Abstract
The mechanical phenotype of cardiomyocytes is essential to their function as the motor unit of the heart. Traditionally, testing of drugs for cardiotoxic effects has relied on primary cardiomyocytes from animal models and focused on short-term, electrophysiological and arrhythmogenic effects. However, primary cardiomyocytes present challenges arising from their limited viability in culture, and tissue from animal models suffers from a mismatch in their physiology to that of human heart muscle. Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) can address these challenges. They also offer the potential to study not only electrophysiological effects but also changes in cardiomyocyte contractile and mechanical function in response to cardiotoxic drugs. With growing recognition of the long-term cardiotoxic effects of some drugs on subcellular structure and function, there is increasing interest in using hiPSC-CMs for in vitro cardiotoxicity studies. In this review, we provide a brief overview of techniques that can be used to quantify changes in the active force cardiomyocytes generate and variations in their inherent stiffness in response to cardiotoxic drugs. We conclude by discussing the application of these tools in understanding how cardiotoxic drugs directly impact the mechanobiology of cardiomyocytes and how cardiomyocytes sense and respond to mechanical load at the cellular level.
Keywords: mechanobiology, cardiotoxicity, Human induced pluripotent stem cell, cardiomyocytes, heart failure, mechanotransduction
Introduction
The ability of the heart to contract and pump blood into the pulmonary and systemic circulations is driven by the contractile force cardiomyocytes (CMs) generate. Under normal conditions, these cells are dynamic and have an intrinsic ability to alter force production in response to changes in hemodynamic pressures and adrenergic stimulation to maintain cardiac output. At the molecular level, this unique ability is driven by the interaction between the sarcomeric proteins actin and myosin. As more myosin heads bind to actin filaments, force production increases. Conversely, a reduction in myosin heads bound to actin filaments will result in less than optimal contractile force. While this is true for healthy hearts, cardiac dysfunction resulting from heart attacks, cardiomyopathies, or cardiotoxicity can lessen the ability of CMs to modulate contractile force in response to environmental changes.[1–5] With most of these defects, CMs are unable to produce enough contractile force to maintain cardiac output leading to heart failure. In some cases, such as hypertrophic cardiomyopathy, cardiomyocytes become hypercontractile and generate higher than normal contractile forces.[6, 7]
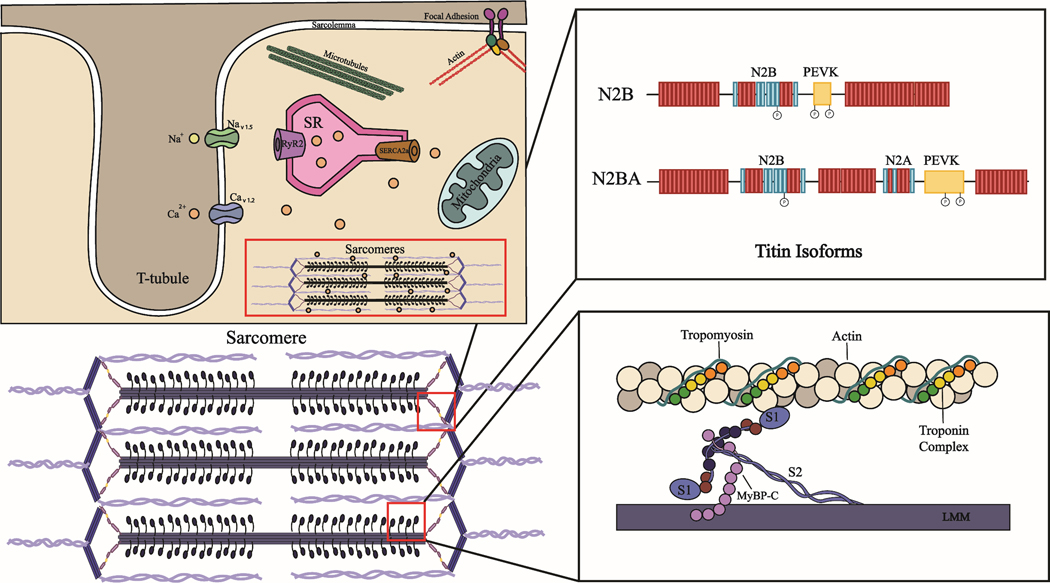
In addition to contracting and creating force to move blood, CMs must be able to relax and maintain an appropriate resting tension that allows for proper ventricular filling and cardiac function.[8] In CMs, resting tension is mainly governed by the elastic sarcomeric protein titin, but is also impacted by cytoskeletal proteins including microtubules, and extracellular proteins such as collagen.[9–13] When CMs are stretched beyond their resting tension, they develop a passive force that impacts cellular stiffness. Reports have shown that titin is responsible for up to 90% of the passive forces CMs experience.[9, 14–16] Thus, this sarcomeric protein is a primary determinant of CM stiffness. Titin’s impact on cellular stiffness is mainly dictated by the expression ratio of the small (N2B) to large (N2BA) isoforms of the protein. CMs that experience high passive stiffness correlate with a higher expression ratio of the smaller stiffer N2B isoform, whereas more compliant CMs have a higher ratio of the larger more compliant N2BA isoform [17–19] While the impact of heart attacks and cardiomyopathies that result in heart failure on cardiac stiffness and contractile force has been extensively studied,[20–23] it remains unclear how cardiotoxic drugs alter these properties.
One of the challenges when conducting drug screening assays for cardiotoxicity is the availability of cellular models that can recapitulate the physiology of adult human CMs in vitro. While primary human CMs can be isolated from explanted hearts for these assays, the propagation of these cells in vitro has been difficult. The use of cell lines from animal models that over-express human cardiac ion channels have also been applied to cardiotoxicity studies,[24] but these models are often inaccurate at predicting human cardiac drug responses as the physiology of these cells differ from humans. Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) overcome many of the challenges with current models as they closely recapitulate the physiology of adult human CMs, can be readily differentiated and can be maintained for months in culture.[25–27] Moreover, with growing recognition of the long-term cardiotoxic effects of some drugs on subcellular structure and function, there is increasing interest in using hiPSC-CMs for cardiotoxicity studies. hiPSC-CMs not only address many challenges of physiology mismatch with animal models and primary cell viability, they also offer the potential to study electrophysiological effects and changes in cardiomyocyte contractile function and mechanical in response to cardiotoxic drugs.
In this review, we first define cardiotoxic processes and agents. Then, we provide a brief overview of techniques that can be used to quantify changes in the forces CMs actively generate and passively experience in response to cardiotoxic drugs. We will focus on assays that have utilized live cell models because the advent of hiPSC-CMs promises a source of CMs that can be matched to the genetic background of patients and can display similar physiology to adult human CMs.[28–30] We conclude by discussing the application of these tools in understanding how cardiotoxic drugs impact the mechanobiology of CMs, or how CMs sense and respond to mechanical load at the cellular level.
Cardiotoxic agents and CM function
The cardiotoxic side-effects of drug therapies were first described in the 1940s when it was noted that treatment with digitalis and local anesthetics adversely impacted cardiac function.[31] Today it is widely known that treatment with chemotherapies such as anthracyclines (e.g., doxorubicin), and other drugs including anti-arrhythmic (e.g., Quinidine), anti-psychotic (e.g., chlorpromazine), and anti-histamines (e.g., Astemizole) can cause cardiotoxic side-effects.[32–35] Amongst these agents, the most concerning are chemotherapies, as there is increasing recognition that traditional (e.g., doxorubicin and paclitaxel) and novel (e.g., trastuzumab, sunitinib, Pembrolizumab) cancer drugs are leading to cardiovascular toxicity and heart failure.[36–40] While the exact mechanism of cancer drug-induced cardiotoxicity is not well understood, it is clear that chemotherapies can adversely impact several aspects of cardiac function, including electrophysiology, mitochondrial function, contractility and viability (Table 1).[41–46] Changes to these structural and functional components can, in turn, alter the intrinsic ability of CMs to contract and generate active force independent of external load or applied stretch.
Table 1.
Example CiPA and cancer drugs that have been identified as cardiotoxic
| CiPA drugs | Mechanism of action in cardiomyocytes | Effects on electrophysiology | Effects on contractility | Effects on viability/ O2 consumption |
|---|---|---|---|---|
| Dofetilide | Block hERG encoded IKr (delayed rectifier potassium) channels | Prolong QT interval and Increase APD [41, 49] | Enhance contractility in rat ventricular cells [50] | No reports |
| Quinidine | Blocks fast Ina, slow Ca2+, and hERG encoded IKr channels [51] | Increase APD in patients [52] | Reduce beating amplitudes in hiPSC-CMs monolayer [53] | Reduces oxygen consumption in rat heart slices [54]. Cardiotoxic to hiPSC-CMs [55] |
| Sotalol | Non-selective beta-adrenergic blocker [56] Blocks hERG encoded IKr channels [57] | Increase APD in patients [58] | Lower contractility in dog hearts [59] | No reports |
| Cisapride | Blocks hERG encoded IKr channels [60] | Increase QT interval and ADP in patients [61] | Do not alter contractility in human primary cells [62] | Cardiotoxic, reduces cell viability [63] |
| Terfenadine | Blocks hERG encoded IKr [64], and INa [65] channels | Prolongs QT interval in hiPSC-CM monolayer [66] | No effect on contractility in hiPSC-CM monolayer [66] | Reduce cell viability in hiPSC-CM monolayer [67] |
| Verapamil | Ca2+ channel blocker | Low doses prolong APD. High doses shorten action potential duration [68] | Reduce contractility and sarcomere dynamics in hiPSC-CM monolayer. [69]. Depress myofibril formation [70] | Increase myocardial oxygen [71] |
| Cancer drugs | Mechanism of action in cardiomyocytes | Effects on electrophysiology | Effects on contractility/ myofibrillar structure | Effects on viability/ O2 consumption |
| Trastuzumab | Monoclonal antibody that targets HER2 receptor | May induce arrhythmias in patients [72] | Decrease left ventricular ejection fraction in patients [73] | Reduce cell viability [74] |
| Sunitinib | Tyrosine kinase inhibitor | Prolongs QT interval [75] | Declines contractile force in engineered heart tissues made with rat heart cells [76] | Reduces mitochondrial function, and is cardiotoxic [77] |
| Sorafenib | Tyrosine kinase inhibitor | Reduce Ca2+ content, and re-uptake in the sarcoplasmic reticulum in patient cardiac tissue [78] | Decrease force development in patient cardiac samples [78] | Cardiotoxic [78] Reduce oxygen consumption in rat hearts [79] |
| Imatinib | Tyrosine kinase inhibitor | No effect on APD in hiPSC-CM monolayer [39] | Declines contractile force in engineered heart tissues made with rat heart cells [76] | Does not alter cell viability in hiPSC-CMs [39] |
| Paclitaxel | Stabilizes tubulin polymerization | Increase spontaneous Ca2+ oscillations in mice [80] | Reduced mechanical output, depressed myofibril formation [45, 46] | No effect on cell viability in rodents [80] |
| Doxorubicin | Prevents DNA re-ligation and double-stranded break repair | Impair Ca2+ handling, [81] increase intracellular Ca2 in hiPSC-CM monolayer [82] | Reduce contractility, degrades myofibrils [36, 44] | Impair metabolic function, reduces O2 consumption in hiPSC-CM monolayer [81] |
ADP = Action potential duration
hERG = human ether-a-go-go-related gene potassium channel
Proarrhythmic drugs are also of concern, several of these drugs have led to altered electrophysiological function in CMs that can be deadly. With the formation of the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, there has been an increase in studies examining the impact of cardiotoxic agents on electrophysiological properties (e.g., calcium handling, ion channel conductance) of CMs. The CiPA initiative was established as a new paradigm to detect and remove proarrhythmic drugs from patient care that can lead to a fatal arrhythmia such as Torsade de Pointes.[47] In addition to impacting the electrophysiological function of CMs, these proarrhythmic drugs can also alter contractility and mitochondrial function (Table 1).
While the impact of proarrhythmic and cancer drugs on CM function is evident, it is less clear how these cardiotoxic agents impact the structural components and extracellular environment around CMs that can alter passive forces. These studies are needed because cardiovascular disease related to oncology therapies is of increasing concern for emerging cancer therapies.[48] Such structure-function studies will provide a more complete picture of how cancer drugs and other toxic compounds alter the active and passive forces of CMs and enable therapeutic interventions to detect and mitigate the secondary effects of cardiac dysfunction and heart failure.
Assays used in cardiotoxicity studies to measure active force
The active force CMs generate is driven by the interaction between the sarcomeric proteins actin and myosin. These proteins are able to interact and produce force when Ca2+ ions bind the troponin complex which through a series of events opens binding sites on actin filaments that allow myosin heads to attach and pull actin filaments inward to contract the cell and generate force (Figure 1). Several assays have been reported to measure changes in active forces in response to cardiotoxic compounds in vitro at the cellular and tissue levels. The functional readout of contractile “force” is commonly reported (Table 2). Importantly, force is always derived from displacement measurements coupled with mechanical models and underlying assumptions about material properties. For example, traction force microscopy (TFM) tracks the displacement of fiducials (beads) in a substrate using high-speed video to determine changes in the amplitude of a contractile field exerted by a cell. These displacements are then mapped to forces by assumptions about mechanical properties and boundary conditions.[83–87] From these forces and deformations, one can estimate the work or strain energy in a contractile cycle. Several other assays focus on ensemble cell motions or edge displacements and estimate a more limited set of biophysical parameters like rates and connectivity. Below, we review in order of prevalence, how several such mechanobiology assays and parameters have or could be used to answer questions about the impact of cardiotoxic agents on active force.
Figure 1.
Cellular structures that impact passive stiffness and force generation in CMs. Representative CM with cellular organelles, ions, and protein structures (top left). The two isoforms of titin found in CMs (top right). Proteins within the sarcomere that are involved in force generation (bottom right).
Table 2.
Assays to assess changes in active force in response to cardiotoxic agents in live-cell models
| Technique | Mechanical Readouts | Electrophysiological readouts | Structural readouts | Applicable Platforms |
|---|---|---|---|---|
| Video Microscopy | Beating frequency Time to peak contraction Time from peak to 50% and 90% relaxation |
Ca2+ transient amplitude Ca2+ transient duration Ca2+ transient rise and decay times Same readouts can be reported using voltage-sensitive dyes for other ions |
Sarcomere length Sarcomere registry Myofibril alignment |
Single-cell 2D monolayer 2D organized sheet 3D tissue constructs |
| Traction force microscopy (TFM) | Beating frequency Beat displacement Relative force Times of contraction/ relaxation Velocity of contraction/ relaxation Power Force-frequency |
*Ca2+ transient amplitude *Ca2+ transient duration *Ca2+ transient rise and decay times *Same readouts can be reported using voltage-sensitive dyes for other ions |
*Sarcomere length *Sarcomere registry *Myofibril alignment |
Single-cell 2D monolayer 2D organized tissue 3D tissue constructs |
| Micropost | Beating frequency Beat displacement Relative force Times of contraction/ relaxation Velocity of contraction/ relaxation Power Force-frequency |
*Ca2+ transient amplitude *Ca2+ transient duration *Ca2+ transient rise and decay times *Same readouts can be reported using voltage-sensitive dyes for other ions |
*Sarcomere length *Sarcomere registry *Myofibril alignment |
Single cells Monolayers 3D tissue constructs |
| Atomic force microscopy | Beating frequency Vertical force |
*Ca2+ transient amplitude *Ca2+ transient duration *Ca2+ transient rise and decay times *Same readouts can be reported using voltage-sensitive dyes for other ions |
*Sarcomere length *Sarcomere registry *Myofibril alignment |
Single-cell |
In combination with video microscopy and cells with voltage-sensitive dyes or fluorescent labels
A. Video microscopy
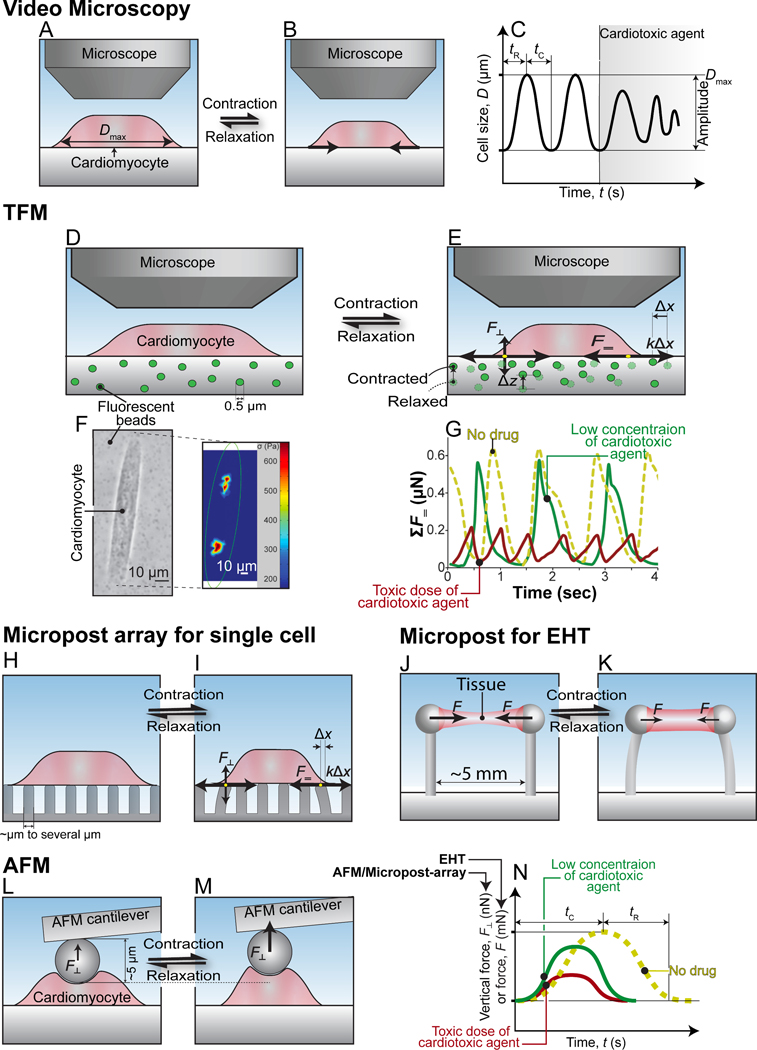
High-speed (typically >40 Hz) video capture of the contraction and relaxation cycles is a commonly used technique to assess changes contractile force in response to cardiotoxic agents in CMs. These measurements are frequently performed in drug screening and development assays in conjunction with electrophysiological measurements.[33, 88] Although the recording of videos for these assays is straightforward, data analysis is more complex. In contrast to isolated primary CMs from animal and human heart tissue, unstructured hiPSC-CMs lack a defined cellular membrane, sarcomere organization, and direction of contraction. [89, 90] Macro-scale motions can be mapped using particle tracking algorithms [91–93], and while motion maps can be extracted from such data, many material and boundary assumptions must be made to transform to force estimates. Since, monolayers of hiPSC-CMs or unstructured single hiPSC-CM lack well defined morphological features, these motion analysis methods have difficulty accurately tracking and quantifying changes in displacement in these models. With the adoption of hiPSC-CMs as models for drug development and screening,[94–96] newer analysis methods seek to account for the lack of organized sarcomere, non-uniformity of contraction, and lack of pronounced membrane edges.[89, 90, 97–99] Such algorithms track displacement in hiPSC-CMs either by monitoring changes in pixel intensity or applying particle image velocimetry methods to generate vector maps that track displacement in all directions regardless of sarcomere organization or detectable edges.[91, 99, 100] This is fundamentally different from analysis tools that relied on raster-line scanning of periodic features and edges (as found in primary cells) to detect changes in sarcomere motion or the displacement of the edges of CMs.[101–103]
Several labs have deployed advanced video microscopy analyses of drug-induced changes in contractile function of hiPSC-CMs, from single cells to 3D tissue constructs. [81, 88, 98, 104–106] Common mechanical readouts include changes in peak contraction/ relaxation amplitudes, contraction/relaxation velocities, and beat rate (Table 2). For example, Sala et al. deployed their “MUSCLEMOTION” analysis software to study the cardiotoxic agent Nifedipine (a Ca2+ channel blocker used to treat hypertension) and learn that it reduces contraction amplitudes in single-cell hiPSC-CM, hiPSC-CM monolayers, and 3D tissue constructs.[98] Similarly, Maddah et al. used their “Pulse” video analysis tool to observe a reduction in the beat rate of hiPSC-CMs monolayers exposed to the cardiotoxic agents Verapamil (Ca2+ blocker) and Cisapride (a drug used to treat gastric-emptying disorders).[97]
Live cell models can also be coupled with Ca2+ dyes,[107] genetically encoded voltage sensors,[108] and fluorescently labeled organelles (e.g., mitochondria) or structural proteins (e.g., sarcomeres). Video analysis tools can then correlate structural parameters with Ca2+ handling, electrophysiological function, structural damage or disorganization, and mechanical parameters (See Table 2). Changes to electrophysiological function, Ca2+ handling, or cellular damage can greatly impact the amount of active force CMs can generate. “SarcTrack” is one such video analysis tool that tracks the displacement of fluorescently labeled sarcomeric proteins to reveal changes to sarcomere length and shortening.[109] Not surprisingly, sarcomere length changes have been correlated with contractile force.[110] Toepfer et al. used of SarcTrack to detect changes to sarcomere function in response to drug treatment using a small molecule that alters myosin function.[109] Monitoring such changes at the level of sarcomere structure and function will enable new insights into mechanisms of action of cardiotoxic compounds. With continued improvements in imaging tools and analysis software, video microscopy analysis will continue to be a cornerstone of drug discovery and screening assays. When combined with quantitative assays of hiPSC-CM contractile work output, such assays offer the potential for semi-automated pipelines to assess change to contractile function under drug treatments.
In the following sections, we focus on biomechanical assays that when combined with molecular biology manipulations and video microscopy, enable insight into the mechanobiology of cardiotoxic responses. By mechanobiology, we mean the changes in biophysical phenotype and the role of mechanical load in cell signaling and in exacerbating cell responses to cardiotoxic compounds.
B. Traction force microscopy
TFM is a technique that is used to quantitatively determine the amount of force CMs exert against a substrate by monitoring the displacements of fiducials embedded in the substrate (Figure 2). Video microscopy alone provides data on changes in contractile displacements and rates (Table 2) by measuring the displacement of CMs over time. By capturing an additional video channel of fluorescent bead motions in a deformable substrate under a cell, TFM can be used to quantify active force, power, and strain energy that CMs exert on a substrate. We refer interested readers to several excellent reviews on the instrumentation and device preparation required, image-processing algorithms and subsequent post-processing analysis principles for TFM.[111–114]
Figure 2.
Mechanobiological techniques for active force measurements in CMs. (A-C) Video microscopy of relaxed (A) and contracted (B) CMs. Representative trace used to determinate functional changes (C). (D-G) Traction force microscopy (TFM) of relaxed (D) and contracted (E) CM on hydrogel substrate with fiducials. Representative brightfield and traction force map (F) of CM. (G) Representative traces of the summed traction forces CMs generate in response to drug treatment. Micropost platforms (H-K). (H, I) micropost array with contracted (H) and relaxed (I) CM. (J) Relaxed and (K) contracted engineered heart tissue (EHT) on micropost. (L, M) Atomic force microscopy (AFM) cantilever on a relaxed (L) and contracted (M) CM. (N) Representative traces used in the determination of functional changes for EHT, AFM, and micropost array measurements.
TFM can be used to determine the active forces generated by single cells and 2D sheets of CMs, and with appropriate model construction and confocal imaging, within 3D tissue constructs.[115–119] Because of the post-processing computation required, TFM has not been widely adopted for drug discovery and screening. However, unlike high-throughput video microscopy assays performed on plastic or glass, TFM offers advantages for research into mechanobiology mechanisms because the substrate stiffness can be matched to physiological stiffness in disease and development. Substrate stiffness is a key factor in functional measurements on CMs, as the stiffness of the substrate can significantly impact the amount of active forces hiPSC-CMs generate.[110, 120, 121] Thus, performing TFM using substrate stiffnesses similar to the physiological environment can be advantageous for examining the impact of drugs on CM mechanical function. TFM is also readily combined with protein patterning techniques to constrain hiPSC-CM size and shape,[110] such cell patterning enhances the subcellular structure, maturity, and biophysical function.
TFM has been deployed in drug screening assays to demonstrate the impact of several compounds on CM function.[90, 98] For example, Kijlstra et al. used TFM and reported a reduction in single-cell hiPSC-CM active force production in response to verapamil, and dofetilide (anti-arrhythmic drug), two known cardiotoxic agents.[90] We have also used TFM to show the application of the technique in assessing the effects of contractile agonists and stimulants on force generation in single-cell hiPSC-CMs.[122] With the advantages TFM offers over traditional video microscopy assays in terms of quantitative measurements and substrate stiffness manipulation TFM holds the potential to examine underlying mechanisms and the impact of cardiotoxic agents on the active forces CMs produce.
C. Microposts for single-cell and engineered heart tissue force measurements
Micropost platforms use vertical, deformable beams to estimate the force of attached CMs (Figure 2). This mechanobiological assay converts micropost motion (imaged at the top of the post) to force using a simpler mechanical model for beam bending and assumptions about the material properties of the microposts. Pairs of posts have been used with 3D cardiac tissue constructs encircling the posts; while arrays of microposts have been used to study monolayers and single CMs attached to the top of the posts (Figure 2). Micropost arrays have been used by several labs to measure the contractile forces generated by single-cell CMs.[123–129] This technique is very similar to single-cell TFM assays in that the traction forces generated by CMs create displacement in the substrate. These displacements are used to calculate the amount of active force from the known spring constant of the beams.[123] Micropost fabrication does require specialized tools or access to a lithographically defined mold. Once manufactured, however, microposts are relatively shelf-stable compared to TFM hydrogel substrates that change stiffness appreciably in about a week.[130] Another advantage is that post displacement is directly related to the force CMs apply to that individual post, making force calculations straightforward and less computationally intensive.[111] However, microposts also present challenges. Matching the stiffness of microposts to physiological conditions is not straightforward because they have an effective out of plane stiffness that is an order of magnitude stiffer than in-plane stiffness. They also present a discontinuous bio-interface where cells can sag or engulf the microposts depending on the protein functionalization strategy.
Microposts are also used to measure changes in the active forces generated by 3D cardiac tissue-like constructs commonly referred to as engineered heart tissues (EHTs). Unlike the micropost arrays used in single-cell measurements (Figure 2), microposts used in EHTs studies use two posts that serve as a scaffold to hold and measure the displacement of the EHT. The EHT is typically cast from a cell-matrix suspension on the substrate and forms a continuous tissue around the microposts. The EHT creates a preload with tissue compaction and slides to the top of the posts, thus, the posts typically include features at the top to keep the EHT from slipping off the top of the posts during contraction. The use of EHTs in drug screening and developmental assays is of increasing interest with commercial systems now coming to market.[131, 132] The hiPSC-CMs in EHTs “trained” with intervals of electrical pacing more closely resemble CMs in an intact heart compared to CMs in 2D monolayer and unstructured single-cell models.[133] In the past decade, there have been several studies that have reported changes in the contractile forces produced by EHTs in response to cardiotoxic agents identified by the CiPA initiative as well as cancer drugs using micropost displacements (Table 1).[134–139] For example, Hansen et al. showed that the cardiotoxic agents quinidine, chromanol, erythromycin, and doxorubicin all negatively impacted CM function using EHTs.[139] With continued improvements in EHT micropost, their use is likely to grow in drug screening assays.
D. Atomic force microscopy
Atomic force microscopy (AFM) is a quantitative assay that was developed to probe nanoscale features of solid materials but is increasingly being applied to biology. AFM measures the interaction forces of a cantilever tip with a surface from the deflection of a cantilever with a known spring constant. The cantilever can be brought into contact with a cell to measure cell biomechanical properties. However, beating hiPSC-CMs also exert force on the free end of the cantilever, the active vertical force produced by the CM causes the displacement of the cantilever, and the lateral forces of contraction can be measured as well as changes in cell stiffness.[140] Several studies have used AFM to study the mechanical function of CMs.[141–145] However, few have used AFM to examine the impact of cardiotoxic agents on the active forces CMs produce. For example, AFM was used to study cardiotoxic reductions in CM force induced by the chemotherapeutic agent doxorubicin.[144, 145]
Although AFM has been used to study CMs and cells in general, it is not a widely adopted technique for assessing changes to the active forces these cells produce. AFM is not high-throughput and force measurements on CMs are challenging and require specialized equipment and technical expertise. The beating nature of CMs can also create fluidic disturbances that can impair accurate force measurements.[143] Traditional AFM can only measure one cell at a time, and it is not trivial to reposition the cantilever accurately for measurements on multiple cells. Thus, while AFM has potential for probing mechanisms linked to changes in cell mechanical properties, it has limited application for high-throughput drug development or screening assays.
Measuring changes to CM stiffness in response to cardiotoxic agents
CMs have an inherent stiffness that is mainly governed by the expression ratios of the N2BA (more compliant) and N2B (stiffer) isoforms of the elastic sarcomeric protein titin (Figure 1).[146] In healthy adult hearts, the expression ratios of N2BA: N2B in CMs are 30:70 to 40:60 which provides the relatively high tensile stiffness needed for proper cardiac function.[147] Studies have shown that the expression ratio of these two isoforms shifts during developmental and diseased states, with the titin-based passive tension getting higher/stiffer throughout development (switch to more N2B) and more compliant (switch to more N2BA) with disease.[20, 148–150] In addition to isoform switching, mutations in the titin protein that leads to truncation or altered phosphorylation can also alter CM stiffness and lead to cardiac dysfunction.[151–154] Changes to the stiffness individual CMs to a lesser degree can also be impacted by microtubules, as these cytoskeletal proteins have been shown to alter the passive stiffness of CMs especially in response to post-translational modifications during disease states.[13, 155]
Several techniques have been used to measure the stiffness of live individual CMs in vitro including AFM, carbon fibers, and microfluidics-based and traditional micropipette aspiration assays.[143, 156–160] Of these methods, AFM is the most commonly used to assess stiffness changes in CMs. Similar to the active force measurements mentioned earlier, stiffness measurements using AFM are performed with a cantilever. To measure CM stiffness using AFM, the cantilever is used to create a precise indentation in the CM membrane that creates a force that is tracked along a force vs. indentation curve. The data from this curve are then fitted to estimate a bulk elastic modulus for the cell (this procedure typically uses a Hertz model, though we note that cells are neither elastic nor homogeneous). Through a similar indentation method, the carbon fiber technique has also been used to determine the stiffness of CMs. However, instead of using a cantilever as in AFM, a small microsphere attached to the carbon fiber is used to create the indentation.[161] Micropipette aspiration is the oldest and simplest of these techniques. The technique is conducted by placing the tip of a pulled glass pipette on an individual cell and, by controlling applied suction, deforming the cell as it is pulled into the micropipette. The elastic properties of the cell are then inferred by Laplace’s law and the changes in cell geometry. Micropipette aspiration was first used by Brady et al. in the 1970s to measure the mechanical properties of living individually isolated rat CMs using a single barrel pipette.[160] The technique has since evolved from using a single barrel pipettes to double-barrel pipettes (offers better cell attachment and wider scope of mechanical measurements) and in some applications, to semi-automated microfluidics with controllers to increase the precision and throughput.[157, 162–164]
Of these, only AFM has been reported for assessing the impact of cardiotoxic agents on CM stiffness. For example, Yue et al. used the technique to show that the cancer drug doxorubicin reduced stiffness of isolated mouse primary CMs.[145] It was not reported in the study whether the reduction in stiffness was due changes to titin, microtubules or other cellular structures. However, patient data from breast cancer patients shows that cancer drugs can cause frameshift mutation in titin, reducing cardiac compliance and ultimately leading to cardiomyopathy.[165] More studies are needed to understand the cardiotoxic effects of chemotherapeutic and other cardiotoxic agents on CM stiffness to understand the mechanisms by which these compounds induce these changes.
Mechanotransduction and Cardiotoxicity
CMs must be able to sense mechanical loads in order to respond to their environment (changes in hemodynamics, ECM content, etc.). The mechanobiology processes by which these cells sense and transmit mechanical signals to initiate a biomechanical response are referred to as mechanosensing and mechanotransduction respectively.[166] There have been several proteins and complexes identified as mechanical sensors, signal transducers, and structural transmission conduits of mechanical loads in CMs, including integrins, the dystrophin-dystroglycan complex, the sarcomere, talin and other elements of the cytoskeleton.[167–169] In CMs, mechanotransduction of mechanical loads are essential to cellular function, development, and maladaptive responses, as the transmission of these signals throughout the cell can significantly alter cellular structure and function.[167] For example, Jian et al. have shown that mechanical load can significantly impact the action potential duration, calcium transients, and contractility of single CMs.[170]
The impact of cardiotoxic agents on the ability of CMs to sense and transmit stress and strain is not fully understood. However, reports from studies in rabbits and humans have demonstrated altered expression of integrins and titin genes, respectively, in response to cancer drug treatment.[165, 171] In both cases, rabbits and humans developed heart failure. While it is unclear if the changes in the gene expression of integrins and titin resulted in altered protein function leading to any causal effects, it is widely known that changes in the function of integrins and/or titin can impact contractility and the transmission of mechanical signals.[167, 172] More research in this area is needed to determine the effects of cardiotoxic agents on CMs ability to sense and transmit mechanical signals.
To address these questions, mechanobiological techniques will play an invaluable role as these assays can provide insights into the mechanisms CMs use to sense and transmit mechanical loads. For example, Pandey et al. used nanopillars (nanoscale post arrays) to show that CMs can sense matrix rigidity through a combination of muscle and non-muscle myosin activity.[173] Using the same technique, they also demonstrated that the stretching of the protein talin which can impact mechanotransduction of mechanical signals depends on matrix stiffness, myofibrillar and non-myofibrillar tension.[173] While these investigators did not apply any cardiotoxic agents to the CMs in their studies, their work provides experimental data that mechanobiological techniques like post arrays can be used to answer questions on how CMs sense and respond to their environment and cardiotoxic agents.
Future Outlook
The use of hiPSC-CMs for cardiotoxicity studies will continue to grow as these cells more closely recapitulate the physiology and function of human adult CMs than in vitro cell models from animals. Although electrophysiological assays to identify arrhythmogenic effects of cardiotoxic agents remains a primary standard for pre-clinical cardiotoxicity screening, assessing concomitant changes in the mechanical phenotype of CMs during the screening process is also needed to unravel mechanisms of disease progression.[174] As discussed in this review, one of the main challenges for adopting the current mechanobiological techniques in a pre-clinical drug screening process is their low throughput. Though techniques such as TFM and AFM can be used to probe CMs for mechanistic discovery, as well as provide a quantitative assessment of changes to CM function, they still require significant technical expertise. However, with the continued development and automation of these techniques, as well as other methods such as the EHT/micropost systems, mechanobiological assays to assess changes in CM function will eventually make their way in the drug screening and development process. Future work is also needed to bridge single cell, tissue, and organism level data to rationalize the outputs across these different scales.
Acknowledgments
Funding:
American Heart Association 17CSA33590101
NIH 1 UG3 TR002588
NIH NIGMS RM1GM131981
Footnotes
Financial conflicts of interest: None
Reference
- 1.Mulieri LA, et al. , Altered myocardial force-frequency relation in human heart failure. Circulation, 1992. 85(5): p. 1743–50. [DOI] [PubMed] [Google Scholar]
- 2.Vikhorev PG and Vikhoreva NN, Cardiomyopathies and Related Changes in Contractility of Human Heart Muscle. International journal of molecular sciences, 2018. 19(8): p. 2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ujfalusi Z, et al. , Dilated cardiomyopathy myosin mutants have reduced force-generating capacity. J Biol Chem, 2018. 293(23): p. 9017–9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witjas-Paalberends ER, et al. , Mutations in MYH7 reduce the force generating capacity of sarcomeres in human familial hypertrophic cardiomyopathy. Cardiovascular Research, 2013. 99(3): p. 432–441. [DOI] [PubMed] [Google Scholar]
- 5.Cuomo A, et al. , Heart Failure and Cancer: Mechanisms of Old and New Cardiotoxic Drugs in Cancer Patients. Cardiac failure review, 2019. 5(2): p. 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spudich JA, Three perspectives on the molecular basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Pflugers Arch, 2019. 471(5): p. 701–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adhikari AS, et al. , β-Cardiac myosin hypertrophic cardiomyopathy mutations release sequestered heads and increase enzymatic activity. Nature Communications, 2019. 10(1): p. 2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiels HA, White E, and J, The Frank-Starling mechanism in vertebrate cardiac myocytes. Biol, 2008. 211: p. 2005–13. [DOI] [PubMed] [Google Scholar]
- 9.Granzier HL and Irving TC, Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J, 1995. 68(3): p. 1027–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollen IAE, et al. , Cardiomyocyte Hypocontractility and Reduced Myofibril Density in End-Stage Pediatric Cardiomyopathy. Frontiers in Physiology, 2017. 8(1103). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaturvedi Rajiv R, et al. , Passive Stiffness of Myocardium From Congenital Heart Disease and Implications for Diastole. Circulation, 2010. 121(8): p. 979–988. [DOI] [PubMed] [Google Scholar]
- 12.Anderson BR and Granzier HL, Titin-based tension in the cardiac sarcomere: molecular origin and physiological adaptations. Progress in biophysics and molecular biology, 2012. 110(2–3): p. 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison P, et al. , Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science, 2016. 352(6284): p. aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brady AJ, Mechanical properties of isolated cardiac myocytes. Physiol Rev, 1991. 71(2): p. 413–28. [DOI] [PubMed] [Google Scholar]
- 15.Granzier HL and Labeit S, The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res, 2004. 94(3): p. 284–95. [DOI] [PubMed] [Google Scholar]
- 16.Freiburg A, et al. , Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res, 2000. 86(11): p. 1114–21. [DOI] [PubMed] [Google Scholar]
- 17.Cazorla O, et al. , Differential Expression of Cardiac Titin Isoforms and Modulation of Cellular Stiffness. Circulation Research, 2000. 86(1): p. 59–67. [DOI] [PubMed] [Google Scholar]
- 18.Prado LG, et al. , Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol, 2005. 126(5): p. 461–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda N, et al. , Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. The Journal of physiology, 2003. 553(Pt 1): p. 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neagoe C, et al. , Titin Isoform Switch in Ischemic Human Heart Disease. Circulation, 2002. 106(11): p. 1333–1341. [DOI] [PubMed] [Google Scholar]
- 21.Makarenko I, et al. , Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res, 2004. 95(7): p. 708–16. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, et al. , Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation, 2004. 110(2): p. 155–62. [DOI] [PubMed] [Google Scholar]
- 23.Borbely A, et al. , Cardiomyocyte stiffness in diastolic heart failure. Circulation, 2005. 111(6): p. 774–81. [DOI] [PubMed] [Google Scholar]
- 24.Fermini B, et al. , A New Perspective in the Field of Cardiac Safety Testing through the Comprehensive In Vitro Proarrhythmia Assay Paradigm. J Biomol Screen, 2016. 21(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 25.Burridge PW, et al. , Chemically defined generation of human cardiomyocytes. Nat Methods, 2014. 11(8): p. 855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian X, et al. , Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc, 2013. 8(1): p. 162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mummery CL, et al. , Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res, 2012. 111(3): p. 344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mummery CL, Perspectives on the Use of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Biomedical Research. Stem Cell Reports, 2018. 11(6): p. 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Protze SI, Lee JH, and Keller GM, Human Pluripotent Stem Cell-Derived Cardiovascular Cells: From Developmental Biology to Therapeutic Applications. Cell Stem Cell, 2019. 25(3): p. 311–327. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Pabon L, and Murry CE, Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res, 2014. 114(3): p. 511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung R, Ghosh AK, and Banerjee A, Cardiotoxicity: precision medicine with imprecise definitions. Open Heart, 2018. 5(2): p. e000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colatsky T, et al. , The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative — Update on progress. Journal of Pharmacological and Toxicological Methods, 2016. 81: p. 15–20. [DOI] [PubMed] [Google Scholar]
- 33.Magdy T, et al. , Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cells to Assess Drug Cardiotoxicity: Opportunities and Problems. Annu Rev Pharmacol Toxicol, 2018. 58: p. 83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L, et al. , Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci, 2013. 136(2): p. 581–94. [DOI] [PubMed] [Google Scholar]
- 35.Li X, et al. , Cardiotoxicity screening: a review of rapid-throughput in vitro approaches. Arch Toxicol, 2016. 90(8): p. 1803–16. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee K, et al. , Doxorubicin Cardiomyopathy. Cardiology, 2010. 115(2): p. 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, et al. , Doxorubicin-induced cardiomyopathy 17 years after chemotherapy. Texas Heart Institute journal, 2012. 39(3): p. 424–427. [PMC free article] [PubMed] [Google Scholar]
- 38.Florescu M, Cinteza M, and Vinereanu D, Chemotherapy-induced Cardiotoxicity. Maedica, 2013. 8(1): p. 59–67. [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma A, et al. , High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med, 2017. 9(377). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varricchi G, et al. , Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr Med Chem, 2018. 25(11): p. 1327–1339. [DOI] [PubMed] [Google Scholar]
- 41.Crumb WJ Jr., et al. , An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J Pharmacol Toxicol Methods, 2016. 81: p. 251–62. [DOI] [PubMed] [Google Scholar]
- 42.Mercurio V, et al. , Models of Heart Failure Based on the Cardiotoxicity of Anticancer Drugs. Journal of Cardiac Failure, 2016. 22(6): p. 449–458. [DOI] [PubMed] [Google Scholar]
- 43.Babiker HM, et al. , Cardiotoxic effects of chemotherapy: A review of both cytotoxic and molecular targeted oncology therapies and their effect on the cardiovascular system. Crit Rev Oncol Hematol, 2018. 126: p. 186–200. [DOI] [PubMed] [Google Scholar]
- 44.Wang G, et al. , Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med, 2014. 20(6): p. 616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toyama Y, et al. , Effects of taxol and Colcemid on myofibrillogenesis. Proc Natl Acad Sci U S A, 1982. 79(21): p. 6556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howarth FC, et al. , Effect of the microtubule polymerizing agent taxol on contraction, Ca2+ transient and L-type Ca2+ current in rat ventricular myocytes. J Physiol, 1999. 516 ( Pt 2): p. 409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong Y-N, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169:286–300.e16. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilchrist Susan C, et al. , Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A Scientific Statement From the American Heart Association. Circulation, 2019. 139(21): p. e997–e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milnes JT, et al. , Investigating dynamic protocol-dependence of hERG potassium channel inhibition at 37 degrees C: Cisapride versus dofetilide. J Pharmacol Toxicol Methods, 2010. 61(2): p. 178–91. [DOI] [PubMed] [Google Scholar]
- 50.Zhang XP, et al. , Dofetilide enhances the contractility of rat ventricular myocytes via augmentation of Na+-Ca 2+ exchange. Cardiovasc Drugs Ther, 2009. 23(3): p. 207–14. [DOI] [PubMed] [Google Scholar]
- 51.Harris K, et al. , Comparison of Electrophysiological Data From Human-Induced Pluripotent Stem Cell–Derived Cardiomyocytes to Functional Preclinical Safety Assays. Toxicological Sciences, 2013. 134(2): p. 412–426. [DOI] [PubMed] [Google Scholar]
- 52.Nademanee K, et al. , Frequency-dependent effects of quinidine on the ventricular action potential and QRS duration in humans. Circulation, 1990. 81(3): p. 790–6. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, et al. , Multi-parametric assessment of cardiomyocyte excitation-contraction coupling using impedance and field potential recording: A tool for cardiac safety assessment. Journal of Pharmacological and Toxicological Methods, 2016. 81: p. 201–216. [DOI] [PubMed] [Google Scholar]
- 54.Hess Marilyn E. and Haugaard N, Studies of the Effect of Antiarrhythmic Drugs on Carbohydrate Metabolism of Rat Heart Muscle in Vitro. Circulation Research, 1958. 6(3): p. 256–259. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Q, et al. , Cardiotoxicity evaluation using human embryonic stem cells and induced pluripotent stem cell-derived cardiomyocytes. Stem cell research & therapy, 2017. 8(1): p. 54–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El‐Battrawy I, et al. , Modeling Short QT Syndrome Using Human‐Induced Pluripotent Stem Cell–Derived Cardiomyocytes. Journal of the American Heart Association. 7(7): p. e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson JL and Prystowsky EN, Sotalol: An important new antiarrhythmic. American Heart Journal, 1999. 137(3): p. 388–409. [DOI] [PubMed] [Google Scholar]
- 58.Campbell TJ, Gavaghan TP, and Morgan JJ, Intravenous sotalol for the treatment of atrial fibrillation and flutter after cardiopulmonary bypass. Comparison with disopyramide and digoxin in a randomised trial. British Heart Journal, 1985. 54(1): p. 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puri PS and Bing RJ, Effects on Myocardial Contractility, Hemodynamics and Cardiac Metabolism of a New Beta-Adrenergic Blocking Drug, Sotalol. CHEST, 1969. 55(3): p. 235–239. [DOI] [PubMed] [Google Scholar]
- 60.Drolet B, et al. , Block of the Rapid Component of the Delayed Rectifier Potassium Current by the Prokinetic Agent Cisapride Underlies Drug-Related Lengthening of the QT Interval. Circulation, 1998. 97(2): p. 204–210. [DOI] [PubMed] [Google Scholar]
- 61.Tack J, et al. , Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Alimentary pharmacology & therapeutics, 2012. 35(7): p. 745–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen N, et al. , Adult Human Primary Cardiomyocyte-Based Model for the Simultaneous Prediction of Drug-Induced Inotropic and Pro-arrhythmia Risk. Frontiers in physiology, 2017. 8: p. 1073–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Z, et al. , Allosteric Modulation of Kv11.1 (hERG) Channels Protects Against Drug-Induced Ventricular Arrhythmias. Circulation: Arrhythmia and Electrophysiology, 2016. 9(4): p. e003439. [DOI] [PubMed] [Google Scholar]
- 64.Woosley RL, et al. , Mechanism of the cardiotoxic actions of terfenadine. Jama, 1993. 269(12): p. 1532–6. [PubMed] [Google Scholar]
- 65.Lu Y. and Wang Z, Terfenadine block of sodium current in canine atrial myocytes. J Cardiovasc Pharmacol, 1999. 33(3): p. 507–13. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe H, et al. , Usefulness of cardiotoxicity assessment using calcium transient in human induced pluripotent stem cell-derived cardiomyocytes. J Toxicol Sci, 2017. 42(4): p. 519–527. [DOI] [PubMed] [Google Scholar]
- 67.He J, Ma J, and Anson B, Use of pluripotent stem cell-derived cardiomyocytes to understand mechanisms of cardiotoxic compounds. 2009. [Google Scholar]
- 68.Zhang S, et al. , Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ Res, 1999. 84(9): p. 989–98. [DOI] [PubMed] [Google Scholar]
- 69.Pointon A, et al. , Assessment of Cardiomyocyte Contraction in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Toxicological Sciences, 2014. 144(2): p. 227–237. [DOI] [PubMed] [Google Scholar]
- 70.Sharp W, Simpson DG, Terracio L, & Borg TK, Signal Transduction and Myofibrillogenesis in Isolated Neonatal Heart Myocytes In Vitro In Biomechanics of Active Movement and Division of Cells (2013). 1994: Springer Berlin Heidelberg. [Google Scholar]
- 71.Mohan IK, et al. , Cardioprotection by HO-4038, a novel verapamil derivative, targeted against ischemia and reperfusion-mediated acute myocardial infarction. American journal of physiology. Heart and circulatory physiology, 2009. 296(1): p. H140–H151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buza V, Rajagopalan B, and Curtis Anne B, Cancer Treatment–Induced Arrhythmias. Circulation: Arrhythmia and Electrophysiology, 2017. 10(8): p. e005443. [DOI] [PubMed] [Google Scholar]
- 73.Barac A, Quo Vadis Trastuzumab? Navigating Cardiac Safety Risk Estimates With Complex Cancer Treatments, 2019. 7(3): p. 225–227. [DOI] [PubMed] [Google Scholar]
- 74.Dogan I, et al. , Inhibition of ErbB2 by herceptin reduces viability and survival, induces apoptosis and oxidative stress in Calu-3 cell line. Mol Cell Biochem, 2011. 347(1–2): p. 41–51. [DOI] [PubMed] [Google Scholar]
- 75.Bello CL, et al. , Electrocardiographic characterization of the QTc interval in patients with advanced solid tumors: pharmacokinetic- pharmacodynamic evaluation of sunitinib. Clin Cancer Res, 2009. 15(22): p. 7045–52. [DOI] [PubMed] [Google Scholar]
- 76.Jacob F, et al. , Analysis of Tyrosine Kinase Inhibitor-Mediated Decline in Contractile Force in Rat Engineered Heart Tissue. PLoS One, 2016. 11(2): p. e0145937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim DM, et al. , Identification of a Mitochondrial DNA Polymerase Affecting Cardiotoxicity of Sunitinib Using a Genome-Wide Screening on S. pombe Deletion Library. Toxicol Sci, 2016. 149(1): p. 4–14. [DOI] [PubMed] [Google Scholar]
- 78.Schneider C, et al. , The Anti-Cancer Multikinase Inhibitor Sorafenib Impairs Cardiac Contractility by Reducing Phospholamban Phosphorylation and Sarcoplasmic Calcium Transients. Sci Rep, 2018. 8(1): p. 5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Will Y, et al. , Effect of the Multitargeted Tyrosine Kinase Inhibitors Imatinib, Dasatinib, Sunitinib, and Sorafenib on Mitochondrial Function in Isolated Rat Heart Mitochondria and H9c2 Cells. Toxicological Sciences, 2008. 106(1): p. 153–161. [DOI] [PubMed] [Google Scholar]
- 80.Zhang K, et al. , Paclitaxel accelerates spontaneous calcium oscillations in cardiomyocytes by interacting with NCS-1 and the InsP3R. Journal of molecular and cellular cardiology, 2010. 49(5): p. 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burridge PW, et al. , Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med, 2016. 22(5): p. 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maillet A, et al. , Modeling Doxorubicin-Induced Cardiotoxicity in Human Pluripotent Stem Cell Derived-Cardiomyocytes. Scientific reports, 2016. 6: p. 25333–25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwarz US and Soine JR, Traction force microscopy on soft elastic substrates: A guide to recent computational advances. Biochim Biophys Acta, 2015. 1853(11 Pt B): p. 3095–104. [DOI] [PubMed] [Google Scholar]
- 84.Plotnikov SV, et al. , High-resolution traction force microscopy. Methods Cell Biol, 2014. 123: p. 367–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Style RW, et al. , Traction force microscopy in physics and biology. Soft Matter, 2014. 10(23): p. 4047–4055. [DOI] [PubMed] [Google Scholar]
- 86.Polacheck WJ and Chen CS, Measuring cell-generated forces: a guide to the available tools. Nat Methods, 2016. 13(5): p. 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kraning-Rush CM, et al. , Quantifying traction stresses in adherent cells. Methods Cell Biol, 2012. 110 SRC - GoogleScholar: p. 139–78. [DOI] [PubMed] [Google Scholar]
- 88.Sharma A, et al. , Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat Protoc, 2018. 13(12): p. 3018–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahola A, et al. , Video image-based analysis of single human induced pluripotent stem cell derived cardiomyocyte beating dynamics using digital image correlation. Biomed Eng Online, 2014. 13: p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kijlstra JD, et al. , Integrated Analysis of Contractile Kinetics, Force Generation, and Electrical Activity in Single Human Stem Cell-Derived Cardiomyocytes. Stem Cell Reports, 2015. 5(6): p. 1226–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Estruch-Samper D, Particle Image Velocimetry RJ Adrian and Westerweel J, Cambridge University Press, The Edinburgh Building, Shaftesbury Road, Cambridge, CB2 2RU, UK. 2011. 558pp. £75. ISBN 978–0-521–44008-0. The Aeronautical Journal (1968), 2012. 116(1176): p. 219–220. [Google Scholar]
- 92.Rajasingh S, et al. , Contactless Particle Image Velocimetry (PIV) Method of Screening Drugs Using Human iPSC-derived Cardiomyocytes. Journal of Molecular and Cellular Cardiology, 2017. 112: p. 145. [Google Scholar]
- 93.Taylor ZJ, et al. , Long-Duration Time-Resolved PIV to Study Unsteady Aerodynamics. IEEE Transactions on Instrumentation and Measurement, 2010. 59(12): p. 3262–3269. [Google Scholar]
- 94.Smith AS, et al. , Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol Adv, 2017. 35(1): p. 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J, et al. , Combining hiPSCs and Human Genetics: Major Applications in Drug Development. Cell Stem Cell, 2017. 21(2): p. 161–165. [DOI] [PubMed] [Google Scholar]
- 96.Suh W, A new era of disease modeling and drug discovery using induced pluripotent stem cells. Arch Pharm Res, 2017. 40(1): p. 1–12. [DOI] [PubMed] [Google Scholar]
- 97.Maddah M, et al. , A non-invasive platform for functional characterization of stem-cell-derived cardiomyocytes with applications in cardiotoxicity testing. Stem cell reports, 2015. 4(4): p. 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sala L, et al. , MUSCLEMOTION: A Versatile Open Software Tool to Quantify Cardiomyocyte and Cardiac Muscle Contraction In Vitro and In Vivo. Circ Res, 2018. 122(3): p. e5–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huebsch N, et al. , Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng Part C Methods, 2015. 21(5): p. 467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maddah M. and Loewke K, Automated, non-invasive characterization of stem cell-derived cardiomyocytes from phase-contrast microscopy. Med Image Comput Comput Assist Interv, 2014. 17(Pt 1): p. 57–64. [DOI] [PubMed] [Google Scholar]
- 101.Steadman BW, et al. , A video system for measuring motion in contracting heart cells. IEEE Trans Biomed Eng, 1988. 35(4): p. 264–72. [DOI] [PubMed] [Google Scholar]
- 102.Delbridge LM and Roos KP, Optical methods to evaluate the contractile function of unloaded isolated cardiac myocytes. J Mol Cell Cardiol, 1997. 29(1): p. 11–25. [DOI] [PubMed] [Google Scholar]
- 103.Bazan C, et al. , Image Processing Techniques for Assessing Contractility in Isolated Adult Cardiac Myocytes. International Journal of Biomedical Imaging, 2009. 2009: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pointon A, et al. , From the Cover: High-Throughput Imaging of Cardiac Microtissues for the Assessment of Cardiac Contraction during Drug Discovery. Toxicol Sci, 2017. 155(2): p. 444–457. [DOI] [PubMed] [Google Scholar]
- 105.Takeda M, et al. , Development of In Vitro Drug-Induced Cardiotoxicity Assay by Using Three-Dimensional Cardiac Tissues Derived from Human Induced Pluripotent Stem Cells. Tissue Engineering Part C: Methods, 2017. 24(1): p. 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feaster TK, et al. , Matrigel Mattress: A Method for the Generation of Single Contracting Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res, 2015. 117(12): p. 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lock JT, Parker I, and Smith IF, A comparison of fluorescent Ca2+ indicators for imaging local Ca2+ signals in cultured cells. Cell Calcium, 2015. 58(6): p. 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suzuki J, Kanemaru K, and Iino M, Genetically Encoded Fluorescent Indicators for Organellar Calcium Imaging. Biophys J, 2016. 111(6): p. 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toepfer Christopher N, et al. , SarcTrack. Circulation Research, 2019. 124(8): p. 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ribeiro AJ, et al. , Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A, 2015. 112(41): p. 12705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roca-Cusachs P, Conte V, and Trepat X, Quantifying forces in cell biology. Nature Cell Biology, 2017. 19: p. 742. [DOI] [PubMed] [Google Scholar]
- 112.Mulligan JA, et al. , Traction Force Microscopy for Noninvasive Imaging of Cell Forces, in Biomechanics in Oncology, Dong C, Zahir N, and Konstantopoulos K, Editors. 2018, Springer International Publishing: Cham; p. 319–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fleissner F. and Parekh SH, Imaging mechanotransduction: Seeing forces from molecules to cells. Current Opinion in Biomedical Engineering, 2018. 5: p. 58–65. [Google Scholar]
- 114.Colin-York H. and Fritzsche M, The future of traction force microscopy. Current Opinion in Biomedical Engineering, 2018. 5: p. 1–5. [Google Scholar]
- 115.Steinwachs J, et al. , Three-dimensional force microscopy of cells in biopolymer networks. Nature Methods, 2015. 13: p. 171. [DOI] [PubMed] [Google Scholar]
- 116.Polio SR, et al. , Topographical control of multiple cell adhesion molecules for traction force microscopy. Integr Biol (Camb), 2014. 6(3): p. 357–65. [DOI] [PubMed] [Google Scholar]
- 117.Maskarinec SA, et al. , Quantifying cellular traction forces in three dimensions. Proc Natl Acad Sci U S A, 2009. 106(52): p. 22108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zundel M, Ehret AE, and Mazza E, Factors influencing the determination of cell traction forces. PLoS One, 2017. 12(2): p. e0172927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han SJ, et al. , Traction microscopy to identify force modulation in subresolution adhesions. Nat Methods, 2015. 12(7): p. 653–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hazeltine LB, et al. , Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol, 2012. 2012: p. 508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wheelwright M, et al. , Investigation of human iPSC-derived cardiac myocyte functional maturation by single cell traction force microscopy. PLoS One, 2018. 13(4): p. e0194909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ribeiro AJS, et al. , Multi-Imaging Method to Assay the Contractile Mechanical Output of Micropatterned Human iPSC-Derived Cardiac Myocytes. Circ Res, 2017. 120(10): p. 1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beussman KM, et al. , Micropost arrays for measuring stem cell-derived cardiomyocyte contractility. Methods, 2016. 94: p. 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao Y. and Zhang X, Cellular mechanics study in cardiac myocytes using PDMS pillars array. Sensors and Actuators A: Physical, 2006. 125(2): p. 398–404. [Google Scholar]
- 125.Tanaka Y, et al. , Demonstration of a PDMS-based bio-microactuator using cultured cardiomyocytes to drive polymer micropillars. Lab Chip, 2006. 6(2): p. 230–5. [DOI] [PubMed] [Google Scholar]
- 126.Ribeiro AJS, et al. , Stable, Covalent Attachment of Laminin to Microposts Improves the Contractility of Mouse Neonatal Cardiomyocytes. ACS Applied Materials & Interfaces, 2014. 6(17): p. 15516–15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taylor RE, et al. , Sacrificial layer technique for axial force post assay of immature cardiomyocytes. Biomedical Microdevices, 2013. 15(1): p. 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim K, et al. , Calibrated micropost arrays for biomechanical characterisation of cardiomyocytes. Micro Nano, 2011. 6 SRC - GoogleScholar: p. 317–322. [Google Scholar]
- 129.Rodriguez ML, et al. , Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J Biomech Eng, 2014. 136(5): p. 051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Denisin AK and Pruitt BL, Tuning the Range of Polyacrylamide Gel Stiffness for Mechanobiology Applications. ACS Appl Mater Interfaces, 2016. 8(34): p. 21893–902. [DOI] [PubMed] [Google Scholar]
- 131.Feric NT, et al. , Engineered Cardiac Tissues Generated in the Biowire II: A Platform for Human-Based Drug Discovery. Toxicological Sciences, 2019. 172(1): p. 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brown GE and Khetani SR, Microfabrication of liver and heart tissues for drug development. Philos Trans R Soc Lond B Biol Sci, 2018. 373(1750). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zimmermann WH, et al. , Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circulation Research, 2002. 90(2): p. 223–230. [DOI] [PubMed] [Google Scholar]
- 134.Mannhardt I, et al. , Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Reports, 2016. 7(1): p. 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schaaf S, et al. , Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One, 2011. 6(10): p. e26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takeda M, et al. , Development of In Vitro Drug-Induced Cardiotoxicity Assay by Using Three-Dimensional Cardiac Tissues Derived from Human Induced Pluripotent Stem Cells. Tissue Eng Part C Methods, 2018. 24(1): p. 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mannhardt I, et al. , Piezo-bending actuators for isometric or auxotonic contraction analysis of engineered heart tissue. J Tissue Eng Regen Med, 2019. 13(1): p. 3–11. [DOI] [PubMed] [Google Scholar]
- 138.Conant G, et al. , High-Content Assessment of Cardiac Function Using Heart-on-a-Chip Devices as Drug Screening Model. Stem Cell Rev Rep, 2017. 13(3): p. 335–346. [DOI] [PubMed] [Google Scholar]
- 139.Hansen A, et al. , Development of a Drug Screening Platform Based on Engineered Heart Tissue. Circulation Research, 2010. 107(1): p. 35–44. [DOI] [PubMed] [Google Scholar]
- 140.Hutter JL and Bechhoefer J, Calibration of atomic‐force microscope tips. Review of Scientific Instruments, 1993. 64(7): p. 1868–1873. [Google Scholar]
- 141.Domke J, et al. , Mapping the mechanical pulse of single cardiomyocytes with the atomic force microscope. European Biophysics Journal, 1999. 28(3): p. 179–186. [DOI] [PubMed] [Google Scholar]
- 142.Shroff SG, Saner DR, and Lal R, Dynamic micromechanical properties of cultured rat atrial myocytes measured by atomic force microscopy. Am J Physiol, 1995. 269(1 Pt 1): p. C286–92. [DOI] [PubMed] [Google Scholar]
- 143.Liu J, et al. , Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS One, 2012. 7(5): p. e37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang W-T, et al. , Characterization of the Mechanodynamic Response of Cardiomyocytes with Atomic Force Microscopy. Analytical Chemistry, 2013. 85(3): p. 1395–1400. [DOI] [PubMed] [Google Scholar]
- 145.Yue T, et al. , Quantifying Drug-Induced Nanomechanics and Mechanical Effects to Single Cardiomyocytes for Optimal Drug Administration To Minimize Cardiotoxicity. Langmuir, 2016. 32(7): p. 1909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Linke WA, Titin Gene and Protein Functions in Passive and Active Muscle. Annu Rev Physiol, 2018. 80: p. 389–411. [DOI] [PubMed] [Google Scholar]
- 147.Neagoe C, et al. , Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. Journal of Muscle Research & Cell Motility, 2003. 24(2): p. 175–189. [DOI] [PubMed] [Google Scholar]
- 148.Opitz CA, et al. , Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ Res, 2004. 94(7): p. 967–75. [DOI] [PubMed] [Google Scholar]
- 149.Lahmers S, et al. , Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res, 2004. 94(4): p. 505–13. [DOI] [PubMed] [Google Scholar]
- 150.LeWinter MM and Granzier HL, Cardiac titin and heart disease. Journal of cardiovascular pharmacology, 2014. 63(3): p. 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Herman DS, et al. , Truncations of titin causing dilated cardiomyopathy. N Engl J Med, 2012. 366(7): p. 619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schafer S, et al. , Titin-truncating variants affect heart function in disease cohorts and the general population. Nat Genet, 2017. 49(1): p. 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Borbely A, et al. , Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res, 2009. 104(6): p. 780–6. [DOI] [PubMed] [Google Scholar]
- 154.Hidalgo C, et al. , PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res, 2009. 105(7): p. 631–8, 17 p following 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen CY, et al. , Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nature Medicine, 2018. 24(8): p. 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sugiura S, et al. , Carbon fiber technique for the investigation of single-cell mechanics in intact cardiac myocytes. Nat Protoc, 2006. 1(3): p. 1453–7. [DOI] [PubMed] [Google Scholar]
- 157.Davidson PM, et al. , High-throughput microfluidic micropipette aspiration device to probe time-scale dependent nuclear mechanics in intact cells. Lab Chip, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hochmuth RM, Micropipette aspiration of living cells. J Biomech, 2000. 33(1): p. 15–22. [DOI] [PubMed] [Google Scholar]
- 159.Zhang C, et al. , Dynamic Model for Characterizing Contractile Behaviors and Mechanical Properties of a Cardiomyocyte. Biophys J, 2018. 114(1): p. 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brady AJ, Tan ST, and Ricchiuti NV, Contractile force measured in unskinned isolated adult rat heart fibres. Nature, 1979. 282(5740): p. 728–729. [DOI] [PubMed] [Google Scholar]
- 161.Nishimura S, et al. , Microtubules modulate the stiffness of cardiomyocytes against shear stress. Circ Res, 2006. 98(1): p. 81–7. [DOI] [PubMed] [Google Scholar]
- 162.Copelas L, et al. , A method for recording isometric tension development by isolated cardiac myocytes: transducer attachment with fibrin glue. Pflügers Archiv, 1987. 408(3): p. 315–317. [DOI] [PubMed] [Google Scholar]
- 163.Bazan C, et al. , Contractility assessment in enzymatically isolated cardiomyocytes. Biophysical reviews, 2012. 4(3): p. 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Siedlik MJ, Varner VD, and Nelson CM, Pushing, pulling, and squeezing our way to understanding mechanotransduction. Methods, 2016. 94: p. 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Linschoten M, et al. , Truncating Titin (TTN) Variants in Chemotherapy-Induced Cardiomyopathy. J Card Fail, 2017. 23(6): p. 476–479. [DOI] [PubMed] [Google Scholar]
- 166.Iskratsch T, Wolfenson H, and Sheetz MP, Appreciating force and shape — the rise of mechanotransduction in cell biology. Nature Reviews Molecular Cell Biology, 2014. 15: p. 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lyon Robert C, et al. , Mechanotransduction in Cardiac Hypertrophy and Failure. Circulation Research, 2015. 116(8): p. 1462–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Saucerman JJ, et al. , Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nature Reviews Cardiology, 2019. 16(6): p. 361–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sheehy SP, Grosberg A, and Parker KK, The contribution of cellular mechanotransduction to cardiomyocyte form and function. Biomech Model Mechanobiol, 2012. 11(8): p. 1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jian Z, et al. , Mechanical Load Effects on Cardiomyocyte Action Potential, Cacium Transient, and Contraction Revealed by using a Novel Patch-Clamp-in-Gel Technology. Biophysical Journal, 2018. 114(3, Supplement 1): p. 620a. [Google Scholar]
- 171.Ichikawa Y, et al. , Modulation of caveolins, integrins and plasma membrane repair proteins in anthracycline-induced heart failure in rabbits. PLoS One, 2017. 12(5): p. e0177660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Israeli-Rosenberg S, et al. , Integrins and integrin-associated proteins in the cardiac myocyte. Circ Res, 2014. 114(3): p. 572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pandey P, et al. , Cardiomyocytes Sense Matrix Rigidity through a Combination of Muscle and Non-muscle Myosin Contractions. Dev Cell, 2018. 44(3): p. 326–336.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang X. and Papoian T, Moving beyond the comprehensive in vitro proarrhythmia assay: Use of human-induced pluripotent stem cell-derived cardiomyocytes to assess contractile effects associated with drug-induced structural cardiotoxicity. J Appl Toxicol, 2018. [DOI] [PubMed] [Google Scholar]