Abstract
Autophagy is an evolutionary conserved catabolic process devoted to the removal of unnecessary and harmful cellular components. In its general form, autophagy governs cellular lifecycle through the formation of double membrane vesicles, termed autophagosomes, that enwrap and deliver unwanted intracellular components to lysosomes. In addition to this omniscient role, forms of selective autophagy, relying on specialized receptors for cargo recognition, exert fine-tuned control over cellular homeostasis. In this regard, xenophagy plays a pivotal role in restricting the replication of intracellular pathogens, thus acting as an ancient innate defense system against infections. Recently, selective autophagy of the endoplasmic reticulum (ER), more simply ER-phagy, has been uncovered as a critical mechanism governing ER network shape and function. Six ER-resident proteins have been characterized as ER-phagy receptors and their orchestrated function enables ER homeostasis and turnover overtime. Unfortunately, ER is also the preferred site for viral replication and several viruses hijack ER machinery for their needs. Thus, it is not surprising that some ER-phagy receptors can act to counteract viral replication and minimize the spread of infection throughout the organism. On the other hand, evolutionary pressure has armed pathogens with strategies to evade and subvert xenophagy and ER-phagy. Although ER-phagy biology is still in its infancy, the present review aims to summarize recent ER-phagy literature, with a special focus on its role in counteracting viral infections. Moreover, we aim to offer some hints for future targeted approaches to counteract host-pathogen interactions by modulating xenophagy and ER-phagy pathways.
Keywords: Autophagy, ER-phagy, Xenophagy, Virophagy, Virus
1. A sneak-peek into the complexity of macroautophagy
The term autophagy refers to a non-selective lysosome-dependent degradation process that cells normally exploit for intracellular housekeeping and macromolecule recycling. Specifically, autophagy ensures a baseline removal of damaged or proteotoxic intracellular structures (i.e. organelles and protein aggregates, respectively) as well as the availability of a ready-to-use pool of amino acids, lipids and other precursors that cells can direct towards biosynthetic pathways. Autophagic flux is cell specific and is mainly dictated by the needs of each cell and tissue [1]. Indeed, cells from post-natal tissues can modulate their basal autophagic flux to properly respond to different stimuli [2,3] including unbalance of nutrient availability [4], growth factor fluctuations [5,6] and pathogen infections [7]. This degradative process relies on the capability of double-membraned vesicles, known as autophagosomes, to encapsulate cytoplasmic materials prior to their delivery to lysosomes [8]. In higher eukaryotes, mature autophagosomes are formed by the closure of cup-shaped phagophores, mainly originating at specialized ER sites, called omegasomes [9]. Here, phagophore expansion occurs and cytosolic portions are progressively trapped as cargo. Finally, the double-membraned autophagosome fuses with a lysosome where the engulfed materials are processed by hydrolytic enzymes [10].
From a molecular point of view, autophagy induction requires the participation of multiple protein partners and complexes that in concert cooperate to facilitate phagophore nucleation, elongation and closure. Inhibition of the mechanistic target of rapamycin (mTOR) is considered to be the main intracellular signal responsible for autophagy induction [4,11]. Upon mTOR blockade, the Unc-51-like kinase 1/2 (ULK1/2) complex, consisting of ULK1/2, autophagy-related protein 13 (ATG13), FAK family kinase interacting protein of 200 kDa (FIP200) and ATG101 [12,13], is activated and is recruited to the omegasome [14]. Here, ULK1/2 complex triggers phagophore nucleation by recruiting components of the class III phosphatidylinositol-3-kinase (PI3KC3) complex I [15]. Once activated, PI3KC3 complex I components, consisting of class III PI3K, Beclin 1, ATG14, vacuolar protein sorting 34 (VPS34) and activating molecule in Beclin 1-regulated autophagy protein 1 (AMBRA1), cooperate to increase local concentrations of phosphatidylinositol-3-phosphate (PI3P), which in turn acts to recruit PI3P-binding autophagy effector proteins to the newly formed phagophore membrane [[15], [16], [17]]. Among them is WIPI2, that acts as a docking site pivotal for the formation of the ATG5–12–16 complex on the outer phagophore membrane [[18], [19], [20]]. The ATG5–12–16 complex then promotes ATG3-mediated conjugation of activated ubiquitin-like ATG8 family members [21]. Phosphatidylethanolamine (PE)-anchored (i.e. lipidated) forms of microtubule-associated protein light chain 3 (LC3) proteins and γ-aminobutyric acid receptor-associated proteins (GABARAPs) are present on the inner and outer membranes of newly formed phagophores, thus contributing to the elongation and then sealing of the phagophore [22,23]. At this stage, Golgi complexes, plasma membrane, recycling endosomes, mitochondria and ATG9-enriched vesicles provide additional sources of membrane for growing autophagosomes [3]. The final stages of autophagy, autophagosome maturation and cargo destruction, require lysosomal delivery and fusion [24] which are accomplished with the help of specialized machinery.
2. Selective forms of autophagy
Starvation-induced autophagy processes large amounts of cytosolic materials that are subsequently disassembled in-bulk. This cellular task appears to lack selectively, and the encapsulated materials are randomly trapped during phagophore elongation and closure. However, our understanding of autophagy has significantly expanded over the past several years, uncovering highly regulated and somewhat interconnected signaling pathways governing targeted forms of autophagy [25]. Selective autophagy relies on the existence of specialized autophagy receptors that drag labelled cargos into autophagosomes [25,26]. Receptors directly bind to LC3/GABARAP on autophagosomal membranes and are themselves degraded along with their cargo within lysosomes. Specifically, autophagy receptors identify an “eat-me” signal, mostly composed of ubiquitin chains, on cargo (e.i, p62, OPTN) [[27], [28], [29]] [[27], [28], [29]] [[27], [28], [29]] or are themselves resident proteins of the targeted organelle (e.i, NIX, FAM134B) [30,31]. Cargo receptors also possess an LC3-interacting region (LIR, with a consensus [W/F/Y]‐X1‐X2‐[I/L/V]) or GABARAP interaction motif (GIM, [W/F]‐[V/I]‐X2‐V), which is often located in the long unstructured sequences of these receptors, and are recognized by LC3s and GABARAPs respectively [26]. As a result, autophagy receptors and cargos are simultaneously engulfed by nascent autophagosome and then degraded in the hydrolytic milieu of the lysosome [32]. Therefore, by coupling a rich repertoire of autophagy receptors with a highly conserved core autophagic machinery, cells exploit this process for the selective removal of defined cellular or subcellular components including misfolded protein aggregates (aggrephagy), damaged mitochondria (mitophagy), peroxisomes (pexophagy), ribosomes (ribophagy) and endoplasmic reticulum (ER-phagy) [26]. The existence of a precise and selective autophagic process depends on several regulatory mechanisms which are devoted to control the expression, localization and activity of autophagy receptors. Specifically, to maintain an appropriate basal level of autophagy receptors without triggering their accumulation over time, most autophagy receptors and adaptors undergo constant turnover by autophagy even in their unloaded state [33]. Moreover, post-translational modifications (PTMs) are involved in these regulatory circuits too. Consistently, phosphorylation is a highly conserved and rapidly deployable PTM that cells can use to control autophagy. For example, phosphorylation of the LIR domain of BNIP3 promotes its binding with LC3 modifiers [34]. Similarly, phosphorylation of p62 and OPTN increases their affinity for ubiquitin chains and LC3 during salmonella infection [28]. Now, great research efforts are concentrated on the identification of PTMs that govern autophagy selectivity and regulation in different contexts [35].
In addition to its role as a scavenger, autophagy is also considered an ancient defense system that cells use to counteract bacterial and viral infections. Xenophagy is a specialized form of autophagy dedicated to “eating” foreign organisms that are potentially harmful for cells [36,37]. Some autophagy receptors, like p62/SQSTM1 and OPTN, can mediate the selective recognition and removal of intracellular bacteria [28,29]. In the same way, ER-phagy, atop of its intrinsic role in controlling ER shape and homeostasis, acts to limit the spread of viruses [38,39]. This is a particularly valuable cellular defense strategy since viruses tend to use the ER as their primary site for replication and accumulation. In the next sections, we will focus our discussion on xenophagy and ER-phagy as first lines of the innate immune response, emphasizing the molecular strategies that pathogens have evolved to escape these cytoprotective controls.
3. Xenophagy: eating the unknown
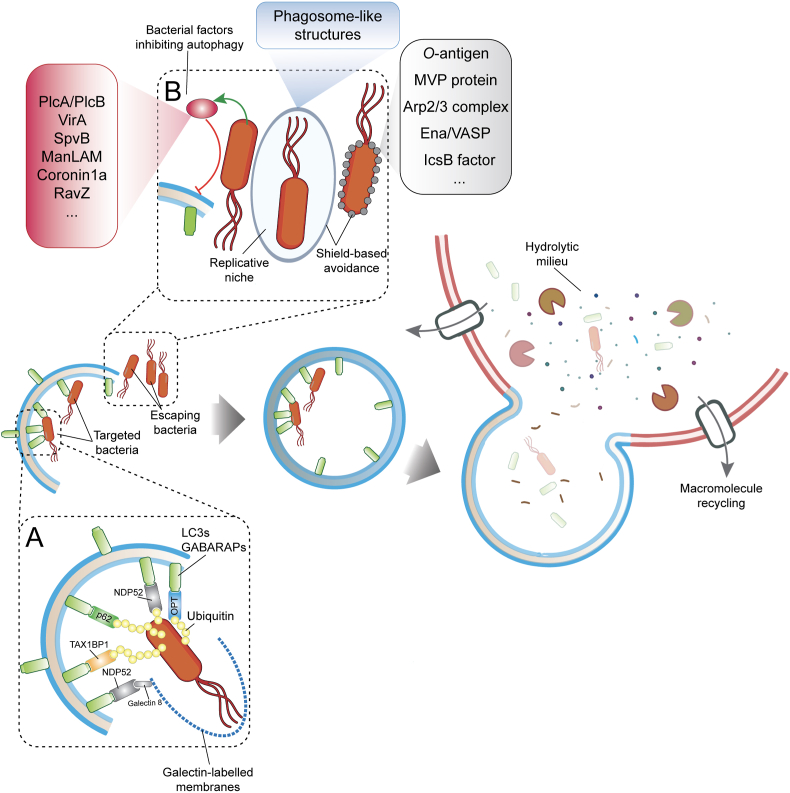
Xenophagy targets intracellular bacteria for lysosomal degradation, and acts as an important innate immune defense that mammalian cells can adopt to cope with potential life-threatening pathogens. Xenophagy is controlled in time and space and its activation requires surveillance mechanisms that are able to recognize invading organisms. Intracellular membrane rupture is one of the first events that expose bacteria to the host cytosol. Once exposed, pathogens are promptly labelled with ubiquitin chains, while the disrupted membranes are recognized by Galectin-8. Such post-translational modifications act as docking sites for specialized autophagy receptors: p62/SQSTM1, OPTN, NDP52 and TAX1BP1 [28,[40], [41], [42]](Fig. 1A). These autophagy receptors interact with LC3/GABARAP to link the decorated membrane and the recognized bacteria to the autophagy machinery and deliver them to lysosomes for clearance (Fig. 1A). Consistently, deletion of autophagy genes (i.e. ULK1, Beclin 1, ATG5, ATG7) causes the loss of xenophagy-dependent control of bacterial replication in cultures cells and animal models [[43], [44], [45], [46]]. Murine strains, where Atg5 is conditionally deleted in myeloid-derived cells (Atg5fl/fl LysM-Cre), succumb by Mycobacterium tuberculosis infection within a few days of infection [45,47]. Similarly, C. elegans with defective autophagy genes exhibit vulnerability to bacterial infections [48].
Fig. 1.
Xenophagy restricts intracellular pathogen infections. (A) Autophagy adaptors are devoted to the recognition of ubiquitin chains and galectins that mark invading bacteria and damaged bacterial phagosome-like membranes, respectively. (B) Intracellular pathogens can escape the autophagy-dependent destruction either eluding the autophagy machinery or inhibiting autophagosome maturation.
While xenophagy provides the first line of innate defense, many bacteria evolved anti-autophagy strategies to efficiently replicate inside cells and spread across tissues undetected and unscathed. Such strategies can involve avoidance of cellular surveillance mechanisms, impairment of phagophore formation as well as subversion of autophagy machinery (Fig. 1B).
A common strategy used by several pathogens to escape xenophagy is to shield themselves from being targeted by ubiquitin chains or being recognized by autophagy proteins. Francisella tularensis is a clear-cut example of this shield-base escape. The surface of F. tularensis is coated with polysaccharidic O-antigens that protect it against poly-ubiquitination and xenophagy-dependent destruction [49]. Furthermore, the antioxidant super-oxide dismutase of F. tularensis can inhibit ROS-dependent induction of xenophagy, while impairing LC3-associated phagocytosis (LAP) during infection of cultured murine macrophages [50]. In a similar manner, Listeria monocytogenes can avoid xenophagy by decorating its surface with the host's Major vault protein (MVP) through the activity of the factor InlK [51]. Moreover, by exploiting the virulence factor ActA, L. monocytogenes can recruit the Arp2/3 complex and Ena/VASP to the bacterial surface, thus preventing its ubiquitination and autophagy [52,53] (Fig. 1B). Although slower than their preferred cytosolic replication, L. monocytogenes can proliferate in spacious Listeria-containing phagosomes (SLAPs) that are decorated by LC3 and LAMP1 [54]. Using the same strategy, Shigella flexneri is able to escape xenophagy by secreting bacterial IcsB effector, which disguises a region of the IcsA protein on the bacterial surface [[55], [56], [57], [58]]. In this way, IcsA cannot be recognized by ATG5, thereby blocking S. flexneri recruitment to the phagophore [55].
While the above reported examples demonstrate the advantages of stealth-mode in escaping intracellular surveillance mechanisms, a successful bacterial replication requires additional strategies that interfere with xenophagy pathways at multiple levels. In this context, coupled to their shield-based strategy, L. monocytogenes dampens autophagosome formation and maturation by lowering local concentrations of PIP3 through the induction of bacterial PlcA and PlcB phospholipases [59,60] (Fig. 1B). Similarly, S. flexneri, can prevent early steps of phagophore nucleation and elongation through the bacterial protein VirA. VirA is a GTPase-activating protein (GAP) that inhibits Rab1, whose activity is crucial for phagophore nucleation and for the recruitment of LC3 to the surface of S. flexneri [58]. Similar to L. monocytogenes and S. flexneri, Salmonella thyphimurium can also suppress autophagosome formation by depolymerizing actin through the activity of the bacterial protein SpvB [61].
Another strategy implemented by pathogens is to interfere with autophagosome maturation while subverting the autophagic machinery to help increase their chances for survival and replication. In the case of Mycobacterium tuberculosis infection, several avoidance strategies are set in motion in order to ensure successful colonization of the host organism. M. tuberculosis efficiently prevents autophagosome fusion by lowering PI3P levels through the activity of mannose-capped lipoarabinomannan (ManLAM) [62] (Fig. 1B). Moreover, in a ManLAM-dependent mechanism, M. tuberculosis inhibits Ca2+ influx thereby impairing upstream mTOR and ULK1 signaling that is required in the early steps of autophagy [62]. Notably, M. tuberculosis also exploits the bacterial factor Coronin1a to impair autophagosome formation [63]. Using similar subversion strategies, Legionella pneumophila blocks autophagosome acidification by irreversibly deconjugating LC3s/GABARAPs via the effector protein RavZ [64]. In parallel, by translocating the effector protein sphingosine-1 phosphate lyase (LpSpl), L. pneumophila can also limit host sphingosine biosynthesis thereby curtailing autophagy/xenophagy [65]. To obtain a continuous flux of nutrients, Anaplasma phagocytophilum enhances autophagosome formation, via the effector Anaplasma translocated substrate 1 (Ats-1), while replicating inside nutrient rich double-lipid bilayer membranes associated with LC3, Beclin 1 and Atg6 but devoid of lysosomal markers [66]. Likewise, Coxiella burnetii survives and replicates in acidified vesicles that colocalize with LC3 during infection of epithelial cells and macrophages [67,68]. Similarly, Yersinia pseudotuberculosis, Y. pestis, Staphylococcus aureus, Serratia marcescens and Brucella abortus replicate intracellularly in autophagosomes or autophagosome-like structures and prevent their maturation and fusion with lysosomes [[69], [70], [71], [72], [73]]. Of note, cell colonization by invading bacteria is likely the result of combined “espionage techniques” against xenophagy.
4. Targeting intracellular pathogens by modulating the autophagy/xenophagy flux
Modulation of the autophagy flux is emerging as a feasible strategy to counteract microbial infections and some pharmacological agents with these properties have entered clinical trials. Inhibiting autophagy with 3-methyladenine arrests the growth of Anaplasma phagocytophilum [74]. Using an opposite modulation, the PDK1 inhibitor AR-12 induces autophagy and efficiently eliminates Salmonella typhimurium in murine macrophages and Francisella tularensis in human leukemic THP-1 macrophages [75,76]. The EGFR inhibitor Gefitinib, used as anti-cancer agent, is also effective in limiting the spread of M. tuberculosis infection by forcing autophagy activation [77]. However, the evident disadvantage of these pharmacological approaches is their promiscuity towards other molecular targets, therefore more-specific agents that more directly modulate autophagy are continuously explored. Of note, cell-permeable autophagy-inducing peptides are promising candidates. Among them Tat–Beclin 1, which is known to stimulate autophagy by interfering with the negative autophagy regulator GAPR-178. Tat–Beclin 1 administration boosts autophagy whilst limiting the replication of the ΔActA strain of L. monocytogenes in infected cultured macrophages [78].
While these approaches are promising for counteracting intracellular bacterial replication by modulating autophagy in opportune in vitro scenarios and murine strains, future research should clarify potential translational applications of these treatments. Future efforts will be also focused on accelerating research platforms involved in the discovery of more-effective targets in host-directed therapies that can be coupled with newly engineered perturbagens that limit side-target effects in system-wide autophagy manipulation approaches.
5. Autophagy as a first line of defense against viral infections
Viruses are generally considered to be non-living organisms capable of infecting hosts from every branch of the tree of life. Approximately 200 species of viruses are known to infect and cause disease in humans [79]. To counteract potentially lethal viral illness, cells have evolved virus‐specific controls and innate immune responses aimed at counteracting and eliminating viruses [80]. In the past decade autophagy has emerged as a pivotal cellular defense mechanism that limits viral infection and this selective form of autophagy is called virophagy [81]. Some autophagy-dependent strategies against viruses have been characterized in-depth in Drosophila melanogaster, since autophagy is one of the major components governing flies’ immunity [82]. Here, the autophagic machinery processes and delivers antigens to Toll-Like Receptors (TLRs) upon vesicular stomatitis virus (VSV) infection, thus stimulating an immune response [83]. Using a similar mechanism Toll-7 and MyD88 signaling have been demonstrated to hamper the replication of the Rift Valley fever virus (RVFV) in flies and mammals, respectively [84]. In mammals, one of the best characterized antiviral roles, directed by the autophagy pathway, is the processing and subsequent delivery of intracellular viral antigens to the peptide grooves of the major histocompatibility complex (MHC) class I upon infection by herpes simplex virus type 1 (HSV-1) [85]. Autophagy also delivers cytosolic proteins for MHC class II presentation which enhances T cell activation [86]. However, similarly to bacteria, viruses have evolved subversive mechanisms against autophagy, increasing their chances for replication and viral pathogenesis. In fact, influenza virus [87], coronaviruses [88], coxsackievirus [89], poliovirus [90], hepatitis C virus (HCV) [91,92] and DENV [93] are known to largely affect autophagic machinery. Influenza A virus (IAV) inhibits autophagosome-to-lysosome fusion [87]. As a consequence, autophagosomes containing viruses accumulate in infected cells allowing viral replication and virion assembly [87]. Newcastle disease virus (NDV) can trigger autophagy in U251 glioma cells to enhance viral replication [94]. Of note, pharmacological or genetic manipulation of the autophagy pathway efficiently halts NDV replication [95]. In contrast, HIV exerts a double-fronted assault on autophagy. During the initial stages of infection, HIV induces autophagosome formation while blocking their maturation and fusion during the late stages of viral replication [96,97]. This results in a massive accumulation of immature autophagosomes, providing membranes to be used as scaffolds for HIV virion assembly [98]. A similar strategy is used by the measles virus (MeV), where increasing autophagic flux results in increased viral replication inside cells [99,100]. Interestingly, MeV utilizes autophagy receptors NDP52 and TAX1BP1, possibly through direct binding by viral effector proteins, for the maturation of a subset of MeV-containing autophagosomes that are required for viral replication. With this in mind, our understanding of the autophagic mechanisms restricting viral infections is far from complete and new intrinsic anti-viral mechanisms continue to be uncovered. Recently, a selective form of autophagy that specifically targets the ER (ER-phagy), has been implicated in controlling cellular fitness and the ability of cells to adapt to different stressful stimuli. Notably, the ER is also one of the favorite sites for viral replication, accumulation, and virion assembly [101]. Thus, ER-phagy can represent a particularly valuable form of anti-virus response through the combination of a highly specialized receptor repertoire coupled with generic autophagy machinery. In the next section, we will discuss ER-phagy and its anti-viral role more in-depth.
6. ER-phagy as a specialized innate immune response
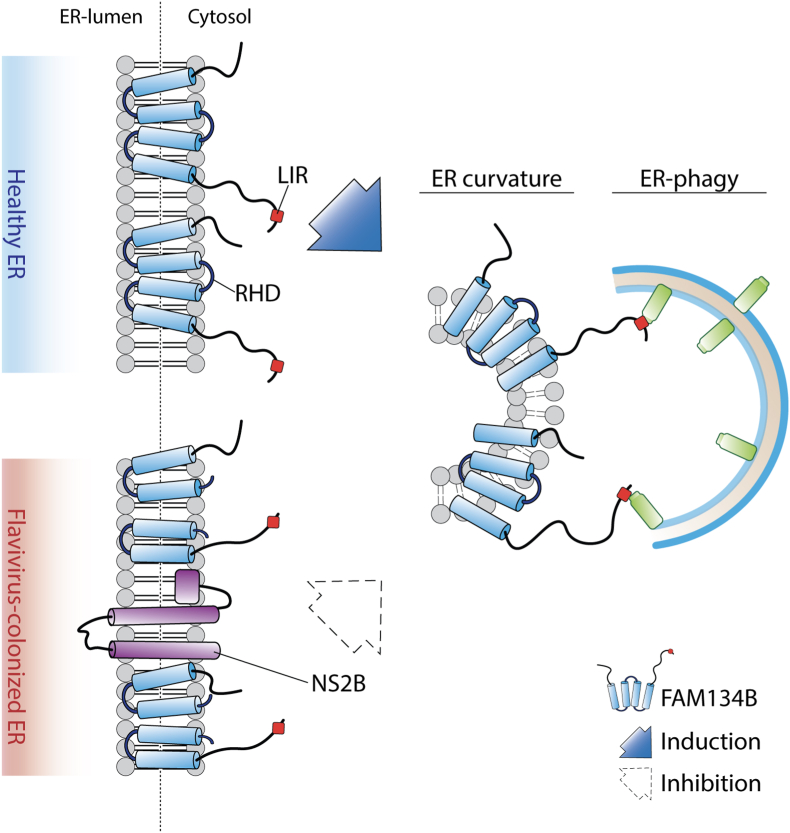
The ER is a membranous organelle, which develops a dynamic and elaborate network, originating from the nuclear membrane, and spanning throughout the cytosol [102]. Its morphology is complex, subject to constant remodeling and, dependent on function, assumes distinct shapes. While ER tubules are very dynamic and constantly elongate, retract, fuse and slide along the cytoskeleton [[102], [103], [104]], ER sheets are less mobile but enlarge in response to ER stress (like calcium imbalance, accumulation of protein aggregates, viral infections) and shrink back to their original size after the stress is resolved [105]. Cells that fail to properly support ER remodeling and turnover are unable to respond to cellular needs or resolve ER stress. This is a common reason for the development of human diseases [106,107]. ER remodeling as well as its constant turnover is mediated by a cellular recycling pathway known as ER-phagy. The mammalian proteins FAM134B, SEC62, RTN3, CCPG1, ATL3 and TEX264 have been identified as autophagy receptors that mediate the coupling of ER fragments to autophagic membranes, thereby participating in the basal turnover of ER, re-shaping after ER expansion upon stress, as well as lysosomal degradation of ER protein aggregates [31,[108], [109], [110], [111], [112], [113]]. These ER-phagy receptors are specialized for their respective ER subdomains and/or specific stress responses. FAM134B is an intra-membrane ER-resident protein which plays a key role in the basal turnover of ER sheets. It harbors a reticulon homology domain (RHD) that can curve ER membranes (Fig. 2) and generates small vesicles. Its LIR domain enables it to bind to mATG8s which promotes the delivery of ER membranes to lysosomes. Absence of FAM134B promotes ER expansion and results in ER stress, affecting neuron survival, and leading to neuropathies [31,114]. During infection, ER associated degradation is exploited by viruses for replication and immune response evasion. Viruses assemble and mature in ER compartments during their infection cycles; therefore, the elimination of specific portions of the ER, via the lysosomal system, is an innate antiviral strategy adopted by host cells to counteract viruses and directly eliminate their proliferative site [115]. Absence of FAM134B results in ER expansion [31] which is also a consequence of flavivirus infection, as these viruses use the ER as a proliferative niche [116,117] (Fig. 2). However, how FAM134B specifically recognizes viral replicative sites within the ER to mediate their elimination remains uncharacterized. Nevertheless, Zika (ZIKV), Dengue (DENV) and West Nile viruses appear to antagonize ER-phagy by directly targeting FAM134 B[38,39] (Fig. 2). Their NS3 virally-encoded protease and its cofactor NS2B cleave FAM134B within its RHD [39], thus impairing FAM134B's intrinsic ability to generate ER vesicles [114] (Fig. 2). Ablation of FAM134B supports Dengue and Zika virus infections, providing direct proof for its pivotal role in mitigating viral infections. Therefore, the cleavage of FAM134B directly benefits flavivirus proliferation [38,118]. Indeed, depletion of the autophagy regulator BPIFB3 enhances FAM134B ER-phagy and impairs flavivirus replication [119]. This suggests that BPIFB3 may have an indirect role in ER remodeling and could regulate DENV and ZIKV proliferation. Of note, BPIFB3 also negatively influences enterovirus replication [119]. In addition, Flavivirus also escapes ER-phagy by targeting RTN3, which is another ER-phagy receptor that can directly fragment ER membranes. Consistently, Flavivirus-encoded NS3A protein which binds RTN3 in order to hijack ER membranes, can generate a replication permissive niche [120]. In addition to flavivirus, FAM134B and RTN3 can affect the replicative status of other types of pathogens. In fact, Ebola virus replication is limited by FAM134B-dependent ER-phagy [38], while RTN3 binding to the NS4B protease of hepatitis C impairs its viral proliferation [121]. Of note, especially in the current days, positive-strand RNA viruses, like SARS-CoV, can also reorganize host cell membranes to build their replicative niche and likely hide RNA replication from antiviral defense mechanisms. Coronaviruses generate a large number of isolated “double-membrane vesicles” (DMVs) whose origin remains obscure. They could derive from ER, late endosome, autophagosome or the secretory pathway [122,123]. However, DMVs are not isolated structures and tend to integrate into a unique reticulo-vesicular network of shaped ER membranes. Therefore, ER is a major source for viral membrane networks, in the case of coronavirus as well [124]. A recent proteomic analysis unraveled the mouse hepatitis virus (MHV), a murine coronavirus, microenvironment and highlighted the functional importance of the ER-Golgi trafficking pathway, ubiquitin and autophagy catabolic systems and translation initiation factors [125]. Even if coronaviruses replication is not clearly affected by macro-autophagy, DMVs are labelled with nonlipidated LC3-I [88]. Of note, FAM134B and its transcriptional regulator TFEB [126] were among the host proteins identified to significantly impact MHV replication [125]; therefore, ER-phagy could play a role in the replication of coronavirus. Viruses are not the only infectious pathogens that target ER membranes. Host cells activate ER-phagy as a response mechanism to alleviate ER stress after Gram-positive bacterial infection. Although the molecular mechanisms are not completely elucidated, upon infection c-di-AMP/STING dependent ER stress seems to be apical to mTORC1 inactivation and consequent autophagy induction [127]. Of note, the SidE enzyme family of the Gram-negative bacteria, Legionella pneumophila, promote the non-canonical phosphoribosyl-linked (PR) serine ubiquitination of several ER proteins in order to remodel the ER membrane network and create a proliferative vacuole. Intriguingly, among the PR-ubiquitinated proteins, the ER-phagy receptors RTN3 and Tex264 as well as FAM134C have been recently identified, implicating their direct involvement in ER remodeling in response to pathogen infection [109,111,113,128].
Fig. 2.
Flavivirus efficiently replicate in ER subdomains by subverting FAM134B-dependent ER-phagy. NS2B protein efficiently cleaves the reticulum homology domain of FAM134B, thus impairing the ER-phagy dependent clearance of virally-colonized ER subdomains.
7. Conclusions
Several types of viruses and bacteria remodel ER membranes and hijack ER machinery in order to generate an auspicious proliferative environment. The molecular mechanisms adopted by pathogens to conquer the ER are not sufficiently understood, creating obstacles for the development of new/alternative therapeutic approaches. In some cases, like the SARS-CoV-2 pandemic, there are no established therapies nor vaccines to counteract the infection. A major hurdle is our lack of understanding of SARS-CoV-2 biology. Coronaviruses remodel the ER of host cells in order to create their proliferation niche; however, their adopted molecular mechanisms still remain elusive. Further studies are needed to elucidate the role of autophagy and ER-phagy in host cell immunity. These studies will improve our knowledge of how cells counteract viral and bacterial infections and will contribute to the development of novel molecules that specifically act to control ER membrane dynamics to tackle pathogen infections.
CRediT authorship contribution statement
Alessio Reggio: Writing - review & editing. Viviana Buonomo: Writing - review & editing. Paolo Grumati: Conceptualization, Writing - review & editing.
Declaration of competing interest
There are no conflict of interest.
Acknowledgments
We kindly acknowledge Dr. Anna Vainshtein (https://craftscience.ca) for the editing and critical reading. This work has been supported by Telethon Foundation and Roche Foundation. Alessio Reggio is supported by Fondazione Umberto Veronesi. Figures were created with Adobe Illustrator and Biorender.com.
References
- 1.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N., Levine B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abada A., Elazar Z. Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep. 2014;15:839–852. doi: 10.15252/embr.201439076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González A., Hall M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017;36:397–408. doi: 10.15252/embj.201696010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T.Y., Lin S.Y., Lin S.C. Mechanism and physiological significance of growth factor-related autophagy. Physiology. 2013;28:423–431. doi: 10.1152/physiol.00023.2013. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma V., Verma S., Seranova E., Sarkar S., Kumar D. Selective autophagy and xenophagy in infection and disease. Front Cell Dev Biol. 2018;6:1–17. doi: 10.3389/fcell.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yorimitsu T., Klionsky D.J. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y., Liu M., Li X., Liu J., Li H. Origin of the autophagosome membrane in mammals. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/1012789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 11.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y. Nutrient-dependent mTORC1 association with the ULK1–Atg13–FIP200 complex required for autophagy. Mol. Biol. Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung C.H., Jun C.B., Ro S.-H., Kim Y.-M., Otto N.M., Cao J. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura T., Tamura N., Kono N., Shimanaka Y., Arai H., Yamamoto H. Autophagosome formation is initiated at phosphatidylinositol synthase‐enriched ER subdomains. EMBO J. 2017;36:1719–1735. doi: 10.15252/embj.201695189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell R.C., Tian Y., Yuan H., Park H.W., Chang Y.-Y., Kim J. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maria Fimia G., Stoykova A., Romagnoli A., Giunta L., Di Bartolomeo S., Nardacci R. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 17.Di Bartolomeo S., Corazzari M., Nazio F., Oliverio S., Lisi G., Antonioli M. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J. Cell Biol. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dooley H.C., Razi M., Polson H.E.J., Girardin S.E., Wilson M.I., Tooze S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell. 2014;55:238–252. doi: 10.1016/j.molcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fracchiolla D., Chang C., Hurley J.H., Martens S. A PI3K-WIPI2 positive feedback loop allosterically activates LC3 lipidation in autophagy. J. Cell Biol. 2020:219. doi: 10.1083/jcb.201912098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann A., Beier V., Franquelim H.G., Wollert T. Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell. 2014;156:469–481. doi: 10.1016/j.cell.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N., Yamamoto A., Hatano M., Kobayashi Y., Kabey Y., Suzuki K. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001;152:657–667. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weidberg H., Shpilka T., Shvets E., Abada A., Shimron F., Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev. Cell. 2011;20:444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 24.Ballabio A., Bonifacino J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020;21:101–118. doi: 10.1038/s41580-019-0185-4. [DOI] [PubMed] [Google Scholar]
- 25.Gatica D., Lahiri V., Klionsky D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkin V., Rogov V.V. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell. 2019;76:268–285. doi: 10.1016/j.molcel.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Lim J., Lachenmayer M.L., Wu S., Liu W., Kundu M., Wang R. Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 2015;11:1–28. doi: 10.1371/journal.pgen.1004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild P., Farhan H., McEwan D.G., Wagner S., Rogov V.V., Brady N.R. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto G., Wada K., Okuno M., Kurosawa M., Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol. Cell. 2011;44:279–289. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Novak I., Kirkin V., McEwan D.G., Zhang J., Wild P., Rozenknop A. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaminets A., Heinrich T., Mari M., Grumati P., Huebner A.K., Akutsu M. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 32.Yim W.W.Y., Mizushima N. Lysosome biology in autophagy. Cell Discov. 2020;6 doi: 10.1038/s41421-020-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stolz A., Ernst A., Dikic I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y., Massen S., Terenzio M., Lang V., Chen-Lindner S., Eils R. Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J. Biol. Chem. 2013;288:1099–1113. doi: 10.1074/jbc.M112.399345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwan D.G., Dikic I. The three musketeers of autophagy: phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011;21:195–201. doi: 10.1016/j.tcb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwan D.G. Host-pathogen interactions and subversion of autophagy. Essays Biochem. 2017;61:687–697. doi: 10.1042/EBC20170058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandia Rekha, Dadar Maryam, Munjal Ashok, Dhama Kuldeep, Tiwari Kumaragurubaran Karthik Ruchi, Mohd, Iqbal Yatoo, Hafiz M., Iqbal N., Singh Karam Pal, Sunil K., Joshi andWanpen C. Various roles in infectious , non-infectious , and Lifestyle Diseases : current knowledge and prospects for disease prevention. Novel Drug. Cells. 2019;8:674. doi: 10.3390/cells8070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiramel A.I., Dougherty J.D., Nair V., Robertson S.J., Best S.M. FAM134B, the selective autophagy receptor for endoplasmic reticulum turnover, inhibits replication of Ebola virus strains makona and mayinga. J. Infect. Dis. 2016;214:S319–S325. doi: 10.1093/infdis/jiw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lennemann N.J., Coyne C.B. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy. 2017;13:322–332. doi: 10.1080/15548627.2016.1265192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y.T., Shahnazari S., Brech A., Lamark T., Johansen T., Brumell J.H. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
- 41.Thurston T.L.M., Wandel M.P., Von Muhlinen N., Á Foeglein, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012;482:414–418. doi: 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tumbarello D.A., Manna P.T., Allen M., Bycroft M., Arden S.D., Kendrick-Jones J. The autophagy receptor TAX1BP1 and the molecular motor myosin VI are required for clearance of Salmonella typhimurium by autophagy. PLoS Pathog. 2015;11:1–26. doi: 10.1371/journal.ppat.1005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jayaswal S., Kamal M.A., Dua R., Gupta S., Majumdar T., Das G. Identification of host-dependent survival factors for intracellular Mycobacterium tuberculosis through an siRNA screen. PLoS Pathog. 2010;6:1–15. doi: 10.1371/journal.ppat.1000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J.J., Lee H.M., Shin D.M., Kim W., Yuk J.M., Jin H.S. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. © 2012 Elsevier Inc. Cell Host Microbe. 2012;11:457–468. doi: 10.1016/j.chom.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Watson R.O., Manzanillo P.S., Cox J.S. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J., Yang K., Zhou L., Wu M., Wu Y., Zhu M. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting rheb. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo E.F., Dekonenko A., Arko-Mensah J., Mandell M.A., Dupont N., Jiang S. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc. Natl. Acad. Sci. U. S. A. 2012;109 doi: 10.1073/pnas.1210500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia K., Thomas C., Akbar M., Sun Q., Adams-Huet B., Gilpin C. Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14564–14569. doi: 10.1073/pnas.0813319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Case E.D.R., Chong A., Wehrly T.D., Hansen B., Child R., Hwang S. The Francisella O-antigen mediates survival in the macrophage cytosol via autophagy avoidance. Cell Microbiol. 2014;16:862–877. doi: 10.1111/cmi.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabadi S.M., Sanchez B.C., Varanat M., Ma Z., Catlett S.V., Melendez J.A. Antioxidant defenses of Francisella tularensis modulate macrophage function and production of proinflammatory cytokines. J. Biol. Chem. 2016;291:5009–5021. doi: 10.1074/jbc.M115.681478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dortet L., Mostowy S., Louaka A.S., Gouin E., Nahori M.A., Wiemer E.A.C. Recruitment of the major vault protein by inlk: a listeria monocytogenes strategy to avoid autophagy. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P.L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 53.Yoshikawa Y., Ogawa M., Hain T., Yoshida M., Fukumatsu M., Kim M. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat. Cell Biol. 2009;11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- 54.Lam G.Y., Cemma M., Muise A.M., Higgins D.E., Brumell J.H. Host and bacterial factors that regulate LC3 recruitment to Listeria monocytogenes during the early stages of macrophage infection. Autophagy. 2013;9:985–995. doi: 10.4161/auto.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa M., Yoshimori T., Suzuki T., Sagara H., Mizushima N., Sasakawa C. Escape of intracellular Shigella from autophagy. 2005;307:727–731. doi: 10.1126/science.1106036. Sci (Nefile///Users/alessio/Dropbox/Letteratura/Authophagy/Research/Baxt2014PDFw York, NY) [DOI] [PubMed] [Google Scholar]
- 56.Baxt L.A., Goldberg M.B. Host and bacterial proteins that repress recruitment of LC3 to Shigella early during infection. PloS One. 2014;9 doi: 10.1371/journal.pone.0094653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kayath C.A., Hussey S., El hajjami N., Nagra K., Philpott D., Allaoui A. Escape of intracellular Shigella from autophagy requires binding to cholesterol through the type III effector, IcsB. Microb. Infect. 2010;12:956–966. doi: 10.1016/j.micinf.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Campbell-Valois F.X., Sachse M., Sansonetti P.J., Parsot C. Escape of actively secreting shigella flexneri from ATG8/LC3-Positive vacuoles formed during cell-to-cell spread is facilitated by IcsB and VirA. mBio. 2015;6:1–11. doi: 10.1128/mBio.02567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tattoli I., Sorbara M.T., Yang C., Tooze S.A., Philpott D.J., Girardin S.E. Listeria phospholipases subvert host autophagic defenses by stalling pre-autophagosomal structures. EMBO J. 2013;32:3066–3078. doi: 10.1038/emboj.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell G., Ge L., Huang Q., Chen C., Kianian S., Roberts M.F. Avoidance of autophagy mediated by PlcA or ActA is required for listeria monocytogenes growth in macrophages. Infect. Immun. 2015;83:2175–2184. doi: 10.1128/IAI.00110-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu S.Y., Chu Y.Y., Yang Y.R., Li Y.Y., He P.Y., Zheng Y.J. Inhibition of macrophage autophagy induced by Salmonella enterica serovar typhi plasmid. Front Biosci - Landmark. 2014;19:490–503. doi: 10.2741/4220. [DOI] [PubMed] [Google Scholar]
- 62.Vergne I., Chua J., Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca 2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 2003;198:653–659. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seto S., Tsujimura K., Koide Y. Coronin-1a inhibits autophagosome formation around Mycobacterium tuberculosis-containing phagosomes and assists mycobacterial survival in macrophages. Cell Microbiol. 2012;14:710–727. doi: 10.1111/j.1462-5822.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- 64.Choy A., Dancourt J., Mugo B., O'Connor T.J., Isberg R.R., Melia T.J. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolando M., Escoll P., Nora T., Botti J., Boitez V., Bedia C. Legionella pneumophila S1P-lyase targets host sphingolipid metabolism and restrains autophagy. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1901–1906. doi: 10.1073/pnas.1522067113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niu H., Xiong Q., Yamamoto A., Hayashi-Nishino M., Rikihisa Y. Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection. Proc. Natl. Acad. Sci. U. S. A. 2012;109:20800–20807. doi: 10.1073/pnas.1218674109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berón W., Gutierrez M.G., Rabinovitch M., Colombo M.I. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect. Immun. 2002;70:5816–5821. doi: 10.1128/IAI.70.10.5816-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newton H.J., Kohler L.J., McDonough J.A., Temoche-Diaz M., Crabill E., Hartland E.L. A screen of Coxiella burnetii mutants reveals important roles for dot/icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pujol C., Klein K.A., Romanov G.A., Palmer L.E., Cirota C., Zhao Z. Yersinia pestis can reside in autophagosomes and avoid xenophagy in murine macrophages by preventing vacuole acidification. Infect. Immun. 2009;77:2251–2261. doi: 10.1128/IAI.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moreau K., Lacas-Gervais S., Fujita N., Sebbane F., Yoshimori T., Simonet M. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell Microbiol. 2010;12:1108–1123. doi: 10.1111/j.1462-5822.2010.01456.x. [DOI] [PubMed] [Google Scholar]
- 71.Liu P., Cheng J., Sy C., Huang W.-C., Yang H.-C., Gallo R.L. IsaB inhibits autophagic flux to promote host transmission of methicillin-resistant Staphylococcus aureus. J. Invest. Dermatol. 2015;135:2714–2722. doi: 10.1038/jid.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fedrigo G.V., Campoy E.M., Di Venanzio G., Colombo M.I., Véscovi E.G. Serratia marcescens is able to survive and proliferate in autophagic-like vacuoles inside non-phagocytic cells. PloS One. 2011;6 doi: 10.1371/journal.pone.0024054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Starr T., Child R., Wehrly T.D., Hansen B., Hwang S., López-Otin C. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe. 2012;11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niu H., Yamaguchi M., Rikihisa Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cell Microbiol. 2008;10:593–605. doi: 10.1111/j.1462-5822.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 75.Chiu H.C., Kulp S.K., Soni S., Wang D., Gunn J.S., Schlesinger L.S. Eradication of intracellular Salmonella enterica serovar typhimurium with a small-molecule, host cell-directed agent. Antimicrob. Agents Chemother. 2009;53:5236–5244. doi: 10.1128/AAC.00555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiu H.C., Soni S., Kulp S.K., Curry H., Wang D., Gunn J.S. Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J. Biomed. Sci. 2009;16:110. doi: 10.1186/1423-0127-16-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanley S.A., Barczak A.K., Silvis M.R., Luo S.S., Sogi K., Vokes M. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shoji-Kawata S., Sumpter R., Leveno M., Campbell G.R., Zou Z., Kinch L. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woolhouse M., Scott F., Hudson Z., Howey R., Chase-Topping M. Human viruses: discovery and emeraence. Philos Trans R Soc B Biol Sci. 2012;367:2864–2871. doi: 10.1098/rstb.2011.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi Y., Bowman J.W., Jung J.U. Autophagy during viral infection - a double-edged sword. Nat. Rev. Microbiol. 2018;16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shelly S., Lukinova N., Bambina S., Berman A., Cherry S. Autophagy plays an essential anti-viral role in Drosophila against Vesicular Stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakamoto M., Moy R.H., Xu J., Bambina S., Yasunaga A., Shelly S.S. Virus recognition by toll-7 activates antiviral autophagy in Drosophila. Immunity. 2012;36:658–667. doi: 10.1016/j.immuni.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moy R.H., Gold B., Molleston J.M., Schad V., Yanger K., Salzano M.V. Antiviral autophagy restricts rift valley fever virus infection and is conserved from flies to mammals. Immunity. 2014;40:51–65. doi: 10.1016/j.immuni.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.English L., Chemali M., Duron J., Rondeau C., Laplante A., Gingras D. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmid D., Pypaert M., Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gannagé M., Dormann D., Albrecht R., Dengjel J., Torossi T., Rämer P.C. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe. 2009;6:367–380. doi: 10.1016/j.chom.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reggiori F., Monastyrska I., Verheije M.H., Calì T., Ulasli M., Bianchi S. Coronaviruses hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe. 2010;7:500–508. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong J., Zhang J., Si X., Gao G., Mao I., McManus B.M. Autophagosome supports coxsackievirus B3 replication in host cells. J. Virol. 2008;82:9143–9153. doi: 10.1128/JVI.00641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor M.P., Kirkegaard K. Modification of cellular autophagy protein LC3 by poliovirus. J. Virol. 2007;81:12543–12553. doi: 10.1128/JVI.00755-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sir D., Chen W., Choi J., Wakita T., Yen T.S.B., Ou J.J. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology. 2008;48:1054–1061. doi: 10.1002/hep.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ke P., Chen S.S.-L. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 2011;121:37–56. doi: 10.1172/JCI41474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee Y.R., Lei H.Y., Liu M.T., Wang J.R., Chen S.H., Jiang-Shieh Y.F. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008;374:240–248. doi: 10.1016/j.virol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meng C., Zhou Z., Jiang K., Yu S., Jia L., Wu Y. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch. Virol. 2012;157:1011–1018. doi: 10.1007/s00705-012-1270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun Y., Yu S., Ding N., Meng C., Meng S., Zhang S. Autophagy benefits the replication of newcastle disease virus in chicken cells and tissues. J. Virol. 2014;88:525–537. doi: 10.1128/JVI.01849-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kyei G.B., Dinkins C., Davis A.S., Roberts E., Singh S.B., Dong C. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 2009;186:255–268. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou D., Spector S.A. Human immunodeficiency virus type-1 infection inhibits autophagy. Aids. 2008;22:695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saribas A.S., Khalili K., Sariyer I.K. Dysregulation of autophagy by HIV-1 Nef in human astrocytes. Cell Cycle. 2015;14:2899–2904. doi: 10.1080/15384101.2015.1069927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petkova D.S., Verlhac P., Rozières A., Baguet J., Claviere M., Kretz-Remy C. Distinct contributions of autophagy receptors in measles virus replication. Viruses. 2017;9 doi: 10.3390/v9050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richetta C., Grégoire I.P., Verlhac P., Azocar O., Baguet J., Flacher M. Sustained autophagy contributes to measles virus infectivity. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inoue T., Tsai B. How viruses use the endoplasmic reticulum for entry, replication, and assembly. Cold Spring Harb Perspect Biol. 2013;5:1–17. doi: 10.1101/cshperspect.a013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shibata Y., Hu J., Kozlov M.M., Rapoport T.A. Mechanisms shaping the membranes of cellular organelles. Annu. Rev. Cell Dev. Biol. 2009;25:329–354. doi: 10.1146/annurev.cellbio.042308.113324. [DOI] [PubMed] [Google Scholar]
- 103.Goyal U., Blackstone C. Untangling the web: mechanisms underlying ER network formation. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:2492–2498. doi: 10.1016/j.bbamcr.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nixon-Abell J., Obara C.J., Weigel A.V., Li D., Legant W.R., Xu C.S. Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science. 2016:354. doi: 10.1126/science.aaf3928. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Westrate L.M., Lee J.E., Prinz W.A., Voeltz G.K. Form follows function: the importance of endoplasmic reticulum shape. Annu. Rev. Biochem. 2015;84:791–811. doi: 10.1146/annurev-biochem-072711-163501. [DOI] [PubMed] [Google Scholar]
- 106.Grumati P., Dikic I., Stolz A. ER-phagy at a glance. J. Cell Sci. 2018;131:1–6. doi: 10.1242/jcs.217364. [DOI] [PubMed] [Google Scholar]
- 107.Wilkinson S. ER-phagy: shaping up and destressing the endoplasmic reticulum. FEBS J. 2019;286:2645–2663. doi: 10.1111/febs.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith M.D., Harley M.E., Kemp A.J., Wills J., Lee M., Arends M. CCPG1 is a non-canonical autophagy cargo receptor essential for ER-phagy and pancreatic ER proteostasis. Dev. Cell. 2018;44:217–232. doi: 10.1016/j.devcel.2017.11.024. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grumati P., Morozzi G., Hölper S., Mari M., Harwardt M.L.I.E., Yan R. Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. Elife. 2017;6:1–32. doi: 10.7554/eLife.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fumagalli F., Noack J., Bergmann T.J., Presmanes E.C., Pisoni G.B., Fasana E. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat. Cell Biol. 2016;18:1173–1184. doi: 10.1038/ncb3423. [DOI] [PubMed] [Google Scholar]
- 111.Chino H., Hatta T., Natsume T., Mizushima N. Intrinsically disordered protein TEX264 mediates ER-phagy. Mol. Cell. 2019;74:909–921.e6. doi: 10.1016/j.molcel.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 112.Chen Q., Xiao Y., Chai P., Zheng P., Teng J., Chen J. ATL3 is a tubular ER-phagy receptor for GABARAP-mediated selective autophagy. Curr. Biol. 2019;29:846–855.e6. doi: 10.1016/j.cub.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 113.An H., Ordureau A., Paulo J.A., Shoemaker C.J., Denic V., Harper J.W. TEX264 is an endoplasmic reticulum-resident ATG8-interacting protein critical for ER remodeling during nutrient stress. Mol. Cell. 2019;74:891–908. doi: 10.1016/j.molcel.2019.03.034. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhaskara R.M., Grumati P., Garcia-Pardo J., Kalayil S., Covarrubias-Pinto A., Chen W. Curvature induction and membrane remodeling by FAM134B reticulon homology domain assist selective ER-phagy. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-10345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ravindran M.S., Bagchi P., Cunningham C.N., Tsai B. Opportunistic intruders: how viruses orchestrate ER functions to infect cells. Nat. Rev. Microbiol. 2016;14:407–420. doi: 10.1038/nrmicro.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Westaway E.G., Mackenzie J.M., Kenney M.T., Jones M.K., Khromykh A.A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Junjhon J., Pennington J.G., Edwards T.J., Perera R., Lanman J., Kuhn R.J. Ultrastructural characterization and three-dimensional Architecture of replication sites in dengue virus-infected mosquito cells. J. Virol. 2014;88:4687–4697. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiramel A.I., Best S.M. Role of autophagy in Zika virus infection and pathogenesis. Virus Res. 2018;254:34–40. doi: 10.1016/j.virusres.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Evans A.S., Lennemann N.J., Coyne C.B. BPIFB3 regulates endoplasmic reticulum morphology to facilitate flavivirus replication. J. Virol. 2020;94 doi: 10.1128/jvi.00029-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aktepe T.E., Liebscher S., Prier J.E., Simmons C.P., Mackenzie J.M. The host protein reticulon 3.1A is utilized by flaviviruses to facilitate membrane remodelling. Cell Rep. 2017;21:1639–1654. doi: 10.1016/j.celrep.2017.10.055. [DOI] [PubMed] [Google Scholar]
- 121.Wu M.J., Ke P.Y., Hsu J.T.A., Yeh C.T., Horng J.T. Reticulon 3 interacts with NS4B of the hepatitis C virus and negatively regulates viral replication by disrupting NS4B self-interaction. Cell Microbiol. 2014;16:1603–1618. doi: 10.1111/cmi.12318. [DOI] [PubMed] [Google Scholar]
- 122.Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Knoops K., Kikkert M., Van Den Worm S.H.E., Zevenhoven-Dobbe J.C., Van Der Meer Y., Koster A.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:1957–1974. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.V’kovski P., Gerber M., Kelly J., Pfaender S., Ebert N., Braga Lagache S. Determination of host proteins composing the microenvironment of coronavirus replicase complexes by proximity-labeling. Elife. 2019;8:1–30. doi: 10.7554/eLife.42037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cinque L., Leonibus C De, Iavazzo M., Krahmer N., Intartaglia D., Salierno F.G. MiT /TFE factors control ER-phagy via transcriptional regulation of. FAM. 2020;134 B:1–22. doi: 10.15252/embj.2020105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moretti J., Roy S., Bozec D., Martinez J., Chapman J.R., Ueberheide B. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell. 2017;171:809–823.e13. doi: 10.1016/j.cell.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shin D., Mukherjee R., Liu Y., Gonzalez A., Bonn F., Liu Y. Regulation of phosphoribosyl-linked serine ubiquitination by deubiquitinases DupA and DupB. Mol. Cell. 2020;77:164–179.e6. doi: 10.1016/j.molcel.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]