ABSTRACT
Despite being one of the continents with the least greenhouse gas emissions, no continent is being struck as severely by climate change (CC) as Africa. Mosquito-borne diseases (MBD) cause major human diseases in this continent. Current knowledge suggests that MBD range could expand dramatically in response to CC. This study aimed at assessing the relationship between CC and MBD in Africa. Methods For this purpose, a systematic peer review was carried out, considering all articles indexed in PubMed, Scopus, Embase and CENTRAL. Search terms referring to MBD, CC and environmental factors were screened in title, abstract and keywords.Results A total of twenty-nine studies were included, most of them on malaria (61%), being Anopheles spp. (61%) the most commonly analyzed vector, mainly in Eastern Africa (48%). Seventy-nine percent of these studies were based on predictive models. Seventy-two percent of the reviewed studies considered that CC impacts on MBD epidemiology. MBD prevalence will increase according to 69% of the studies while 17% predicted a decrease. MBD expansion throughout the continent was also predicted. Most studies showed a positive relationship between observed or predicted results and CC. However, there was a great heterogeneity in methodologies and a tendency to reductionism, not integrating other variables that interact with both the environment and MBD. In addition, most results have not yet been tested. A global health approach is desirable in this kind of research. Nevertheless, we cannot wait for science to approve something that needs to be addressed now to avoid greater effects in the future.
KEYWORDS: Mosquito-borne diseases, malaria, dengue, climate change, environmental factors, Africa
Introduction
The United Nations (UN) defined CC as a change in the statistical properties of the climate system when considered over long periods of time, regardless of cause [1,2].
The Earth temperatures are fit to allow life thanks to the so-called ‘greenhouse effect’, which is a natural process by which radiation from the Earth’s atmosphere warms its surface to a temperature above what it would be without this atmosphere [3]. The ‘greenhouse gases’ have an influence on the Earth’s energy balance; an increase in their emissions led to anthropogenic global warming. Emissions of several greenhouse gases have increased substantially since large-scale industrialization began in the mid-1800 s, especially due to population and economic development and growth, which motivated an increased consumption of burning fossil fuels, agriculture, land clearing and the impact of cattling and use of fertilizers containing nitrogen. In fact, emissions registered between 2000 and 2010 were among the highest in history [4].
CC affects all regions and their populations´ health [5], mostly negatively, impacting on social and environmental health determinants (clean air, potable drinking water, food safety and safe housing, among others) [6].
Albeit producing only 2% to 3% of the global greenhouse gas emissions [7,8], Africa is suffering major CC repercussions [4,9,10]. The Intergovernmental Panel on Climate Change (IPCC), which is the United Nations body for assessing the science related to CC, already stated in 2001 that Africa is highly vulnerable to the various manifestations of CC, mainly due to six situations: (a) the limited water resources; (b) food security at risk from declines in agricultural production; (c) natural resources productivity at risk and biodiversity; (d) vector- and water-borne diseases, especially in areas with inadequate health infrastructure; (e) coastal zones vulnerable to sea-level rise; and (f) exacerbation of desertification [4].
According to different meteorological models, Africa will face an increased tendency of warming and variable rainfall patterns, although the impact of CC in the health of African populations still poses many unanswered questions [11]. Current projections foresee a rise of more than 2ºC in the mean annual temperature for this continent. Moreover, African ecosystems are already being affected by climate change [4].
Over 80% of the global population is at risk of a vector-borne disease. Mosquito-borne diseases (MBD), such as malaria, dengue, chikungunya and zika, are the largest contributor to human vector-borne disease burden, particularly in the African continent [12,13]. For instance, 93% of all malaria cases worldwide occur in Africa. In comparison, South East Asia accounts for 3.4% and the Eastern Mediterranean region for 2.1% of all cases [14]. Regarding dengue infections, Africa’s burden seems to be nearly equivalent to that of the Americas, even in the absence of strong surveillance systems for MBD in most African countries. In fact, although half of the countries with chikungunya and zika infections are located in Africa, there are not much data on the epidemiology of these MBD in Africa [15,16].
According to several international scientific reports, MBD will expand in parallel with CC [86; 1,9]. Climate patterns influence the lifespan of mosquitos, their rate and frequency of reproduction, mosquito blood-feeding patterns, as well as extrinsic incubation periods [17,18]. Despite these certainties, the impact of CC on MBD still raises controversy and debate [19–21], as many other cross-cutting global processes are not usually taken into account when assessing the impact of CC in MBD dynamics.
The present systematic review is aimed at assessing the effects of CC in the epidemiology of the most prevalent MBD and their vectors in Africa.
Methods
A systematic review was performed on MBD and CC in Africa following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews and meta-analyses [22]. The analyzed MBD included some of the most prevalent MBD in Africa, i.e. malaria, dengue, zika, chikungunya, Rift Valley fever (RVF), West Nile virus (WNV), yellow fever and lymphatic filariasis (LF), as well as their vectors Anopheles spp, Aedes spp, Coquillettidia spp, Ochlerotatus spp, Haemagogus spp and Culex spp.
Search strategy
Two main reviewers searched for indexed articles published in the PubMed, Scopus, Embase and CENTRAL databases. The following search terms were screened in title, abstract and keywords using the AND Boolean logic operator:
Vector borne diseases OR Mosquito borne diseases OR Malaria OR Lymphatic Filariasis OR Yellow fever OR Dengue OR West Nile OR Zika OR Chikungunya OR Rift Valley fever
Vector mosquitoes OR Mosquitoes OR Aedes OR Anopheles OR Culex OR Coquillettidia OR Ochlerotatus OR Haemagogus
Climate variability OR Climate change OR Weather OR Climate OR Temperature change OR Environment OR Temperature OR Warming OR Meteorology OR Rainfall OR Humidity OR Altitude
Africa OR Algeria OR Angola OR Benin OR Botswana OR Burkina Faso OR Burundi OR Cabo Verde OR Cameroon OR Central African Republic OR Chad OR Comoros OR Congo OR Côte d’Ivoire OR Democratic Republic of the Congo OR Djibouti OR Egypt OR Equatorial Guinea OR Eritrea OR Eswatini OR Swaziland OR Ethiopia OR Gabon OR Gambia OR Ghana OR Guinea OR Guinea-Bissau OR Kenya OR Liberia OR Lesotho OR Libya OR Madagascar OR Malawi OR Mali OR Mauritania OR Mauritius OR Mayotte OR Morocco OR Niger OR Nigeria OR Réunion OR Rwanda OR Saint Helena OR Sao Tome and Principe OR Senegal OR Sierra Leone OR Somalia OR South Africa OR South Sudan OR Sudan OR Togo OR Tunisia OR Uganda OR United Republic of Tanzania OR Tanzania OR Western Sahara OR Zambia OR Zimbabwe
Full-text articles were read to evaluate them according to the inclusion criteria. Two reviewers examined all citations in the study selection process. If it was uncertain whether to include a study, a third reviewer assessed whether the article should be included or not. The reference lists of all included articles were also cross-checked for relevant studies. Besides, possible relevant information was also checked in gray literature sources (such as Google Advanced search and key institutional websites) and was included when it met the inclusion criteria.
Eligibility criteria
The search was performed in English, French, Portuguese, German, Italian and Spanish languages. Only original research studies with quantitative analysis were considered, thereby excluding reviews, short communications, posters and conference abstracts. Studies were included if the impact of CC on MBD was analyzed. CC was considered as a generic term covering environmental, climatic and meteorological variables. Inclusion and exclusion criteria for selected studies are listed in Table 1.
Table 1.
Eligibility criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Research studies | Other type of study |
| Studies must refer to climate change (and not just single environmental and/or climatic variables) | Not assessing the impact of climate change on MBD or their vectors |
| Published between 1 January 2004 and 31 December 2018 | Published before 1 January 2004 or after 31 December 2018 |
| Languages: English, French, Portuguese, German, Italian and Spanish | Not carried out in Africa. |
The selected papers were systematically and thematically analyzed. To summarize the state of the art on MBD and CC in Africa, data were extracted into evidence tables under the following headings: year of publication, type of MBD, vector, place of the study, time frame of observed data, type of environmental and/or climatic factor and data sources, analytical approach, summary of the results, CC impact on MBD incidence (Yes/No), projected prevalence and main limitations.
A 13-items quality assessment tool was developed, based on literature and a similar study carried out in Europe (yet to be published), to evaluate internal and external validity of the selected publications. The external validity of a study is related to the study’s generalizability and the applicability of the study’s findings and therefore takes into account the study’s purpose. The internal validity refers to the bias that could occur in a study and compromise its findings [23]. The quality of each manuscript was assessed by reviewing study objective/s, study design, data presentation and discussion, granting 1 if the criterion was met, and zero if it was not. The maximum achievable total score was 13 points (supplementary table 1).
A standardized Excel (Version 2010, Microsoft Corporation, Richmond, WA, USA) spreadsheet was used to extract information from included studies. References retrieved were saved in Zotero software 5.0.67 (www.zotero.org). We chose this open-source software in order to share complete libraries among all reviewers and contributors, for purposes of transparency and standardization. The analyzed countries from the published literature were projected onto a map of Africa using QGIS software version 2.18.13.
Results
The systematic search strategy yielded a total of 907 citations (707 Pubmed, 65 Scopus, 42 CENTRAL, 62 Embase, 31 other sources) published between 1 January 2004 and 31 December 2018. That number was reduced to 856 after excluding 51 duplicate records. After screening the titles and the abstracts, i.e. reading them to see if they fit the inclusion criteria, we retained 124 articles for full-text screening. A final set of 29 articles met all inclusion criteria and were included in our review. Seven hundred and thirty-two articles were excluded, mostly because of not dealing with either MBD (n = 68), CC (n = 438) or Africa (n = 226). Figure 1 presents the PRISMA chart of the study selection process and the main reasons for exclusion.
Figure 1.
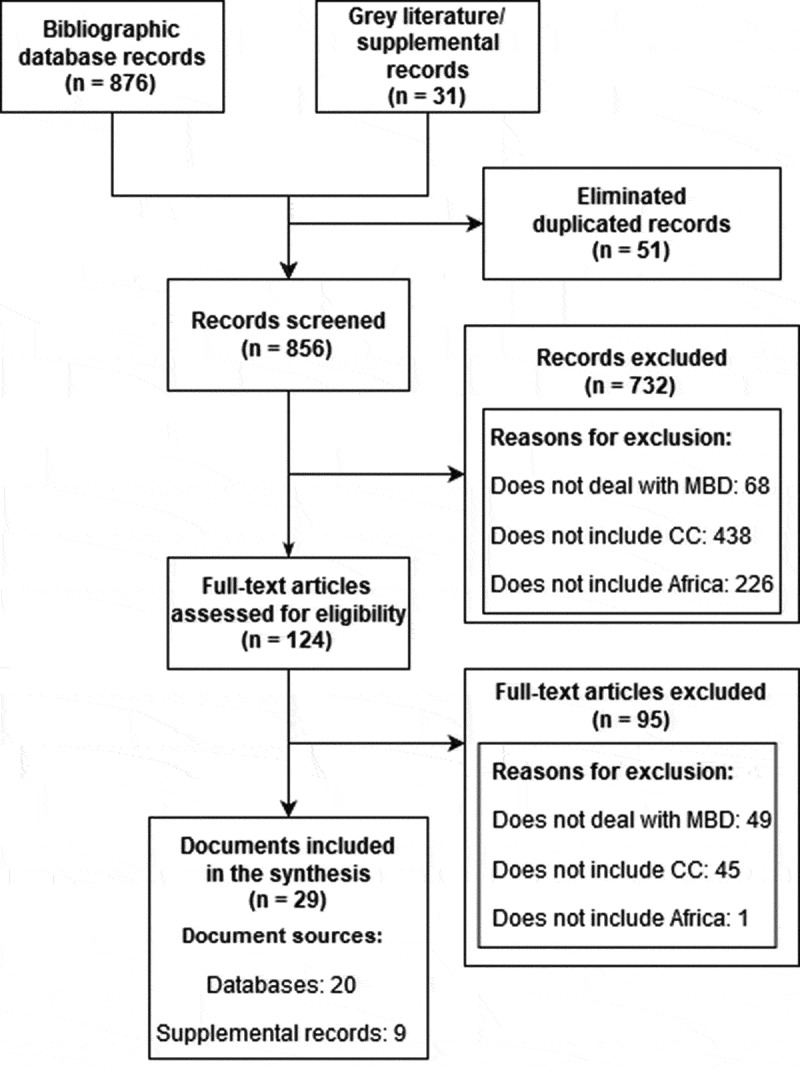
Study selection process.
Descriptive characteristics of the studies
The descriptive characteristics are summarized in Tables 2–4. Nineteen studies dealt with CC and malaria and four with CC and dengue. Other analyzed diseases included RVF (n = 3), WNV (n = 2), LF (n = 1), zika (n = 1) and chikungunya (n = 1). Two studies targeted more than one MDB that share the same vector: dengue and chikungunya, and WNV and LF. Impact of CC in Anopheles spp distribution was dealt with in 19 studies, while 7 studies examined Aedes spp and 5 studies examined Culex spp (Tables 2–4).
Table 2.
Summary of analyzed geographical areas, vectors, environmental factors and main findings of studies dealing with malaria.
| Geographical area |
Vector |
Analyzed environmental
factors |
Affected by climate
change |
Changes in prevalence or incidence
due to climate change |
Expansion due to climate
change |
First author (year of
publication) |
|
|---|---|---|---|---|---|---|---|
| Main findings | |||||||
| East [Kenia) | Anopheles spp | Average temperature increase | Yes | Increases | No | 19, | |
| An increase of 1ºC has been registered over the last 30 years, which coincides with an increase in malaria outbreaks | |||||||
| East [Kenia] | Anopheles spp | Average temperature increase | Uncertain | Uncertain | Uncertain | 17, | |
| Parasitary development may be underestimated under cold conditions if only average monthly temperatures are taken into account. On the contrary, under warm weather conditions it may be overestimated | |||||||
| East [Kenia] | Anopheles spp | Variations in rainfall patterns, altitude changes | Yes | Decreases | No | 42, | |
| Decreasing trends in malaria at both low and high altitudes. Increases in rainfall increment significantly malaria incidence | |||||||
| East [Kenia] | Anopheles spp | Average temperature variations | No | Decreases | No | 45, | |
| Predicted relative increases in the larvarian development rate due to climate change are smaller since water temperature does not increase | |||||||
| East [Kenia] | Anopheles spp | Average temperature increase, variations in diurnal temperature | No | Increases | No | 18, | |
| If diurnal temperature range increases, extrinsic incubation period sensitivity decreases. Relative effects of average temperature increases are smaller than predicted if daily temperature fluctuations are taken into account | |||||||
| East [Tanzania] | Anopheles gambiae, Anopheles stephensi | Average temperature increase | No | Decreases | No | 44, | |
| After mosquito exposure to different temperatures (27ºC optimal temperature, 30ºC and 33ºC future projections] and diurnal variability (0,6–9ºC), it is verified that average temperature increases induce a decrease in oocyte prevalence and intensity and sporozoite prevalence | |||||||
| East (Tanzania) | Anopheles spp | Average temperature increase, increased rainfall | Yes | Increases | Uncertain | [32] | |
| 32–33ºC endemic transmission frame. Effects of rainfall are more unpredictable and difficult to quantify | |||||||
| East (Tanzania) | Anopheles spp | Average temperature increase, increased rainfall | Yes | Increases | Yes | [33] | |
| Extinction depends more on rainfall than on temperature. Optimal temperature for endemic transmission and progress into previously free zones: 32–33ºC. In 2080, with 4–5ºC increases, Rukwa and Kigoma (near Democratic Republic of Congo). Southern Morogoro, Iringa, Ruvuma and Mtwara near Malawi and Mozambique will be affected | |||||||
| East [Kenia, Uganda, Rwanda, Burundi) | Anopheles gambiae | Average temperature increase | Yes | Increases | Yes | 20, | |
| Temperature changes significantly amplified by mosquito population dynamics | |||||||
| East [Ethiopia] | Anopheles spp | Average temperature increase, increased altitude | Yes | Increases | Yes | 37, | |
| Increases in temperature extend spatial distribution to higher altitudes | |||||||
| East [Ethiopia, Kenia, Uganda] | Anopheles spp | Average temperature increase, increased rainfall | Yes | Increases | No | 41, | |
| Significant changes in climatic variability coincide with increased magnitude and frequency of malaria epidemics since 1989 | |||||||
| South, East and West | Anopheles gambiae, Anopheles arabiensis | Average temperature increase | Yes | Increases | Yes | 34, | |
| Future malaria suitability will decrease in Western Africa and Sahel due to increases in average annual temperatures and will increase in Eastern and Southern Africa because of 1.5–2.7ºC increases | |||||||
| West [Niger, Benin and Mali] | Anopheles gambiae | Average temperature variations, increased rainfall | Uncertain | Increases | Yes | 24, | |
| If tropical meteorological data from Benin were applied on the Niger Sahel, mosquito abundance will increase, whereas it may decrease with Malian data | |||||||
| Africab | Anopheles spp | Average temperature increase, diminished rainfall | Yes | Increases | Yes | 27, | |
| Current occurrence is restricted by deserts and highlands, epidemics in the Sahel and some Highland regions. Future projections show a decrease in most tropical areas in Africa due to increasing temperatures and decreasing annual rainfall. Increasing epidemics in southern Sahel. Increasing intensity in Eastern Africa and Highland areas | |||||||
| Africab | Anopheles gambiae | Net effect of climate change | Yes | Uncertain | Yes | 35, | |
| All year transmission suitable areas shift from Central and Western Africa to Uganda, Angola, Gabon and Cameroon. High season transmission [4–8 months] expands into Southern Africa and Madagascar | |||||||
| Africab | Anopheles gambiae, Anopheles arabiensis | Average temperature increase, variations in summer and Winter rainfall | Yes | Increases | Yes | 39, | |
| Shift toward Southern and Eastern Africa. Western and Central Africa might lose suitability for both Anopheles species | |||||||
| Africab | Anopheles gambiae, Anopheles arabiensis | Average temperature increase, variations in rainfall patterns | Yes | Increases | Yes | 40, | |
| Anopheles gambiae expansion toward Angola, Burundi, Comoro Islands, Ethiopia, Kenia, Malawi, Mali, South Africa, Tanzania, Zambia; and of Anopheles arabiensis toward Angola Botswana, Burundi, Democratic Republic of Congo, Djibouti, Ethiopia, Kenia, Malawi, Namibia, Rwanda, South Africa, Sudan, Swaziland, Gambia, Uganda, Zimbabwe with 2ºC temperature increase in all Africa and changes in rainfall patterns. 0.1ºC increases lead to expansion of Anopheles gambiae toward Angola, Cameroon, Ethiopia, Guinea, Mozambique, Niger, Sierra Leona, South Africa, Uganda, Zambia and Zimbabwe. | |||||||
| Worldwidea | Anopheles spp | Net effect of climate change | Yes | Increases | No | 47, | |
| Increase in malaria in East African highlands, South Africa, central Angola and the Madagascar plateau. Decreases in tropical areas, including Western Africa. Net increase of suitability and population at risk, but with uncertainties | |||||||
| Worldwidea | Anopheles spp | Net effect of climate change, climate trends since 1900 | Yes | Decreases | No | 28, | |
| Future effects are smaller than those observed since 1900. Contradiction between observed and predicted effects | |||||||
aPredictive models that analyze disease incidence at worldwide level.
bPredictive models that analyze disease incidence throughout the whole African continent.
Table 4.
Summary of analyzed geographical areas, vectors, environmental factors and main findings of studies dealing with RVF, chikungunya, zika, WNV and LF.
| Geographical area |
Disease (Vector) |
Analyzed environmental
factors |
Affected by climate
change |
Changes in prevalence or incidence
due to climate change |
Expansion due to climate
change |
First author (year of
publication) |
|
|---|---|---|---|---|---|---|---|
| Main findings | |||||||
| South and center | Dengue, chikungunya [Aedes aegypti, Aedes albopictus) | Net effect of climate change | Yes | Increases | Yes | 25, | |
| Potential expansion of Aedes aegypti into Southern Africa, occurrence extended into Central Africa. Increased expansive potential than Aedes albopictus, which may also raise | |||||||
| East (Tanzania] | RVF [Culex pipiens) | Average temperature variations, increased rainfall | Yes | Unknown | Yes | 31, | |
| Rift valley fever expansion into zones close to Tanganyika, Malawi and Victoria lakes (Western, Southwestern and Northern Tanzania] in 2020 and 2050 | |||||||
| West (Senegal) | RVF [Aedes vexans, Culex poicilipes) | Average minimum and maximum temperature variations, increased rainfall | No | Increases | No | 38, | |
| Decreases in minimum temperature promote the occurrence of Culex poicilipes. Aedes vexans shows a negative correlation with relative humidity and minimum and maximum temperatures. Proximity to water ponds increases risk of becoming a spot zone for both vectors | |||||||
| West (Senegal] | RVF [Aedes vexans, Culex poicilipes) | Increased rainfall | No | Increases | No | 11, | |
| Aedes vexans distribution density depends on total rainfall and pond dynamics | |||||||
| East (Southern Sudan] | Zika [Aedes aegypti, Aedes albopictus, Aedes africanus) | Net effect of climate change | Yes | Increases | Yes | 26, | |
| Possible occurrence of zika virus in Southern Sudan | |||||||
| South (South Africa] | WNV [Culex univittatus) | Increased summer rainfall | Yes | Increases | No | 43, | |
| Total summer rainfall, previous summer rainfall and interannual rainfall variations are related to infection rates | |||||||
| Worldwidea | WNV, LF [Culex quinquefasciatus] | Net effect of climate change | Yes | Unknown | Yes | 36, | |
| Ideal conditions in narrow zones of Northern Africa and Western Europe. Future transmission similar to current, including Central and Southern Africa. High uncertainty about Northern and Central Africa | |||||||
aPredictive models that analyze disease incidence at worldwide level.
The geographic zones under study were Eastern Africa (n = 14), Western Africa (n = 4) and Southern Africa (n = 3). Six studies analyzed the impact of CC on MBD from a worldwide perspective and four on a continental African basis (Table 2, Figure 2).
Figure 2.
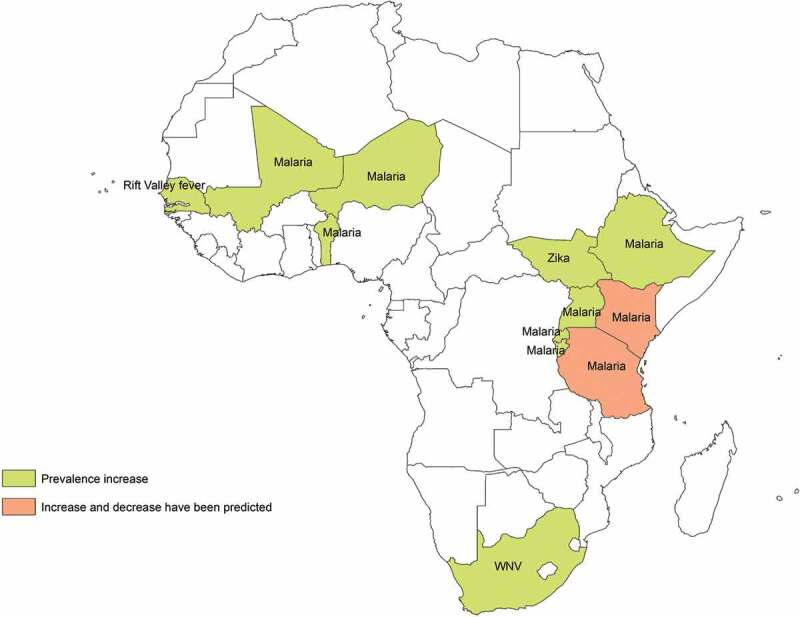
African countries with expected increase/decrease of mosquito-borne diseases prevalence under the effects of climate change. Footnote: Changes in Rift Valley Fever prevalence in Tanzania were uncertain.
About two-thirds (n = 18) of the papers were published in the second half of the study’s timeframe (2012–2018).
All were observational studies, mostly based on predictive models (n = 23) [17–21,24-41]. Time series analysis was performed in three studies [41–43] and two studies were designed experimentally [44,45]. All studies except for one focused on future estimations about climate change. In this paper, a model based on retrospective data was developed, predicting changes for the last decades, and later authors compared these projections with the real occurrences [19].
The main analyzed environmental factors included average temperature increases and/or variations (n = 18), changes in rainfall patterns (n = 12) and the net effect of CC (n = 8), while two studies focused on altitude variations and another two on variations in the diurnal temperature range (Tables 2–4). Whereas the data source to assess the CC was not described in detail in one study [28], WorldClim datasets were the most commonly used dataset to compute the net effect based on average temperatures and rainfall [25,26,31,32,35,36]. Those studies that used WorldClim datasets used data recorded between 1950 and 2000. One study used a different meteorological dataset – CliMond – also based on temperature and precipitation data from 1950 to 2000 [29], while other studies focused on datasets from previously published studies [11,17,20,27,28,33,34,37,38,46]. Yet another study used temperature and rainfall data from 1960 to 1999 from different national and international institutions [47]. Two studies used CLIMEX datasets [39,40], while other studies used meteorological data from local weather stations [18,19,24,41–43,45]. Two studies did not describe their climate data sources [30,44].
A summary of the studies’ main findings is provided in Tables 2–4. Overall, 72% (n = 21) of the included studies found a significant relationship between CC and MBD prevalence, while 6 studies found no such association. Only two studies found uncertainties in this link. Sixty-nine percent of the studies pointed out that MBD prevalence will increase as a consequence of CC (13 for malaria, 2 for dengue, 1 for dengue and chikungunya, 2 for RVF, 1 for WNV and 1 for zika), while 14 papers concluded that some of the analyzed MBD will expand (8 for malaria, 2 for dengue, 1 for dengue and chikungunya, 1 for zika, 1 for RVF and 1 for WNV and LF). According to three studies, the analyzed disease’s occurrence area will contract for malaria (n = 2) and dengue (n = 1).
Overall, variations in meteorological variables were linked to altered MBD dynamics in 21 studies. Changes in temperature were related to increased MBD prevalence in 12 studies, 10 of whom dealt with malaria, 1 with dengue and 1 with RVF. The net effect of CC was linked to increases in MBD prevalence in four studies, of which two analyzed dengue, one malaria, one zika and one chikungunya. In these studies, net effect of climate change was defined as combinations of different and interlinked environmental factors (i.e. temperature and rainfall) over a long period of time. Variations in precipitation patterns were linked to increase MBD prevalence in eight studies, six dealing with malaria, one with RVF and one with WNV. Changes in the terrain’s altitude and variations in the diurnal temperature range were linked to increased malaria and dengue prevalence, respectively. Two studies showed decreases in malaria prevalence as a result of the net effect of CC, altered precipitation patterns and changes in the terrain’s altitude (Tables 2–4).
Effect of climate change on malaria in Africa
According to 74% of the studies targeting malaria (n = 14), this disease prevalence will be affected by CC in several African countries, specifically the East African highlands. Environmental factors that may influence malaria occurrence in Africa include changes in temperature (n = 14) and rainfall patterns (n = 8), as well as the vectors’ capacity to adapt and survive at higher altitudes (n = 2) and diurnal temperature ranges (n = 1) or the net effect of CC. Eleven of these studies predicted an increase in disease prevalence and/or geographical distribution due to CC, whereas a decrease was predicted by two researches, specifically in Kenia and on a worldwide range. One research showed uncertain results. Some of these findings were contradictory, as they referred to the same countries, for example, Kenia [19,42] (Figure 2). Regarding disease spread, according to nine studies, CC will expand the disease across the continent, principally through Eastern Africa (Table 2, Figure 3).
Figure 3.
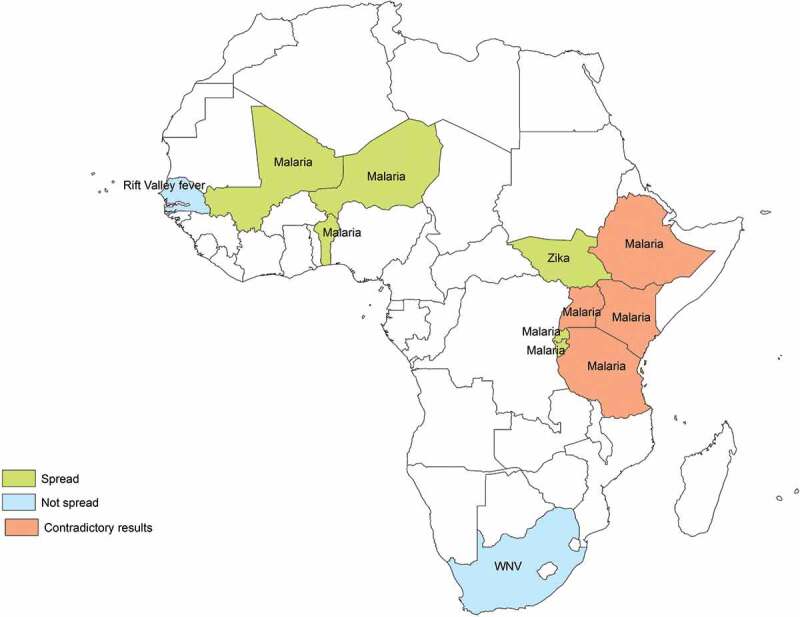
African countries with expected spread/contract of mosquito-borne diseases incidence under the effects of climate change.
Effect of climate change on dengue in Africa
The impact of CC on dengue epidemiology was assessed on a worldwide range and in Southern and Central Africa. According to 75% of the studies (n = 3), dengue prevalence will increase due to the effects of CC in several African countries in Southern and Central Africa (Figure 2). Temperature changes as well as variations in rainfall patterns and relative humidity were pointed out as possible influencing factors in the increase of disease’s prevalence and disease spread. On the other hand, Khormi et al. predicted marginal areas suitable for dengue vectors’ expansion in Northern Africa under future CC scenarios, specifically Mauritania (Table 3, Figure 3).
Table 3.
Summary of analyzed geographical areas, vectors, environmental factors and main findings of studies dealing with dengue.
| Geographical area |
Vector |
Analyzed environmental
factors |
Affected by climate
change |
Changes in prevalence or incidence
due to climate change |
Expansion due to climate
change |
First author [year of
publication] |
|
|---|---|---|---|---|---|---|---|
| Main findings | |||||||
| Worldwidea | Aedes aegypti | Net effect of climate change | No | Decreases | No | 29, | |
| In 2030 Democratic Republic of the Congo, Congo, Gabon, the southern coast of Benin, Nigeria, Togo, Ghana and Ivory Coast will be climatically suitable. Marginal zones include the Western coast of Mauritania and Morocco. In 2070, expansion especially into Lybia and Egypt | |||||||
| Worldwidea | Aedes aegypti | Average temperature increase, variations in diurnal temperature | Yes | Increases | Yes | 30, | |
| Dengue epidemic potential decreases at more than 29ºC. Increased risk in the Northern hemisphere and parts of Southern Africa | |||||||
| Worldwidea | Aedes aegypti, Aedes albopictus | Net effect of climate change | Yes | Increases | Yes | 46, | |
| Changes in dengue distribution in 2080. Limiting factor is the absence of Aedes aegypti. | |||||||
| South and center | Aedes aegypti, Aedes albopictus | Net effect of climate change | Yes | Increases | Yes | 25, | |
| Potential expansion of Aedes aegypti into Southern Africa, occurrence extended into Central Africa. Increased expansive potential than Aedes albopictus, which may also raise | |||||||
aPredictive models that analyze disease incidence at worldwide level.
Effect of climate change on other MBDs in Africa
Two studies predicted a positive impact of CC in RVF prevalence (essentially due to variations in rainfall patterns and average temperatures), while one study showed uncertain results about the relationship between CC and RVF epidemiology in Tanzania (Table 4).
According to the time series analysis developed by Uejio et al., the WNV prevalence will increase as a result of changing rainfall patterns, getting expanded into the highlands and deserts of South Africa. In contrast, one study showed uncertainty about these changes in disease prevalence, in particular in Northern Africa and parts of Central Africa (Table 4, Figure 2).
According to Campbell et al. chikungunya will expand into Southern Africa due to an increased presence of its vector Aedes aegypti owing to an increased temperature (Table 4, Figure 3).
In the study on zika and CC by Carlson et al., it was predicted that zika distribution will be altered as a result of changing rainfall patterns and variable diurnal temperature ranges, which will have an impact on the distribution of Aedes africanus, A. aegypti and A. albopictus. Moreover, according to this paper zika cases will be registered in previously naïve areas, such as Southern Sudan (Table 4).
Quality assessment
Overall, the studies were of medium or good quality. The main reasons for scoring lower were lack of pointing out the study’s objectives or the study period (Figure 4).
Figure 4.
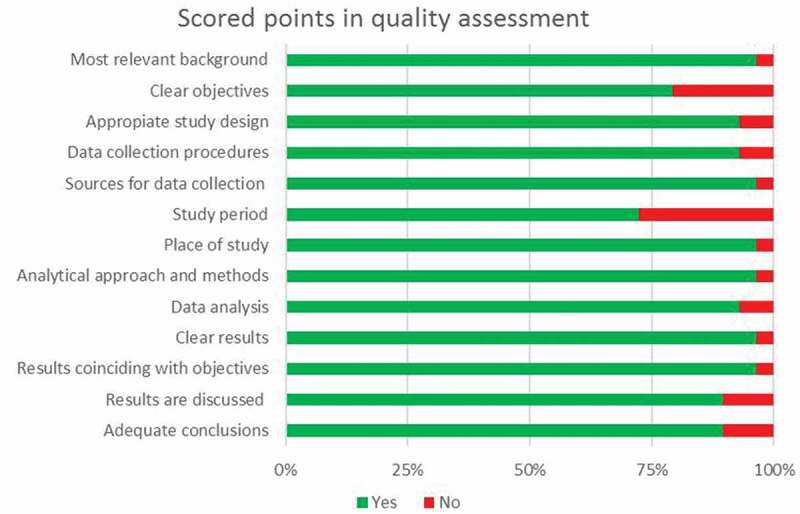
Scored points in quality assessment.
The most frequent limitation identified by the studies’ authors was not including other possible factors that may affect MBD occurrence (n = 14) [17–19,21,24,27,28,31,39–41,45-47] or focusing on one single vector when more than one species can transmit the disease (n = 4) [31,34,36,38].
Discussion
The impact of CC on MBD raises great controversy and several studies have been performed to address this incognita with contradictory results [17–21,24,25,27–35,37,39–47]. In our review, 69% of the included studies found a positive impact of CC on MBD [20,24–27,30-32,34–40,46], while others foresaw a decrease [20,28,29,44,45] or reached uncertain results [17,31,35,36]. Probably, these contradictory results may be related to the difficulties arising when trying to define CC properly.
It is difficult to measure the impact of climate change, since several indicators could be used, e.g. greenhouse gas emissions, atmospheric concentrations or changes in different environmental factors. Meteorological data like median temperatures, rainfall patterns, sea level and volcanic eruptions are usually used, while long-term indicators such as decadal variations in temperature and precipitation are not taken into account [4]. Moreover, the lack of other relevant environmental factors is common. According to the National Aeronautics and Space Administration (NASA), some of the potential future effects of global CC include more frequent wildfires, longer periods of drought in some regions and an increase in the number, duration and intensity of tropical storms [48]. However, measuring all these variables is difficult, especially if there are insufficient meteorological stations and the existing ones are located near major cities and, therefore, exposed to a phenomenon called urban heat island, which consists of a metropolitan area that is warmer than its surrounding rural areas as a result of human activities [49]. Furthermore, the meteorological data used in the different studies came from different sources and the collection period also differed from one study to another; therefore, our results depend strongly on the input data and how this data was used in the study design.
Africa is the continent that is most threatened by climate change [50]. Current predictions foresee a major rise in mean temperatures compared to the global average for the end of the twenty-first century, ranging from 3ºC to 6ºC when compared to the reference period 1986 to 2005 according to the scenario RCP8.5. By the middle of the twenty-first century, the mean temperature may exceed 2ºC in most parts of Africa and 4ºC by the end of the century. These changes may vary by geographical zone; Coastal Africa, as well as Central Africa, may experience smaller increases, whereas North Africa is expected to suffer warmer summers [4]. According to the World Bank, the East African coast and Madagascar might be also less affected than other regions. Precipitation might decrease by up to 30% across Africa [51], although it might increase by up to 10% in East Africa, the Gulf of Guinea and by smaller percentages in West Africa and the Sahel zone [52].
Malaria was the most frequent assessed MBD in this systematic review, particularly in Eastern Africa [17–20,32-34,37,41,42,44,45]. Malaria is the most prevalent and frequently analyzed disease in this continent [53]. Furthermore, unlike other of the analyzed MBD in this paper, malaria is not a neglected tropical disease (NTD), thus receiving major investment on control and research [54]. Regarding the regional distribution of the selected paper, it should be taken into account that huge disparities exist across Africa in terms of research investment [55,56]. These research gaps may also be influenced by the distribution and preferences of the international collaborations, which may affect the representation of countries in African health research [57].
Regarding the analyzed environmental factors, temperature was the most frequently analyzed climatic variable in the selected studies. In the context of CC, heatwaves and extreme maximum and minimum temperatures are expected to become more frequent [58]. A rise of 1ºC during the last decades has already been registered in Eastern African regions [19]. Temperature may affect both vectors and parasites [9,86]. Culex spp mosquitoes have been shown to be sensitive to increased temperatures, therefore increasing the risk of WNV infections [59]. However, Anopheles spp mosquito populations decrease at 40ºC, while Plasmodium spp parasites seem to have an optimal temperature window of 30–32ºC [60]. Nevertheless, it should be taken into account that an adaptation to changing environmental conditions of the pathogen, the vector or both may take place in the future [61].
Rainfall was the second most frequently analyzed climatic variable. The majority of the analyzed papers showed an increase in MBD prevalence and/or incidence due to increased rainfall [32,33,39–41] whereas one study showed increases in MBD prevalence/incidence as a result of diminished rainfall [27]. Changes in rainfall due to abnormal El Niño-Southern Oscillation (ENSO) and other oscillations in temperature, rainfall and extreme weather events have been described in the context of CC [11]. For instance, low peaks of rainfall have been linked to increased malaria prevalence, whereas high peaks of rainfall combined with lower maximum temperatures have been linked to decreased malaria prevalence in Kenya. Moreover, delays of one to two months in rainfall increased malaria prevalence in Kenya [62].
Mosquito appearance at naïve higher altitudes due to increased temperatures was analyzed in two studies in Eastern Africa [37,42]. Although malaria risk is believed to be low at altitudes above 1500 m [63], favorable meteorological conditions may lead to disease appearance at altitudes up to 3000 m [63–65]. CC plays an important role in malaria appearance at higher altitudes by changing the vectors’ ability to survive at higher altitudes [65].
Only few studies address other key ecological, vectoral or demographical factors, i.e., attack rates, R0 or per capita density of vectors [66,67]. Moreover, in some particular cases (such as RVF and LF) it should have been taken into account (and analyzed) that the disease can be transmitted by different mosquito species, on which the impact of CC may differ.
Effect of climate change on malaria, dengue, chikungunya, zika, Rift Valley Fever and West Nile Fever
In the last two decades, an important decrease in worldwide malaria prevalence has been observed, but in recent years there has been a stagnation, not only due to lack of finance but also owing to other factors, such as political instability, mistreatment and self-medication or lacking coverage or drug resistance [53,68–71].
According to WHO estimations, CC has already been responsible for 3% of all malaria deaths worldwide (WHO), especially in children aged under 5 years [53,72]. Warm and humid meteorological conditions, such as those registered in tropical habitats, are ideal for mosquito breeding [27]. Changing climatic conditions in Rwanda led to an epidemic in the 1980 s [73]. Recent vector appearance in Kenyan highlands suggests that weather and environmental conditions have become favorable for malaria proliferation in this country [74].
There is also some evidence against an impact of CC on malaria distribution [28]; even pointing to a prevalence decrease [44,45] as a consequence of the CC in Kenya and Tanzania [17,44].
If there is something in common among such contradictory studies, it is that important factors were missing in the analysis of all of them, i.e. deforestation [12,75,76], group immunity [77], lack of precise historical and current data concerning wildlife species [78] and the use of climatic data based exclusively on temperature [12,17,19,35]. In addition, migration plays an important role in all vector-borne diseases (VBD) [79]. Furthermore, under- and misdiagnosis, as well as delayed diagnosis and treatment as a result of superstitious beliefs, poor healthcare assistance and political instability pose challenges to malaria control and elimination [80,81]. The problem here is how to integrate this variety and diversity of factors in complex mathematical models. We believe that a multisectoral global health approach might be a possibility.
According to the WHO, 3.8% of dengue deaths are due to CC [72]. The positive impact of CC on its epidemiology observed in most analyzed researches is consistent with recent dengue outbreaks in Cape Verde, Madeira and Angola [82]. The disease has also spread all over the world, which has been particularly related to increased globalization and migration [52,79,83]. Moreover, dengue is frequently under or misdiagnosed as a consequence of insufficient laboratories or lack of knowledge among healthcare workers [84]. On the other hand, the absence of serotype distinction [30] contributes to conflicting results. This also happens with most febrile mosquito-borne diseases in Africa, mainly clinically diagnosed (and treated) as malaria [12,85,86]. Because of this, it could be expected that dengue figures will increase in the future thanks to an increased investment in dengue and other NTD research and diagnosis, thus increasing their prevalence, while malaria prevalence rates may decrease. These changes are not only because of the impact of CC but also due to other variables, such as optimized healthcare facilities and, thus, optimized diagnosis [12].
Worldwide, chikungunya and zika have also seen a recent expansion. While previously confined to Africa, chikungunya outbreaks are now being reported in India and Indic Ocean islands, as well as Europe and the Americas [79]. It seems that as a consequence of drought, chikungunya has risen on the East African coasts [87,88]. The Atlantic and Indian Ocean coasts, as well as an area spreading from West Africa to South Sudan, have been identified as suitable regions for chikungunya spread under future climate change scenarios [89]. The problem is that most of these studies regret the lack of entomological information [25,90]. For example, Aedes albopictus has only been reported in some parts of West Africa, Madagascar and South Africa [90]. Zika, which is also widely underdiagnosed, has been reported in Asia, the Americas and the Pacific region recently [91]. According to our review, South Sudan is at risk, although differing virulence among lineages has to be taken into account. Furthermore, lacking historical data on the virus’ distribution contributes to unspecific projections [26,90].
While changes in RVF distribution in Senegal seem not to be influenced by CC [21,31,38], the epidemiology of this virus is largely altered as a result of a warming climate in Tanzania [31]. Projected hotspots for RVF include regions in Eastern Africa, especially in Kenya, Tanzania, Uganda, Rwanda and Burundi [92]. Although the relationship between CC and the ENSO phenomenon raises controversy [11], major RVF outbreaks in Kenia, Somalia and Tanzania in the late 1990 s after flooding due to ENSO have been reported [4]. Moreover, changes in rainfall due to abnormal El Niño-Southern Oscillation (ENSO) and other oscillations in rainfall and temperature or extreme weather events have been described in the context of CC and thus increasing RVF prevalence in Senegal. In addition, rainfall also plays an important role in MBD dynamics, both in parasite and vectoral densities [11]. Just as a matter of interest, Ehrenkranz et al. considered that the 10 Biblical plagues were RVF outbreaks which were triggered by aberrant ENSO which led to climate warming on the Mediterranean coast [93]. As with RVF, WNV also needs to be studied taking into account all the possible vector species. Besides, bird migration has been described as a focal point for WNV epidemics, i.e. birds act as a host for the virus, which might then spread to previously naïve regions when the birds fly to warmer breeding sites during the European winter [94].
Limitations and conclusions
First, we performed a search that was bound to certain inclusion criteria. Second, publication bias was another possible limitation, since we do not know whether some studies with contrary results regarding the topic of CC and MBD have not been published. In addition, selection bias might also affect our study. To decrease the risk of not selecting important studies, the selection process was done independently by two reviewers. All these bias are major limitations of systematic reviews [95]. Finally, the African continent and all African countries were included as terms search. As a consequence, some papers assessing the impact of CC on MBD from a worldwide perspective might have gone missing. To handle this limitation, we broaden the search to enrich the discussion section.
Regarding the principal selected papers limitations, we observed that main findings (of modeling approaches) were depending on the climate data set and time period used. All models using WorldClim as a data source predicted a positive impact of CC on the analyzed MBD [25,26,31,32,35,36]. Therefore, it would be recommendable to homogenize study methods and data sources or, at least, compare the results of studies that use the same methods and data by meta-analysis. Moreover, key MBD determinants were missing in most analyses probably due to the complexity of the applied mathematical approaches. Moreover, CC is just one part of an overall mechanism that is changing the epidemiology of MBD. Further studies are necessary to clarify cross-cutting issues on the impact of CC on MBD, as well as the impact of other factors like land cover change or socio-economic factors. A nation’s gross domestic product (GDP) is one of the many factors that determine a higher or lower risk of acquiring these diseases. If GDP is high, the adverse effects of CC can be counteracted more easily [96]. Since low-income countries are among the most affected by CC [10,12,97], we believe that the effects on MBD could be larger than in other contexts. Besides, pathogens and their natural habitat, as well as vectors, need to be identified and characterized and longitudinal monitoring programs need to be established to better describe diseases´ epidemiology on humans, hosts and vectors. Under this paradigm, a global health approach that encompasses qualitative and quantitative studies is strongly recommendable to disentangle the link between CC and MBD epidemiology in Africa. These research gaps may also be influenced by the distribution and preferences of the international collaborations, which may affect the representation of countries in African health research [57]. In particular, further investigation is necessary to fill in the gaps in those regions where the relationship between climate change and MBD dynamics has not been elucidated so far, e.g. Central, South and Northern Africa.
In conclusion, the problems resulting as a consequence of CC and MBD transcend national borders and thus have a global impact. Therefore, health must be improved worldwide, disparities on all levels need to be reduced, and health threats have to be addressed by all countries, especially since mosquitoes know no borders. The implementation of these measurements also needs to be evaluated by applied research. Finally, even in the absence of strong evidence, the authors believe that it cannot be waited for science to approve something that needs to be addressed now to avoid greater effects in the future.
Acknowledgments
The corresponding author’s affiliation center belongs to the ISCIII-Sub. Gral. Redes-Network Biomedical Research on Tropical Diseases (RICET in Spanish) grant RD16CIII/0003/0001, RD16/0027/0020, RD16CIII/0003/0001 and the European Regional Development Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors would also like to thank Laura Díez-Izquierdo and Edrit Franquiz for their valuable inputs and review.
Funding Statement
The authors received no specific funding for this work.
Disclosure statement
The authors declare they have no actual or potential competing financial interests.
References
- [1].IPCC (Intergovernmental Panel on Climate Change) . 2001. The scientific basis. [cited 2019 December26]. Available from: https://www.ipcc.ch/report/ar3/wg1/
- [2].WMO (World Meteorological Organization) . 2016. FAQs—climate. [cited 2019 December26]. Available from: https://public.wmo.int/en/about-us/FAQs/faqs-climate
- [3].Ingeoexpert . Causas del cambio climático y el calentamiento global; 2017. [cited 2019 December26]. Available from: https://ingeoexpert.com/blog/2017/12/07/causas-cambio-climatico/
- [4].IPCC (Intergovernmental Panel on Climate Change) . Climate Change 2014, Working Group II: Impacts, Adaptation and Vulnerability, 22.2.1; 2014.
- [5].UNO (United Nations Organization) . Climate change. United nations sustainable development; 2019. [cited 2019 December26]. Available from: https://www.un.org/sustainabledevelopment/climate-change/
- [6].WHO (World Health Organization . 2019a. Climate change and health. [cited 2019 December26]. Available from: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health
- [7].UNO (United Nations Organization) . United nations fact sheet on climate change - Africa is particularly vulnerable to the expected impacts of global warming; 2006. [cited 2020 April28]. Available from: https://unfccc.int/files/press/backgrounders/application/pdf/factsheet_africa.pdf
- [8].WRI (World Resources Institute) . 4 charts explain greenhouse gas emissions by countries and sectors; 2020. [cited 2020 April28]. Available from: https://www.wri.org/blog/2020/02/greenhouse-gas-emissions-by-country-sector
- [9].Costello A, Abbas M, Allen A, et al. Managing the health effects of climate change. Lancet. 2009;373(9676):1693–1733. [DOI] [PubMed] [Google Scholar]
- [10].King AD, Harrington LJ.. The inequality of climate change from 1.5 to 2°C of global warming. Geophys Res Lett. 2018;45(10):5030–5033. [Google Scholar]
- [11].Tourre YM, Lacaux JP, Vignolles C, et al. Climate impacts on environmental risks evaluated from space: A conceptual approach to the case of Rift Valley Fever in Senegal. Glob Health Action. 2009;2(1):2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Franklinos LHV, Jones KE, Redding DW, et al. The effect of global change on mosquito-borne disease. The Lancet. Infect Dis (Auckl). 2019;19(9):e302–e312. [DOI] [PubMed] [Google Scholar]
- [13].Omodior O, Luetke MC, Nelson EJ.. Mosquito-borne infectious disease, risk-perceptions, and personal protective behavior among U.S. international travelers. Prev Med Rep. 2018;12:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].WHO (World Health Organization) . World malaria report 2019b. Geneva, Switzerland: World Health Organization; 2019b. [Google Scholar]
- [15].Simo FBN, Bigna JJ, Well EA, et al. Chikungunya virus infection prevalence in Africa: a contemporaneous systematic review and meta-analysis. Public Health. 2019. January;166:79–88. Epub 2018 Nov 21. [DOI] [PubMed] [Google Scholar]
- [16].WHO (World Health Organization) . 2019c. Zika epidemiology update. [cited 2020 April28]. Available from: https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf?ua=1
- [17].Blanford JI, Blanford S, Crane RG, et al. Implications of temperature variation for malaria parasite development across Africa. Sci Rep. 2013;3(1):1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Paaijmans KP, Read AF, Thomas MB.. Understanding the link between malaria risk and climate. Proc Nat Acad Sci. 2009;106(33):13844–13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alonso D, Bouma MJ, Pascual M. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proc R Soc B. 2011;278(1712):1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pascual M, Ahumada JA, Chaves LF, et al. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Nat Acad Sci. 2006;103(15):5829–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thomas CJ, Davies G, Dunn CE. Mixed picture for changes in stable malaria distribution with future climate in Africa. Trends Parasitol. 2004;20(5):216–220. [DOI] [PubMed] [Google Scholar]
- [22].Moher D, Shamseer L, Clarke M, et al., PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cochrane . 2020. Assessing risk of bias in included studies: introduction. [cited 2020 April28]. Available from: https://handbook-5-1.cochrane.org/chapter_8/8_1_introduction.htm
- [24].Bomblies A, Eltahir EAB. Assessment of the impact of climate shifts on malaria transmission in the Sahel. EcoHealth. 2009;6(3):426–437. [DOI] [PubMed] [Google Scholar]
- [25].Campbell LP, Luther C, Moo-Llanes D, et al. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc B. 2015;370(1665):20140135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carlson CJ, Dougherty ER, Getz W. An ecological assessment of the pandemic threat of Zika Virus. PLoS Negl Trop Dis. 2016;10(8):e0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ermert V, Fink AH, Morse AP, et al. The impact of regional climate change on malaria risk due to greenhouse forcing and land-use changes in tropical Africa. Environ Health Perspect. 2012;120(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gething PW, Smith DL, Patil AP, et al. Climate change and the global malaria recession. Nature. 2010;465(7296):342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khormi HM, Kumar L. Climate change and the potential global distribution of Aedes aegypti: spatial modelling using geographical information system and CLIMEX. Geospat Health. 2014;8(2):405. [DOI] [PubMed] [Google Scholar]
- [30].Liu-Helmersson J, Stenlund H, Wilder-Smith A, et al. Vectorial capacity of aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS ONE. 2014;9(3):e89783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mweya CN, Mboera LEG, Kimera SI. Climate influence on emerging risk areas for rift valley fever epidemics in Tanzania. Am J Trop Med Hyg. 2017;97(1):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Parham PE, Michael E. Modelling climate change and malaria transmission. Adv Exp Med Biol. 2010a;673:184–199. [DOI] [PubMed] [Google Scholar]
- [33].Parham PE, Michael E. Modeling the effects of weather and climate change on malaria transmission. Environ Health Perspect. 2010b;118(5):620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Peterson AT. Shifting suitability for malaria vectors across Africa with warming climates. BMC Infect Dis. 2009;9(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ryan SJ, McNally A, Johnson LR, et al. Mapping physiological suitability limits for malaria in Africa under climate change. Vector-borne Zoonotic Dis. 2015;15(12):718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Samy AM, Elaagip AH, Kenawy MA, et al. Climate change influences on the global potential distribution of the mosquito culex quinquefasciatus, vector of West Nile Virus and Lymphatic Filariasis. Plos One. 2016;11(10):e0163863. doi:10.1371/journal.pone.0163863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Siraj AS, Santos-Vega M, Bouma MJ, et al. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science. 2014;343(6175):1154–1158. [DOI] [PubMed] [Google Scholar]
- [38].Talla C, Diallo D, Dia I, et al. Modelling hotspots of the two dominant Rift Valley fever vectors (Aedes vexans and Culex poicilipes) in Barkédji, Sénégal. Parasit Vectors. 2016;9(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tonnang HE, Kangalawe RY, Yanda PZ. Predicting and mapping malaria under climate change scenarios: the potential redistribution of malaria vectors in Africa. Malar J. 2010;111:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tonnang HE, Tchouassi DP, Juarez HS, et al. Zoom in at African country level: potential climate induced changes in areas of suitability for survival of malaria vectors. Int J Health Geogr. 2014;13(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou G, Minakawa N, Githeko AK, et al. Association between climate variability and malaria epidemics in the East African highlands. Proc Nat Acad Sci. 2004;101(8):2375–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chaves LF, Hashizume M, Satake A, et al. Regime shifts and heterogeneous trends in malaria time series from Western Kenya Highlands. Parasitology. 2012;139(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Uejio CK, Kemp A, Comrie AC. Climatic controls on west nile virus and sindbis virus transmission and outbreaks in South Africa. Vector-borne Zoonotic Dis. 2012;12(2):117–125. [DOI] [PubMed] [Google Scholar]
- [44].Murdock CC, Sternberg ED, Thomas MB. Malaria transmission potential could be reduced with current and future climate change. Sci Rep. 2016;6(1):27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Paaijmans KP, Imbahale SS, Thomas MB, et al. Relevant microclimate for determining the development rate of malaria mosquitoes and possible implications of climate change. Malar J. 2010;9(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rogers DJ. Dengue: recent past and future threats. Philos Trans R Soc B. 2015;370(1665):20130562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Caminade C, Kovats S, Rocklov J, et al. Impact of climate change on global malaria distribution. Proc Nat Acad Sci. 2014;111(9):3286–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].NASA (National Aeronautics and Space Adminstration) . The effects of climate change. Climate Change: Vital Signs of the Planet; 2019. [cited 2019 December27]. Available from: https://climate.nasa.gov/effects
- [49].Rogers DJ, Randolph SE. Climate change and vector-borne diseases. Adv Parasitol. 2006;62:345–381. [DOI] [PubMed] [Google Scholar]
- [50].IPCC (Intergovernmental Panel on Climate Change) . Climate Change 2007, Working Group II: Impacts, Adaptation and Vulnerability, Executive Summary; 2007.
- [51].World Bank . Turn down the heat: climate extremes, regional impacts, and the case for resilience. A report for the World Bank by the potsdam institute for climate impact research and climate analytics. Washington, DC: World Bank; 2013. [Google Scholar]
- [52].James R, Washington R. Changes in African temperature and precipitation associated with degrees of global warming. Clim Change. 2012;117(4):859–872. [Google Scholar]
- [53].WHO (World Health Organization) . World malaria report 2018; 2018. [cited 2019 December26]. Available from: https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1
- [54].WHO (World Health Organization) . Neglected diseases; 2019d. [cited 2019 December27]. Available from: http://www.who.int/neglected_diseases/diseases/en/
- [55].Head MG, Goss S, Gelister Y, et al. Global funding trends for malaria research in sub-Saharan Africa: A systematic analysis. Lancet Global Health. 2017;5(8):e772–e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McPake B. Inequalities in investment in malaria research in sub-Saharan Africa: are they inequities? Lancet Global Health. 2017;5(8):e730–e731. [DOI] [PubMed] [Google Scholar]
- [57].Hedt-Gauthier BL, Jeufack HM, Neufeld NH, et al. Stuck in the middle: A systematic review of authorship in collaborative health research in Africa, 2014–2016. BMJ Glob Health. 2019;4(5):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].US EPA (United States Environmental Agency) . Climate change indicators: high and low temperatures [Reports and Assessments] 2016; 2016. [cited 2019 December26]. Available from: https://www.epa.gov/climate-indicators/climate-change-indicators-high-and-low-temperatures
- [59].Morin CW, Comrie AC. Regional and seasonal response of a West Nile virus vector to climate change. Proc Nat Acad Sci. 2013;110(39):15620–15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ngarakana-Gwasira ET, Bhunu CP, Mashonjowa E. Assessing the impact of temperature on malaria transmission dynamics. Afrika Matematika. 2014;4(25):1095–1112. [Google Scholar]
- [61].Parham PE, Waldock J, Christophides GK, et al. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philos Trans R Soc B. 2015;370:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kipruto EK, Ochieng AO, Anyona DN, et al. Effect of climatic variability on malaria trends in Baringo County, Kenya. Malar J. 2017;16(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].WHO (World Health Organization) . Malaria; 2019e. [cited 2019 December27]. Available from: https://www.who.int/ith/diseases/malaria/en/
- [64].Bødker R, Akida J, Shayo D, et al. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. J Med Entomol. 2003;40(5):706–717. [DOI] [PubMed] [Google Scholar]
- [65].Chappuis F, Loutan L. Malaria et altitude. Revue Médicale Suisse,1, 23002; 2003.
- [66].Macacu A, Bicout DJ. Effect of the epidemiological heterogeneity on the outbreak outcomes. Mathematical Biosciences and Engineering: MBE. 2017;14(3):735–754. [DOI] [PubMed] [Google Scholar]
- [67].Zimmer C, Yaesoubi R, Cohen T. A likelihood approach for real-time calibration of stochastic compartmental epidemic models. PLoS Comput Biol. 2017;13(1):e1005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Galactionova K, Smith TA, de Savigny D, et al. State of inequality in malaria intervention coverage in sub-Saharan African countries. BMC Med. 2017;15(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kivumbi GW, Nangendo F, Ndyabahika BR. Financial management systems under decentralization and their effect on malaria control in Uganda. Int J Health Plann Manage. 2004;19(Suppl 1):S117–131. [DOI] [PubMed] [Google Scholar]
- [70].Voice of Nigeria . Lack of funds contributes to challenges of Malaria elimination-NMEP; 2018. [cited 2019 December26]. Available from: https://www.von.gov.ng/lack-of-funds-contributes-to-challenges-of-malaria-elimination-nmep/
- [71].WHO (World Health Organization) . Malaria; 2019f. [cited 2019 December27]. Available from: http://www.who.int/malaria/en/
- [72].WHO (World Health Organization) . Global health risks: mortality and burden of disease attributable to selected major risks. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- [73].Loevinsohn ME. Climatic warming and increased malaria incidence in Rwanda. Lancet. 1994;343(8899):714–718. [DOI] [PubMed] [Google Scholar]
- [74].Chen H, Githeko AK, Zhou G, et al. New records of Anopheles arabiensis breeding on the Mount Kenya highlands indicate indigenous malaria transmission. Malar J. 2006;5(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Afrane YA, Lawson BW, AK G, et al. Effects of microclimatic changes caused by land use and land cover on duration of gonotrophic cycles of Anopheles gambiae (Diptera: culicidae) in western Kenya highlands. J Med Entomol. 2005;42(6):974–980. [DOI] [PubMed] [Google Scholar]
- [76].Manga L, Toto JC, Carnevale P. Malaria vectors and transmission in an area deforested for a new international airport in southern Cameroon. Ann Soc Belg Med Trop. 1995 Mar;75(1):43-9. [PubMed] [Google Scholar]
- [77].Hay SI, Cox J, Rogers DJ, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415(6874):905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Thomas CD, Franco AMA, Hill JK. Range retractions and extinction in the face of climate warming. Trends Ecol Evol. 2006;21(8):415–416. [DOI] [PubMed] [Google Scholar]
- [79].Rezza G. Dengue and chikungunya: long-distance spread and outbreaks in naïve areas. Pathog Glob Health. 2014;108(8):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].FSG . Political instability and malaria; 2011. [cited 2019 December26]. Available from: https://www.fsg.org/blog/political-instability-and-malaria
- [81].Soofi K, Khanjani N, Kamiabi F. The challenges of the malaria elimination program in the South East of Iran: a qualitative study. J Arthropod Borne Dis. 2019;13(1):94–103. [PMC free article] [PubMed] [Google Scholar]
- [82].Silvano J, Abreu C. [Dengue fever in Portuguese speaking countries: which epidemiological links may we set?]. Acta Medica Portuguesa. 2014;27(4):503–510. [PubMed] [Google Scholar]
- [83].Jaenisch T, Junghanss T, Wills B, et al. Dengue expansion in Africa—not recognized or not happening? Emerg Infect Dis. 2014;20(10). DOI: 10.3201/eid2010.140487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Amarasinghe A, Kuritsk JN, Letson GW, et al. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17(8):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chong SE, Mohamad Zaini RH, Suraiya S, et al. The dangers of accepting a single diagnosis: case report of concurrent Plasmodium knowlesi malaria and dengue infection. Malar J. 2017;16(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fagbami AH, Onoja AB. Dengue haemorrhagic fever: an emerging disease in Nigeria, West Africa. J Infect Public Health. 2018;11(6):757–762. [DOI] [PubMed] [Google Scholar]
- [87].Chretien JP, Anyamba A, Bedno SA, et al. Drought-associated chikungunya emergence along coastal East Africa. Am J Trop Med Hyg. 2007;76(3):405–407. PMID: 17360859. [PubMed] [Google Scholar]
- [88].Patz JA, Olson SH, Uejio CK, et al. Disease emergence from global climate and land use change. Med Clin North Am. 2008;92(6):1473–1491, xii. [DOI] [PubMed] [Google Scholar]
- [89].Tjaden NB, Suk JE, Fischer D, et al. Modelling the effects of global climate change on Chikungunya transmission in the 21st century. Sci Rep. 2017;7(3813). DOI: 10.1038/s41598-017-03566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kraemer MU, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].WHO (World Health Organization) . Zika virus; 2019g. [cited 2019 December26]. Available from: https://www.who.int/news-room/fact-sheets/detail/zika-virus
- [92].Taylor D, Hagenlocher M, Jones AE, et al. Environmental change and Rift Valley fever in eastern Africa: projecting beyond HEALTHY FUTURES. Geospat Health. 2016;11(s1):387. [DOI] [PubMed] [Google Scholar]
- [93].Ehrenkranz NJ, Sampson DA. Origin of the old testament plagues: explications and Implications. Yale J Biol Med. 2008;81(1):31–42. PMID: 18604309. [PMC free article] [PubMed] [Google Scholar]
- [94].Epstein PR. Climate change and human health. N Engl J Med. 2005;353(14):1433–1436. [DOI] [PubMed] [Google Scholar]
- [95].Delgado-Rodríguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med Intensiva. 2018;42(7):444–453. [DOI] [PubMed] [Google Scholar]
- [96].Aström C, Rocklöv J, Hales S, et al. Potential distribution of dengue fever under scenarios of climate change and economic development. EcoHealth. 2012;9(4):448–454. [DOI] [PubMed] [Google Scholar]
- [97].Harrington LJ, Frame DJ, Fischer EM, et al. Poorest countries experience earlier anthropogenic emergence of daily temperature extremes. Environ Res Lett. 2016;11(5). http://centaur.reading.ac.uk/65737/. [Google Scholar]


