Abstract
The effect of systemic corticosteroids on clinical outcomes in patients with coronavirus disease 2019 (COVID-19) remains controversial. While the use of corticosteroids raises concerns regarding delayed viral clearance, secondary infections, and long-term complications that can lead to increased mortality, corticosteroids have the potential to reduce mortality if used appropriately. Herein, we report good outcomes in two patients with COVID-19 who received systemic corticosteroids as adjunctive therapy. An 83-year-old man with hypertension and smoking history and a 62-year-old man with a drinking habit were transferred to our hospital with a diagnosis of COVID-19. The patients developed general malaise and loss of appetite with persistent high fever. Despite the prescription of antiviral drugs, their hypoxemia progressed rapidly. However, after the introduction of systemic corticosteroids, their symptoms improved as the fever decreased, and their hypoxemia gradually improved. These results suggest that some patients with COVID-19 may benefit from the appropriate use of systemic corticosteroids as adjunctive therapy.
Keywords: Acute respiratory distress syndrome, Coronavirus disease 2019, Corticosteroids, Methylprednisolone, Severe acute respiratory syndrome coronavirus 2
1. Introduction
Coronavirus disease 2019 (COVID-19) commonly does not produce symptoms or manifests with only mild fever and upper respiratory tract symptoms. While the disease often resolves spontaneously, in some cases, aggravation of pneumonia may lead to life-threatening acute respiratory distress syndrome (ARDS). Systemic administration of corticosteroids may have an inhibitory effect on lung injury associated with inflammation; however, various issues have been reported, including delayed viral clearance, secondary infections, and long-term complications, and no consensus has yet been reached regarding the effects of such a treatment on patients with COVID-19. In this communication, we report our experience with two cases of severe COVID-19 in which good clinical courses were achieved after systemic administration of methylprednisolone.
2. Case report
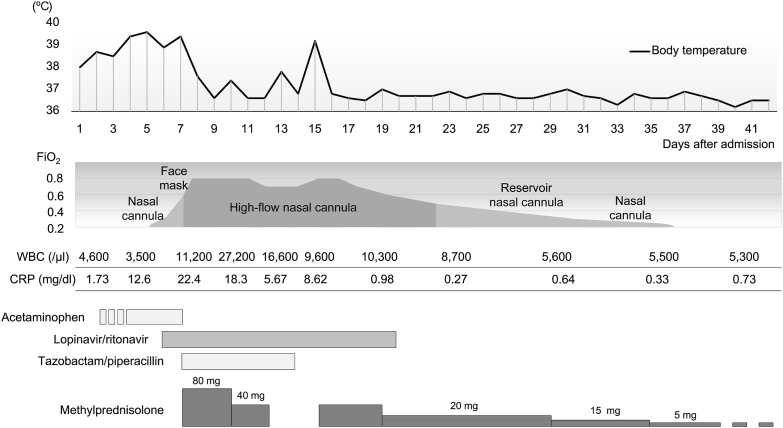
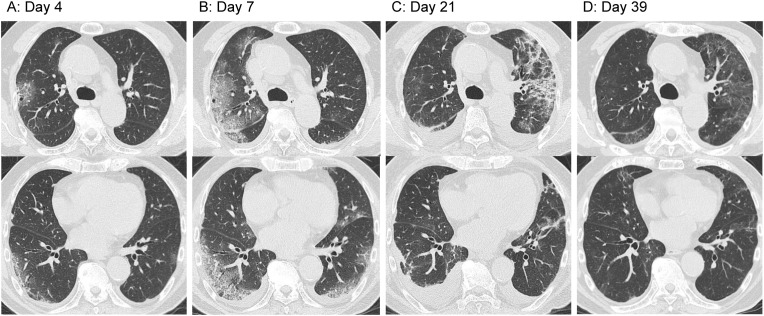
After clinical findings confirmed the presence of COVID-19 in some individuals among the passengers of a large cruise ship traveling around Asia, quarantine started at the port of Yokohama, Japan. Patient 1 was an 83-year-old man who had been taking oral calcium channel blockers for hypertension and had a 38 pack-years’ smoking history. The patient developed fever while on the ship and, therefore, was tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by polymerase chain reaction (PCR) of throat swab samples on the day after symptom onset. The result was positive; thus, the patient was transported to our hospital (hospital day 1). On admission, a body temperature of 38 °C and a respiratory rate of 18 breaths per minute, with an oxygen saturation of 97% at room air, were recorded. The patient was conscious and lucid, and auscultation of the chest showed no abnormal findings. Blood tests performed on admission showed a white blood cell count of 4600/μl and a C-reactive protein level of 1.73 mg/dl, indicating a mildly increased inflammatory response; however, liver and kidney functions showed no abnormal findings. Chest radiographs taken on admission showed clear areas with increased density in the right-middle and lower lung fields as well as in the left lower lung field. The clinical course after admission is shown in Fig. 1 . The patient was treated with oral acetaminophen, but fever exceeding 38 °C persisted and was later accompanied by cough and sputum. Computed tomography (CT) scans taken on hospital day 4 showed mottled ground-glass opacities (GGOs) in the lung periphery (Fig. 2 A). Upon approval by our hospital's ethics review board and obtaining consent from the patient, treatment using lopinavir/ritonavir (400 mg/100 mg) (one tablet per dose, taken twice daily for 14 days) was started on hospital day 6. Owing to the persistently high fever, the patient also developed general malaise, loss of appetite, and delirium. On hospital day 7, the GGOs increased in size (Fig. 2B) and hypoxemia progressed rapidly; therefore, high-flow nasal cannula oxygen therapy was started. As a low-dose corticosteroid therapy at early-phase ARDS, methylprednisolone was administered at a dose of 80 mg/day, and in consideration of the possibility of mixed infections, tazobactam/piperacillin treatment was started at a dose of 13.5 g/day. The fever resolved promptly, and there were improvements in general malaise, loss of appetite, and delirium. However, the hypoxemia persisted; therefore, high-concentration oxygen therapy was continued with a fraction of inspiratory oxygen of 0.8, and the use of mechanical ventilation was considered. Starting on hospital day 8, the patient's fever disappeared. As this may have been due to the effects of lopinavir/ritonavir, corticosteroid treatment was stopped on hospital day 13. The patient developed fever again, and chest radiographs showed a clear area with increased density in the left upper lung field; CT scans taken 5 days later showed consolidation (Fig. 2C). Based on the clinical course, ARDS and organizing pneumonia were suspected rather than complication by secondary infection; therefore, methylprednisolone 40 mg/day was started on hospital day 16. The fever resolved promptly, and the patient's respiratory status tended to improve. Supplemental oxygen treatment was terminated safely on hospital day 34. Corticosteroids were changed from intravenous methylprednisolone to oral prednisolone and terminated by reducing the doses gradually. On hospital day 19, nasopharyngeal swab samples showed negative results in SARS-CoV-2 PCR analysis. Rehabilitation allowed for gradual recovery of the decreased activities of daily living; findings confirmed that the opacities in both lungs had virtually disappeared (Fig. 2D), and the patient was therefore discharged on hospital day 42.
Fig. 1.
Clinical course of Patient 1 after admission. The FiO2 values during the use of nasal cannula, face mask, and reservoir nasal cannula are estimates. FiO2: fraction of inspiratory oxygen, WBC: white blood cell count, CRP: C-reactive protein.
Fig. 2.
Computed tomography (CT) scans of Patient 1. (A) Day 4. Mottled ground-glass opacities (GGOs) are visible at the lung periphery. (B) Day 7. Increased GGO dimensions. (C) Day 21. The GGO in the right lung has virtually disappeared but consolidation developed in the upper lobe of the left lung after discontinuation of methylprednisolone therapy. Pleural effusions caused by congestive heart failure have also developed. (D) Day 39. The opacities in both lungs have virtually disappeared.
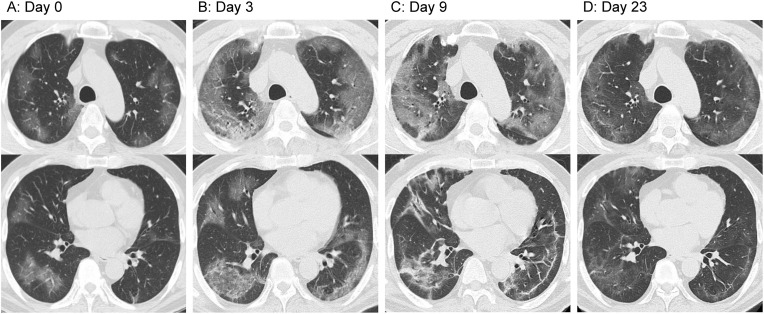
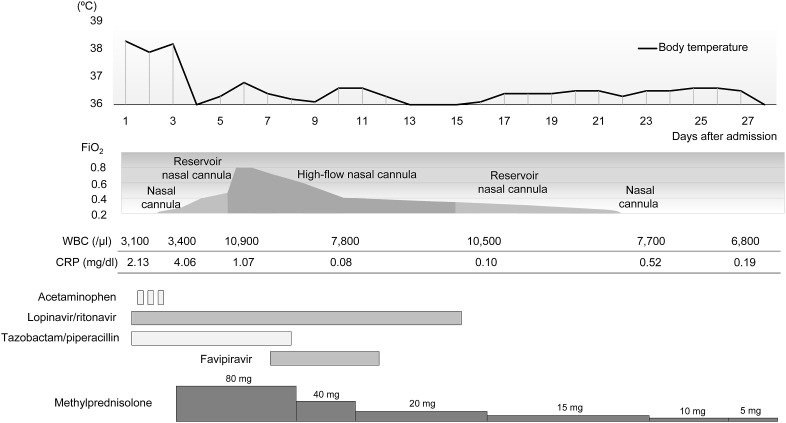
Patient 2 was a 62-year-old man with a history of early-stage gastric cancer and alcohol consumption. The patient had developed fever, general malaise, polyarticular pain, and diarrhea the day after returning from a 6-day business trip in a region of Japan where the number of confirmed COVID-19 cases was relatively high. As the symptoms showed no improvement for four days after onset, the patient consulted a previous physician. CT scans revealed GGOs disseminated mainly at the periphery of both lungs (Fig. 3 A); therefore, nasopharyngeal swab samples were subjected to a SARS-CoV-2 PCR test. The results were positive; thus, the patient was transported to our hospital six days after symptom onset (hospital day 1). The findings on admission included a body temperature of 38.3 °C, a respiratory rate of 24 breaths per minute, and an oxygen saturation of 94% at room air. The patient was conscious and lucid. Chest auscultation findings showed no abnormal findings. Blood tests on admission revealed a white blood cell count of 3100/μl and a C-reactive protein level of 2.13 mg/dl, indicating a mildly increased inflammatory response. Assessments of liver and kidney functions showed no abnormal findings; however, lactate dehydrogenase (417 U/l) and creatine kinase (800 U/l) concentrations were elevated. Chest radiographs at admission showed clear areas with increased density at the periphery of both lungs. The clinical course after admission is shown in Fig. 4 . Upon approval by the hospital ethics review board and obtaining consent from the patient, a 14-day treatment of oral lopinavir/ritonavir (400 mg/100 mg) was started at one tablet per dose, twice daily. In addition, considering mixed infection, tazobactam/piperacillin at 13.5 g/day was also started. Nonetheless, the patient's general malaise worsened and appetite declined. CT scans showed that the GGO in both lungs rapidly worsened (Fig. 3B); therefore, methylprednisolone 80 mg/day was started on hospital day 3. The fever resolved rapidly, and the patient's general malaise and appetite also improved; however, on hospital day 5, the hypoxemia progressed rapidly. Therefore, high-flow nasal cannula oxygen therapy was started. Based on our experience with Patient 1, corticosteroid therapy was terminated by decreasing the doses gradually. The patient's respiratory status started to improve on hospital day 6, and upon approval by our hospital's ethics review board and obtaining consent from the patient, 5-day oral favipiravir therapy (200 mg) was started on hospital day 7 according to the following regimen: eight tablets per dose twice daily on the first day and three tablets per dose twice daily from the second to fifth days. CT scans obtained on hospital day 9 showed a tendency toward GGO improvement accompanied by subpleural curvilinear shadows (Fig. 3C). On hospital day 21, oxygen administration was terminated without incident, and nasopharyngeal swab samples subjected to SARS-CoV-2 PCR testing on the same day showed negative results. After examinations confirmed the disappearance of virtually all abnormal findings in both lungs (Fig. 3D), the patient was discharged on hospital day 28. In both patients, there was no relapse of symptoms after the termination of the corticosteroid treatment.
Fig. 3.
Computed tomography (CT) scans of Patient 2. (A) Day 0. Mottled ground-glass opacities (GGOs) are visible at the lung periphery. (B) Day 3. Rapid exacerbation of the GGOs in both lungs. (C) Day 9. Tendency toward GGO improvement, accompanied by subpleural curvilinear shadows. (D) Day 23. The opacities in both lungs have virtually disappeared.
Fig. 4.
Clinical course of Patient 2 after admission. The FiO2 values during the use of nasal cannula and reservoir nasal cannula are estimates. FiO2: fraction of inspiratory oxygen, WBC: white blood cell count, CRP: C-reactive protein.
3. Discussion
Both cases reported in this study had developed severe pneumonia due to SARS-CoV-2. Patient 1 was treated with lopinavir/ritonavir in combination with methylprednisolone, while Patient 2 was treated with lopinavir/ritonavir in combination with favipiravir and methylprednisolone. In both cases, improvement was achieved without the use of advanced medical technology such as mechanical ventilation and extracorporeal membrane oxygenation. The COVID-19 pandemic has caused shortage of intensive care unit beds and mechanical ventilators necessary for critically ill patients in medical institutions. Given that there are countries and regions with poorly equipped healthcare systems, treatment strategies will be needed to minimize the disease's severity.
Thus far, no antiviral agent has been proven effective for COVID-19, although clinical trials of various drugs are currently being performed worldwide. Such drugs include lopinavir/ritonavir, which were originally developed as anti-human immunodeficiency viral agents, and favipiravir, which was developed as an anti-influenza viral agent. Lopinavir/ritonavir has been used in the treatment of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [1]; however, it was not effective in a randomized controlled trial performed on 199 patients with COVID-19 [2]. The results of clinical trials of favipiravir have not yet been published. Clinical trials are underway for each of the drugs, the results of which are awaited to evaluate the drugs’ effectiveness. However, we could not directly determine the effectiveness of the antiviral agents in the two cases described in our study.
Given the current absence of antiviral drugs with confirmed effectiveness, the question is whether respiratory and circulatory management through mechanical ventilation and extracorporeal membrane oxygenation is the only treatment option for COVID-19 patients with rapidly worsening conditions. In SARS and MERS patients, corticosteroids have often been administered in combination with other therapeutic agents; similarly, they have been administered to COVID-19 patients in the hope that they may have inhibitory effects on inflammation [3]. However, Russell et al. reported that corticosteroids should not be used in the treatment of lung injury and septic shock due to SARS-CoV-2 [4]. Furthermore, the WHO Interim Guidelines on the management of severe acute respiratory infection due to SARS-CoV-2 stated that routine corticosteroid administration should be avoided unless there are specific reasons such as septic shock or exacerbation of asthma or chronic obstructive pulmonary disease [5]. The recommendations were based on reports suggesting that corticosteroid treatment in patients with SARS, MERS, or influenza may delay viral clearance from the respiratory tract and blood, increase mortality, and lead to secondary infections or complications of mental illness or diabetes [[6], [7], [8]]. Yang et al. conducted a systematic review and meta-analysis of the effects of corticosteroid treatment on patients with coronavirus infection and found that corticosteroid treatment was associated with higher mortality, longer hospital stays, and a higher rate of bacterial infection [9]. However, Shang et al. pointed out that most reports published thus far on the management of COVID-19 with corticosteroids are observational studies; thus, mortality in the corticosteroid-treated groups was likely to be higher due to selection bias and confounding factors [10]. Previous reports have shown increased serum inflammatory cytokine levels in SARS and MERS patients; findings in COVID-19 patients have also confirmed increased proinflammatory cytokine levels, especially in patients requiring management in the intensive care unit. The serum levels of granulocyte colony-stimulating factor, interferon gamma-induced protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1A, and tumor necrosis factor-alpha were significantly higher in these patients, suggesting an association between a cytokine storm and disease severity [3]. Chen et al. previously reported a decreased mortality rate, no secondary infections or other complications, and decreased length of hospital stay with proper use of corticosteroids in SARS patients [11]. Furthermore, in their systematic review and meta-analysis, Siemieniuk et al. reported that corticosteroid therapy could reduce the mortality rate and the need for mechanical ventilation and could shorten the length of hospital stay in patients with community-acquired pneumonia [12]. In a previous retrospective cohort study of COVID-19 pneumonia patients, Wu et al. showed that even though the number of severe cases was higher in the methylprednisolone-treated group than in the untreated group, the mortality rate was lower in the treated group [13]. Experts from the Chinese Thoracic Society have issued a consensus statement that, although administration of corticosteroids for treating SARS-CoV-2 pneumonia requires careful consideration, in cases requiring corticosteroid administration, the doses need to be low or moderate (equivalent of 0.5–1 mg methylprednisolone/kg/day) and administered only over a short period (7 days or less) [14]. Based on these reports, we hypothesize that corticosteroid treatment may not be necessary in mild cases but may be considered in moderate to severe cases. Although this medication is not covered by insurance in Japan, it would be ethically acceptable to administer corticosteroids for patients with ARDS due to COVID-19 according to these reports and clinical practice guidelines for the management of ARDS in Japan [15].
In the two cases described here, persistently high fever was accompanied by fatigue and loss of appetite. In addition, the hypoxemia progressed rapidly, and mechanical ventilation was therefore considered. Informed consent was obtained for the administration of low-dose corticosteroids, which can cause side effects such as secondary infections or complications of mental illness or diabetes, but may contribute to symptomatic improvement by reducing excessive inflammation. After administration of methylprednisolone, the fever resolved immediately, and the fatigue and appetite improved. If corticosteroids had not been administered, more oxygen may have been required due to the high fever, and oxygenation may have been impossible to maintain. While corticosteroids themselves may not have a direct ameliorating effect on oxygenation, they appeared to be at least indirectly effective in maintaining oxygenation. However, caution is needed concerning mental illness as a complication of corticosteroid use and, as in Patient 1, careful assessment is needed when using corticosteroids in older adults with delirium. Regarding delayed viral clearance as a potential negative aspect of corticosteroid therapy, negative PCR test results were obtained on hospital day 20 for Patient 1 and on hospital day 21 for Patient 2. Previous reports have shown that SARS-CoV-2 viral genes can be detected in upper respiratory tract samples over a relatively long period after the disappearance of symptoms [16]. While viral clearance could have been achieved earlier if corticosteroids had not been administered in the two cases reported in this study, the most important goal in the management of COVID-19 is to reduce mortality. In the two cases described in our study, the administration of methylprednisolone was followed by improvement in symptoms such as fever, fatigue, and loss of appetite, and the patients' conditions improved without the development of fatal hypoxemia.
We reported our experiences with two cases of COVID-19 in which corticosteroids, used as adjunctive therapy, appeared to achieve a certain effect. No consensus has yet been reached regarding the administration of corticosteroids for the treatment of COVID-19; however, when used properly, they may be beneficial in some cases. In conclusion, well-designed randomized controlled trials are needed to evaluate the efficacy and safety of corticosteroid use in such cases.
Authors’ contributions
All authors meet the ICMJE authorship criteria. MK and YY treated both patients, and drafted the manuscript. SS participated in the treatment of the patient 2. TM and KS made intellectual contributions and helped in patient management and writing the manuscript. All authors critically reviewed the manuscript and approved the final version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The publication of this case report was approved by the institutional ethics review board (approval number: 2–12) after written informed consent was obtained from the patients.
Declaration of competing interest
None.
References
- 1.Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K., et al. Middle East respiratory syndrome. N Engl J Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (n-CoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected
- 6.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., et al. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 7.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. Epub 2006 Sep. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23:99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81:e13–e20. doi: 10.1016/j.jinf.2020.03.062. Epub 2020 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q., et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siemieniuk R.A., Meade M.O., Alonso-Coello P., Briel M., Evaniew N., Prasad M., et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- 13.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. Epub 2020 March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J.P., Hu Y., Du R.H., Chen Z.S., Jin Y., Zhou M., et al. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Zhonghua Jiehe He Huxi Zazhi. 2020;43:E007. doi: 10.3760/cma.j.issn.1001-0939.2020.0007. Epub 2020 Feb 8. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto S., Sanui M., Egi M., Ohshimo S., Shiotsuka J., Seo R., et al. The clinical practice guideline for the management of ARDS in Japan. J Intensive Care. 2017;5:50. doi: 10.1186/s40560-017-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]