ABSTRACT
Neglected tropical diseases affect over 1 billion people, and cause 170,000 deaths each year. They result in disability, stigma and disfigurement, and also push families into poverty. Tropical infections can involve the kidney, presenting as a wide variety of ways, varying from transient urinary abnormalities to severe acute kidney injury (AKI). It is important to assess renal function in patients with tropical infections for earlier detection of AKI, appropriate treatment and prevention of Chronic Kidney Disease (CKD) outcome in some of them. There was an exponential increase in research on new kidney biomarkers that were earlier and specific for renal damage but few in the scope of tropical infections. In this review, we focus on kidney biomarkers that are being studied in some of the most prevalent tropical infections such as visceral leishmaniasis, leptospirosis, malaria, schistosomiasis and leprosy. Further studies are needed to evaluate the usefulness of renal biomarkers in the early diagnosis of renal diseases associated with tropical infections.
KEYWORDS: Kidney biomarkers, neglected tropical diseases, acute kidney injury, chronic kidney disease, diagnosis
Introduction
Neglected tropical diseases affect over 1 billion people, and cause 170,000 deaths each year. They are most prevalent in the so-called tropical zone that traverse the globe from Central America, Mexico, parts of South America, the Caribbean, Africa, the Middle East to Southeast Asia and result in disability, stigma and disfigurement, and also push families into poverty [1,2]. Tropical infections are caused by a variety of bacteria, viruses and parasitic organisms across varying geographical regions and the most of them are acute, presenting as a febrile illness with involvement of multiple organ systems, including the kidney. It is can involve the glomerular, tubulointerstitial and vascular compartments of the kidney, presenting as a wide variety of ways, varying from transient urinary abnormalities to severe acute kidney injury (AKI) [2].
According to the World Health Organization (WHO), biomarker is any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease [3]. Another definition of biomarkers includes any measurement reflecting an interaction between a biological system and a potential hazard, which may be chemical, physical, or biological. The measured response may be functional and physiological, biochemical at the cellular level, or a molecular interaction [4].
Serum creatinine has been used as a biomarker of chronic kidney disease (CKD) and acute kidney injury for more than one century, but it is well known to be a late rising marker in the blood of patients, being these clinical conditions often diagnosed only when the disease is established. For this reason, there was an exponential increase in research on new kidney biomarkers that were earlier and specific for renal damage [5].
In this review, we focus on kidney biomarkers that are being studied in tropical infections. We discuss here the following tropical infections: visceral leishmaniasis, leptospirosis, malaria, schistosomiasis and leprosy; and the already studied kidney biomarkers in these diseases: neutrophil gelatinase associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), monocyte chemoattractant protein 1 (MCP-1), cystatin C, transcription factor nuclear factor kappa B (NF-κB) and microalbuminuria.
Kidney biomarkers in visceral leishmaniasis
Visceral Leishmaniasis (VL)-related nephropathy is known both in humans and animals. However, in humans, most studies are based on very few cases, which showed different manifestations such as acute proliferative glomerulonephritis, collapsing focal segmental glomerulosclerosis, acute interstitial nephritis, tubular cell necrosis, and tubulitis, as well as acute kidney injury [6]. AKI is a frequent manifestation in (up to 46%), being related with amphotericin B use, secondary infections, hemodynamic abnormalities and the parasitic infection itself [7].
Meneses et al determined serum and urinary NGAL (sNGAL and uNGAL), urinary KIM-1 (uKIM-1) and urinary MCP-1 (uMCP-1) levels in 50 VL hospitalized patients. The AKI development was observed in 23 patients (46%), and three of them had AKI stage 2 (6%), whereas 20 cases had AKI stage 1 (40%), defined by the Kidney Disease Improving Global Outcome (KDIGO) criteria. The AKI group had significant longer hospital stay and higher levels of sNGAL, uNGAL, uKIM-1 and uMCP-1. Overall, sNGAL, uKIM-1 and uMCP-1 showed correlations with important clinical renal abnormalities, such as proteinuria, albuminuria, serum creatinine and glomerular filtration rate (GFR). In a logistic regression model, only sNGAL levels at admission showed an early association with AKI development. Therefore, sNGAL levels showed good sensitivity and specificity to predict AKI and it is a potential candidate to be included in the clinical care of such patients [8].
In a prospective study from the same group, the association between renal abnormalities and inflammation biomarkers in VL (uMCP-1 and urinary malondialdehyde-uMDA) was investigated in 16 VL adult patients evaluated before treatment compared with a group of 13 healthy volunteers and 5 VL patients evaluated after treatment. Urinary MCP-1 as well as uMDA showed significant differences between VL patients and control [9]. Urinary MCP-1 is a biomarker that has been associated with kidney damage and inflammation in chronic and acute diseases [10] but there are few data in the literature that report the association between infectious and parasitic diseases and MCP-1. Urinary MCP-1 and MDA was elevated in patients with VL, which may suggest inflammation and incipient renal damage, although other classical markers, such as creatinine, was not changed [9].
Although the aim was not to evaluate the role of uNGAL in visceral leishmaniasis-associated AKI, another Brazilian study evaluated the role of uNGAL in the early diagnosis of drug nephrotoxicity, in the case of amphotericin B-induced AKI, most patients received amphotericin B (AmB) predominantly for the treatment of leishmaniasis (22 of 24 patients, five patients had visceral and 17 were cutaneous leishmaniasis). Twelve patients received AmB deoxycholate and 12 received liposomal AmB. AKI was also defined according to KDIGO criterion. Determination of the uNGAL level was able to significantly shorten the time to detection of AmB-induced AKI, even compared to the use of the most sensitive serum creatinine (sCr)-based criteria. The diagnostic performance of the uNGAL level against the sCr-based criterion was moderate when looking at the entire group but excellent in the AmB deoxycholate subgroup. Finally, uNGAL levels were higher in recipients of AmB deoxycholate than in those of liposomal AmB [11].
Aiming at reducing patient morbimortality, NGAL levels as biomarkers can be used in clinical practice to improve clinical management strategies and prevent the immediate exposure to additional nephrotoxic drugs in hospitalized VL patients with a high risk of AKI development [8].
Cystatin C, used in clinical practice some parts of the world, has advantages in relation to creatinine as a marker of renal function because is formed at a constant rate and freely filtered by the healthy kidney and is not affected by factors such as muscle mass, nutrition, age and sex, factors that have been shown to affect creatinine. In contrast to creatinine that requires a fall in GFR of > 50% before the levels start to increase, cystatin C seems to be particularly useful in cases with minor impairments of GFR [12].
To our knowledge, only one study evaluated renal function in VL patients by cystatin measurement. Renal function was studied in visceral leishmaniasis (VL) and post-kala-azar dermal leishmaniasis (PKDL) by means of the specific marker cystatin C and related to circulating immune complexes and cytokine production. Cystatin C was measured in plasma in subjects with VL and PKDL and in control subjects (40, 17 and 22, respectively). The levels in VL were significantly raised compared with subjects with PKDL and also compared with healthy subjects. In VL, cystatin C levels were positively correlated to circulating immune complexes and production of granulocyte-macrophage colony stimulating factor (GM-CSF), a pro-inflammatory cytokine. Thus, cystatin C appears to be a superior marker of glomerular function in subjects with VL and immune complex deposition and GM-CSF are two functions, which most likely are causally involved in the mechanisms leading to glomerular dysfunction in this disease [6].
Another Sudanese study assessed the prevalence of renal injury in a cohort of 88 parasitologically confirmed VL patients and evaluate it according to the presence of microalbuminuria and urinary retinol binding protein (uRBP). Microalbuminuria is an early marker of glomerulonephritis. On the other hand, tubulointerstitial nephritis and proximal tubulopathies are associated with excretion of uRBP, a low molecular weight protein that is completely reabsorbed by the kidneys. All of the study patients revealed within normal range serum urea and creatinine levels. The routine urinalysis showed no albuminuria or increased pus cells. However, 35/88 (40%) of the study VL patients had microalbuminuria. Urinary RBP could not be detected in any of the patients. The authors suggest that glomerular involvement is the main renal damage in patients with VL [13].
Kidney biomarkers in malaria
Malaria is a major public health problem in tropical countries, with an estimated 228 million cases and 405,000 deaths worldwide by 2018 [14]. Renal involvement may be seen in infections due to bites of Plasmodium falciparum, P. vivax and P. malariae [15] however is more common in malaria caused by P. falciparum (62.5%) than in P. vivax infection (28.6%) [2]. Malaria was the first parasitic infection to be clearly associated with glomerular diseases in tropical areas [16]. Severe malaria can cause disease in glomeruli, tubules and in the interstitial region. Kidney disease in malaria is primarily due to erythrocyte abnormalities. AKI is a known complication of malaria and can occur in around 40% of patients with severe disease by P. falciparum in endemic regions, contributing to high mortality rate, around 75% of cases [17].
A study conducted in a general hospital, located in Rotterdam, The Netherlands evaluated the predictive performance of NGAL and KIM-1 for AKI in 39 travelers with imported P. falciparum infection. AKI was defined using the KDIGO criteria. Of 39 of these patients, six had AKI. Serum NGAL and uNGAL were significantly higher in the AKI group. Five out of six patients with AKI presented with elevated sNGAL and uNGAL was elevated in all six. In contrast, only one of the AKI patients showed elevated uKIM-1 at presentation. All patients that were classified as KDIGO stage 3, either at presentation or later during admission, already showed elevated concentrations of both sNGAL and uNGAL at presentation. The authors concluded that NGAL and KIM-1 have a good diagnostic performance for AKI. Particularly, uNGAL was found to have an excellent predictive performance [18].
Bangladeshi study evaluated the ability of AKI biomarkers included uNGAL to predict the later requirement for renal replacement therapy (RRT) in 163 patients with severe falciparum malaria. The measurement of plasma creatinine on admission in these patients was able to predict the later requirement for RRT as accurately as the more complicated and expensive tests used to diagnose AKI. Creatinine is safe and relatively inexpensive. To the authors, there appears little to recommend the use uNGAL in the resource poor settings where most patients with severe malaria will be managed [19].
The relationship between plasma soluble urokinase–type plasminogen activator receptor (suPAR), as a proxy-measure of mononuclear cell immune activation, and the plasma concentration of Plasmodium falciparum histidine-rich protein 2 (PfHRP2), as a measure of sequestered parasite burden, with AKI in 137 adult patients with falciparum malaria was assessed in another Bangladeshi study. Severity of kidney dysfunction and damage was measured quantitatively by creatinine clearance and uNGAL, respectively. Admission plasma suPAR and PfHRP2 were strongly and independently associated with AKI, and a later requirement for RRT. Plasma PfHRP2 independently correlated with uNGAL, and a requirement for RRT [20].
Another kidney biomarker already studied in malaria is the cystatin C. A retrospective study of stored sera and patient files assessment of renal function by serum concentration of creatinine and cystatin C and comparison of the results from both. Were included 108 adult patients with falciparum malaria. Elevated cystatin C was more frequent than elevated creatinine. Patients older than 50 years developed renal dysfunction more often than younger ones. Results from cystatin C and creatinine were concordant in 58% and contradictory in 41% of cases. Four patients had elevated creatinine but normal cystatin C levels, hence 58% of patients showed elevation of at least one indicator of GFR. According to the authors cystatin C allows a more accurate monitoring of renal function than creatinine in malaria [21]. Burchard et al conducted a study using cystatin C to estimate the frequency of renal dysfunction in 78 children with uncomplicated Plasmodium falciparum malaria and compared with a group of 23 children who had fever but appeared aparasitaemic or had very light parasitaemias (fever group). Elevated plasma concentrations of cystatin C were observed in 13 (17%) of the 78 children in the malaria group but not in any child in the fever group, indicating subclinical impairment of renal function. However, the mean plasma concentration of cystatin C in the malaria group was not significantly different from that in the fever group. There was no significant association between cystatin C concentration and level of parasitemia [22].
Acute kidney injury (AKI) is a well recognized complication of severe malaria in adults, but the incidence and clinical importance of AKI in pediatric severe malaria (SM) is not well documented. Recent study evaluated 108 children aged 1 to 10 years with severe malaria. KDIGO-defined AKI occurred in 45.5% of children. Were evaluated biomarkers of kidney function (creatinine, BUN and cystatin C) in children with or without AKI. Admission BUN and cystatin C were significantly higher in children with AKI compared with those without with levels increasing across stages of AKI. Besides that cystatin C was positively correlated over hospitalization, with the strongest associations observed in children with stage 3 AKI. Admission levels of cystatin C and BUN were associated with all-cause 14-day and 6-month mortality. Elevated cystatin C is predictor of death, and it was better than creatinine at discriminating between survivors and nonsurvivors children with severe malaria. Overall, CysC may be a more accurate measure of renal function, and a better prognostic marker than creatinine [23].
It has recently been revealed that activation of transcription factor nuclear factor kappa B (NF-κB) induces pro-inflammatory gene expression involved in the development of progressive renal inflammatory diseases. A thai study investigated whether urinary sediment NF-κB p65 can act as a biomarker for AKI in patients with P. falciparum malaria. Thirty-nine patients were evaluated and divided into 4 groups: P. vivax malaria, uncomplicated P. falciparum malária, complicated P. falciparum malaria without AKI and complicated P. falciparum malaria with AKI. Urinary sediment NF-κB p65 levels were significantly increased on the day of admission (day 0) and on day 7 post-treatment in complicated P. falciparum malaria patients with AKI, compared to the other groups. Furthermore, within the complicated P. falciparum malaria with AKI group, a significant positive correlation was found between NF-κB p65 in the urinary sediment cells and serum creatinine. Therefore, the urinary level of NF-κB p65 has a potential role as a disease biomarker in estimating damage to renal tubular epithelial cells and subsequent progression of AKI among complicated P. falciparum malaria patients [24].
Kidney biomarkers in leptospirosis
Leptospirosis has importance for public health and is considered as neglected zoonotic disease by the the World Health Organization [25]. Renal involvement in leptospirosis has been reviewed comprehensively in the past decade. In addition to inflammatory and immune-mediated mechanisms, leptospira can be directly nephrotoxic. The tubulointerstitial damage is also due in part to hemodynamic alterations, hypotension, hyperbilirubinemia and rhabdomyolysis. Although tubulointerstitial involvement is the most common cause of AKI, pre-renal AKI due to dehydration/hypotension is often seen. On rare occasions glomerular involvement due to inflammatory mediators and immune complex deposits may be seen [2]. Since AKI is one of the most serious complications of leptospirosis, AKI biomarkers are being studied more frequently in this disease than other tropical infections.
A multicenter study in Thailand assessed the role of NGAL as an early marker and an outcome predictor of leptospirosis associated AKI. KDIGO criteria were used for AKI diagnosis. Of the 113 cases with confirmed leptospirosis diagnosis, about one third (37.2%) developed AKI. AKI patients had significantly higher urine and plasma NGAL than non-AKI patients. However, neither uNGAL nor sNGAL were significantly different between patients recovering from versus those not recovery [26].
Some studies have suggested a role of endothelial activation in leptospiral pathogenesis [27,28]. In this sense, Libório et al performed a cohort study in an outbreak of leptospirosis among military personnel to evaluate the association between the presence and severity of renal lesions with biomarkers representative of glycocalyx and endothelial injury (intercellular adhesion molecule-1 [ICAM-1] and syndecan-1). AKI was diagnosed in 14 of 46 (30.4%) patients. Leptospirosis was associated with higher levels of ICAM-1 and syndecan-1. Patients with leptospirosis-associated AKI had increased level of syndecan-1 and ICAM-1 compared with leptospirosis patients with no AKI. Although not the main focus of the study, one point that also deserved consideration was the lack of correlation between NGAL and AKI, contrary to what has been shown by many other studies. Although there was no correlation between serum NGAL and the presence of AKI, there was a positive correlation between syndecan-1 and serum NGAL levels. A trend was observed in the positive correlation between ICAM-1 and serum NGAL levels. This association remained even after multivariate analysis including other AKI associated characteristics [29].
A retrospective study was performed in Philippines with 112 confirmed leptospirosis patients, 23 non-leptospirosis patients, and eight healthy controls. To identify urinary markers associated with Leptospira infection, was performed a proteomic analysis using two urinary samples, which revealed defensin in the leptospirosis sample. Because the major source of defensin may be leukocytes, the levels of urine defensin α1 (uDA1) and other known urinary proteins such as uNGAL and urine N-acetyl-β-D-glucosidase (uNAG), which are markers of AKI and tubular dysfunction, respectively, were measured in samples obtained from patients. The levels of uDA1/Cr, uNGAL/Cr, and uNAG/Cr were positive in 46%, 90%, and 80% of leptospirosis patients, and 69%, 70%, and 70% of non-leptospirosis patients, respectively. uDA1 and uNGAL levels were significantly associated with serum creatinine levels in leptospirosis patients, however, no correlation was found with uNAG levels. The authors suggest that elevation of uDA1 and uNGAL levels reflects various types of kidney damage and can be used to track kidney injury in leptospirosis patients [30].
Besides protean acute presentations of leptospirosis, the possibility of chronic human infection and asymptomatic colonization have been reported. However, renal involvement in those with leptospira chronic exposure remains undetermined [31,32].
To explore the relationship between leptospirosis and Mesoamerican Nephropathy (MeN), condition of unknown etiology and not associated with common causes of CKD, a recent study evaluated the prevalence of Leptospira seropositivity among 282 workers employed in a region where MeN is common in Nicaragua; estimated incident cases of leptospirosis among sugarcane workers within one harvest season; and determined whether Leptospira seropositivity was associated with biomarkers of kidney function and injury: NGAL, interleukin-18 (IL-18) and NAG. Leptospira seroprevalence differed among job categories and was highest among sugarcane cutters (59%). Biomarkers of kidney injury were significantly elevated among seropositive sugarcane workers at late-harvest when adjusting only for sex, age, and years worked. This association was attenuated by 15–22% when the model additionally included job category as a covariate, suggesting that job category is acting as a proxy for an unmeasured exposure that is causing the elevation in biomarkers of kidney injury. However, Leptospira infection may be acting as a kidney disease susceptibility factor, given the higher concentrations of biomarkers among seropositive workers [33].
The relationship between risk of developing CKD in leptospirosis seropositive patients was evaluated in another study using serum and urine NGAL, KIM-1 and MCP-1. The individuals with previous leptospira exposure disclosed a lower estimated GFR and a higher percentage of CKD, particularly at stage 3a-5, than those without leptospira exposure. The kidney biomarkers was tested in the 88 subjects, which were divided into three groups according to anti-leptospira antibody titers. Urinary KIM1 was higher in cases with antibody titer above 400 as compared with that in the other two groups. The level of uNGAL, sNGAL and sMCP-1 did not show a significant difference among three groups [34]. There are studies showing persistent tubular dysfunction in leptospirosis. Daher et al reported that, after leptospirosis AKI, renal function recovery is fast and complete after 6 months, except for urinary concentration capacity evidenced by the ratio urinary to plasma osmolality (U/Posm) that remained lower than normal [35].
Kidney biomarkers in schistosomiasis
Renal involvement in schistosomiasis is described mainly by glomerular involvement. The immunological nature of glomerular involvement in schistosomiasis is well established [36–38]. Schistosomal glomerulopathy is associated with the hepatosplenic form of the disease; however, it has also been observed in the hepatointestinal form [39]. Its incidence varies between 5–6%, increasing to 15% when considering only patients with the hepatosplenic form [40]. Only one study revealed that schistosomiasis infection may present with renal tubular dysfunction demonstrated by the loss of the ability of urinary acidification and concentration [41]
Few studies have evaluated the role of renal biomarkers in schistosomiasis. Hanemann et al investigated, for the first time, the role of uMCP-1 e microalbuminuria in patients with subclinical schistosomiasis. They compared microalbuminuria levels between treated and untreated patients infected by S. mansoni with a healthy control group and found no difference between them. However, although there was no difference among the three groups in relation to urinary albumin excretion rate, an increase in uMCP-1 was observed in patients with active or treated schistosomiasis, suggesting that infection can induce a chronic renal inflammatory status that is not resolved by the specific treatment of the offending agent [42].
A second study also demonstrated an association between uMCP-1 and schistosomiasis, but with hepatosplenic patients. Microalbuminuria >30 mg/day was found in 15% of patients and there was no correlation between microalbuminuria and GFR. Urinary MCP-1 levels were higher in schistosomiasis patients than in the control group. Moreover, was found a positive correlation between uMCP-1 levels and microalbuminuria and 24-h proteinuria in patients with schistosomiasis, suggesting a role of MCP-1 in the early detection of renal damage associated with schistosomiasis [41].
Kidney biomarkers in leprosy
Renal abnormalities in leprosy have been largely described in medical literature, but there are few studies evaluating renal function in these patients. Mitsuda and Ogawa were the first to report renal lesions related to this disease, through autopsy findings [43]. Leprosy patients are at risk for developing kidney disease. Identification of these patients is difficult in part due to lack of sensitivity of diagnostic tests used in clinical practice to detect incipient renal disease [44].
Meneses et al, evaluated uMCP-1 and oxidative stress through urinary malondialdehyde (uMDA) in 44 leprosy patients with no clinical kidney disease in comparison with a healthy control group and correlated them with traditional, but less sensitive markers of renal disease. In addition, they compared patients according to bacilli smear positive cases and polar leprosy clinical picture. Leprosy patients had higher urinary protein excretion, uMCP-1and uMDA levels than healthy controls. There was a positive correlation between uMCP-1 and bacteriological index in skin smears, urinary protein excretion, albumin excretion rate and uMDA. After adjusting for high-sensitivity C-reactive protein uMCP-1 remained correlated with albumin excretion rate and MDA levels, suggesting these patients are at increased risk of developing clinical kidney disease [44].
Microalbuminuria has been evaluated more frequently in leprosy. Kirsztajn et al have evaluated laboratory and clinical manifestations of renal disease in 96 patients with leprosy, looking for a sensitive and early marker for detection and possibly follow-up of nephropathy in these patients. Abnormal microalbuminuria was found in 15.8% of the cases. The authors also found a high frequency of hematuria and elevation of urinary beta 2-microglobulin in these patients still with normal serum creatinine [45]. Posteriorly, a cross-sectional study in 59 consecutive paucibacillary (PB) and multibacillary (MB) leprosy patients found only 8.5% of the patients presented microalbuminuria, with no difference between MB and PB patients. There was also no correlation between microalbuminuria and GFR. Microalbuminuria was not a predictor for GFR decrease and no association with low creatinine clearance was observed among patients [46]. Most recent study evaluated clinical and laboratory manifestations of renal disease in 189 patients with all forms of leprosy, before the onset of specific treatment with the multidrug therapy (MDT) regimen currently adopted worldwide. Microalbuminuria was detected in 9.6% of the cases and was more common in hypertensive patients the authors conclude that relevant renal disease in leprosy is currently uncommon, and this secondary involvement is self-limited once specific effective treatment is performed [47].
Figure 1 shows a summary of the main tropical infections and renal biomarkers that have already been investigated in these infections.
Figure 1.
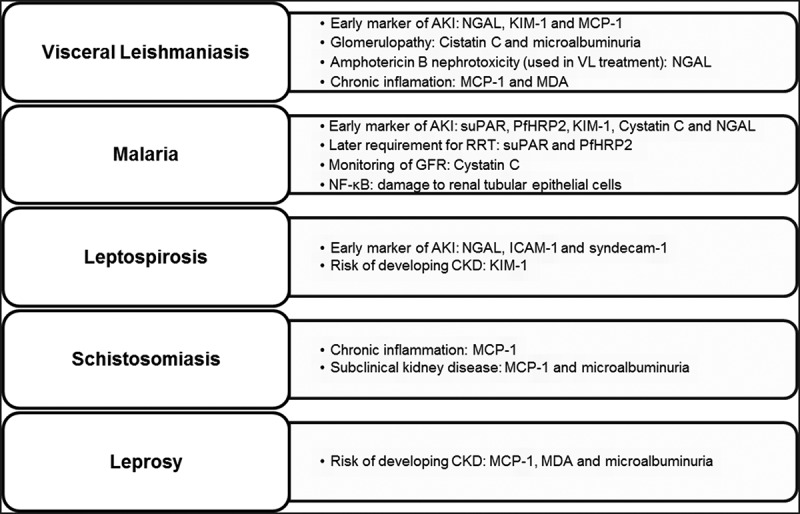
Main tropical infections and renal biomarkers that have already been investigated.
Conclusion
Renal involvement in tropical infections is frequent and occurs in a variety of ways, from asymptomatic cases to more severe forms of kidney disease. It is important to assess renal function in patients with tropical infections for earlier detection, appropriate treatment and prevention of CKD outcome in some of them. Serum creatinine, the most widely used marker for clinical renal function assessment, has limitations. New renal biomarkers have been increasingly studied in order to improve the detection of renal diseases, but few in the scope of tropical infections. Further studies are needed to evaluate the usefulness of renal biomarkers in the early diagnosis of renal diseases associated with tropical infections and its application in clinical practice.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Watts C. Neglected tropical diseases: A DFID perspective. Walson JL, ed. PLoS Negl Trop Dis. 2017;11(4):e0005492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kamath N, Iyengar A.. Infections and the kidney: a tale from the tropics. Pediatr Nephrol. 2018;33(8):1317–1326. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization . International Programme on Chemical Safety. Biomarkers in risk assessment: validity and validation. Geneva: WHO; 2001. Available from http://www.inchem.org/documents/ehc (Accessed in July 2020).
- [4].World Health Organization. International Programme on Chemical Safety. Biomarkers and risk assessment: concepts and principles. Vol. 155. Geneva: WHO; 1993. Available from http://www.inchem.org/documents/ehc/ehc/ehc155.htm (Accessed in April 2020). [Google Scholar]
- [5].McCullough PA, Bouchard J, Waikar SS, et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: executive summary from the tenth consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol. 2013;182:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Elshafie AI, Elghazali G, Rönnelid J, et al. C as a marker of immune complex-associated renal impairment in a sudanese population with visceral leishmaniasis. Am J Trop Med Hyg. 2006;75(5):864–868. [PubMed] [Google Scholar]
- [7].Oliveira MJC, Silva GB, Abreu KLS, et al. Risk factors for acute kidney injury in visceral leishmaniasis (Kala-Azar). Am J Trop Med Hyg. 2010;82(3):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meneses GC, De Francesco Daher E, da Silva Junior GB, et al. Visceral leishmaniasis-associated nephropathy in hospitalised Brazilian patients: new insights based on kidney injury biomarkers. Trop Med Int Heal. 2018;23(10):1046–1057. [DOI] [PubMed] [Google Scholar]
- [9].Oliveira MJC, Silva JGB, Sampaio AM, et al. Short report: preliminary study on tubuloglomerular dysfunction and evidence of renal inflammation in patients with Visceral leishmaniasis. Am J Trop Med Hyg. 2014;91(5):908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grandaliano G, Gesualdo L, Ranieri E, et al. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: A pathogenetic role in interstitial monocytes recruitment. J Am Soc Nephrol. 1996;7(6):906–913. [DOI] [PubMed] [Google Scholar]
- [11].Rocha PN, Macedo MN, Kobayashi CD, et al. Role of urine neutrophil gelatinase-associated lipocalin in the early diagnosis of amphotericin B-induced acute kidney injury. Antimicrob Agents Chemother. 2015;59(11):6913–6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34. [DOI] [PubMed] [Google Scholar]
- [13].Elnojomi NA, Musa AM, Younis BM, et al. Surrogate markers of subtle renal injury in patients with visceral leishmaniasis. Saudi J Kidney Dis Transpl. 2010;21(5):872–875. [PubMed] [Google Scholar]
- [14].World Health Organization . Malaria; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria (Accessed in July 2020).
- [15].Mishra SK, Das BS.. Malaria and acute kidney injury. Semin Nephrol. 2008;28(4):395–408. [DOI] [PubMed] [Google Scholar]
- [16].Elsheikha HM, Sheashaa HA. Epidemiology, pathophysiology, management and outcome of renal dysfunction associated with plasmodia infection. Parasitol Res. 2007;101(5):1183–1190. [DOI] [PubMed] [Google Scholar]
- [17].Da Silva Junior GB, Pinto JR, Barros EJG, et al. Kidney involvement in malaria: an update. Rev Inst Med Trop Sao Paulo. 2017;59(December2016):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Van Wolfswinkel ME, Koopmans LC, Hesselink DA, et al. Neutrophil gelatinase-associated lipocalin (NGAL) predicts the occurrence of malaria-induced acute kidney injury. Malar J. 2016;15(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hanson J, Hasan MM, Royakkers AA, et al. Laboratory prediction of the requirement for renal replacement in acute falciparum malaria. Malar J. 2011;10(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Plewes K, Royakkers AA, Hanson J, et al. Correlation of biomarkers for parasite burden and immune activation with acute kidney injury in severe falciparum malaria. Malar J. 2014;13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Günther A, Burchard GD, Slevogt H, et al. Renal dysfunction in falciparum - Malaria is detected more often when assessed by serum concentration of cystatin C instead of creatinine. Trop Med Int Heal. 2002;7(11):931–934. [DOI] [PubMed] [Google Scholar]
- [22].Burchard GD, Ehrhardt S, Mockenhaupt FP, et al. Renal dysfunction in children with uncomplicated, Plasmodium falciparum malaria in Tamale, Ghana. Ann Trop Med Parasitol. 2003;97(4):345–350. [DOI] [PubMed] [Google Scholar]
- [23].Conroy AL, Hawkes M, Elphinstone RE, et al. Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis. 2016;3(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Punsawad C, Viriyavejakul P. Nuclear factor kappa B in urine sediment: A useful indicator to detect acute kidney injury in Plasmodium falciparum malaria. Malar J. 2014;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].World Health Organization . Neglected tropical diseases; 2020. Available from: https://www.who.int/neglected_diseases/zoonoses/infections_more/en/ (Accessed in July 2020).
- [26].Srisawat N, Praditpornsilpa K, Patarakul K, et al. Neutrophil gelatinase associated lipocalin (NGAL) in leptospirosis acute kidney injury: a multicenter study in Thailand. PLoS One. 2015;10(12):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gómez RM, Vieira ML, Schattner M, et al. Putative outer membrane proteins of Leptospira interrogans stimulate human umbilical vein endothelial cells (HUVECS) and express during infection. Microb Pathog. 2008;45(56):315–322. [DOI] [PubMed] [Google Scholar]
- [28].Vieira ML, D’Atri LP, Schattner M, et al. A novel leptospiral protein increases ICAM-1 and E-selectin expression in human umbilical vein endothelial cells. FEMS Microbiol Lett. 2007;276(2):172–180. [DOI] [PubMed] [Google Scholar]
- [29].Libório AB, Braz MBM, Seguro AC, et al. Endothelial glycocalyx damage is associated with leptospirosis acute kidney injury. Am J Trop Med Hyg. 2015;92(3):611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chagan-Yasutan H, Chen Y, Lacuesta TL, et al. Urine levels of defensin α1 reflect kidney injury in leptospirosis patients. Int J Mol Sci. 2016;17(10):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nicolescu M, Andreescu N. May human leptospirosis develop as a chronic infection? Zentralblatt fur Bakteriol Mikrobiol und Hyg - Abt 1 Orig A. 1984;257(4):530. [PubMed] [Google Scholar]
- [32].Ganoza CA, Matthias MA, Saito M, et al. Asymptomatic renal colonization of humans in the Peruvian Amazon by Leptospira. PLoS Negl Trop Dis. 2010;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Riefkohl A, Ramírez-Rubio O, Laws RL, et al. Leptospira seropositivity as a risk factor for Mesoamerican nephropathy. Int J Occup Environ Health. 2017;23(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang HY, Hung CC, Liu SH, et al. Overlooked risk for chronic kidney disease after leptospiral infection: a population-based survey and epidemiological cohort evidence. PLoS Negl Trop Dis. 2015;9(10):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].De Francesco Daher E, Zanetta DMT, Abdulkader RCRM. Pattern of renal function recovery after leptospirosis acute renal failure. Nephron Clin Pract. 2004;98(1):c8–c14. [DOI] [PubMed] [Google Scholar]
- [36].el-Sherif AK, Befus D. Predominance of IgA deposits in glomeruli of Schistosoma mansoni infected mice. Clin Exp Immunol. 1988;71(1):39–44. [PMC free article] [PubMed] [Google Scholar]
- [37].Martinelli R, Rocha H. Nefrologia clínica: envolvimento glomerular na esquistossomose mansônica. J Bras Nefrol. 1996;18(3):279–282. [Google Scholar]
- [38].Barsoum R. The changing face of schistosomal glomerulopathy. Kidney Int. 2004;66(6):2472–2484. [DOI] [PubMed] [Google Scholar]
- [39].Abensur H, Nussenzveig I, Saldanha LB, et al. Nephrotic syndrome associated with hepatointestinal schistosomiasis. Rev Inst Med Trop Sao Paulo. 1992;34(4):273–276. [DOI] [PubMed] [Google Scholar]
- [40].Rodrigues VL, Otoni A, Voieta I, et al. Glomerulonephritis in schistosomiasis mansoni: a time to reappraise. Rev Soc Bras Med Trop. 2010;43(6):638–642. [DOI] [PubMed] [Google Scholar]
- [41].Duarte DB, Vanderlei LA, De Azevêdo Bispo RK, et al. Renal function in hepatosplenic schistosomiasis - an assessment of renal tubular disorders. PLoS One. 2014;9(12):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hanemann ALP, Libório AB, Daher EF, et al. Monocyte chemotactic protein-1 (MCP-1) in patients with chronic schistosomiasis mansoni: evidences of subclinical renal inflammation. PLoS One. 2013;8(11):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].MITSUDA K, OGAWA M. A study of one hundred and fifty autopsies on cases of leprosy. Int J Lepr. 1937;5:53–60. [Google Scholar]
- [44].Meneses GC, Libório AB, de Daher EF, et al. Urinary monocyte chemotactic protein-1 (MCP-1) in leprosy patients: increased risk for kidney damage. BMC Infect Dis. 2014;14(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kirsztajn GM, Nishida SK, Silva MS, et al. Renal abnormalities in leprosy. Nephron. 1993;65(3):381–384. [DOI] [PubMed] [Google Scholar]
- [46].Oliveira RA, Silva GB, Souza CJ, et al. Evaluation of renal function in leprosy: A study of 59 consecutive patients. Nephrol Dial Transplant. 2008;23(1):256–262. [DOI] [PubMed] [Google Scholar]
- [47].Polito MG, Moreira SR, Nishida SK, et al. It is time to review concepts on renal involvement in leprosy: pre- and post-treatment evaluation of 189 patients. Ren Fail. 2015;37(7):1171–1174. [DOI] [PubMed] [Google Scholar]


