Abstract
Context: Autonomic dysreflexia is a potentially lethal condition experienced by spinal cord injury (SCI) patients. It has a wide range of causes, most of which are genitourinary in nature. However, there has been no documented case of primary bladder cancer as a trigger for recurrent autonomic dysreflexia.
Findings: We present a case of a 51-year-old spinal cord injury patient with multiple presentations of autonomic dysreflexia. Work up revealed locally invasive bladder cancer, and following definitive surgery, his symptoms were alleviated.
Conclusions: In SCI patients with recurrent AD, comprehensive work up with involvement of a specialized Urologist is required as AD may be the only presenting symptom of a serious underlying medical condition.
Keywords: Autonomic dysreflexia, Spinal cord injury, Bladder cancer, Hydronephrosis
Introduction
Autonomic dysreflexia (AD) is a potentially catastrophic condition seen in spinal cord injury (SCI) patients. AD is a condition of unopposed sympathetic response to stimuli occurring below the level of the spinal injury. It can present rapidly and have potentially life threatening complications.1,2 An estimated 60% of patients with an SCI above the level of T6 will experience AD, which is located above the spinal level of splanchnic outflow.3 Patients typically present with symptoms of excessive para-sympathetic response above the lesion and unopposed sympathetic response below. The common complaints are a pounding headache, perspiration and flushing of the face and body above the lesion, while they will be found to have cool extremities and increased spasticity below the lesion. The most common signs are increased blood pressure by at least 20% and bradycardia.1
Urologists require a detailed understanding of the condition, as up to 81-87% of the precipitants are genitourinary in nature.1 Bladder over-distension is the most common genitourinary precipitant of AD. Other urologic causes include urinary tract infections, sexual activity or vibrostimulation, catheterization, cystoscopy, urodynamics and upper or lower urinary tract pathology (e.g. calculi).4,5 Non-urologic causes of AD include constipation and rectal distension, appendicitis or other acute surgical abdomens, hemorrhoids, anal fissures, pressure sores, skin infections, pregnancy, long bone fractures and even ingrown toenails.1,5 The management of AD is primarily focused on lowering blood pressure and relieving the precipitant in order to avoid catastrophic consequences such as cerebral hemorrhage.6
While AD is a relatively well-understood condition with many identified causes, there are currently no recorded cases of AD secondary to bladder cancer within the literature. The true risk of bladder cancer in neurogenic bladder patients, particularly in those with chronic indwelling catheters is unknown.7,8 A recent study found that patients with SCI present with bladder cancer at a significantly younger age (median 29 years younger), and with more invasive disease (79% presented with T2 or greater) than a representative sample of the general population. The authors suggest that this indicates SCI influences bladder cancer risk and prognosis, but the exact attributable risk and physiologic mechanism are still unknown.8
Herein we present a case of recurrent AD secondary to previously unrecognized and otherwise asymptomatic muscle invasive bladder cancer with associated hydroureteronephrosis.
Case summary
A 51-year-old male quadriplegic (AIS A) secondary to a C5 injury suffered in 2002 was referred to Urology for recurrent UTIs over the previous year. He was not using a catheter and was a lifelong smoker at 1 pack per day. An initial ultrasound 3 months prior to the clinic visit, revealed normal kidneys bilaterally. He complained of urinary urgency, frequency, and dysuria, and denied gross hematuria. Urine cultures were consistently positive, and were treated accordingly. Video urodynamics (VUDS) were arranged following his clinic visit.
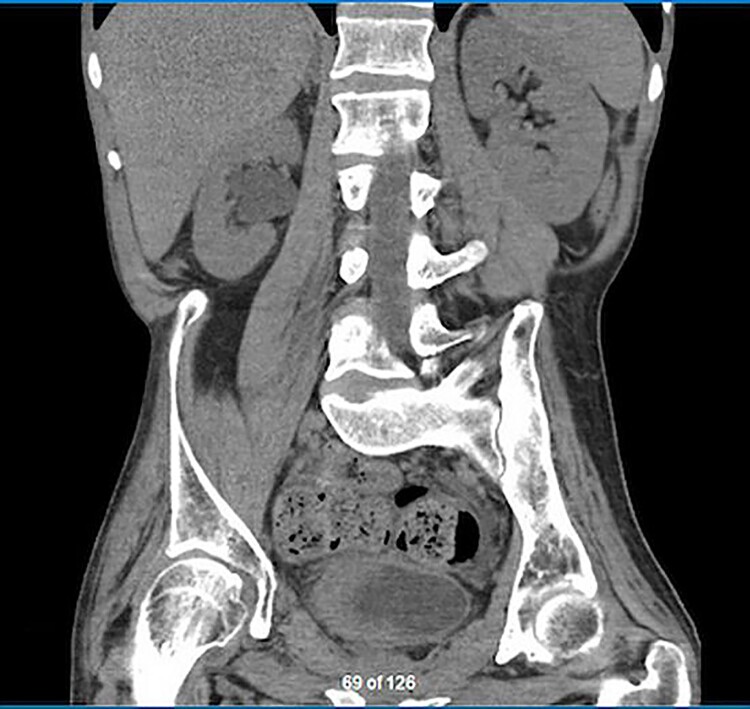
Five months later, while still awaiting the VUDS, he presented to the emergency department (ED) with severe bladder spams associated with perspiration. An indwelling catheter was placed in the ED with the belief that retention may be the cause of his AD and he was discharged home. He returned to the ED the next day with recurrent spasms, perspiration and severe headache despite the catheter, which was confirmed to be draining well. Other possible sources of recurrent AD were ruled out (constipation, pressure sores). Unfortunately, he returned to the ED a week later with similar complaints, and was found to have a blood pressure of 220/109 mmHg. An ultrasound revealed severe right hydroureteronephrosis, and was promptly followed up with a CT scan, which demonstrated a lobulated soft tissue thickening along the right bladder wall suspicious for a mass (Figure 1).
Figure 1.
Coronal view of the lobulated soft tissue thickening of the right bladder wall and upstream right hydronephrosis.
He was admitted to the Urology service and was taken for urgent transurethral resection of bladder tumor (TURBT). A large tumor was identified on the right bladder wall and was removed. His ureter could not be stented as the orifice couldn’t be identified, so a nephrostomy tube (NT) was placed post-operatively. He remained in hospital as he continued to have intermittent episodes of AD, despite the NT and TURBT. Pathology revealed muscle invasive, high-grade urothelial carcinoma. His staging was negative for any distant metastatic disease, so he was taken back to the operating room for a radical cystectomy and ileal conduit. Final pathology revealed pT4 urothelial carcinoma with local invasion into the prostate, negative margins and negative lymph nodes. Following his definitive surgery, he had no further episodes over 6 months of follow up.
Discussion
Autonomic dysreflexia is a well-known and devastating consequence of SCI. Cerebral hemorrhage is the most common catastrophic complication of AD, and patients must be warned of the early signs of AD so they can seek urgent medical treatment to lower blood pressure and remove causative stimuli.9
There have been numerous studies on the precipitating factors for AD, with most focused on genitourinary sources. It is well understood that hollow organ distension, seen in urinary retention and constipation, is a common precipitant of AD. However, there is only one case in the literature that discusses AD secondary to hydronephrosis.10 It is also documented within the literature that lower urinary tract pathology, such as bladder calculi, can lead to AD. It stands to reason that a bladder calculus causing recurrent bladder spasms leading to AD could behave in similar manner to a large bladder tumor.
SCI patients who present with recurrent AD with no obvious source identified on history or physical examination need to have imaging of their abdomen and pelvis to rule out any structural abnormality of the genitourinary or gastrointestinal system. This case highlights the need to repeat imaging that is only months old as the aggressiveness of the tumour led to the hydronephrosis and lateral wall thickening in just a few months. Our approach to the workup of possible GU causes of AD is to start with a renal / bladder ultrasound (US) to look for big and obvious abnormalities such as bladder stones, foreign bodies or hydronephrosis. CT scan of the abdomen and pelvis would be the next step if abnormal findings were seen on the screening US to better define them such as in this case with new onset hydronephrosis and a thickened wall. If there is still no identified source after imaging, but there is suspicion that the urinary system is involved, they should be referred to a urologist. Video-urodynamics are typically arranged to rule out the possibility of detrusor muscle causes such as impaired compliance or uninhibited contractions, while cystoscopy can also be performed at the urologist’s discretion for further assessment of the lower urinary tract especially if concerns such as gross hematuria, difficult catheterization or possible retained foreign body have been identified. Urine cytology is notoriously confusing in the setting of indwelling catheters or intermittent catheters and is rarely useful.8
Our case is unique to the literature in that it is the first documented case in which a patient experienced recurrent AD secondary to bladder cancer. In our case, the patient had local resection of his tumor, and a nephrostomy tube placed to alleviate his hydronephrosis. This decreased the frequency of his episodes of AD, but it was not until he had definitive surgery with removal of his bladder and creation of a urinary reservoir that his episodes of AD completely resolved. Based on the resolution of his symptoms after his bladder was removed, and not after the hydronephrosis was alleviated with the nephrostomy tube, it is our opinion that the primary precipitant of his episodes of AD was the local irritation and spasms of the bladder secondary to the large, invasive tumor. This case also highlights the aggressiveness of bladder cancer in that ultrasound imaging was normal with no bladder wall thickening and no hydronephrosis just months before his presentation with recurrent AD suggesting that repeating the imaging is paramount to detection.
Unfortunately, there is a paucity of literature regarding bladder cancer risk in patients with neurogenic bladders. Bothig et al. found that SCI patients with bladder cancer presented at a younger age and with more invasive disease than control patients from the general population. They also demonstrated an increased risk of the histologic variant Squamous Cell Carcinoma (SCC) in SCI patients who are catheter dependent. Their findings support the notion that neurogenic bladder plays a role in the long term risk for bladder cancer, but to what extent remains unknown.8 Unfortunately, no protocol for bladder cancer screening, using cystoscopy, urine tumor markers or urinary cytology has been developed despite a recent systematic review.11 A study by Sammer et al. found that surveillance cystoscopies may be warranted in patients with neurogenic bladder, but the exact frequency and initiation of screening remain unknown.12 It does appear that the time since injury influences bladder cancer risk, as the vast majority of SCI patients that developed bladder cancer did so more than ten years after their initial injury.8
Conclusion
Our patient represents the first documented case of bladder cancer as a cause of autonomic dysreflexia, and brings to light the importance of fully investigating patients with recurrent episodes of AD. Episodes of autonomic dysreflexia may be a blessing in disguise for SCI patients, as they may indicate there is underlying pathology, such as a ureteric calculus or even a malignancy, in patients who are otherwise deprived of normal sensation. Our case highlights the importance of a thorough workup of patients with recurrent AD to identify rare but potentially serious precipitants that may otherwise remain clinically silent. In the absence of an accepted surveillance protocol, patients with recurrent unexplained AD require a full work up to rule out non genitourinary causes, as well as a cystoscopy, urodynamics and upper tract imaging (ultrasound, CT, MRI) by a specialized Urologist to rule out an urinary tract causes.
Disclaimer statements
Contributors: None
Conflict of Interest Statement: Both authors have no conflicts of interest to disclose.
Ehics approval: None
Funding Statement
The authors received no financial support in the writing of this paper.
References
- 1.Bycroft J, Shergill IS, Choong EAL, Arya N, Shah P.. Autonomic dysreflexia: a medical emergency. Postgrad Med J 2005;81(954):232-5. doi: 10.1136/pgmj.2004.024463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shergill IS, Arya M, Hamid R, et al. The importance of autonomic dysreflexia to the Urologist. Br J Urol 2004;93:923–6. doi: 10.1111/j.1464-410X.2003.04756.x [DOI] [PubMed] [Google Scholar]
- 3.Trop CS, Bennett C.. Autonomic dysreflexia and its urological implications: a review. J Urol 1991;146:1461-9. doi: 10.1016/S0022-5347(17)38140-5 [DOI] [PubMed] [Google Scholar]
- 4.Vaidyanathan S, Soni BM, Sett P, et al. Pathophysiology of autonomic dysreflexia: long-term treatment with terazosin in adult and paediatric spinal cord injury patients manifesting recurrent dysreflexic episodes. Spinal Cord 1998;36:761-70. doi: 10.1038/sj.sc.3100680 [DOI] [PubMed] [Google Scholar]
- 5.Wein A, Kavoussi L, Partin A, Peters C.. Campbell-Walsh Urology. 11th ed. Elsevier; 2016. [Google Scholar]
- 6.Karlsson A. Autonomic dysreflexia. Spinal Cord 1999;37:383-91. doi: 10.1038/sj.sc.3100867 [DOI] [PubMed] [Google Scholar]
- 7.Sugimura T, Arnold E, English S, Moore J.. Chronic suprapubic catheterization in the management of patients with spinal cord injuries: Analysis of upper and lower urinary tract complications. BJU Int 2008;101(11):1396-1400. doi: 10.1111/j.1464-410X.2007.07404.x [DOI] [PubMed] [Google Scholar]
- 8.Böthig R, Kurze I, Fiebag K, et al. Clinical characteristics of bladder cancer in patients with spinal cord injury: the experience from a single centre. Int Urol Nephrol 2017;49(6):983-94. doi: 10.1007/s11255-017-1570-6 [DOI] [PubMed] [Google Scholar]
- 9.Wan D, Krassioukov AV.. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J Spinal Cord Med 2014;37(1):2-10. doi: 10.1179/2045772313Y.0000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaidyanathan S, Soni BM, Oo T, Hughes PL, Singh G, Mansour P.. Delayed complications of discontinuation of intrathecal baclofen therapy: Resurgence of dyssynergic voiding, which triggered off autonomic dysreflexia and hydronephrosis. Spinal Cord 2004;42(10):598-602. doi: 10.1038/sj.sc.3101631 [DOI] [PubMed] [Google Scholar]
- 11.Cameron AP, Rodriguez GM SK.. Systematic review of urological followup after spinal cord injury. J Urol 2012;187:391-7. doi: 10.1016/j.juro.2011.10.020 [DOI] [PubMed] [Google Scholar]
- 12.Sammer U, Walter M, Knüpfer SC, Mehnert U, Bode-Lesniewska B KT. Do we need surveillance ure- thro-cystoscopy in patients with neurogenic lower urinary tract dysfunction? PLoS One 2015;10:e0140970. [DOI] [PMC free article] [PubMed] [Google Scholar]