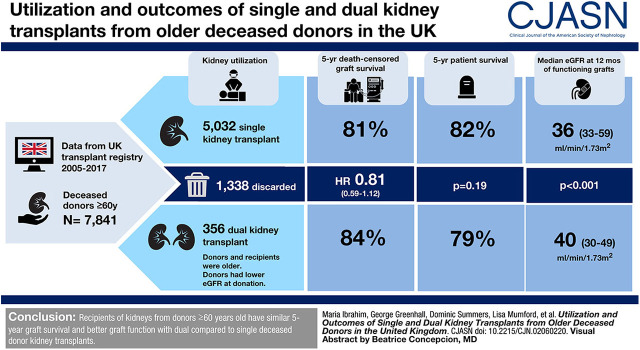
Visual Abstract
Keywords: transplant outcomes, transplantation, survival, renal transplantation, organ transplant, kidney transplantation, kidney donation, kidney donor, utilization, deceased donor
Abstract
Background and objectives
Kidneys from elderly deceased donors are often discarded after procurement if the expected outcomes from single kidney transplantation are considered unacceptable. An alternative is to consider them for dual kidney transplantation. We aimed to examine the utilization of kidneys from donors aged ≥60 years in the United Kingdom and compare clinical outcomes of dual versus single kidney transplant recipients.
Design, setting, participants, & measurements
Data from the United Kingdom Transplant Registry from 2005 to 2017 were analyzed. We examined utilization rates of kidneys retrieved from deceased donors aged ≥60 years, and 5-year patient and death-censored graft survival of recipients of dual and single kidney transplants. Secondary outcomes included eGFR. Multivariable analyses and propensity score analysis were used to correct for differences between the groups.
Results
During the study period, 7841 kidneys were procured from deceased donors aged ≥60 years, of which 1338 (17%) were discarded; 356 dual and 5032 single kidneys were transplanted. Donors of dual transplants were older (median, 73 versus 66 years; P<0.001) and had higher United States Kidney Donor Risk Indices (2.48 versus 1.98; P<0.001). Recipients of dual transplants were also older (64 versus 61 years; P<0.001) and had less favorable human leukocyte antigen matching (P<0.001). After adjusting for confounders, dual and single transplants had similar 5-year graft survival (hazard ratio, 0.81; 95% CI, 0.59 to 1.12). No difference in patient survival was demonstrated. Similar findings were observed in a matched cohort with a propensity score analysis method. Median 12-month eGFR was significantly higher in the dual kidney transplant group (40 versus 36 ml/min per 1.73 m2; P<0.001).
Conclusions
Recipients of kidneys from donors aged ≥60 years have similar 5-year graft survival and better graft function at 12 months with dual compared with single deceased donor kidney transplants.
Introduction
The shortage of kidneys for transplantation has led to global efforts to increase the utilization of deceased donor kidneys. This has resulted in the increased use of kidneys from older, more marginal donors (1). One approach to make use of such kidneys is dual adult kidney transplantation. This technique is based on the concept of increased nephron mass, because glomerular function declines with age (2–5). Given the increasing age of the deceased donor population and concerns about high discard rates (1,6,7), the appropriate utilization of organs from older donors is of growing importance.
Deciding which kidneys from older donors should be implanted as two single transplants, as a dual transplant, or which should be discarded is challenging. Although some centers in the United Kingdom and internationally use preimplantation histologic evaluation to help identify which kidneys should be implanted as duals (2,8,9), no clinical or histologic parameters have been widely accepted to aid utilization decisions (10–16). There is also little clinical guidance regarding recipient selection, and many offering policies for kidneys from older donors are based on donor criteria alone (17,18). Additional considerations for dual kidney transplantation include increased technical difficulty and longer time under anesthesia (16,19,20). These factors must be weighed against the significant morbidity and mortality associated with remaining on the transplant waiting list (21).
The United Kingdom transplants a higher percentage of kidneys as dual transplants than the United States (4% versus 2% in 2017) (7), in keeping with the higher proportion of transplanted kidneys from elderly donors (11% versus 0.6% in 2005–2017) (7). The increasing use of deceased donor kidneys from older donors and donation after circulatory death (DCD) donors (22,23) renders analysis of current United Kingdom outcome data increasingly important. Acceptable outcomes after dual kidney transplantation would support the expanded use of organs otherwise perceived to be highly marginal and might reduce unnecessary discard of these organs.
Our study characterizes the practice of dual kidney transplantation in the United Kingdom in a recent cohort of deceased donors aged ≥60 years. We aimed to examine the utilization rate of kidneys procured from older donors and compare the clinical outcomes of dual and single kidney transplants from such donors. Two statistical techniques were used to adjust for baseline differences between dual and single transplant groups. Subgroup analyses were used to determine if donor criteria could be used to identify organs best used as dual transplants.
Materials and Methods
Study Population
We performed a retrospective cohort study of patients with either a single or dual kidney transplant in the United Kingdom from January 1, 2005 to December 31, 2017. Our data source was the United Kingdom Transplant Registry (UKTR) held by National Health Service Blood and Transplant (NHSBT). To reduce confounding, we restricted our cohort for all analyses to donors aged ≥60 years. We included recipients aged ≥18 years who received their first kidney(s)-only graft. Patients with previous extrarenal solid organ grafts were included.
There are no United Kingdom organ offering pathways that necessitate the use of kidneys from adult donors as dual transplants. The UKTR does not record the reasons for use of kidneys as dual transplants, or whether a preimplantation biopsy was performed.
Study Parameters and Definitions
Organ discard rates were examined by analyzing kidneys from donors aged ≥60 years, procured for the purposes of transplantation. We defined dual kidney transplants as the implantation of two kidneys from the same donor into a recipient. Calculated reaction frequency reflects the human leukocyte antigen (HLA) sensitization of a recipient and is the percentage of blood group–identical, HLA antibody–incompatible donors in the last 10,000 deceased donors in the United Kingdom. Patients were considered highly sensitized if their calculated reaction frequency was ≥85%. Cold ischemia time was defined as the duration between the time the organ was flushed with cold preservation fluid in situ to the time of organ reperfusion with recipient blood. For dual kidney transplants, the time of perfusion of the first kidney implanted was used to calculate the cold ischemia time.
If ethnicity was unknown, patients were assumed to be non-Black when calculating eGFR. Only recipients with a functioning transplant at the relevant time point were considered in analyses of graft function. Primary nonfunction was defined as a graft that never functioned sufficiently to allow independence from dialysis, irrespective of cause. Loss of one kidney in a dual kidney transplant was not considered primary nonfunction. Delayed graft function was defined as the requirement for dialysis in the first postoperative week, regardless of cause. “Graft survival” refers to death-censored graft survival and was defined as the time from transplantation to return to long-term KRT or retransplantation, whichever occurred first, with data censored at the time of death or at last known follow-up. Patient survival was the time from transplantation to death or last known follow-up.
eGFR, expressed in ml/min per 1.73 m2, was calculated using the four‐variable Modification of Diet in Renal Disease formula (24), which is in keeping with the eGFR recorded on the United Kingdom donor data forms at the time of organ offering. Donor eGFR was also calculated using the Cockcroft–Gault equation to account for differences in donor size, which may be representative of nephron mass (25). Because the United Kingdom Kidney Donor Risk Index does not consider changes in age-related donor risk in donors aged >60 years (26), the United States Kidney Donor Risk Index (USKDRI) was used in this study as per the original Rao index, without scaling to the normalization factor (27). The United States Kidney Donor Profile Indices (USKDPIs) were calculated from the appropriate KDRIRAO mapping table using data from 2007 to 2017 (27). A discarded kidney was defined as an organ that had been retrieved for the purposes of transplantation but not subsequently implanted.
Statistical Analyses
Categorical data were summarized using proportions, and unadjusted analyses were performed using chi-squared testing. Continuous data were reported using medians and interquartile ranges (IQRs), and unadjusted analyses were performed using Wilcoxon testing.
Analyses of graft survival were conducted using Kaplan–Meier survival estimates and compared using the log-rank test. We then performed multivariable analyses by fitting a Cox proportional hazards model to account for factors known to affect graft outcome, based on a combination of clinical knowledge and factors previously shown to affect graft survival (6,26). These included donor age, type (donation after brain death [DBD] or DCD), and history of hypertension; recipient age, waiting time to transplant, ethnicity, and primary kidney disease; and HLA mismatch and time period (6). Time period was arbitrarily categorized into three groups of equal duration (2006–2009, 2010–2013, and 2014–2017). Exploratory analyses were conducted to identify a donor age or USKDRI value at which graft survival was statistically significantly better in the dual cohort than the single kidney transplant cohort, suggesting a means of identifying organs more suitable for implantation as duals. We examined the effect of dual and single kidney transplantation on 12-month eGFR using a multivariable linear regression model, adjusting for the same covariates that were used to assess graft survival.
We also used propensity score analysis to correct for covariates and to crosscheck the findings of the Cox model (28–30). After identifying potentially confounding factors that differ significantly between the two cohorts, a propensity score was obtained for all patients using a logistic regression model, which represents the probability of undergoing a dual versus single kidney transplant. For each dual transplant recipient, a recipient of single transplants was then matched, and the covariates tested in the adjusted sample to ensure sufficient balance was obtained. From this data set, survival analysis was conducted using a Kaplan–Meier survival estimate. The matched cohort was adjusted for donor age, type, sex, and cause of death; recipient age, waiting time to transplant, ethnicity, primary kidney disease, and calculated reaction frequency; and donor/recipient HLA mismatch and time period.
For the variable “recipient primary kidney disease,” we created a “not reported” group because a high proportion of data were missing, in keeping with the NHSBT annual report (26).
A two-tailed P value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS software, version 7.1 of the SAS Enterprise Guide (SAS Institute Inc., Cary, NC).
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Results
United Kingdom Trends in Usage of Kidney Transplants from Older Donors
During the study period, 7841 kidneys were procured from deceased donors aged ≥60 years in the United Kingdom (Supplemental Figure 7). Of these, 1338 (17%) were discarded. The number of discarded organs from donors aged ≥60 years increased over the study period; however, the proportion has remained stable (Figure 1). Of the organs that were implanted, there were 356 dual transplants and 5032 single transplants (Table 1). The median (IQR) donor age of duals was significantly higher than that of single kidney transplants (73 [68–76] versus 66 [63–70] years; P<0.001). Dual transplant donors were more likely to be DCD donors (74% versus 41%; P<0.001), male, have hypertension, a noncerebrovascular accident cause of death, and have diabetes. USKDRI was significantly higher in the dual group than the single transplant group (2.48 [2.23–2.74] versus 1.98 [1.77–2.23]; P<0.001), corresponding with a USKDPI of 99% versus 97%. Donor kidneys used for dual transplantation had lower eGFRs at donation than those used for single transplants. Kidneys that were discarded had a median (IQR) age of 69 (65–74) years, with a lower median (IQR) USKDRI when compared with those of dual transplants (2.24 [1.93–2.60] versus 2.48 [2.23–2.74]); the USKDPI was 99% (95%–100%) versus 99% (99%–100%). Similar retrieval kidney function (median [IQR] serum creatinine, 0.9 [0.7–1.2] versus 0.9 [0.7–1.1] mg/dl) was observed.
Figure 1.
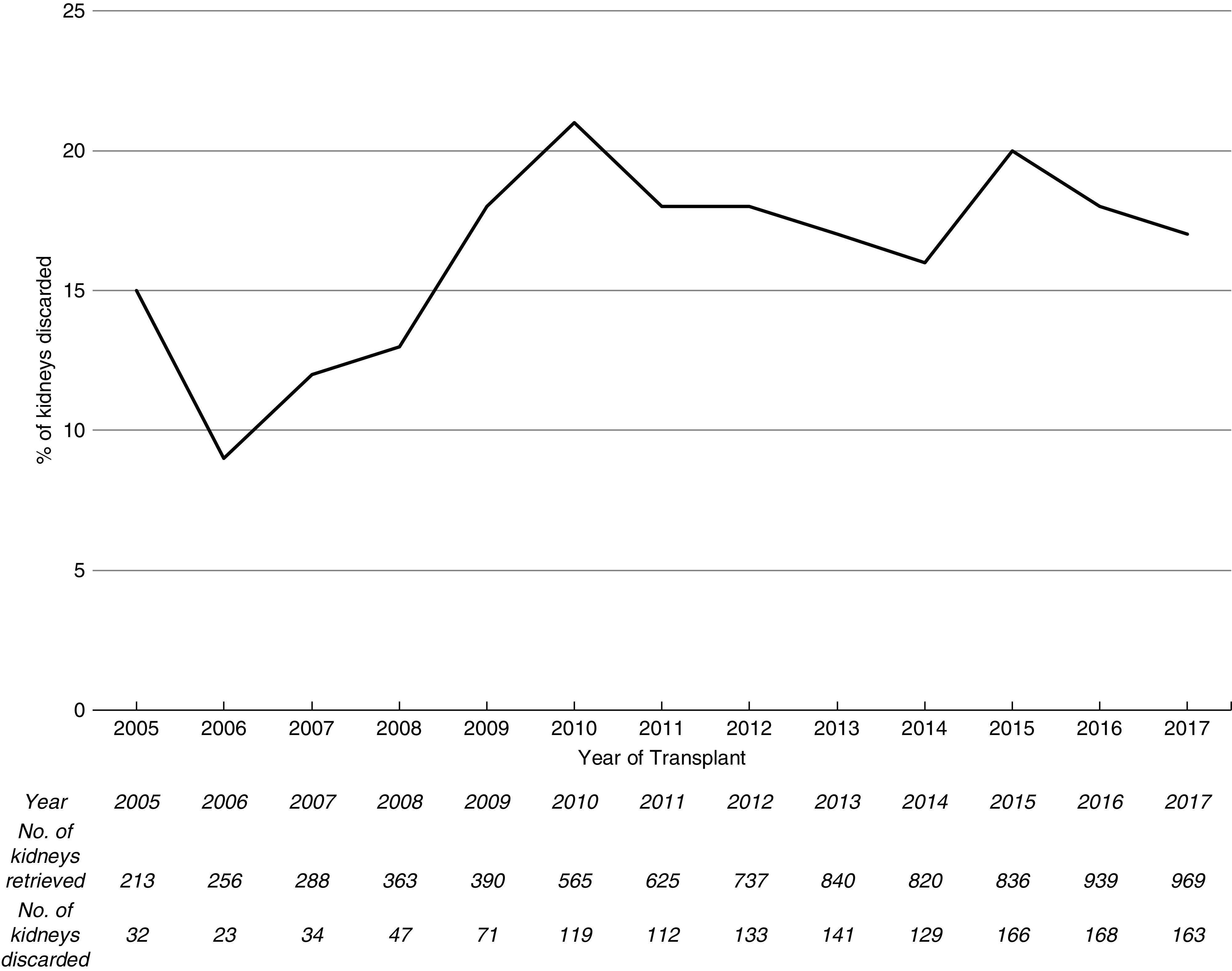
The percentage of kidneys retrieved from United Kingdom deceased donors aged ≥60 years that were discarded over the study time period has remained fairly stable. The absolute number of kidneys retrieved and number of kidneys discarded from donors aged ≥60 years and above are depicted in the table.
Table 1.
Donor, recipient, and operative variables for single and dual kidney transplants from donors aged ≥60 years
| Variable | Single Kidney Transplants (n=5032) | Dual Kidney Transplants (n=356) |
|---|---|---|
| Donor age (yr) | 66 (63–70) | 73 (68–76) |
| Donor type | ||
| DBD | 2870 (59%) | 84 (26%) |
| DCD | 2162 (41%) | 272 (74%) |
| Donor sex | ||
| Male | 2515 (49%) | 199 (55%) |
| Female | 2517 (51%) | 157 (45%) |
| Donor hypertension | ||
| Yes | 2223 (45%) | 219 (62%) |
| No | 2727 (55%) | 132 (37%) |
| Missing | 82 (0%) | 5 (0%) |
| Donor ethnicity | ||
| White | 4884 (97%) | 343 (96%) |
| Asian | 80 (2%) | 8 (2%) |
| Black | 23 (0.5%) | 3 (0.8%) |
| Other | 28 (0.6%) | 0 (0%) |
| Unknown/missing | 17 (0.3%) | 2 (0.6%) |
| Donor cause of death | ||
| Cerebrovascular accident | 3573 (71%) | 199 (56%) |
| Road traffic accident | 57 (1%) | 10 (0.2%) |
| Miscellaneous | 1263 (25%) | 138 (39%) |
| Other trauma | 139 (3%) | 9 (0.3%) |
| Donor diabetes | ||
| No | 4497 (89%) | 289 (81%) |
| Yes | 433 (9%) | 57 (16%) |
| Unknown/missing | 102 (2%) | 10 (3%) |
| Donor retrieval creatinine (mg/dl) | 0.8 (0.6–1.0) | 0.9 (0.7–1.1) |
| Donor retrieval eGFR (MDRD) (ml/min per 1.73 m2) | 90 (70–114) | 83 (62–107) |
| Donor retrieval eGFR (Cockcroft–Gault) (ml/min) | 89 (70–113) | 75 (58–98) |
| UKKDRI | 1.51 (1.47–1.56) | 1.52 (1.47–1.56) |
| Missing | 54 (1%) | 7 (2%) |
| USKDRI | 1.98 (1.77–2.23) | 2.48 (2.23–2.74) |
| USKDPI (%) | 95 (90–95) | 99 (95–100) |
| Missing | 421 (8%) | 30 (8%) |
| Recipient age (yr) | 61 (52–67) | 64 (58–69) |
| Recipient sex | ||
| Male | 3187 (63%) | 239 (66%) |
| Female | 1842 (37%) | 116 (33) |
| Missing | 3 | 1 |
| Recipient ethnicity | ||
| White | 3808 (76%) | 278 (78%) |
| Asian | 754 (14%) | 44 (12%) |
| Black | 350 (6%) | 25 (7%) |
| Other | 75 (1%) | 8 (2%) |
| Unknown/missing | 120 (2%) | 9 (3%) |
| Recipient BMI (kg/m2) | 26 (23–30) | 27 (24–29) |
| Missing | 1234 (25%) | 79 (22%) |
| Primary kidney disease | ||
| Diabetes | 629 (13%) | 49 (14%) |
| GN | 83 (2%) | 3 (1%) |
| Cystic kidney diseasea | 710 (14%); 2020 (40%) | 29 (8%) |
| Other | 1590 (32%) | 179 (50%) |
| Not reported | 96 (27%) | |
| Recipient waiting time (d) | 990 (417–1418) | 703 (255–1005) |
| Recipient calculated reaction frequency | ||
| 0%–85% | 4775 (91%) | 350 (96%) |
| >85% | 457 (9%) | 14 (4%) |
| HLA mismatch levelb | ||
| 1 | 460 (9%) | 5 (1%) |
| 2 | 1328 (26%) | 58 (16%) |
| 3 | 2696 (54%) | 201 (56%) |
| 4 | 548 (11%) | 92 (26%) |
| Recipient dialysis status at time of transplant | ||
| Hemodialysis | 3007 (65%) | 219 (63%) |
| Peritoneal dialysis | 1045 (23%) | 81 (23%) |
| Pre-emptive | 13 (0%) | 1 (0%) |
| Missing | 561 (12%) | 49 (14%) |
| Cold ischemia time (h) | 14.4 (11.4–17.8) | 14.5 (11.9–17.3) |
| Missing | 38 (0.8%) | 4 (1%) |
| Warm ischemia time (mins)c | 31 (25–43) | 32 (25–45) |
| Missing | 473 (27%) | 64 (23%) |
Continuous data are expressed as median (interquartile ranges), categoric variables as n (%). DBD, donation after brain death; DCD, donation after circulatory death; MDRD, Modification of Diet in Renal Disease; UKKDRI, United Kingdom Kidney Donor Risk Index; USKDRI, United States Kidney Donor Risk Index; USKDPI, United States Kidney Donor Profile Index; BMI, body mass index.
Cystic kidney disease, includes dominant and recessive types.
HLA mismatch level 1 (-A; -B; -DR), 000; HLA mismatch level 2, 100, 010, 110, 200, 210; HLA mismatch level 3, 020, 120, 220, 001, 101, 201, 011, 111, 211; HLA mismatch level 4, 020, 121, 221, 002, 102, 202, 012, 112, 212, 022, 122, 222.
Warm ischemia time includes results from controlled DCD donors only from 2011 onward (1730 single and 278 dual kidney transplants). Calculated as time from donor withdrawal of life-sustaining treatment to donor crossclamp during organ procurement.
Recipients of dual transplants were older than recipients of single transplants (64 versus 61 years; P<0.001) and waited a shorter time for transplantation (703 versus 990 days; P<0.001). Recipients of dual transplants also had less favorable HLA matching than those that received single kidney transplants and were less likely to be highly sensitized.
There was an initial rise in the number of dual transplants performed from donors aged ≥60 years with a peak of 61 per annum in 2013, followed by a decline to 34 per annum in 2017 (Figure 2). There were wide variations in the number of dual transplants performed between United Kingdom adult kidney transplant centers. In nine centers, >5% of all deceased donor transplants from donors ≥60 years were dual transplants; however, five centers performed no dual transplants during the study period (Supplemental Figure 1).
Figure 2.
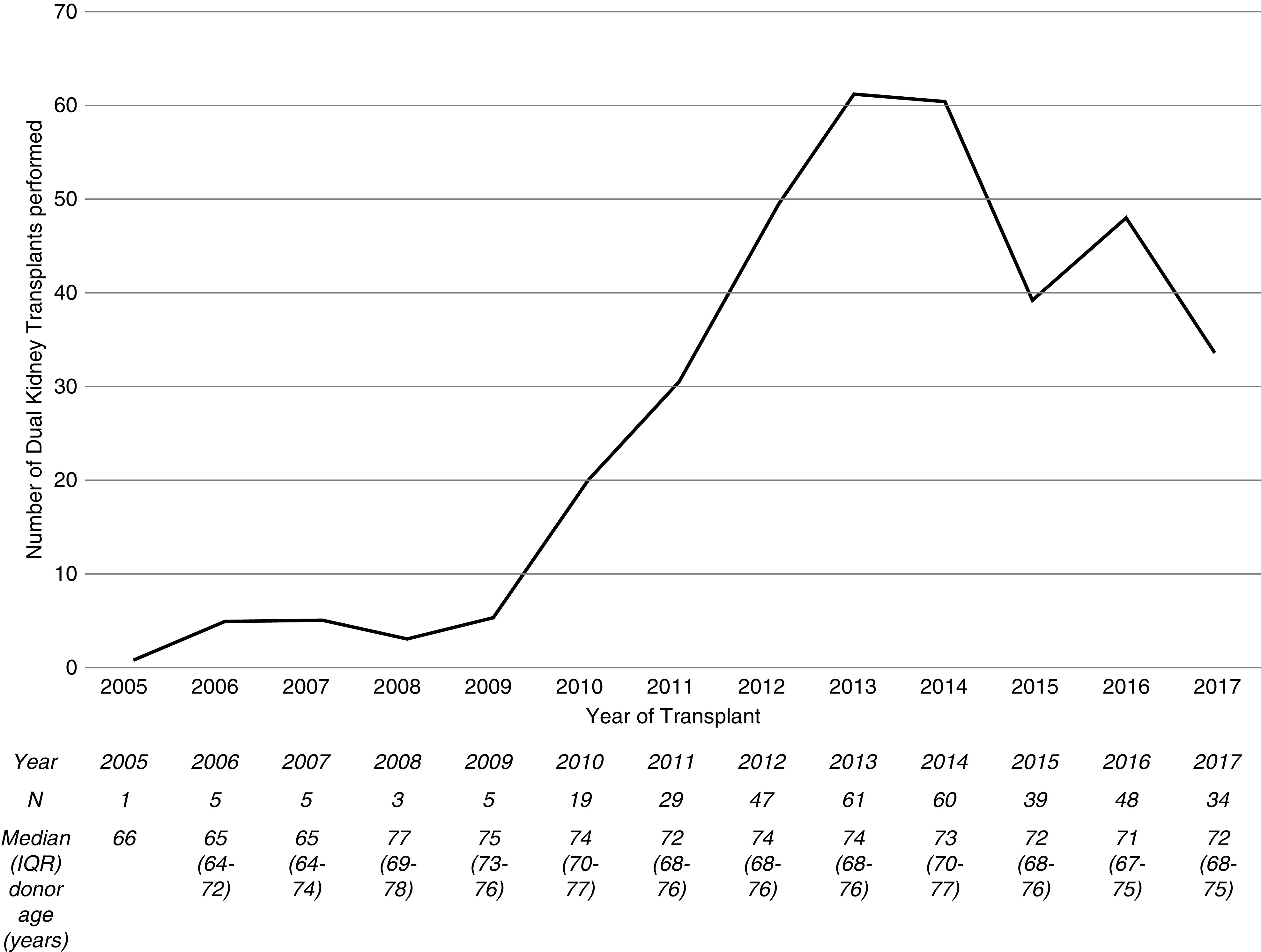
Line graph and table showing the increasing number of dual kidney transplants performed from donors aged ≥60 years in the United Kingdom from 2005 to 2014, followed by a decline in recent years. The median dual transplant donor age has gradually increased. IQR, interquartile range.
Organ utilization data were re-examined to determine if the declining usage of dual transplants since 2013 was due to a reduction in the number of elderly donors or an increase of single versus dual transplants (Supplemental Figure 2). The proportion of duals performed decreased across all donor age groups between the 2 years, whereas the proportion of kidneys discarded was similar for all donor age groups in 2013 and 2017 (excluding donors aged ≥80 years where donor numbers were small).
Graft Function
The rates of delayed graft function were similar in the dual and single kidney transplant groups (36% versus 31%; P=0.31). In DCD donor transplants, delayed graft function occurred in 37% of the single cohort and 35% of the dual transplant cohort (P=0.31). For DBD donor transplants, delayed graft function rates were 30% in both cohorts (P=0.14) (Supplemental Table 1). Overall, patients in the single transplant cohort had a primary nonfunction rate of 4% (13/356) compared with 3% (9/356) in the dual cohort (P=0.34).
For functioning grafts, the median (IQR) 1-year eGFRs were significantly higher in the dual than the single kidney transplant group (46 [33–59] ml/min per 1.73 m2 versus 39 [30–49] ml/min per 1.73 m2; P<0.001). The 3-year eGFRs were also higher (45 [35–58] ml/min per 1.73 m2 versus 40 [30–50] ml/min per 1.73 m2; P≤0.001). Dual kidney transplant recipients had higher median 12-month eGFRs than those with single transplants for all donor age groups (Table 2). The 3-year eGFRs showed a similar pattern of outcomes (Supplemental Table 2). There was no statistically significant difference in median (IQR) 3-year eGFRs of functioning dual kidney transplants (or single kidney transplants) when stratified by DBD/DCD donor type (data not shown). After adjusting for donor and recipient factors by using a multivariable linear regression model, 12-month eGFR remained 9 ml/min per 1.73 m2 higher in the dual kidney transplant group versus singles (95% CI, 7.70 to 11.50; P<0.001).
Table 2.
Median (interquartile range) 12-mo eGFR of functioning single and dual kidney transplants, by donor age
| Donor Age (yr) | Single Kidney Transplants | Dual Kidney Transplants | P Value | ||
|---|---|---|---|---|---|
| 12-mo eGFR (ml/min per 1.73 m2) | Frequency (%) | 12-mo eGFR (ml/min per 1.73 m2) | Frequency (%) | ||
| 60–64 | 42 (32–52) | 1642 (33) | 48 (36–58) | 38 (11) | 0.03 |
| 65–69 | 39 (30–49) | 1426 (28) | 42 (27–54) | 64 (18) | 0.24 |
| 70–74 | 37 (28–46) | 731 (15) | 46 (33–64) | 74 (21) | <0.001 |
| 75–79 | 36 (27–45) | 282 (6) | 47 (32–59) | 100 (28) | <0.001 |
| 80+ | 29 (22–40) | 22 (0) | 43 (27–54) | 20 (6) | 0.02 |
| Failed | 419 (8) | 28 (8) | |||
| Missing | 510 (10) | 32 (9) | |||
To consider whether dual kidney transplants might have been used as two single kidney transplants, we examined the proportion of kidneys with an eGFR ≥60 ml/min per 1.73 m2 at 1 year. There were 197 dual transplants from donors aged ≥60 years where follow-up at 3 years was achieved and the patient was free of dialysis; of these patients, 47 (24%) had an eGFR of ≥60 ml/min per 1.73 m2.
Graft and Patient Survival
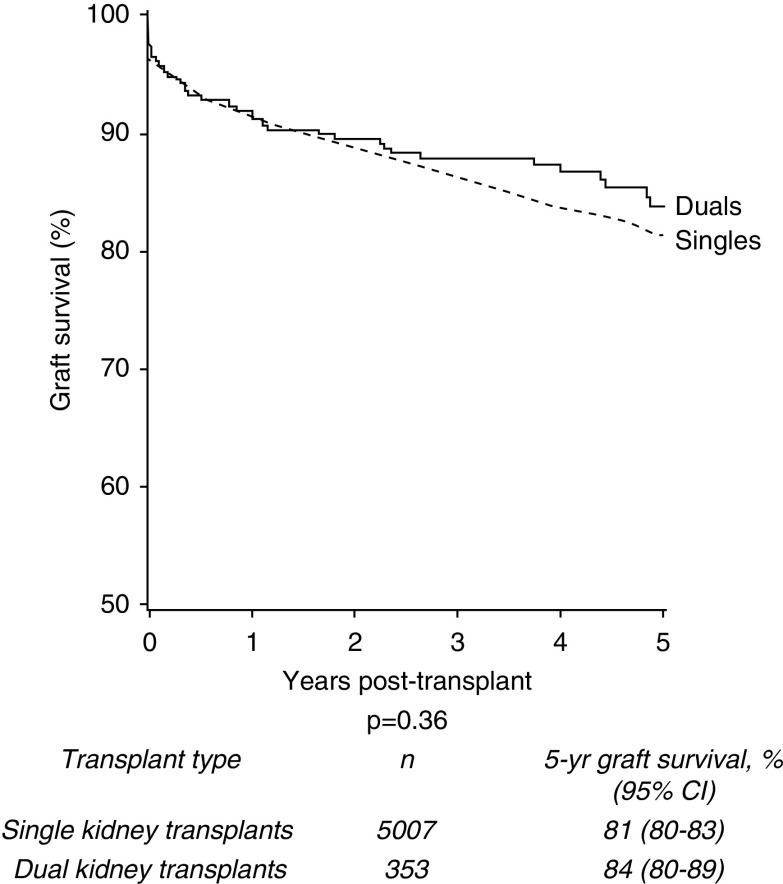
For donors aged ≥60 years, 5-year graft survival in the dual transplant group was 84% compared with 81% in the single kidney transplant cohort (P=0.36) (Figure 3). Donor cohorts were further stratified to explore values at which graft survival was superior in the dual than the single kidney transplant group.
Figure 3.
No significant difference in 5-year death-censored graft survival of single versus dual kidney transplants from deceased donors aged ≥60 years, 2005–2017.
Firstly, groups were stratified by donor age. No statistically significant differences in 5-year graft survival were seen when comparing dual and single transplants from donors aged ≥70 years (85% and 81%, respectively; P=0.25; see Supplemental Figure 3) or donors aged ≥75 years (85% and 81%, respectively; P=0.28; data not shown). Groups were then stratified by USKDRI using the median USKDRI of duals of 2.5 (USKDPI 99%) as an initial cutoff point; however, no statistically significant difference was observed when stratified on unadjusted analysis (P=0.90; Supplemental Figure 4). Further stratification of high-risk USKDRI groups using a higher cutoff of 2.75 demonstrated no statistically significant difference on unadjusted analysis (P=0.40; Supplemental Figure 5). Finally, the cohort was also stratified by recipient age above and below 70 years. Again, no statistically significant difference was demonstrated (P=0.81; Supplemental Figure 6).
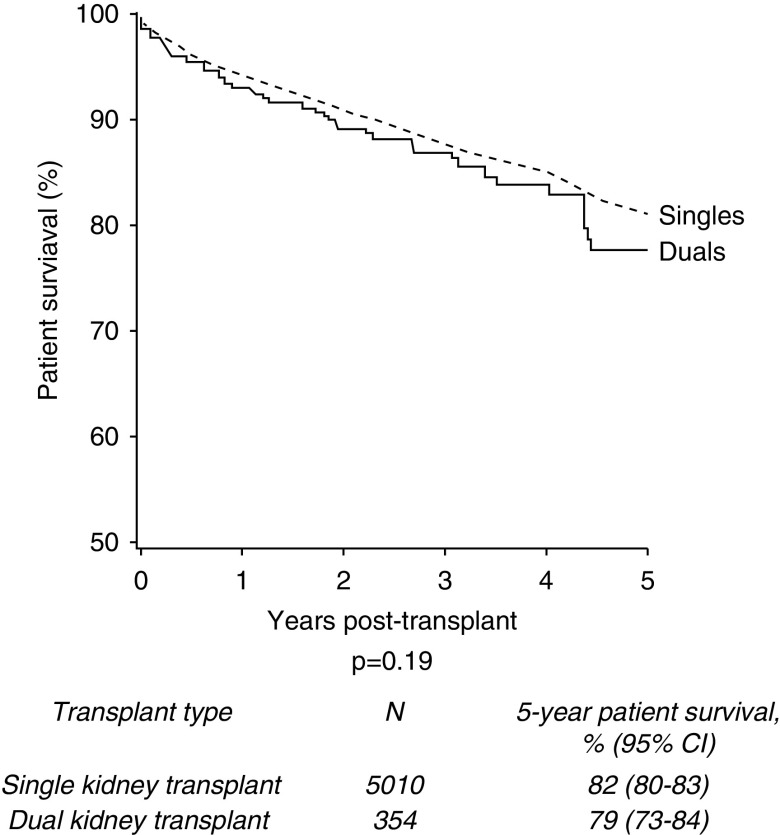
The 5-year patient survival was 82% in those who underwent single kidney transplants (n=4108/5010) compared with 79% in the dual group (n=280/354) (P=0.19; Figure 4). Of the deceased patients, 76% (n=496) of single kidney transplant recipients and 65% (n=36) of dual kidney transplant recipients died with a functioning graft (P=0.08). For all-cause graft loss (where an event was defined as graft failure or death with a functioning graft), 5-year graft survival was 70% for single kidney transplant recipients and 72% for dual kidney transplant recipients (P=0.51).
Figure 4.
No significant difference in 5-year patient survival of single versus dual kidney transplants from donors aged ≥60 years, 2005–2017.
Risk Adjustment of Death-Censored Graft Survival Outcomes
A multivariable Cox proportional hazards model was fitted to adjust for the donor and recipient risk factors described above. On unadjusted testing, factors found to be associated with 5-year death-censored graft survival (P<0.001) were donor age, donor type, recipient primary kidney disease group, donor/recipient HLA mismatch level, recipient age, and recipient waiting time. These variables were then added to a multivariable model. No statistically significant difference was seen between the single and dual kidney transplant groups (hazard ratio, 0.81; 95% CI, 0.59 to 1.12; P=0.21).
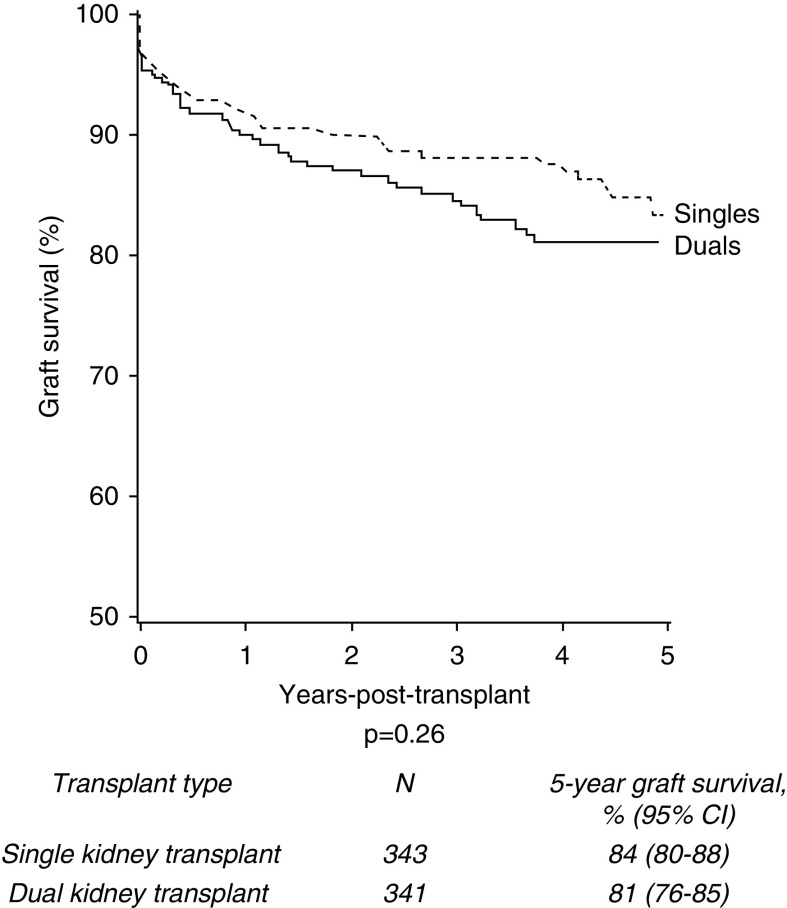
In the propensity score analysis, a matched cohort data set of 353 recipients of dual kidney transplants and an equally matched cohort of single kidney transplants was created. Unadjusted survival analysis demonstrated no difference in 5-year death-censored graft survival between the two groups (Figure 5).
Figure 5.
In a propensity score matched cohort, 5-year death-censored graft survival of single and dual kidney transplants from deceased donors aged ≥60 years remains similar.
Discussion
This is the largest national registry analysis examining outcomes of dual kidney transplants outside of the United States (31). Our study demonstrates acceptable 5-year death-censored graft survival in dual kidney transplants from older donors, despite a very high median USKDRI of 2.48 (USKDPI 100%) (32). For functioning grafts, dual transplants had superior graft function at 1 year and 3 years post-transplantation compared with single kidney transplants from similar donor age groups. In addition, rates of primary nonfunction were low. Patient survival was similar in recipients of dual and single kidney transplants from older donors, despite an older median recipient age.
Outcomes of United Kingdom dual kidney transplants compare favorably with those from United States registry analyses (33). Kidneys implanted in the United States between 2002 and 2012 from donors with USKDPIs between 90% and 100% had a death-censored graft survival of approximately 60% at 5 years (10). In this United Kingdom analysis, 5-year graft survival in the dual transplant group was 84% from donors aged ≥60 years. Other United States registry analyses included dual kidneys transplanted before the year 2000, making comparisons less valid (14,34). The median KDRI of both single and dual kidneys transplanted from older donors in this study was much higher than that of discarded kidneys in the United States of 1.78 (35), suggesting at least some of those kidneys could have been implanted with reasonable outcomes. These findings are of increasing relevance currently given the increasing discard rate of kidneys in the United States (36) and ongoing emphasis on strategies to reduce this (37). In contrast, our study has shown that discard rates of kidneys from older donors have been stable in the United Kingdom for the last 5 years, despite significant increases in the numbers of kidneys procured.
A unique finding of our study is the high proportion of dual transplants (74%) from DCD donors. The reason for this could lie in the differences between the DBD and DCD organ offering systems in the United Kingdom during the study period. Before 2014, both kidneys from DCD donors were offered to the local center to be implanted into the recipient(s) of their choice. It may also reflect a perception early in the study period that kidneys from older DCD donors were of higher risk for poorer long-term graft outcomes than those from DBD donors of an equivalent age. Subsequent studies have shown that these concerns appear to be unfounded in the United Kingdom (23,38). On occasion, surgeons may have implanted two DCD donor kidneys from the same donor as a dual rather than two single transplants if the anticipated cold ischemic time of the single organ for the second recipient was adjudged to have been unacceptable (23,38).
Of note, >20% of recipients with functioning dual kidney transplants in our study had an eGFR of >60 ml/min per 1.73 m2 at 3 years post-transplant, suggesting these kidneys could potentially have been successfully implanted as single kidney transplants into two recipients, which is preferable from an organ utilization perspective. This challenges the adequacy of the current preoperative assessment of deceased donor kidneys (39,40) and the post-transplant eGFR threshold that needs to be achieved to optimize outcomes in older recipients of deceased donor kidneys.
Our data suggest there has been a move away from using kidneys from very elderly deceased donors as dual kidney transplants in recent years in the United Kingdom (Figure 2, Supplemental Figure 2). Dual transplantation activity is highly variable across United Kingdom transplant units. There are at least two possible reasons for the recent decline in dual kidney transplantation. Firstly, falling usage of dual kidney transplantation may reflect ongoing uncertainties regarding identifying kidneys suitable for single kidney transplants, dual kidney transplant, or organ discard. We were unable to identify a donor criterion that was associated with higher death-censored graft survival in dual kidney transplants over single kidney transplants. These findings contrast with those of Klair et al. (34) who showed that kidneys from donors with USKDRI of >2.2 had a superior graft survival when implanted as a dual transplant, and Tanriover et al. (10) who identified a graft survival benefit with a USKDPI threshold of 90%.
Currently, there are no widely accepted criteria that reliably aid utilization decisions of kidneys from older deceased donors. The use of preimplantation kidney biopsies to identify and quantify chronic histologic changes was not widespread in the United Kingdom during the study period (11). The evidence base underpinning this approach remains contested (8,11–13,41–43). A current United Kingdom trial aims to determine if national availability of preimplantation kidney biopsies can increase the utilization of organs from older donors (44). Other clinical thresholds such as donor terminal eGFR <60 ml/min per 1.73 m2 have also been described to aid utilization decisions, although none appear to have been widely accepted and implemented (31).
Secondly, the United Kingdom kidney offering systems underwent changes for both DBD and DCD donor in the study time period (23,45,46). After 2014 (38), kidneys from DCD donors aged <65 years were offered as single kidney transplants for named patients, reducing the ability of centers to implant both kidneys from such donors as dual kidney transplants. This, however, does not explain the changing utilization patterns of organs from DCD donors aged >65 years, which are still offered to centers for implantation as single or dual as they deem appropriate. Recent changes to the United Kingdom deceased donor kidney offering scheme may affect this trend (47).
We acknowledge the limitations of this study. Some important variables are not recorded in the UKTR data set, including the rates of single graft nephrectomy in the dual kidney transplant group, detailed pretransplant recipient comorbidities, second kidney cold ischemia times in dual kidney transplants, post-transplant complications, and post-transplant quality of life. The retrospective nature of this study does not allow the complexities of clinical decision making to be captured, both for the utilization decision between dual and single kidney transplantation and for recipient selection, as well as factors that may have influenced this decision (e.g., donor age, donor kidney function, histologic scores, kidney size, presence of parenchymal scars, etc.). The relatively small number of dual kidney transplants in comparison to single kidney transplants during the study period may have limited our ability to identify a donor criterion to aid organ utilization. Also, it has previously been shown that there is a large cardiovascular burden on recipients of dual kidney transplants (48–50); however, in this study, we were unable to reliably analyze recipient cause of death due to a high proportion of missing data (data not shown).
This study provides reassurance that implantation of kidneys from older deceased donors in the United Kingdom leads to acceptable short- and medium-term outcomes. Despite very high median USKDPIs and apparently highly unfavorable donor risk indices, dual kidney transplants from older donors in our study resulted in higher post-transplant eGFRs than those from single kidney transplants, with similar graft and patient survival rates. These outcomes support the cautious expansion of dual kidney transplantation into selected recipients aged <60 years and are particularly relevant given the increasing age of deceased kidney donors and high discard rate of such organs globally (7).
Robust tools are needed to aid clinical organ utilization decisions, along with the evidence base to support the thresholds for improved quality of life and patient survival on which these tools should be evaluated. Dual kidney transplants in the United Kingdom allow the use of organs that may otherwise be discarded, with acceptable post-transplant results.
Disclosures
All authors have nothing to disclose.
Funding
D.M. Summers reports receiving grants from the National Institute for Health Research during the conduct of the study.
Supplementary Material
Acknowledgments
We thank the United Kingdom deceased donor kidney donation and transplant communities for providing the data on which the UKTR is based.
Dr. Maria Ibrahim carried out statistical analysis and contributed to the writing of this paper; Ms. Lisa Mumford contributed to the statistical analysis and writing of the manuscript; Mr. Niaz Ahmad, Dr. Richard Baker, Prof. John Forsythe, Dr. George Greenhall, Ms. Rachel Johnson, Mr. Gavin Pettigrew, and Mr. Dominic M. Summers contributed to the writing of this manuscript; and Mr. Chris Callaghan coinitiated the project with Mr. Niaz Ahmad, both are joint senior authors and have participated in the writing of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02060220/-/DCSupplemental.
Supplemental Table 1. Early graft function following deceased donor kidney transplantation from donors aged 60 years and older, stratified by donor type.
Supplemental Table 2. Recipient median (IQR) 3-year eGFR of functioning single and dual kidney transplants, by donor age.
Supplemental Figure 1. Percentage of dual kidney transplants performed between 2005 and 2017, by UK adult kidney transplant centre, of the total number of deceased donor kidney transplants (single and dual kidney transplants) from donors aged 60 years and above.
Supplemental Figure 2. Proportion of kidneys implanted as a single kidney transplant, dual kidney transplant, or discarded during 2013 and 2017, by donor age.
Supplemental Figure 3. Five-year death-censored graft survival for single and dual kidney transplants from donors aged 70 years and over.
Supplemental Figure 4. Kaplan-Meier curve showing 5-year death-censored graft survival for single and dual kidney transplants from donors aged 60 years and above, stratified by US KDRI ≥2.5.
Supplemental Figure 5. Kaplan-Meier curve showing 5-year death-censored graft survival for single and dual kidney transplants from donors aged 60 years and above, stratified by US KDRI ≥2.75.
Supplemental Figure 6. Five-year death-censored graft survival for single and dual kidney transplants from donors aged 60 years and over, stratified further by recipient age.
Supplemental Figure 7. Numbers of individuals included in study.
References
- 1.Johnson RJ, Bradbury LL, Martin K, Neuberger J; UK Transplant Registry : Organ donation and transplantation in the UK-the last decade: A report from the UK national transplant registry. Transplantation 97[Suppl 1]: S1–S27, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Grinyò J, Ruggenenti P, Beatini M, Cole EH, Milford EL, Brenner BM: Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG). J Am Soc Nephrol 10: 2591–2598, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Alfrey EJ, Lee CM, Scandling JD, Pavlakis M, Markezich AJ, Dafoe DC: When should expanded criteria donor kidneys be used for single versus dual kidney transplants? Transplantation 64: 1142–1146, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Glassock RJ, Rule AD: Aging and the kidneys: Anatomy, physiology and consequences for defining chronic kidney disease. Nephron 134: 25–29, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003. [DOI] [PubMed] [Google Scholar]
- 6. National Health Service (NHS) Blood and Transplant: Annual report on kidney transplantation. Report for 2017/2018, Filton, United Kingdom, NHS Blood and Transplant, 2018.
- 7.Ibrahim M, Vece G, Mehew J, Johnson R, Forsythe J, Klassen D, Callaghan C, Stewart D: An international comparison of deceased donor kidney utilization: What can the United States and the United Kingdom learn from each other? Am J Transplant 20: 1309–1322, 2020. 31758833 [DOI] [PubMed]
- 8.Remuzzi G, Cravedi P, Perna A, Dimitrov BD, Turturro M, Locatelli G, Rigotti P, Baldan N, Beatini M, Valente U, Scalamogna M, Ruggenenti P; Dual Kidney Transplant Group : Long-term outcome of renal transplantation from older donors. N Engl J Med 354: 343–352, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Kosmoliaptsis V, Salji M, Bardsley V, Chen Y, Thiru S, Griffiths MH, Copley HC, Saeb-Parsy K, Bradley JA, Torpey N, Pettigrew GJ: Baseline donor chronic renal injury confers the same transplant survival disadvantage for DCD and DBD kidneys. Am J Transplant 15: 754–763, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, Tsapepas DS, Crew RJ, Dube GK, Sandoval PR, Samstein B, Dogan E, Gaston RS, Tanriover JN, Ratner LE, Hardy MA: Kidneys at higher risk of discard: Expanding the role of dual kidney transplantation. Am J Transplant 14: 404–415, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallon DH, Riddiough GE, Summers DM, Butler AJ, Callaghan CJ, Bradbury LL, Bardsley V, Broecker V, Saeb-Parsy K, Torpey N, Bradley JA, Pettigrew GJ: Successful transplantation of kidneys from elderly circulatory death donors by using microscopic and macroscopic characteristics to guide single or dual implantation. Am J Transplant 15: 2931–2939, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Casati C, Colombo VG, Perrino M, Rossetti OM, Querques M, Giacomoni A, Binaggia A, Colussi G: Renal transplants from older deceased donors: Use of preimplantation biopsy and differential allocation to dual or single kidney transplant according to histological score has no advantages over allocation to single kidney transplant by simple clinical indication. J Transplant 2018: 4141756, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips BL, Kassimatis T, Atalar K, Wilkinson H, Kessaris N, Simmonds N, Hilton R, Horsfield C, Callaghan CJ: Chronic histological changes in deceased donor kidneys at implantation do not predict graft survival: A single-centre retrospective analysis. Transpl Int 32: 523–534, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AP, Price TP, Lieby B, Doria C: Dual kidney allocation score: A novel algorithm utilizing expanded donor criteria for the allocation of dual kidneys in adults. Ann Transplant 21: 565–576, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Singh D, Kiberd B, Lawen J: Can the outcome of older donor kidneys in transplantation be predicted? An analysis of existing scoring systems. Clin Transplant 18: 351–356, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Rogers J, Farney AC, Orlando G, Harriman D, Reeves-Daniel A, Jay CL, Doares W, Kaczmorski S, Gautreaux MD, Stratta RJ: Dual kidney transplantation from donors at the extremes of age. J Am Coll Surg 228: 690–705, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Khalil MAM, Tan J, Khan TFT, Khalil MAU, Azmat R: Dual kidney transplantation: A review of past and prospect for future. Int Sch Res Notices 2017: 2693681, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariani A, Ferla F, De Carlis R, Rossetti O, Covucci E, Tripepi M, Concone G, Lauterio A, Mangoni I, De Carlis L: Dual kidney transplantation: Evaluation of recipient selection criteria at niguarda hospital. Transplant Proc 48: 315–318, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Seth A, Sharma A, Singh S, Pandey GS, Kenwar DB: A novel technique of dual kidney transplantation (DKT) from adult donors. Urology 130: 201–204, 2019. [DOI] [PubMed] [Google Scholar]
- 20.Ekser B, Furian L, Broggiato A, Silvestre C, Pierobon ES, Baldan N, Rigotti P: Technical aspects of unilateral dual kidney transplantation from expanded criteria donors: Experience of 100 patients. Am J Transplant 10: 2000–2007, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU: Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol 4: 1239–1245, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merion RM, Goodrich NP, Johnson RJ, McDonald SP, Russ GR, Gillespie BW, Collett D: Kidney transplant graft outcomes in 379 257 recipients on 3 continents. Am J Transplant 18: 1914–1923, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Summers DM, Watson CJ, Pettigrew GJ, Johnson RJ, Collett D, Neuberger JM, Bradley JA: Kidney donation after circulatory death (DCD): State of the art. Kidney Int 88: 241–249, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976. [DOI] [PubMed] [Google Scholar]
- 26.Watson CJ, Johnson RJ, Birch R, Collett D, Bradley JA: A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation 93: 314–318, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Austin PC: A tutorial and case study in propensity score analysis: An application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res 46: 119–151, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbour S, Djurdjev O, Gill JS, Dong JJ, Gill J: A propensity score matched analysis shows no adverse effect of early steroid withdrawal in non-diabetic kidney transplant recipients with and without glomerulonephritis. Kidney Int 96: 460–469, 2019. [DOI] [PubMed] [Google Scholar]
- 30.Callaghan CJ, Charman SC, Muiesan P, Powell JJ, Gimson AE, van der Meulen JH; UK Liver Transplant Audit : Outcomes of transplantation of livers from donation after circulatory death donors in the UK: A cohort study. BMJ Open 3: e003287, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snanoudj R, Timsit MO, Rabant M, Tinel C, Lazareth H, Lamhaut L, Martinez F, Legendre C: Dual kidney transplantation: Is it worth it? Transplantation 101: 488–497, 2017. [DOI] [PubMed] [Google Scholar]
- 32.Organ Procurement and Transplantation Network: A guide to calculating and interpreting the Kidney Donor Profle index (KDPI), Richmond, VA, US Department of Health and Human Services, 2018. Available at: https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf. Accessed April 15, 2019 [Google Scholar]
- 33.Gill J, Cho YW, Danovitch GM, Wilkinson A, Lipshutz G, Pham PT, Gill JS, Shah T, Bunnapradist S: Outcomes of dual adult kidney transplants in the United States: An analysis of the OPTN/UNOS database. Transplantation 85: 62–68, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Klair T, Gregg A, Phair J, Kayler LK: Outcomes of adult dual kidney transplants by KDRI in the United States. Am J Transplant 13: 2433–2440, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Mohan S, Chiles MC, Patzer RE, Pastan SO, Husain SA, Carpenter DJ, Dube GK, Crew RJ, Ratner LE, Cohen DJ: Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int 94: 187–198, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Israni AK, Zaun D, Hadley N, Rosendale JD, Schaffhausen C, McKinney W, Snyder JJ, Kasiske BL: OPTN/SRTR 2018 annual data report: Deceased organ donation. Am J Transplant 20[Suppl s1]: 509–541, 2020. [DOI] [PubMed] [Google Scholar]
- 37.Cooper M, Formica R, Friedewald J, Hirose R, O’Connor K, Mohan S, Schold J, Axelrod D, Pastan S: Report of National Kidney Foundation consensus conference to decrease kidney discards. Clin transplant 33: e13419, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA: Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: A cohort study. Lancet 381: 727–734, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Mittal S, Adamusiak A, Horsfield C, Loukopoulos I, Karydis N, Kessaris N, Drage M, Olsburgh J, Watson CJ, Callaghan CJ: A Re-evaluation of discarded deceased donor kidneys in the UK: Are usable organs still being discarded? Transplantation 101: 1698–1703, 2017. [DOI] [PubMed] [Google Scholar]
- 40.Dare AJ, Pettigrew GJ, Saeb-Parsy K: Preoperative assessment of the deceased-donor kidney: From macroscopic appearance to molecular biomarkers. Transplantation 97: 797–807, 2014. [DOI] [PubMed] [Google Scholar]
- 41.D’Arcy FT, O’Connor KM, Shields W, Zimmerman JA, Mohan P, Eng M, Little DM, Power R, Dorman A, Hickey DP: Dual kidney transplantation with organs from extended criteria cadaveric donors. J Urol 182: 1477–1481, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Frutos MA, Mansilla JJ, Cabello M, Soler J, Ruiz P, Lebrón M, Baena V, Hernández D: Optimization of expanded donors using dual kidney transplantation: Case-control study. Transplant Proc 44: 2060–2062, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Naesens M: Zero-time renal transplant biopsies: A comprehensive review. Transplantation 100: 1425–1439, 2016. [DOI] [PubMed] [Google Scholar]
- 44.Ayorinde JO, Summers DM, Pankhurst L, Laing E, Deary AJ, Hemming K, Wilson EC, Bardsley V, Neil DA, Pettigrew GJ: PreImplantation trial of histopathology in renal allografts (PITHIA): A stepped-wedge cluster randomised controlled trial protocol. BMJ Open 9: e026166, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson RJ, Fuggle SV, Mumford L, Bradley JA, Forsythe JL, Rudge CJ; Kidney Advisory Group of NHS Blood and Transplant : A new UK 2006 national kidney allocation scheme for deceased heart-beating donor kidneys. Transplantation 89: 387–394, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Fuggle SV, Johnson RJ, Bradley JA, Rudge CJ; Kidney Advisory Group of NHS Blood and Transplant : Impact of the 1998 UK National Allocation Scheme for deceased heartbeating donor kidneys. Transplantation 89: 372–378, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Mumford L; NHS Blood and Transplant Kidney Offering Scheme Working Group: Endorsement of a new national kidney offering scheme, Filton, United Kingdom, NHS Blood and Transplant, 2018. Available at: https://www.odt.nhs.uk/odt-structures-and-standards/odt-hub-programme/kidney-offering-scheme/. Accessed 31 March, 2019 [Google Scholar]
- 48.Fernández-Lorente L, Riera L, Bestard O, Carrera M, Gomà M, Porta N, Torras J, Melilli E, Gil-Vernet S, Grinyó JM, Cruzado JM: Long-term results of biopsy-guided selection and allocation of kidneys from older donors in older recipients. Am J Transplant 12: 2781–2788, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Pierobon ES, Sandrini S, De Fazio N, Rossini G, Fontana I, Boschiero L, Gropuzzo M, Gotti E, Donati D, Minetti E, Gandolfo MT, Brunello A, Libetta C, Secchi A, Chiaramonte S, Rigotti P: Optimizing utilization of kidneys from deceased donors over 60 years: Five-year outcomes after implementation of a combined clinical and histological allocation algorithm [published correction appears in Transpl Int 26: e109, 2013]. Transpl Int 26: 833–841, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Rigotti P, Capovilla G, Di Bella C, Silvestre C, Donato P, Baldan N, Furian L: A single-center experience with 200 dual kidney transplantations. Clin Transplant 28: 1433–1440, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.