μm) are less influenced by gravity and can travel a distance of about 1 m–1.5 m. Droplet nuclei (the airborne residue of a potentially infectious aerosol from which most of the liquid has evaporated) measuring < 5 μm can remain suspended in the air very long and can be carried even 50 m by air flows as a ‘cloud’. Viruses usually enter the respiratory tract in small droplets and droplet nuclei (Atkinson et al., 2009; Fernstrom & Goldblatt, 2013; Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care, 2014; Liu et al., 2020; Tang et al., 2006).
However, recent work has demonstrated that exhalations, sneezes and coughs are primarily made of a multiphase turbulent gas cloud (a puff) that entrains ambient air which traps and carries within it droplets of all sizes (Bourouiba, 2020). Moreover, the moist and warm atmosphere within the turbulent gas cloud protects the pathogen-bearing droplets against evaporation for much longer than occurs with isolated droplets, hence, the lifetime of droplets within a ‘puff’ could be extended to many minutes. Depending on environmental conditions (temperature and humidity) the gas cloud with pathogen-loaded droplets of all sizes can travel 7m–8m and be either directly inhaled by a susceptible person, contaminate other rooms via the ventilation system or settle on various surfaces and fomites.
Studies conducted in China, in which 75,465 cases of SARS-CoV-2 were analyzed, did not prove airborne transmission of the virus (Ong et al., 2020). It had already been confirmed that transmission of SARS-CoV-2 occurred through small infectious droplets, from close proximity (approx. 1.5 m) to an infected person with respiratory symptoms, through fomites in the immediate vicinity of the infected person (Ong et al., 2020), or from objects which had been infected, such as a thermometer, etc. (Fernstrom & Goldblatt, 2013; Pittet et al., 2006). It seems, therefore, that SARS-CoV-2 can be transmitted by an infected person to the packaging of food or by the food itself. It is possible both during shopping, when an infected person without appropriate protective measures touches food, e.g. bread or fruit, and also during food production.
The German Federal Institute for Risk Assessment (BfR) does not yet have any information on infection with SARS-CoV-2 via bakery goods or fresh fruit and vegetable transmission. Coronaviruses can generally reach bakery products or fruit and vegetables through an infected person sneezing or coughing directly on them. A smear infection to another person then appears to be possible if the virus is transmitted shortly afterwards via the hands or the food itself to the mucous membranes of the mouth, throat or eyes. Nowadays, bakery products in retail stores are usually packed to protect and minimize the risk of contamination. Preparation of fruit and vegetables requires maintenance of the general rules of hygiene, which include thorough washing of the food and frequent hand washing during handling (Can the New Type of Coronavirus Be Transmitted via Food and Objects?, 2020).
7. Stability of SARS-CoV-2 in the environment and on various surfaces
It seems reasonable to consider tightening preventive measures primarily in food stores. There are several factors that can make SARS-CoV-2 less likely to be transmitted via food, even if the virus is present in the food or when the person in contact with the food in the store (the seller) or the person producing the food is infected. First of all, proper hand hygiene, cleaning and disinfection of potentially contaminated surfaces are the basic actions aimed at limiting transmission of the virus, both in healthcare and in the food industry (Ong et al., 2020). Furthermore, several ways to prevent viral contamination can be adjusted particularly to food or their packaging. Firstly, food safety procedures that are already known and used to prevent pathogen contamination, such as washing and disinfecting hands, surfaces and all utensils that come into contact with food or applying appropriate processing temperatures, should still be implemented (COVID-19 and Food Safety, 2020). The staff should also be healthy, and ideally tested for SARS-CoV-2, as presymptomatic and asymptomatic people can spread the virus. It is important that all people who handle food, along with managers, cleaners, maintenance contractors, delivery workers, and food inspectors, should be excluded from work immediately after even suspicion of being a virus transmitter. Personal protective equipment, such as masks and gloves, can be effective in reducing the spread of viruses, and physical distancing should also be introduced within the food industry (COVID-19 and Food Safety, 2020). The impact of environmental conditions such as temperature, relative humidity, absolute humidity and sunlight on virus stability and spread is largely unknown (Aboubakr, Sharafeldin, & Goyal, 2020).
The biology of SARS-CoV-2 is not without significance. In contrast to other pathogens such as noroviruses, SARS-CoV-2 cannot survive a long period of time (weeks) on a surface, and its stability differs depending on surfaces. In addition, viruses do not multiply on food, which means that if infectious droplets with virus settle on the surface of the food or its packaging, the quantity of virus will decrease over time (COVID-19 and Food Safety, 2020).
Previously known human coronaviruses such as: SARS-CoV, MERS-CoV or endemic human coronaviruses are able to survive on various surfaces such as metal, glass or plastic for several days (Otter et al., 2016; Peng et al., 2020; Seto et al., 2003). Apart from the type of surface, ambient temperature and relative humidity can also affect transmission. It has been shown that MERS-CoV is most stable at low temperatures and low humidity; under these conditions the virus remained infectious for up to 48 h ( van Doremalen, Bushmaker, & Munster, 2013). Stability of the SARS virus has been analyzed on various surfaces and in human samples, i.e. blood, sputum, urine and feces (Duan et al., 2003). It was shown that after 60 h incubation the infectivity in serum began to decrease, and after 120 h it was not detectable. In contrast, the infectivity of SARS virus in urine was significantly lower but persisted, albeit slightly decreasing, even after 120 h of incubation. Infections with SARS-CoV in filtered sputum fell slightly faster than in unfiltered sputum, but there was no difference between infectivity of filtered and unfiltered feces (Duan et al., 2003). The authors found that the highest SARS stability was on a glass surface, with decrease after 60 h of incubation at room temperature. The survival of SARS virus on a wooden surface, in the soil or water was the lowest, and lasted for 60 h, whereas after 120 h it became almost undetectable.
According to scientists from the WHO laboratory network, the SARS-CoV-2 virus is more stable under laboratory conditions than previously known coronaviruses. After 21 days of cultivation at two different temperatures (4 °C and 80 °C) only minimal reduction in virus concentration was found. In addition, SARS-CoV-2 is stable in feces and urine at room temperature for at least 1–2 days. It may even remain stable for up to four days in stools of a higher pH ((WHO | First Data on Stability and Resistance of SARS Coronavirus Compiled by Members of WHO Laboratory Network, 2020)).
According to the latest research, the survival of the SARS-CoV-2 virus on plastic surfaces at room temperature and 65% relative humidity was four days, and after seven days it completely lost its ability to become infectious (Chin et al., 2020). Another study showed that SARS-CoV-2 retained its infectivity for three days on a plastic surface at room temperature. Moreover, it has been shown that there is no difference between the persistence of SARS-CoV-2 and SARS-CoV-1 on a plastic surface, and that both virus strains completely lost their infectivity after four days (van Doremalen, Bushmaker, Morris, Holbrook, Gamble & Williamson, 2020). Available studies on the survival of coronaviruses on metal surfaces have shown that survival varies with the type of metal. In general, coronaviruses survive shorter periods on copper, nickel and brass than on stainless steel and zinc surfaces. For example, SARS-CoV-1 persisted on copper for 8 h while it remained infectious for two days on stainless steel with complete degradation after three days (van Doremalen et al., 2020). SARS-CoV-2 showed lower survival on copper than SARS-CoV-1 (van Doremalen et al., 2020).
According to Chin et al. SARS-CoV-2 virus remained infectious on glass at room temperature and 65% relative humidity for two days and became completely undetectable after four days (Chin et al., 2020). SARS-CoV-1 retained its infectivity for a long time (four days) on glass at room temperature. Active virions were also detectable in some cases of 120 h incubation on metal, cloth and filter paper. The relationship between stability and temperature was also demonstrated. SARS viruses were more stable at 4 °C than at room temperature (Duan et al., 2003). Research shows that clothing is not a significant source of virus transmission. Clothes are made of porous materials, but SARS-CoV-2 lasts longer on non-porous surfaces (Aboubakr et al., 2020).
Active virions were also detectable in some cases of 120 h incubation on metal, cloth and filter paper. The relationship between stability and temperature was also demonstrated. SARS viruses were more stable at 4 °C than at room temperature (Duan et al., 2003).
According to the latest research published in The New England Journal of Medicine, SARS-CoV-2 is stable for several hours to several days in aerosols and surfaces (van Doremalen et al., 2020). It has been shown that the virus was detectable in aerosols up to 3 h after infection, survived 4 h on copper, up to 24 h on cardboard, and 2–3 days on plastic and stainless steel (van Doremalen et al., 2020). It was also found that the stability of SARS-CoV-2 appears to be similar to the stability of SARS-CoV under experimental conditions, but there are some differences in the epidemiological characteristics of these viruses. SARS-CoV-2 had a very high titer and higher potential for virus spread by asymptomatic carriers (Bai et al., 2020; Chen, Huang, Chan, Su, Chang & Chang, 2004; van Doremalen et al., 2020). The stability kinetics of SARS-CoV and SARS-CoV-2 were similar, furthermore, SARS-CoV and SARS-CoV-2 half-lives were similar in aerosols and on copper. In contrast, on cardboard, the half-life of SARS-CoV-2 was longer than SARS-CoV. The longest life span of both viruses concerned stainless steel and plastic, the estimated average half-life of SARS-CoV-2 and SARS-CoV was 5.6 h and 4.2 h respectively for stainless steel, and 6.8 h and 7.6 h respectively for plastic (van Doremalen et al., 2020). In summary, studies show that SARS-CoV-2 transmission in aerosol and fomites is plausible as the virus remains viable, it also remains infectious in aerosols for hours and on surfaces for up to several days. Moreover, the results suggest that SARS-CoV-2 can be transmitted not only by droplet transmission but also by touching contaminated objects, e.g. food packaging.
8. Risk minimization – recommendation
Although to date there is no evidence of viruses that cause respiratory illnesses being transmitted via food or food packaging, there is constant consumer concern about food safety in the context of a pandemic. Risk minimization of COVID-19 in relation to the food chain should be considered in two aspects. First, in the context of food safety control systems, and second, in the context of direct consumer protection.
To preserve the integrity of national food safety control systems, support international trade and the food supply chain, each competent authority will need to prioritize critically important services during the ongoing COVID-19 pandemic. The challenges facing national health authorities arise from reduced capacity to maintain a fully functioning food safety inspection program. This results from the reallocation of staff to national COVID-19 emergency response teams or to changes in staff working practices (homeworking). Moreover, national health authorities also need to respond to an increasing number of queries from Government, the food industry, consumers and the media (COVID-19 and Food Safety, 2020).
It is imperative for the food industry to reinforce personal hygiene measures and provide refresher training on food hygiene principles to eliminate or reduce the risk of food surfaces and food packaging materials becoming contaminated with the virus from food workers. Personal protective equipment (PPE), such as masks and gloves, can be effective in reducing the spread of viruses and disease within the food industry, but only if used properly. Moreover, the food industry is strongly advised to introduce physical distancing and stringent hygiene and sanitation measures (COVID-19 and Food Safety; Modes of Transmission of Virus Causing COVID-19, 2020) (FAO/WHO, 7 April 2020). WHO emphasized the great importance of frequent hand hygiene, respiratory etiquette and environmental cleaning and disinfection, as well as the importance of maintaining social distancing and avoidance of close, unprotected contact with people who have fever or respiratory symptoms.
Interim guidance for the food industry (COVID-19 and Food Safety, 2020)recommends that frequent and effective handwashing and sanitation should apply at each stage of food processing, manufacture and marketing. Moreover, available hand sanitizer for consumers is strongly recommended. Consumers should also wear face masks and gloves as well as maintaining social distancing. Consumers should remember not to touch their face. Food products on open self-service displays in retail stores should be placed in plastic/cellophane or paper packaging (COVID-19 and Food Safety). Both the entrance to the store and the exit from it should be preceded by hand disinfection. One of the most important safety precautions should be the sanitization of shopping trolleys and baskets. After completing their shopping shoppers should remove packaging then wash their hands and (if possible) the products purchased.
9. Conclusion
In conclusion, although there is no direct and credible evidence that COVID-19 is a foodborne disease, contact with food cannot be considered as completely safe in the context of an ongoing pandemic. In many countries the only open stores are those selling food. What's more, it is a product of the first need, and therefore there is a demand for it all the time. It is therefore very important to closely monitor the health of staff who handle food, and to test the food as comprehensively as possible for the presence of SARS-CoV-2. It is also important to identify all infected people, especially asymptomatic carriers of the virus, thereby preventing its spread. COVID-19 is not a typical foodborne disease, but we can be infected when handling food without proper hygiene and precautions, just as we can by handling any other item that has come into contact with a person infected by SARS-CoV-2.
Acknowledgement
The work was financed by a subsidy of the Ministry of Science and Higher Education for the University of Agriculture in Krakow for 2020.
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transboundary and Emerging Diseases. 2020:1–17. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infectious Diseases of Poverty. 2020;9(1):29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hosani F., I., Kim L., Khudhair A., Pham H., Al Mulla M., Al Bandar Z.…Gerber S., I. Serologic follow-up of middle east respiratory syndrome coronavirus cases and contacts—Abu Dhabi, United Arab Emirates. Clinical Infectious Diseases. 2018;68(3):409–418. doi: 10.1093/cid/ciy503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaili A., N., Briese T., Mishra N., Kapoor V., Sameroff S., C., de Wit E.…Lipkin W., I. Middle east respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5(2) doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., Fraser C., Ghani A.C., Donnelly C.A., Riley S., Ferguson N.M., et al. Epidemiology, transmission dynamics and control of SARS: The 2002-2003 epidemic. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 2004;359(1447):1091–1105. doi: 10.1098/rstb.2004.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad Ali S., Baloch M., Ahmed N., Arshad Ali A., Iqbal A. The outbreak of Coronavirus Disease 2019 (COVID-19)—an emerging global health threat. Journal of Infection and Public Health. 2020;13(4):644–646. doi: 10.1016/j.jiph.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J., Chartier Y., Pessoa-Silva C.L., Jensen P., Li Y., Seto W.-H., editors. Natural ventilation for infection control in health-care settings. World Health Organization; 2009. http://www.ncbi.nlm.nih.gov/books/NBK143284/ [PubMed] [Google Scholar]
- Auwaerter P.G. Johns Hopkins ABX Guide; 2020. Coronavirus COVID-19 (SARS-CoV-2)https://www.hopkinsguides.com/hopkins/.//view/Johns_Hopkins_ABX_Guide/540747/all/Coronavirus_COVID_19__SARS_CoV_2?refer=true [Google Scholar]
- Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M., et al. Evidence for camel-to-human transmission of MERS coronavirus. New England Journal of Medicine. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. Journal of the American Medical Association. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baric R.S. SARS-CoV: Lessons for global health. Virus Research. 2008;133(1):1–3. doi: 10.1016/j.virusres.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M., Vena A., Giacobbe D.R. The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm. European Journal of Clinical Investigation. 2020;50(3) doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles M., Donaldson E., Baric R. SARS-CoV and emergent coronaviruses: Viral determinants of interspecies transmission. Current Opinion in Virology. 2011;1(6):624–634. doi: 10.1016/j.coviro.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. Journal of the American Medical Association. 2020 doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: Important reservoir hosts of emerging viruses. Clinical Microbiology Reviews. 2006;19(3):531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can the new type of coronavirus be transmitted via food and objects? Bundesinstitut für risikobewertung (BfR) 2020. https://www.bfr.bund.de/en/can_the_new_type_of_coronavirus_be_transmitted_via_food_and_objects_-244090.html
- Cauchemez S., Nouvellet P., Cori A., Jombart T., Garske T., Clapham H., et al. Unraveling the drivers of MERS-CoV transmission. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(32):9081–9086. doi: 10.1073/pnas.1519235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., To K.K.-W., Tse H., Jin D.-Y., Yuen K.-Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends in Microbiology. 2013;21(10):544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Guix S., Paraira M., Galofré B., Sáanchez G., et al. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. MedRxiv. 2020 doi: 10.1101/2020.06.13.20129627. [DOI] [Google Scholar]
- Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochemical and Biophysical Research Communications. 2020;525(1):135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-C., Huang L.-M., Chan C.-C., Su C.-P., Chang S.-C., Chang Y.-Y., et al. SARS in hospital emergency room. Emerging Infectious Diseases. 2004;10(5):782–788. doi: 10.3201/eid1005.030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. Journal of Medical Virology. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong Cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. Elsevier; 2013. Viruses in food and water: Risks, surveillance and control. [Google Scholar]
- Corman V., M., Baldwin H., J., Tateno A., F., Zerbinati R., M., Annan A., Owusu M.…Drexler J., F. Evidence for an ancestral association of human coronavirus 229E with bats. Journal of Virology. 2015;89(23):11858–11870. doi: 10.1128/JVI.01755-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease (COVID-2019) situation report -173 . World Health Organization; 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200803-covid-19-sitrep-196-cleared.pdf?sfvrsn=8a8a3ca4_4 [Google Scholar]
- Corman V., M., Muth D., Niemeyer D., Drosten C. In: Kielian M., Mettenleiter T.C., Roossinck M.J., editors. Vol 100. Academic Press; 2018. Chapter eight - hosts and sources of endemic human coronaviruses; pp. 163–188. (Advances in virus research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus: No evidence that food is a source or transmission route. European Food Safety Authority.; 2020. https://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route [Google Scholar]
- COVID-19 and food safety: Guidance for food businesses. World Health Organisation; 2020. https://www.who.int/publications-detail/covid-19-and-food-safety-guidance-for-food-businesses [Google Scholar]
- CS N°39/2020 - Studio ISS su acque di scarico, a Milano e Torino Sars-Cov-2 presente già a dicembre - ISS. 2020, July 15. https://www.iss.it/primo-piano/-/asset_publisher/o4oGR9qmvUz9/content/id/5422725?_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_o4oGR9qmvUz9_redirect=https%3A%2F%2Fwww.iss.it%2Fprimo-piano%3Fp_p_id%3Dcom_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_o4oGR9qmvUz9%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_o4oGR9qmvUz9_cur%3D0%26p_r_p_resetCur%3Dfalse%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_o4oGR9qmvUz9_assetEntryId%3D5422725
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.M. Middle East respiratory syndrome (MERS) coronavirus: Putting one health principles into practice? Veterinary Journal (London, England. 2017;222:52–53. doi: 10.1016/j.tvjl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes A., Berti V., Tandjaoui-Lambotte Y., Alloui C., Carbonnelle E., Zahar J.R., et al. SARS-CoV-2 was already spreading in France in late December 2019. International Journal of Antimicrobial Agents. 2020;55(6) doi: 10.1016/j.ijantimicag.2020.106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Karesh W.B., Munster V.J. Stability of Middle East respiratory syndrome coronavirus in milk. Emerging Infectious Diseases. 2014;20(7):1263–1264. doi: 10.3201/eid2007.140500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 2013;18(38) doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- Dijkman R., Jebbink M., F., Koekkoek S., M., Deijs M., Jónsdóttir H., R., Molenkamp M.…van der Hoek L. Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. Journal of Virology. 2013;87(11):6081–6090. doi: 10.1128/JVI.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.-M., Zhao X.-S., Wen R.-F., Huang J.-J., Pi G.-H., Zhang S.-X., et al. SARS Research Team Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomedical and Environmental Sciences: Biomedical and Environmental Sciences. 2003;16(3):246–255. [PubMed] [Google Scholar]
- Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biology. 2018;16(8) doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai P., Batool M., Shah M., Choi S. Middle East respiratory syndrome coronavirus: Transmission, virology and therapeutic targeting to aid in outbreak control. Experimental & Molecular Medicine. 2015;47:e181. doi: 10.1038/emm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zowalaty M.E., Järhult J.D. From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans - Call for a one health approach. One Health (Amsterdam, Netherlands) 2020;9 doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The enemy within: How SARS-CoV-2 uses our own proteins to infect our cells. 2020, July 15. https://www.cas.org/blog/covid-19-spike-protein
- Fan Y., Zhao K., Shi Z.-L., Zhou P. Bat coronaviruses in China. Viruses. 2019;11(3):210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom A., Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. Journal of Pathogens. 2013 doi: 10.1155/2013/493960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A.B., Bevins S.N. Spillover of SARS-CoV-2 into novel wild hosts in north America: A conceptual model for perpetuation of the pathogen. The Science of the Total Environment. 2020;733:139358. doi: 10.1016/j.scitotenv.2020.139358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Human coronavirus: Host-pathogen interaction. Annual Review of Microbiology. 2019;73(1):529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- Gao Q.Y., Chen Y.X., Fang J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. Journal of Digestive Diseases. 2020 doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.-Y., Li J.-L., Yang X.-L., Chmura A.A., Zhu G., Epstein J.H., et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Li J., Zhou Q., Xu Z., Chen L., Zhang Y., et al. A new bat-HKU2–like coronavirus in swine, China, 2017. Emerging Infectious Diseases journal – CDC. 2017;23(9):1607–1609. doi: 10.3201/eid2309.170915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacres I., Granda-Albuja M.G., Freire B., Rios-Touma B. First SARS-CoV-2 detection in river water: Implications in low sanitation countries. MedRxiv. 2020 doi: 10.1101/2020.06.14.20131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Han B., Wang J. COVID-19: Gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital Wards, Wuhan, China, 2020. Emerging Infectious Diseases. 2020;26(7):1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney J.M., Hendley J.O. Transmission OF experimental rhinovirus infection BY contaminated surfaces. American Journal of Epidemiology. 1982;116(5):828–833. doi: 10.1093/oxfordjournals.aje.a113473. [DOI] [PubMed] [Google Scholar]
- Han H.-J., Wen H., Zhou C.-M., Chen F.-F., Luo L.-M., Liu J., et al. Bats as reservoirs of severe emerging infectious diseases. Virus Research. 2015;205:1–6. doi: 10.1016/j.virusres.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld R., Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Research. 2013;100(1):286–295. doi: 10.1016/j.antiviral.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L. Human coronaviruses: What do they cause? Antiviral Therapy. 2007;12(4 Pt B):651–658. [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. Washington State 2019-nCoV Case Investigation Team First case of 2019 novel coronavirus in the United States. New England Journal of Medicine. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How COVID-19 Spreads Center of disease control and prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftransmission.html
- Huang I.-C., Bosch B.J., Li F., Li W., Lee K.H., Ghiran S., et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. Journal of Biological Chemistry. 2006;281(6):3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Ge X., Wang L.-F., Shi Z. Bat origin of human coronaviruses. Virology Journal. 2015;12(1):221. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulswit R.J.G., de Haan C.a.M., Bosch B.-J. Coronavirus spike protein and tropism changes. Advances in Virus Research. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Science China. Life Sciences. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J., Li S., Yount B., Smith A., Sturges L., Olsen J.C., et al. Evidence supporting a zoonotic origin of human coronavirus strain NL63. Journal of Virology. 2012;86(23):12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L.-P., Yang X.-L., Ge X.-Y., Zhang W., Li B., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathogens. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. World Health Organization; 2014. http://www.ncbi.nlm.nih.gov/books/NBK214359/ [PubMed] [Google Scholar]
- Jalava K., Kauppinen A., Al-Hello H., Räsänen S. An outbreak of norovirus infection caused by ice cubes and a leaking air ventilation valve. Epidemiology and Infection. 2019;147 doi: 10.1017/S095026881800314X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.L., Mead K.R., Lynch R.A., Hirst D.V.L. Lifting the lid on toilet plume aerosol: A literature review with suggestions for future research. American Journal of Infection Control. 2013;41(3):254–258. doi: 10.1016/j.ajic.2012.04.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killerby M.E., Biggs H.M., Midgley C.M., Gerber S.I., Watson J.T. Middle East respiratory syndrome coronavirus transmission. Emerging Infectious Diseases. 2020;26(2):191–198. doi: 10.3201/eid2602.190697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton S.D., Boles C.L., Perencevich E.N., Diekema D.J., Nonnenmann M.W., CDC Epicenters Program Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrobial Resistance and Infection Control. 2018;7(1):16. doi: 10.1186/s13756-018-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., et al. A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kwok Y.L.A., Gralton J., McLaws M.-L. Face touching: A frequent habit that has implications for hand hygiene. American Journal of Infection Control. 2015;43(2):112–114. doi: 10.1016/j.ajic.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C., Cavanagh D. In: Maramorosch K., Murphy F.A., Shatkin A.J., editors. Vol. 48. Academic Press; 1997. The molecular biology of coronaviruses; pp. 1–100. (Advances in virus research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T.-Y., Shum M.H.-H., Zhu H.-C., Tong Y.-G., Ni X.-B., Liao Y.-S., et al. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. 2020. BioRxiv, 2020.02.13.945485. [DOI] [PubMed]
- Lau S.K.P., Feng Y., Chen H., Luk H.K.H., Yang W.-H., Li K.S.M., et al. Severe acute respiratory syndrome (SARS) coronavirus ORF8 protein is acquired from SARS-related coronavirus from greater horseshoe bats through recombination. Journal of Virology. 2015;89(20):10532–10547. doi: 10.1128/JVI.01048-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Wong E.Y.M., Tsang C.-C., Ahmed S.S., Au-Yeung R.K.H., Yuen K.-Y., et al. Discovery and sequence analysis of four deltacoronaviruses from birds in the Middle East suggest interspecies jumping and recombination as potential mechanism for avian-to-avian and avian-to-mammalian transmission. Journal of Virology. 2018 doi: 10.1128/JVI.00265-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature Microbiology. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annual Review of Virology. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ji F., Wang L., Wang L., Hao J., Dai M., et al. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, xuzhou, China. Emerging Infectious Diseases. 2020;26(7) doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.X., Ng Y.L., Tam J.P., Liu D.X. Human coronaviruses: A review of virus–host interactions. Diseases. 2016;4(3):26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liao X., Qian S., Yuan J., Wang F., Liu Y., et al. Community transmission of severe acute respiratory syndrome coronavirus 2, shenzhen, China, 2020. Emerging Infectious Diseases. 2020;26(6) doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A.C., Steel J., Mubareka S., Palese P. High temperature (30°C) blocks aerosol but not contact transmission of influenza virus. Journal of Virology. 2008;82(11):5650–5652. doi: 10.1128/JVI.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). BioScience Trends, advpub. 2020. [DOI] [PubMed]
- Lu G., Wang Q., Gao G.F. Bat-to-human: Spike features determining “host jump” of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends in Microbiology. 2015;23(8):468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet (London, England) 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Huillier A.G., Tapparel C., Turin L., Boquete-Suter P., Thomas Y., Kaiser L. Survival of rhinoviruses on human fingers. Clinical Microbiology and Infections: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2015;21(4):381–385. doi: 10.1016/j.cmi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.S., Smith D.W. COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don't. Microbiology Australia. 2020. [DOI] [PMC free article] [PubMed]
- Mao K., Zhang H., Yang Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environmental Science & Technology. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- Masters P.S. Vol 66. Academic Press; 2006. The molecular biology of coronaviruses; pp. 193–292. (Advances in virus research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Shirato K., Kawase M., Terada Y., Kawachi K., Fukushi S., et al. Middle East respiratory syndrome coronavirus spike protein is not activated directly by cellular furin during viral entry into target cells. Journal of Virology. 2018;92(19) doi: 10.1128/JVI.00683-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey B., Heymann D.L. SARS to novel coronavirus - old lessons and new lessons. Epidemiology and Infection. 2020;148:e22. doi: 10.1017/S0950268820000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott C.V., Alicic R.Z., Harden N., Cox E.J., Scanlan J.M. Put a lid on it: Are faecal bio-aerosols a route of transmission for SARS-CoV-2? Journal of Hospital Infection. 2020 doi: 10.1016/j.jhin.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K. Coronavirus disease 2019 (COVID-19): Epidemiology, virology, clinical features, diagnosis, and prevention. 2020, August 1. https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-epidemiology-virology-clinical-features-diagnosis-and-prevention
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and Correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environmental Science and Technology Letters. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Memish Z.A., Cotten M., Meyer B., Watson S.J., Alsahafi A.J., Al Rabeeah A.A., et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerging Infectious Diseases. 2014;20(6):1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Huang X., Zhou P., Li C., Wu A. Alert for SARS-CoV-2 infection caused by fecal aerosols in rural areas in China. Infection Control & Hospital Epidemiology. 2020;41(8) doi: 10.1017/ice.2020.114. 987–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. Droplets and aerosols in the transmission of SARS-CoV-2. New England Journal of Medicine. 2020 doi: 10.1056/NEJMc2009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proceedings of the National Academy of Sciences. 2014 doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020, July 9. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- Moon S.-Y., Son J.S. Infectivity of an asymptomatic patient with Middle East respiratory syndrome coronavirus infection. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2017;64(10):1457–1458. doi: 10.1093/cid/cix170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. Journal of Microbiology, Immunology, and Infection. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubareka S., Lowen A.C., Steel J., Coates A.L., García-Sastre A., Palese P. Transmission of influenza virus via aerosols and fomites in the Guinea pig model. The Journal of Infectious Diseases. 2009;199(6):858–865. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Journal of the American Medical Association. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. Journal of Hospital Infection. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. Journal of the American Medical Association. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. International Journal of Oral Science. 2020;12(1):1–6. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlot T., Penninger J.M. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes and Infection. 2013;15(13):866–873. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D., Allegranzi B., Sax H., Dharan S., Pessoa-Silva C.L., Donaldson L., et al. WHO Global Patient Safety Challenge, World Alliance for Patient Safety Evidence-based model for hand transmission during patient care and the role of improved practices. The Lancet Infectious Diseases. 2006;6(10):641–652. doi: 10.1016/S1473-3099(06)70600-4. [DOI] [PubMed] [Google Scholar]
- Pratelli A. Canine coronavirus inactivation with physical and chemical agents. The Veterinary Journal. 2008;177(1):71–79. doi: 10.1016/j.tvjl.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Zhao Y.B., Wang Q., Li J.Y., Zhou Z.J., Liao C.H., et al. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes and Infection. 2020. [DOI] [PMC free article] [PubMed]
- Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Medical Microbiology and Immunology. 2005;194(1):1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Farag E.A., Jonges M., Godeke G.J., El-Sayed A.M., Pas S.D., et al. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 2014;19(23) doi: 10.2807/1560-7917.es2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of Autoimmunity. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New England Journal of Medicine. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir J.S.M., Lam T.T.-Y., Ahmed M.M.M., Li L., Shen Y., Abo-Aba S.E.M., et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science (New York, N.Y.) 2016;351(6268):81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- Saif L.J. Animal coronaviruses: What can they teach us about the severe acute respiratory syndrome? Revue Scientifique Et Technique (International Office of Epizootics) 2004;23(2):643–660. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- Seto W.H., Tsang D., Yung R.W.H., Ching T.Y., Ng T.K., Ho M., et al. Advisors of Expert SARS group of Hospital Authority Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet (London, England) 2003;361(9368):1519–1520. doi: 10.1016/s0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Research. 2008;133(1):74–87. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science (New York, N.Y.) 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizun J., Yu M.W.N., Talbot P.J. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: A possible source ofhospital-acquired infections. Journal of Hospital Infection. 2000;46(1):55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. Journal of Hospital Infection. 2006;64(2):100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Review of aerosol transmission of influenza A virus. Emerging Infectious Diseases. 2006;12(11):1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Aerosol transmission of influenza A virus: A review of new studies. Journal of The Royal Society Interface. 2009;6(6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray L.B., Holmes K.V. Amino acid substitutions and an insertion in the spike glycoprotein extend the host range of the murine coronavirus MHV-A59. Virology. 2004;324(2):510–524. doi: 10.1016/j.virol.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Y., Boquete‐Suter P., Koch D., Pittet D., Kaiser L. Survival of influenza virus on human fingers. Clinical Microbiology and Infections. 2014;20(1):O58–O64. doi: 10.1111/1469-0691.12324. [DOI] [PubMed] [Google Scholar]
- To K.K.-W., Tsang O.T.-Y., Chik-Yan Yip C., Chan K.-H., Wu T.-C., Chan J.M.C., et al. An Official Publication of the Infectious Diseases Society of America; 2020. Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani M., Bigazzi R., Carducci A. Viral contamination of aerosol and surfaces through toilet use in health care and other settings. American Journal of Infection Control. 2014;42(7):758–762. doi: 10.1016/j.ajic.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Dempsey D.M., Dutilh B.E., et al. Changes to virus taxonomy and the international Code of virus classification and nomenclature ratified by the international Committee on Taxonomy of viruses (2019) Archives of Virology. 2019;164(9):2417–2429. doi: 10.1007/s00705-019-04306-w. [DOI] [PubMed] [Google Scholar]
- Walsh E.E., Shin J.H., Falsey A.R. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. The Journal of Infectious Diseases. 2013;208(10):1634–1642. doi: 10.1093/infdis/jit393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Du GG. COVID-19 may transmit through aerosol. Irish Journal of Medical Science (1971-) 2020 doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-F., Anderson D.E. Viruses in bats and potential spillover to animals and humans. Current Opinion in Virology. 2019;34:79–89. doi: 10.1016/j.coviro.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Journal of the American Medical Association. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-F., Shi Z., Zhang S., Field H., Daszak P., Eaton B.T. Review of bats and SARS. Emerging Infectious Diseases. 2006;12(12):1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xia S., Wang Q., Xu W., Li W., Lu L., et al. Broad-spectrum coronavirus fusion inhibitors to Combat COVID-19 and other emerging coronavirus diseases. International Journal of Molecular Sciences. 2020;21(11) doi: 10.3390/ijms21113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Yan M., Xu H., Liang W., Kan B., Zheng B., et al. SARS-CoV infection in a restaurant from palm civet. Emerging Infectious Diseases. 2005;11(12):1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Advances in Virus Research. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO; World Health Organization; 2020, August 1. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network.https://www.who.int/csr/sars/survival_2003_05_04/en/ [Google Scholar]
- Winther B., McCue K., Ashe K., Rubino J.R., Hendley J.O. Environmental contamination with rhinovirus and transfer to fingers of healthy individuals by daily life activity. Journal of Medical Virology. 2007;79(10):1606–1610. doi: 10.1002/jmv.20956. [DOI] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Huang Y., Yuen K.-Y. Coronavirus diversity, phylogeny and interspecies jumping. Experimental Biology and Medicine. 2009;234(10):1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Lam C.S.F., Lau C.C.Y., Tsang A.K.L., Lau J.H.N., et al. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and deltacoronavirus. Journal of Virology. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Yuen K. Infectious diseases emerging from Chinese wet-markets: Zoonotic origins of severe respiratory viral infections. Current Opinion in Infectious Diseases. 2006;19(5):401–407. doi: 10.1097/01.qco.0000244043.08264.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Genome Composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host & Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: What we know. International Journal of Infectious Diseases. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., et al. Systematic Comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.-Y., Huang Y., Ganesh L., Leung K., Kong W.-P., Schwartz O., et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. Journal of Virology. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Zhang Y., Liu Y., Chen Y., Deng H., Ma Z.…Deng F. Angiotensin-converting enzyme 2 (ACE2) from raccoon dog can serve as an efficient receptor for the spike protein of severe acute respiratory syndrome coronavirus. Journal of General Virology. 2009;90(11):2695–2703. doi: 10.1099/vir.0.013490-0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., et al. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. Journal of Autoimmunity. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. International Journal of Biological Sciences. 2020;16(10) doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.-B., Tang G.-D., Zhang L., Corlett R.T. Decoding the evolution and transmissions of the novel pneumonia coronavirus (SARS-CoV-2/HCoV-19) using whole genomic data. Zoological Research. 2020;41(3):247–257. doi: 10.24272/j.issn.2095-8137.2020.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Zhu J., Zhang Z., Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the Incubation Period. The Journal of Infectious Diseases. 2020;221(11):1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R., Castro M.F.G., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Science Immunology. 2020;5(47) doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z.-Q., Chen D.-H., Tan W.-P., Qiu S.-Y., Xu D., Liang H.-X., et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: A study of hospitalized children with acute respiratory tract infection in guangzhou, China. European Journal of Clinical Microbiology & Infectious Diseases. 2018;37(2):363–369. doi: 10.1007/s10096-017-3144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z., et al. The digestive system is a potential route of 2019-nCov infection: A bioinformatics analysis based on single-cell transcriptomes. 2020. BioRxiv, 2020.01.30.927806. [DOI]
- Zhang L., Yang J.-R., Zhang Z., Lin Z. Genomic variations of SARS-CoV-2 suggest multiple outbreak sources of transmission. 2020. MedRxiv, 2020.02.2520027953. [DOI]
- Zheng J. SARS-CoV-2: An emerging coronavirus that causes a global threat. International Journal of Biological Sciences. 2020;16(10):1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li C., Zhao G., Chu H., Wang D., Yan H.H.-N., et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Science Advances. 2017;3(11) doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Vedantham P., Lu K., Agudelo J., Carrion R., Nunneley J.W., et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Research. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]