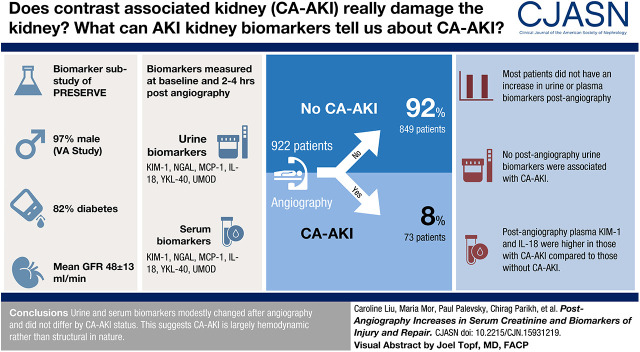
Visual Abstract
Keywords: contrast media, prognostic biomarker, tubular injury, urinary biomarkers, plasma biomarkers, clinical trial, renal hemodynamics, intrinsic injury, creatinine, Biomarkers, Angiography
Abstract
Background and objectives
It is unknown whether iodinated contrast causes kidney parenchymal damage. Biomarkers that are more specific to nephron injury than serum creatinine may provide insight into whether contrast-associated AKI reflects tubular damage. We assessed the association between biomarker changes after contrast angiography with contrast-associated AKI and 90-day major adverse kidney events and death.
Design, setting, participants, & measurements
We conducted a longitudinal analysis of participants from the biomarker substudy of the Prevention of Serious Adverse Events following Angiography trial. We measured injury (kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, IL-18) and repair (monocyte chemoattractant protein-1, uromodulin, YKL-40) proteins from plasma and urine samples at baseline and 2–4 hours postangiography. We assessed the associations between absolute changes and relative ratios of biomarkers with contrast-associated AKI and 90-day major adverse kidney events and death.
Results
Participants (n=922) were predominately men (97%) with diabetes (82%). Mean age was 70±8 years, and eGFR was 48±13 ml/min per 1.73 m2; 73 (8%) and 60 (7%) participants experienced contrast-associated AKI and 90-day major adverse kidney events and death, respectively. No postangiography urine biomarkers were associated with contrast-associated AKI. Postangiography plasma kidney injury molecule-1 and IL-18 were significantly higher in participants with contrast-associated AKI compared with those who did not develop contrast-associated AKI: 428 (248, 745) versus 306 (179, 567) mg/dl; P=0.04 and 325 (247, 422) versus 280 (212, 366) mg/dl; P=0.009, respectively. The majority of patients did not experience an increase in urine or plasma biomarkers. Absolute changes in plasma IL-18 were comparable in participants with contrast-associated AKI (−30 [−71, −9] mg/dl) and those without contrast-associated AKI (−27 [−53, −10] mg/dl; P=0.62). Relative ratios of plasma IL-18 were also comparable in participants with contrast-associated AKI (0.91; 0.86, 0.97) and those without contrast-associated AKI (0.91; 0.85, 0.96; P=0.54).
Conclusions
The lack of significant differences in the absolute changes and relative ratios of injury and repair biomarkers by contrast-associated AKI status suggests that the majority of mild contrast-associated AKI cases may be driven by hemodynamic changes at the kidney.
Introduction
Contrast-associated AKI (CA-AKI) is a common cause of AKI. More than 80 million patients undergo procedures with iodinated contrast media every year (1), with incidence rates of CA-AKI as high as 25% in high-risk patients (2). CA-AKI has been associated with a wide range of adverse outcomes, including cardiovascular events, progressive impairment in kidney function, and mortality (3–5).
There have been a number of proposed mechanisms of CA-AKI, including but not limited to direct cytotoxicity from contrast media (6–9), hypoxia and medullary vasoconstriction (10–12), and oxidative stress (13,14). However, it is unknown whether the small increments in serum creatinine that define CA-AKI are due to intrinsic damage to the kidney parenchyma or to hemodynamic factors. Patients with kidney disease undergoing procedures with intravascular iodinated contrast may have decreased renovascular autoregulation and be at higher risk for renal vasoconstriction, resulting in increases in serum creatinine at the time of angiographic procedures that may not constitute true kidney injury or contribute to adverse long-term outcomes (15). These findings may also reflect ascertainment bias resulting from the use of hospitalized patients without control groups in studies of CA-AKI (16,17).
Determining whether the small increments in serum creatinine that typically define CA-AKI reflect meaningful kidney damage or functional changes related to hemodynamic effects is important in light of mounting data demonstrating that patients with CKD are considerably less likely to undergo contrast-enhanced procedures than those without CKD, presumably due to provider concern about precipitating CA-AKI (18–22). Measurement of urinary biomarkers of injury and repair may allow the differentiation of functional changes versus tubular damage as they are more closely correlated with histologic assessments of acute tubular injury than fluctuations in serum creatinine (23,24). In particular, neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) are injury markers for the distal and proximal tubule (25,26). IL-18 and monocyte chemoattractant protein-1 (MCP-1) are inflammatory proteins released in response to tubular injury of the kidney (27–31). Chitinase-3-like protein (also known as YKL-40) is a sensitive marker of inflammation and repair (32,33). Uromodulin (UMOD) is produced exclusively by the kidney and has been established as a direct marker of the number of intact tubular cells of the ascending limb of the loop of Henle (34). We undertook this study to determine if plasma and urine biomarkers of kidney injury and repair could help elucidate whether CA-AKI reflects true kidney injury or is simply a marker of patients at increased risk for hemodynamic instability at the time of and following contrast administration (35).
Materials and Methods
Study Design
The methods of the Prevention of Serious Adverse Events following Angiography (PRESERVE) trial (36) and of this substudy (37) have been described previously. The trial was a two-by-two factorial design that compared intravenous isotonic sodium bicarbonate with intravenous isotonic saline and oral N-acetyl cysteine with oral placebo in patients with CKD (eGFR<60 ml/min per 1.73 m2 and diabetes or eGFR<45 ml/min per 1.73 m2 without diabetes) who were undergoing coronary or noncoronary angiography. Participants were recruited from February 2013 to March 2017 and were excluded for the following: receiving dialysis; eGFR<15 ml/min per 1.73 m2; unstable baseline blood creatinine; decompensated heart failure; emergent angiogram; having received iodinated contrast in the past 5 days; known allergy to acetylcysteine; known anaphylactic allergy to iodinated contrast; incarceration; age <18 years; pregnancy; unwillingness to comply with outcome assessment; or ongoing participation in an unapproved, concurrent interventional trial (36).
The primary outcome of the parent trial was a composite of major adverse kidney events and death (MAKE-D) within 90 days. Adverse kidney events included the need for dialysis within 90 days or persistent impairment in kidney function defined as a ≥50% increase in serum creatinine 90 days after angiography, confirmed by subsequent testing within 14 days of the initial measurement. CA-AKI, defined as an increase in serum creatinine of ≥25% or ≥0.5 mg/dl (44 μmol/L) from baseline at 3–5 days after angiography, was a secondary outcome. Stages of AKI were defined using Acute Kidney Injury Network criteria and were as follows: stage 1 (increase in serum creatinine to 1.5–1.9 times baseline or increase in serum creatinine by ≥0.3 mg/dl), stage 2 (increase in serum creatinine to 2.0–2.9 times baseline), and stage 3 (increase in serum creatinine to 3.0 times baseline or increase in serum creatinine to ≥4.0 mg/dl) (38).
The trial randomized 5177 patients and was stopped following a prespecified interim analysis (39) indicating a lack of difference between the comparator interventions on study outcomes (36). Participants were excluded if they did not undergo angiography or withdrew consent (n=184). As part of the PRESERVE trial, participants were offered the opportunity to provide plasma and urine prior to and 2–4 hours following angiography. A total of 922 participants from 19 centers participated in the biomarker ancillary substudy (Supplemental Figure 1). Ninety-six participants were missing either baseline serum creatinine (n=13) or 3- to 5-day serum creatinine (n=95) and were presumed to not have CA-AKI. Sixty-two participants were missing 90-day serum creatinine for the MAKE-D outcome; 742 had both preangiography and postangiography urine samples, and 854 had both preangiography and postangiography plasma samples for absolute change and relative ratio calculations. The PRESERVE trial and this ancillary biomarker study were approved by the Veterans Affairs Central Institutional Review Board. Written informed consent was obtained from all participants.
Substudy Sample Collection and Biomarker Measurement
In 19 participating study sites, we collected and measured plasma and urine samples for injury (KIM-1, NGAL, and IL-18) and repair (MCP-1, UMOD, and YKL-40) at baseline and 2–4 hours postangiography, the methods of which have been described previously (37). The biomarkers were chosen a priori for specificity to the nephron, availability of reliable assays, and prior use of these biomarkers in the setting of AKI (40).
All samples were stored at −80°C prior to biomarker measurement. Urine and plasma KIM-1, NGAL, IL-18, MCP-1, UMOD, and YKL-40 were measured using a multiplex assay (Meso Scale Diagnostics [MSD] LLC, Rockville, MD). This assay uses patterned arrays and an electrochemiluminescence detection method, which is quantified using the MSD Quickplex SQ 120 instrument. The interassay coefficients of variation (CVs) for urinary biomarker measurements in laboratory controls were as follows: KIM-1 (CV: 9%), NGAL (CV: 12%), IL-18 (CV: 7%), MCP-1 (CV: 4%), UMOD (CV: 14%), and YKL-40 (CV: 5%). In plasma, the interassay CVs were as follows: KIM-1 (CV: 4%), NGAL (CV: 5%), IL-18 (CV: 3%), MCP-1 (CV: 3%), UMOD (CV: 15%), and YKL-40 (CV: 5%). The lower limits of detection were 6, 12, 6, 32, 6, and 0.6 ng/ml for IL-18, KIM-1, MCP-1, YKL-40, UMOD, and NGAL, respectively. Biomarkers were measured in duplicate and averaged. Biomarkers were measured in one batch (both before and after time points). Samples were collected and stored in a central repository. All personnel measuring the biomarkers were blinded to clinical outcomes.
Statistical Analyses
For this analysis, CA-AKI was the primary outcome of interest, and MAKE-D was the secondary outcome of interest. We compared demographic characteristics and clinical variables by CA-AKI status using t tests for normally distributed continuous variables, the Wilcoxon rank-sum test for continuous variables without a normal distribution, and the chi-squared test for categorical variables. We calculated the absolute change (i.e., Δ) and relative ratio between postangiography and baseline plasma and urine biomarker levels and compared them between patients who did and did not develop CA-AKI, as well as between patients who did and did not develop MAKE-D using the Wilcoxon rank-sum test. The absolute change was defined as postoperative minus preoperative values, and the relative ratio was the ratio of postoperative values to preoperative values.
We reported P values adjusted for urine albumin-creatinine ratio (UACR) and baseline eGFR using van Elteren tests, which are stratified extensions of the Wilcoxon rank-sum test. Urine biomarkers measurements were adjusted for urine creatinine concentration to account for the hydration therapies tested during the clinical trial and their subsequent effects on urine volume and dilution of injury and protein markers. Unadjusted urine biomarker results are shown in Supplemental Tables 2 and 3. A P value of <0.05 was considered statistically significant for all analyses. Statistical analyses were run on SAS software, version 9.4 (SAS Institute) and Stata version 14 (StataCorp LLC).
Results
Study Population
Participants (n=922) were predominately men (97%) with mean ± SD age of 70±8 years and history of diabetes (82%) (Table 1). Overall, 73 (8%) and 60 (7%) patients experienced CA-AKI and MAKE-D, respectively. Follow-up was complete for the substudy. There were no significant differences in demographic, clinical, or procedural variables between patients who did and did not develop CA-AKI (Table 1). Baseline serum creatinine and eGFR were comparable between the two groups. Demographic, clinical, and procedural characteristics of patients in the substudy were comparable with those of the main trial (Supplemental Table 1) (41).
Table 1.
Baseline characteristics in a biomarker ancillary study of the Prevention of Serious Adverse Events following Angiography trial
| Baseline Characteristics | Contrast-Associated Acute Kidney Injury Status | |
|---|---|---|
| No, n=849 | Yes, n=73 | |
| Demographic characteristics | ||
| Age, yr, mean ± SD | 70±8 | 70±8 |
| Men, no. (%) | 824 (97%) | 72 (99%) |
| Race/ethnicity, no. (%)a | ||
| White | 661 (78%) | 57 (79%) |
| Black | 134 (16%) | 10 (14%) |
| Hispanic | 29 (3%) | 3 (4%) |
| Other | 24 (3%) | 2 (3%) |
| Clinical characteristics | ||
| Weight, kg, mean ± SD | 100±22 | 102±21 |
| Median (IQR) baseline serum creatinine, mg/dlb | 1.6 (1.3–1.7) | 1.5 (1.1–1.8) |
| UACR categories, mg/g, no. (%)c | ||
| <30 | 344 (43%) | 27 (38%) |
| 30–300 | 259 (33%) | 27 (38%) |
| ≥300 | 188 (24%) | 17 (24%) |
| Median (IQR) baseline urine creatinine, mg/dld | 96 (65–135) | 84 (64–116) |
| Median (IQR) postoperative urine creatinine, mg/dle | 57 (38–82) | 53 (37–71) |
| Baseline eGFR, ml/min per 1.73 m2, no. (%)f | ||
| 15–30 | 63 (8%) | 6 (8%) |
| 30–45 | 289 (35%) | 24 (33%) |
| ≥45 | 479 (58%) | 42 (58%) |
| Diabetes, no. (%) | 701 (83%) | 59 (81%) |
| Procedural characteristics | ||
| Coronary procedure, no. (%)g | 745 (88%) | 67 (92%) |
| Percutaneous intervention, no. (%)h | 229 (27%) | 26 (36%) |
| Left ventricular end diastolic pressure, mm Hg, mean ± SDi | 19.4±8.3 | 19.2±8.7 |
| Trial arm, no. (%) | ||
| Saline + placebo | 196 (23%) | 15 (21%) |
| Saline + acetylcysteine | 219 (26%) | 17 (23%) |
| Sodium bicarbonate + placebo | 225 (27%) | 17 (23%) |
| Sodium bicarbonate + acetylcysteine | 209 (25%) | 24 (33%) |
| Contrast volume, ml, mean ± SD | 105±66 | 124±74 |
| AKI stagej | ||
| No AKI | 717 (95%) | 5 (7%) |
| Stage 1 | 36 (5%) | 66 (90%) |
| Stage 2 | 0 (0%) | 2 (3%) |
| Stage 3 | 0 (0%) | 0 (0%) |
| MAKE-D | 47 (5%) | 13 (18%) |
| Death | 26 (3%) | 2 (3%) |
| Need for dialysis | 9 (1%) | 3 (4%) |
| 90-d ≥50% in serum creatinine from baseline | 18 (2%) | 8 (11%) |
IQR, interquartile range; UACR, urine albumin-creatinine ratio; MAKE-D, major adverse kidney events or death.
Two participants were missing information on race.
Thirteen participants were missing information on baseline serum creatinine.
Sixty participants were missing information on UACR.
One hundred twenty-five participants were missing information on baseline urine creatinine.
Seventy-eight participants were missing information on postoperative urine creatinine.
Nineteen participants were missing information on eGFR.
Two participants were missing information on angiography type.
Two participants were missing information on percutaneous intervention.
Five hundred eighty-eight participants were missing information on left ventricular end diastolic pressure.
Ninety-six participants were missing either (or both) the baseline or 3- to 5-day serum creatinine and are presumed to have no contrast-associated AKI. Stages of AKI were defined by acute kidney injury network criteria: stage 1: increase in serum creatinine to 1.5–1.9 times baseline or increase in serum creatinine by ≥0.3 mg/dl; stage 2: increase in serum creatinine to 2.0–2.9 times baseline; stage 3: increase in serum creatinine to 3.0 times baseline or increase in serum creatinine to ≥4.0 mg/dl.
Changes in Plasma and Urine Biomarkers by Contrast-Associated AKI Status
No preangiography urine biomarkers were significantly different by CA-AKI status before and after indexing to urine creatinine (Table 2, Supplemental Table 2). Preangiography plasma KIM-1 was significantly higher in those who developed CA-AKI compared with those who did not (Table 2). Postangiography, plasma KIM-1, and IL-18 were significantly higher in participants who developed CA-AKI compared with those who did not. Among urine creatinine–indexed urine biomarkers, postangiography levels of urine UMOD and KIM-1 were slightly higher among patients who developed CA-AKI (Table 2). There were no differences in unadjusted, postangiography urine biomarker levels between patients who did and did not develop CA-AKI (Supplemental Table 2). Postangiography levels of plasma KIM-1 and IL-18 were higher among patients who experienced CA-AKI compared with those who did not, even after adjustment for baseline eGFR and UACR (Table 2).
Table 2.
Plasma and urine creatinine–indexed urine biomarker concentrations according to development of contrast-associated AKI
| Biomarker | Median (Interquartile Range) Plasma Biomarker, pg/ml | Median (Interquartile Range) Urine Creatinine–Indexed Urine Biomarker, pg/mg | ||||||
|---|---|---|---|---|---|---|---|---|
| Contrast-Associated Acute Kidney Injury | No Contrast-Associated Acute Kidney Injury | Unadjusted P Value | Adjusted P Valuea | Contrast-Associated Acute Kidney Injury | No Contrast-Associated Acute Kidney Injury | Unadjusted P Value | Adjusted P Valuea | |
| Preangiography | ||||||||
| KIM-1 | 430 (295, 713) | 319 (190, 593) | 0.002 | 0.004 | 16 (10, 28) | 16 (9, 27) | 0.48 | 0.43 |
| NGAL | 232 (168, 297) | 230 (175, 299) | 0.89 | 0.55 | 0.3 (0.1, 0.4) | 0.2 (0.1, 0.5) | 0.91 | 0.60 |
| IL-18 | 347 (265, 447) | 316 (245, 408) | 0.09 | 0.12 | 0.2 (0.1, 0.4) | 0.2 (0.2, 0.4) | 0.70 | 0.46 |
| MCP-1 | 218 (168, 265) | 218 (181, 271) | 0.46 | 0.31 | 2 (2, 3) | 2 (1, 3) | 0.74 | 0.87 |
| UMOD | 68 (49, 87) | 71 (50, 97) | 0.28 | 0.26 | 32 (16, 57) | 25 (15, 48) | 0.08 | 0.13 |
| YKL-40 | 108,251 (54,743, 205,041) | 94,598 (54,166, 178,090) | 0.41 | 0.50 | 4 (2, 17) | 4 (2, 12) | 0.71 | 0.99 |
| Postangiography | ||||||||
| KIM-1 | 428 (248, 745) | 306 (179, 567) | 0.006 | 0.04 | 16 (10, 26) | 13 (7, 23) | 0.06 | 0.04 |
| NGAL | 225 (158, 271) | 206 (157, 269) | 0.73 | 0.96 | 0.3 (0.2, 0.6) | 0.3 (0.1, 0.6) | 0.74 | 0.46 |
| IL-18 | 325 (247, 422) | 280 (212, 366) | 0.004 | 0.009 | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.4) | 0.39 | 0.15 |
| MCP-1 | 194 (170, 254) | 209 (175, 260) | 0.15 | 0.09 | 2 (2, 4) | 2 (1, 4) | 0.68 | 0.60 |
| UMOD | 66 (49, 87) | 66 (47, 91) | 0.91 | 0.80 | 35 (25, 43) | 30 (21, 39) | 0.02 | 0.04 |
| YKL-40 | 97,570 (55,914, 216,946) | 82,268 (47,238, 154,878) | 0.13 | 0.23 | 9 (4, 33) | 9 (4, 22) | 0.85 | 0.84 |
| Absolute change | ||||||||
| KIM-1 | −18 (−63, 27) | −18 (−51, 5) | 0.63 | 0.43 | −1 (−5, 1) | −1 (−6, 2) | 0.80 | 0.70 |
| NGAL | −23 (−47, −3) | −20 (−45, 1) | 0.62 | 0.43 | 0 (−0, 0) | 0 (−0, 0) | 0.55 | 0.52 |
| IL-18 | −30 (−71, −9) | −27 (−53, −10) | 0.58 | 0.62 | 0 (−0, 0) | 0 (−0, 0) | 0.98 | 0.94 |
| MCP-1 | −6 (−32, 17) | −6 (−40, 32) | 0.37 | 0.30 | −0 (−0, 1) | 0 (−0, 0) | 0.70 | 0.72 |
| UMOD | −4 (−9, 0) | −4 (−11, 0) | 0.52 | 0.28 | −0 (−18, 15) | 1 (−12, 13) | 0.90 | 0.80 |
| YKL-40 | −13,128 (−32,783, −2162) | −10,235 (−24,532,−2189) | 0.34 | 0.37 | 3 (1, 8) | 3 (1, 9) | 0.82 | 0.88 |
| Relative ratio | ||||||||
| KIM-1 | 0.96 (0.87, 1.05) | 0.94 (0.87, 1.02) | 0.50 | 0.41 | 0.89 (0.76, 1.09) | 0.91 (0.66, 1.11) | 0.82 | 0.80 |
| NGAL | 0.90 (0.82, 0.99) | 0.91 (0.81, 1.00) | 0.76 | 0.59 | 1.26 (0.86, 1.93) | 1.15 (0.82, 1.68) | 0.35 | 0.38 |
| IL-18 | 0.91 (0.86, 0.97) | 0.91 (0.85, 0.96) | 0.53 | 0.54 | 1.03 (0.83, 1.48) | 1.02 (0.79, 1.40) | 0.71 | 0.89 |
| MCP-1 | 0.97 (0.84, 1.10) | 0.97 (0.83, 1.17) | 0.34 | 0.33 | 0.99 (0.83, 1.25) | 1.01 (0.81, 1.25) | 0.86 | 0.92 |
| UMOD | 0.94 (0.89, 1.00) | 0.93 (0.85, 1.01) | 0.22 | 0.14 | 0.97 (0.59, 1.71) | 1.07 (0.68, 1.74) | 0.68 | 0.48 |
| YKL-40 | 0.88 (0.78, 0.96) | 0.87 (0.78, 0.96) | 0.66 | 0.79 | 1.77 (1.19, 4.31) | 1.71 (1.16, 4.19) | 0.94 | 0.95 |
KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein-1; UMOD, uromodulin.
Adjusted for baseline eGFR and urine albumin-creatinine ratio
There were no differences in the absolute changes of any of the plasma or urine biomarkers from baseline between patients with and without CA-AKI, including in analyses that adjusted for baseline eGFR and UACR and in analyses that indexed urine biomarkers to urine creatinine concentration (Figures 1, A and B and 2A, Table 2, Supplemental Table 2). Relative ratios of plasma and urine biomarkers were also comparable by CA-AKI status (Figure 1, C and D, Supplemental Table 2). The lack of difference in the relative ratios persisted after indexing urine biomarkers to urine creatinine and adjusting for baseline eGFR and UACR (Figure 2B, Table 2).
Figure 1.
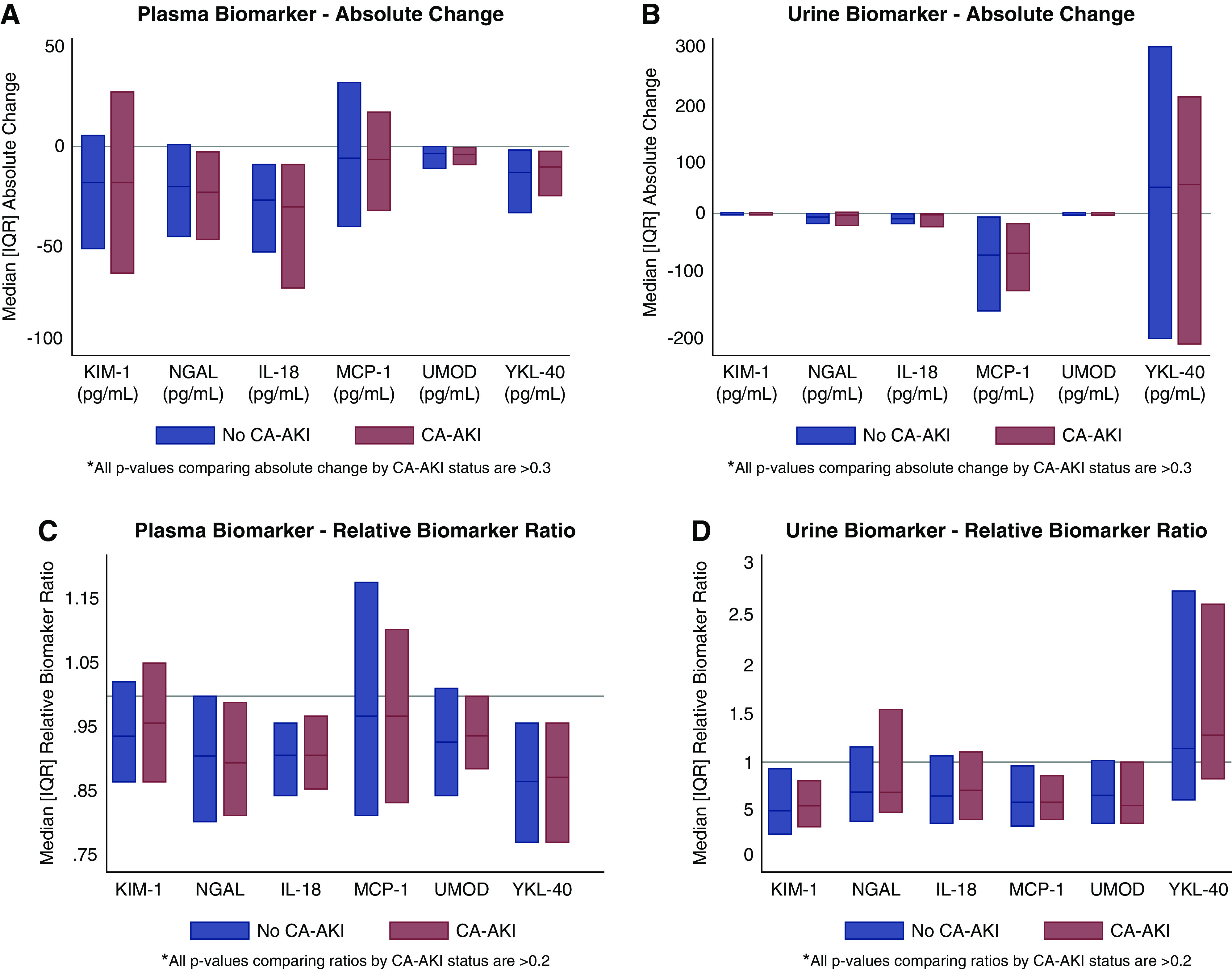
Absolute changes and relative ratios of plasma and unadjusted urine biomarkers are comparable by contrast associated-AKI status. (A) Plasma biomarker—absolute change. (B) Urine biomarker—absolute change. (C) Plasma biomarker—relative biomarker ratio. (D) Urine biomarker—relative biomarker ratio. IQR, interquartile range; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; UMOD, uromodulin.
Figure 2.
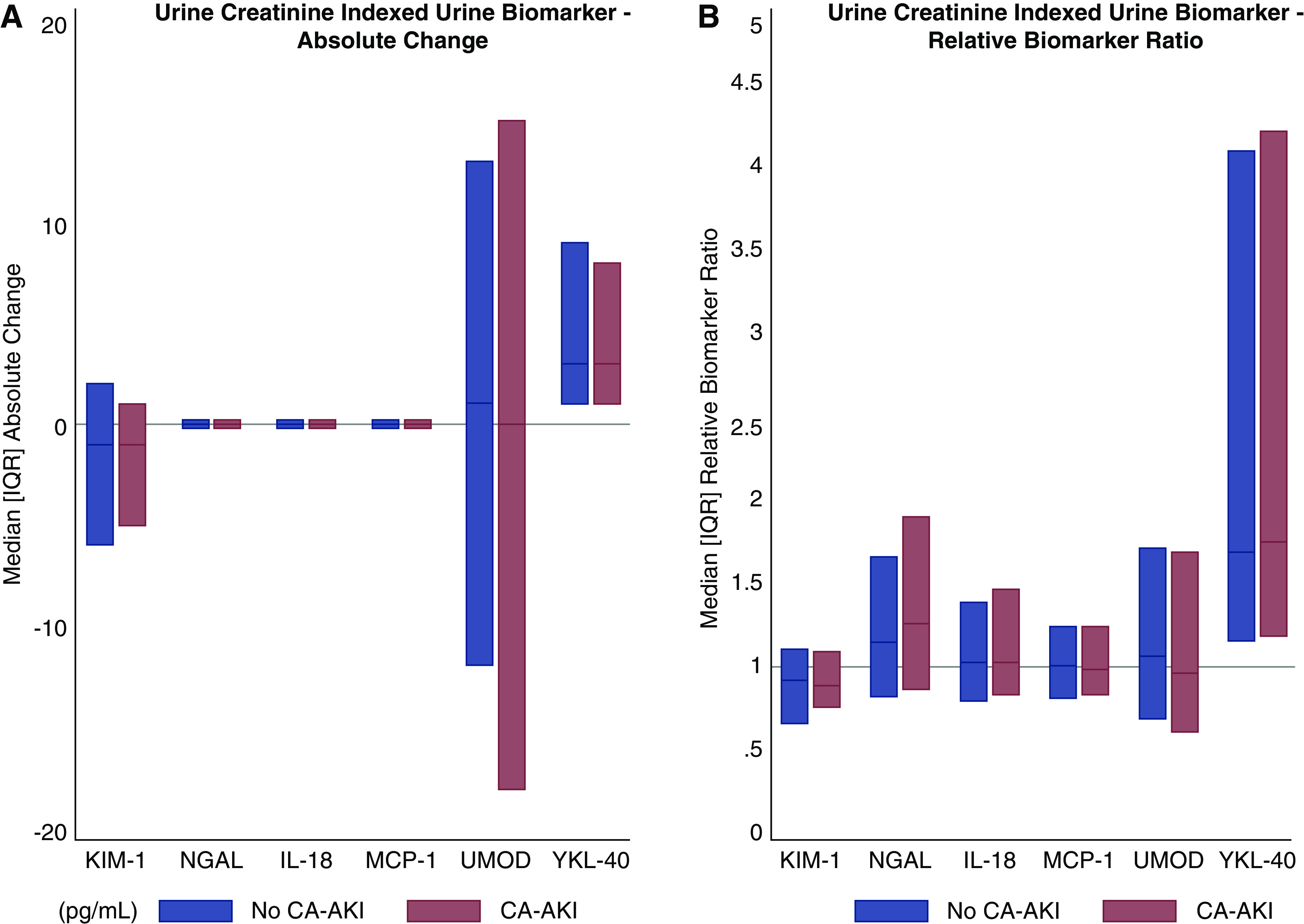
Absolute changes and relative ratios of urine creatinine–indexed urine biomarkers are comparable by contrast associated-AKI status. (A) Urine creatinine–indexed urine biomarker—absolute change. (B) Urine creatinine–indexed urine biomarker—relative biomarker ratio.
Changes in Plasma and Urine Biomarkers by Major Adverse Kidney Events and Death Status
Preangiography urine IL-18, MCP-1, and YKL-40 remained statistically significantly higher in those who developed MAKE-D after indexing to urine creatinine (Table 3). Among plasma biomarkers, preangiography KIM-1, NGAL, UMOD, and MCP-1 remained significantly higher among participants who experienced MAKE-D after adjustment for baseline eGFR and UACR. Postangiography, plasma KIM-1, NGAL, and YKL-40 were higher and UMOD was lower among patients who developed MAKE-D compared with those who did not, including after adjustment for baseline eGFR and UACR (Table 3). Among urine biomarkers indexed to urine creatinine, postangiography urine KIM-1, IL-18, MCP-1, and YKL-40 levels were higher among patients who developed MAKE-D (Table 3).
Table 3.
Plasma and urine creatinine–indexed urine biomarker concentrations according to development of major adverse kidney events or death
| Biomarker | Median (Interquartile Range) Plasma Biomarker, pg/ml | Median (Interquartile Range) Urine Creatinine–Indexed Urine Biomarker, pg/mg | ||||
|---|---|---|---|---|---|---|
| Major Adverse Kidney Events or Death | No Major Adverse Kidney Events or Death | Adjusted P Valuea | Major Adverse Kidney Events or Death | No Major Adverse Kidney Events or Death | Adjusted P Valuea | |
| Preangiography | ||||||
| KIM-1 | 504 (306, 948) | 321 (189, 585) | 0.04 | 18 (12, 41) | 16 (9, 26) | 0.11 |
| NGAL | 259 (209, 420) | 228 (173, 296) | 0.04 | 0.5 (0.2, 2) | 0.2 (0.1, 0.5) | 0.16 |
| IL-18 | 332 (238, 446) | 318 (246, 408) | 0.86 | 0.4 (0.2, 0.7) | 0.2 (0.1, 0.4) | 0.002 |
| MCP-1 | 229 (167, 280) | 217 (181, 270) | 0.94 | 3 (2, 6) | 2 (2, 3) | 0.03 |
| UMOD | 59 (41, 81) | 71 (51, 97) | 0.01 | 29 (14, 39) | 25 (15, 49) | 0.90 |
| YKL-40 | 141,987 (98,750, 284,819) | 91,371 (53,545, 174,700) | 0.001 | 10 (3, 44) | 4 (1, 11) | 0.008 |
| Postangiography | ||||||
| KIM-1 | 505 (299, 885) | 299 (175, 557) | 0.02 | 17 (12, 45) | 13 (7, 22) | 0.03 |
| NGAL | 238 (194, 343) | 202 (156, 265) | 0.02 | 0.4 (0.2, 1.2) | 0.3 (0.1, 0.6) | 0.27 |
| IL-18 | 304 (208, 390) | 284 (216, 367) | 0.69 | 0.4 (0.2, 0.6) | 0.2 (0.2, 0.4) | <0.001 |
| MCP-1 | 205 (166, 235) | 209 (175, 261) | 0.51 | 4 (2, 6) | 2 (1, 3) | 0.01 |
| UMOD | 60 (43, 79) | 67 (48, 91) | 0.04 | 28 (18, 35) | 30 (21, 40) | 0.42 |
| YKL-40 | 126,938 (80,109, 268,256) | 80,593 (46,350, 15,369) | <0.001 | 21 (8, 78) | 8 (4, 21) | 0.02 |
| Absolute change | ||||||
| KIM-1 | −22 (−102, 7) | −17 (−49, 6) | 0.47 | −1 (−5, 4) | −1 (−6, 1) | 0.29 |
| NGAL | −19 (−58, −1) | −20 (−45, 0) | 0.67 | 0.01 (−0.1, 0.1) | 0.03 (−0.04, 0.2) | 0.10 |
| IL-18 | −26 (−59, −6) | −27 (−53, −10) | 0.68 | 0.002 (−0.1, 0.07) | 0.01 (−0.06, 0.08) | 0.89 |
| MCP-1 | −7 (−31, 29) | −6 (−40, 29) | 0.78 | 0.07 (−0.4, 1.1) | 0.01 (−0.4, 0.5) | 0.51 |
| UMOD | −3 (−7, 1) | −4 (−11, 0) | 0.17 | −0.6 (−9, 5) | 1.5 (−13, 13) | 0.50 |
| YKL-40 | −10,513 (−26,799, 2237) | −10,306 (−24,742, −2231) | 0.47 | 5 (0.8, 15) | 3.0 (0.9, 8.3) | 0.63 |
| Relative ratio | ||||||
| KIM-1 | 0.95 (0.90, 1.03) | 0.94 (0.87, 1.02) | 0.76 | 0.95 (0.79, 1.18) | 0.90 (0.66, 1.10) | 0.30 |
| NGAL | 0.92 (0.83, 1.00) | 0.91 (0.81, 1.00) | 0.89 | 1.05 (0.79, 1.26) | 1.16 (0.83, 1.74) | 0.08 |
| IL-18 | 0.92 (0.87, 0.97) | 0.91 (0.85, 0.96) | 0.29 | 1.01 (0.82, 1.43) | 1.03 (0.79, 1.41) | 0.80 |
| MCP-1 | 0.96 (0.83, 1.15) | 0.97 (0.83, 1.16) | 0.79 | 1.02 (0.85, 1.30) | 1.01 (0.81, 1.25) | 0.38 |
| UMOD | 0.95 (0.88, 1.02) | 0.93 (0.85, 1.01) | 0.21 | 0.98 (0.73, 1.30) | 1.08 (0.68, 1.76) | 0.37 |
| YKL-40 | 0.92 (0.84, 1.03) | 0.87 (0.77, 0.96) | 0.007 | 1.30 (1.04, 4.28) | 1.74 (1.18, 4.23) | 0.07 |
KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein-1; UMOD, uromodulin.
Adjusted for baseline eGFR and urine albumin-creatinine ratio.
The absolute changes of plasma biomarkers were comparable by MAKE-D status, including after adjusting for baseline eGFR and UACR (Table 3). Among unadjusted urine biomarkers, only the absolute change of NGAL was larger among patients who developed MAKE-D (Supplemental Table 3). This difference was statistically significant after adjusting for baseline eGFR and UACR but not after indexing urine NGAL to urine creatinine (Table 3).
The relative ratio of plasma YKL-40 was significantly lower among participants who did not experience MAKE-D compared with those who experienced MAKE-D (Table 3). This difference persisted after adjusting P values for baseline eGFR and UACR.
Discussion
Of the six biomarkers measured in plasma and urine in this substudy, only two plasma biomarkers (KIM-1 and IL-18) had higher postangiography levels among those who developed CA-AKI compared with those who did not. The urinary biomarkers, which are more closely related to histologic injury, were not elevated after angiography (30,31). The absolute change and relative ratios of plasma and urine biomarkers were modest and comparable between patients with and without CA-AKI. These findings suggest that CA-AKI, defined by small increments in creatinine following angiography, is not primarily characterized by intrinsic injury to kidney tissue, but rather reflects hemodynamic fluctuations and altered kidney perfusion without cellular damage.
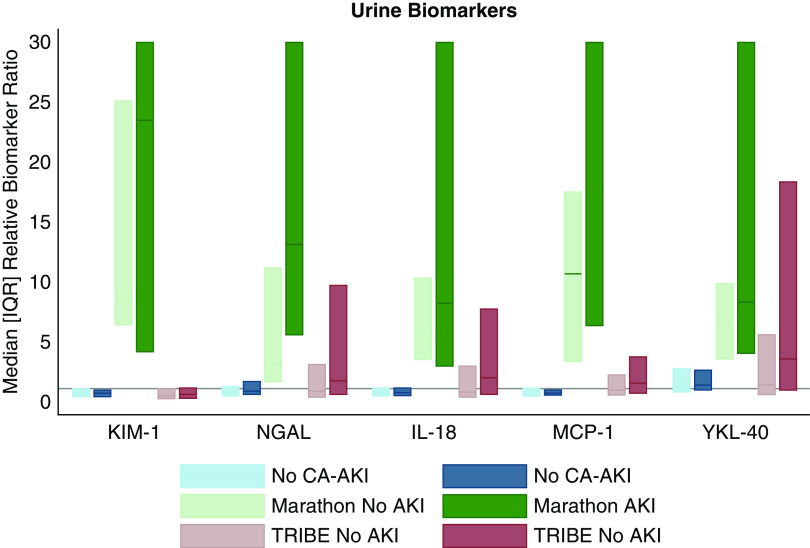
Biomarkers have been shown to change significantly in cases of confirmed AKI (41–45). The direction, magnitude, and timing of biomarker changes depend on the marker of interest and the type and severity of injury. Analyses of pediatric patients who underwent cardiopulmonary bypass demonstrated multifold increases in urine NGAL and plasma NGAL from baseline to 2 hours postprocedure among those with AKI (41). In otherwise healthy marathon runners who had granular casts and kidney tubular cells on urine microscopy following the race, injury and repair biomarkers increased three- to tenfold within 30 minutes postmarathon (42). The magnitudes of the absolute changes of the six plasma and urine biomarkers in this study were much smaller when compared with cohorts of cardiac surgery recipients and marathon runners (Figure 3, Supplemental Figure 2). The lack of substantial absolute changes in biomarkers in our study suggests that the majority of increases in serum creatinine that defined CA-AKI among the PRESERVE trial patients were not due to true cellular injury to kidney tubules.
Figure 3.
Relative ratios of urine biomarkers by CA-AKI status in this study are less than those in the Marathon study and the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) study. Comparison of the relative ratio of urine biomarkers in two cohorts: the Prevention of Serious Adverse Events following Angiography (PRESERVE) trial and the Marathon study. The PRESERVE trial compares urine biomarker levels before and 2–4 hours postangiography in patients who did and did not develop CA-AKI. The Marathon study compares urine biomarker levels 24 hours premarathon to 30 minutes postmarathon. The TRIBE-AKI study compared urine biomarkers before and 0–6 hours after cardiac surgery. The relative ratios of urine biomarkers in the PRESERVE trial are substantially lower than the ratios of urine biomarkers in the marathon runners and in patients undergoing cardiac surgery. The relative biomarker ratio was truncated at 30 for the Marathon study results to facilitate the visual comparison across studies. In the AKI group, the 75th percentiles >30 are urine KIM-1 (105.04), urine NGAL (48.38), urine IL-18 (43.86), and urine MCP-1 (188.71). The median for MCP-1 is 60.84.
Another finding from this study was that there were no significant differences in the absolute changes and relative ratios of the biomarkers between patients who developed CA-AKI and those who did not. These findings remained robust after adjusting for preangiography kidney function and urine albumin excretion and provide further evidence supporting that the majority of these increments in serum creatinine were not due to cellular damage to the kidney.
The findings from this biomarker ancillary may help explain findings from a secondary analysis of the PRESERVE trial. Most PRESERVE trial participants who experienced CA-AKI did not go on to develop MAKE-D. Of the 429 participants who experienced CA-AKI, only 53 (12.3%) later developed the 90-day MAKE-D outcome (46). A mediation analysis found that CA-AKI was not a mediator of the association between preangiography eGFR and 90-day MAKE-D (46). The lack of differences in biomarker changes by CA-AKI status provides a potential mechanistic explanation as to why CA-AKI is merely a marker, and not a mediator, of long-term outcomes.
The findings of this study and other studies from the PRESERVE trial are highly relevant to the current state of contrast-enhanced procedure utilization among patients with CKD. Registry-based studies in the United States (18,20), Canada (22), and Sweden (19) and the Global Registry of Acute Coronary Events (21,22) found lower rates of angiography among patients with CKD compared with patients without CKD, which are hypothesized to be attributed to provider concern for precipitating CA-AKI among those with CKD. However, such underutilization of clinically indicated and potentially life-saving angiographic procedures due to fear of CA-AKI would be considered inappropriate if the incidence of true CA-AKI with intrinsic nephron injury is much lower than previously estimated.
There are several strengths to our study. We indexed urine biomarkers to urine creatinine to account for the intravenous fluids tested in the trial. Furthermore, sample procurement, biomarker assays, reagents, and freeze-thaw methods were standardized across sites.
There are certain limitations to this study. Our analyses were limited to one-time urine and plasma collections taken 2–4 hours following angiography. This time frame may not capture all biomarker changes. In particular, studies of adults undergoing cardiac surgery have shown that urine KIM-1 does not peak until 2 days after surgery. However, statistically significant differences in urine KIM-1 are still expected within 0–6 hours postsurgery between the two groups (45). Furthermore, among injury and repair biomarkers (i.e., NGAL, IL-18, YKL-40, and MCP-1) known to change significantly within 1–2 hours in other settings of AKI, we found insignificant changes that did not differ by CA-AKI status. Additionally, the majority (70%) of participants received diagnostic angiography as opposed to an intervention, thereby limiting the amount of contrast administered and the likelihood of intrinsic nephron injury from contrast media or atheromatous emboli. Our findings may not be applicable to all cases of CA-AKI. In particular, our results may not be generalizable to the most severe cases of CA-AKI or to settings where greater volume or higher-osmolality contrast is used. Finally, the PRESERVE trial participants were predominantly men, potentially limiting the generalizability of our findings.
In conclusion, levels of certain plasma and urine injury and repair biomarkers changed modestly following angiography; yet, these changes did not differ by CA-AKI status. Our findings suggest that most cases of CA-AKI likely reflect hemodynamic fluctuations in the kidney function rather than intrinsic kidney damage and are consistent with prior findings that CA-AKI may not be a mediator of MAKE-D. These findings will help inform the judicious use of preventive care in the setting of angiography and should enhance the use of clinically indicated contrast-enhanced procedures among patients who are considered to be at risk for the development of CA-AKI.
Disclosures
M. Mor reports receiving grants from the National Institutes of Health during the conduct of the study. P. Palevsky receives consulting fees and advisory committee fees from Durect, HealthSpan Dx, and Novartis; serves on the Data and Safety Monitoring Board of Baxter; serves as a member of an end point adjudication committee from GE Healthcare; and reports receiving grants from BioPorto and Dascena outside the submitted work. C. Parikh has received consulting fees from Renalytix; serves on the Data and Safety Monitoring Board of Genfit; reports receiving consulting fees from Akebia Therapeutics, Inc.; reports receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases and grants from the National Heart, Lung, and Blood Institute; and reports receiving consulting fees from Renaltix AI outside the submitted work. S. Weisbord has received consulting fees and advisory fees from Durect and Saghmos Therapeutics and reports receiving personal fees from Cytokinetics and Saghmos Therapeutics outside the submitted work. All remaining authors have nothing to disclose.
Funding
This study was supported by United States Department of Veterans Affairs Office of Research and Development grant VA CSP #578 PRESERVE Trial (Principal Investigator: S.D. Weisbord; co-Principal Investigator: P.M. Palevsky), National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK098214 Biomarker Collection and Analysis in the PRESERVE Trial Cohort (Multiple Principal Investigators: S.D. Weisbord, P.M. Palevsky, and C.R. Parikh), and National Health and Medical Research Council of Australia grant 1011387. C.R. Parikh was additionally supported by George M. O’Brien Kidney Center grant P30DK079310 and National Institutes of Health grant R01HL085757.
Supplementary Material
Acknowledgments
Parts of this study were presented in poster form at the American Society of Nephrology Kidney Week in San Diego, California from October 23–28, 2018.
The funding source had no role in the study design, data collection, analysis, reporting, or decision to submit for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Contrast-Associated Acute Kidney Injury: Will Clarifying Mechanisms Allay Anxiety?“ on pages 1225–1227.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.15931219/-/DCSupplemental.
Supplemental Figure 1. Study flow chart.
Supplemental Figure 2. Median (interquartile range) relative ratios of plasma biomarkers by contrast-associated AKI status and plasma biomarkers from the Marathon study and the TRIBE-AKI cohort.
Supplemental Table 1. Demographic, clinical, and procedural characteristics of the Prevention of Serious Adverse Events following Angiography trial and biomarker ancillary substudy.
Supplemental Table 2. Unadjusted urine biomarker concentrations according to development of contrast-associated AKI.
Supplemental Table 3. Unadjusted urine biomarker concentrations according to development of major adverse kidney events or death.
References
- 1.Katzberg RW, Haller C: Contrast-induced nephrotoxicity: Clinical landscape. Kidney Int Suppl 69: S3–S7, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Rudnick MR, Goldfarb S, Tumlin J: Contrast-induced nephropathy: Is the picture any clearer? Clin J Am Soc Nephrol 3: 261–262, 2008. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA: Radiocontrast-induced acute kidney injury. Nephron, Physiol 109: 61–72, 2008. [DOI] [PubMed] [Google Scholar]
- 4.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW: Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Weisbord SD, Chen H, Stone RA, Kip KE, Fine MJ, Saul MI, Palevsky PM: Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol 17: 2871–2877, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Nazıroğlu M, Yoldaş N, Uzgur EN, Kayan M: Role of contrast media on oxidative stress, Ca(2+) signaling and apoptosis in kidney. J Membr Biol 246: 91–100, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Quintavalle C, Brenca M, De Micco F, Fiore D, Romano S, Romano MF, Apone F, Bianco A, Zabatta MA, Troncone G, Briguori C, Condorelli G: In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis 2: e155, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu ZZ, Schmerbach K, Lu Y, Perlewitz A, Nikitina T, Cantow K, Seeliger E, Persson PB, Patzak A, Liu R, Sendeski MM: Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedback. Am J Physiol Renal Physiol 306: F864–F872, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peer A, Averbukh Z, Berman S, Modai D, Averbukh M, Weissgarten J: Contrast media augmented apoptosis of cultured renal mesangial, tubular, epithelial, endothelial, and hepatic cells. Invest Radiol 38: 177–182, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Heyman SN, Brezis M, Epstein FH, Spokes K, Silva P, Rosen S: Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int 40: 632–642, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Sendeski M, Patzak A, Pallone TL, Cao C, Persson AE, Persson PB: Iodixanol, constriction of medullary descending vasa recta, and risk for contrast medium-induced nephropathy. Radiology 251: 697–704, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liss P, Nygren A, Erikson U, Ulfendahl HR: Injection of low and iso-osmolar contrast medium decreases oxygen tension in the renal medulla. Kidney Int 53: 698–702, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Bakris GL, Lass N, Gaber AO, Jones JD, Burnett JC Jr.: Radiocontrast medium-induced declines in renal function: A role for oxygen free radicals. Am J Physiol 258: F115–F120, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Heyman SN, Rosen S, Khamaisi M, Idée J-M, Rosenberger C: Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 45: 188–195, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Mehran R, Dangas GD, Weisbord SD: Contrast-associated acute kidney injury. N Engl J Med 380: 2146–2155, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP: The controversy of contrast-induced nephropathy with intravenous contrast: What is the risk? Am J Kidney Dis 75c: 105–113, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Rao QA, Newhouse JH: Risk of nephropathy after intravenous administration of contrast material: A critical literature analysis. Radiology 239: 392–397, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD; Acute Coronary Treatment and Intervention Outcomes Network registry : Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 121: 357–365, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, Wallentin L, Jernberg T; SWEDEHEART : Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: Data from the SWEDEHEART register. J Intern Med 268: 40–49, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Han JH, Chandra A, Mulgund J, Roe MT, Peterson ED, Szczech LA, Patel U, Ohman EM, Lindsell CJ, Gibler WB: Chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. Am J Med 119: 248–254, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Medi C, Montalescot G, Budaj A, Fox KA, López-Sendón J, FitzGerald G, Brieger DB; GRACE Investigators : Reperfusion in patients with renal dysfunction after presentation with ST-segment elevation or left bundle branch block: GRACE (Global Registry of Acute Coronary Events). JACC Cardiovasc Interv 2: 26–33, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Wong JA, Goodman SG, Yan RT, Wald R, Bagnall AJ, Welsh RC, Wong GC, Kornder J, Eagle KA, Steg PG, Yan AT; Canadian Acute Coronary Syndromes I and II, and Canadian Global Registry of Acute Coronary Events (GRACE/GRACE) Investigators : Temporal management patterns and outcomes of non-ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J 30: 549–557, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV: Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28: 478–485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F: Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28: 436–440, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C: NGAL: A biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol 42: 141–150, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Wada T, Yokoyama H, Su SB, Mukaida N, Iwano M, Dohi K, Takahashi Y, Sasaki T, Furuichi K, Segawa C, Hisada Y, Ohta S, Takasawa K, Kobayashi K, Matsushima K: Monitoring urinary levels of monocyte chemotactic and activating factor reflects disease activity of lupus nephritis. Kidney Int 49: 761–767, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL: Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noris M, Bernasconi S, Casiraghi F, Sozzani S, Gotti E, Remuzzi G, Mantovani A: Monocyte chemoattractant protein-1 is excreted in excessive amounts in the urine of patients with lupus nephritis. Lab Invest 73: 804–809, 1995. [PubMed] [Google Scholar]
- 30.Kiyici S, Erturk E, Budak F, Ersoy C, Tuncel E, Duran C, Oral B, Sigirci D, Imamoglu S: Serum monocyte chemoattractant protein-1 and monocyte adhesion molecules in type 1 diabetic patients with nephropathy. Arch Med Res 37: 998–1003, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Edelstein CL, Hoke TS, Somerset H, Fang W, Klein CL, Dinarello CA, Faubel S: Proximal tubules from caspase-1-deficient mice are protected against hypoxia-induced membrane injury. Nephrol Dial Transplant 22: 1052–1061, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, Elias JA: The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 182: 918–928, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, Chupp GL, Moeckel GW, Lee CG, Elias JA, Parikh CR, Cantley LG: Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol 24: 309–319, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, Angermann S, Hasenau AL, Stecher L, Heemann U, Renders L, Scherberich J: Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 95: e3011, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu K, Rosenstiel P, Paragas N, Hinze C, Gao X, Huai Shen T, Werth M, Forster C, Deng R, Bruck E, Boles RW, Tornato A, Gopal T, Jones M, Konig J, Stauber J, D’Agati V, Erdjument-Bromage H, Saggi S, Wagener G, Schmidt-Ott KM, Tatonetti N, Tempst P, Oliver JA, Guarnieri P, Barasch J: Unique transcriptional programs identify subtypes of AKI. J Am Soc Nephrol 28: 1729–1740, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, Conner TA, Chertow GM, Bhatt DL, Shunk K, Parikh CR, McFalls EO, Brophy M, Ferguson R, Wu H, Androsenko M, Myles J, Kaufman J, Palevsky PM; PRESERVE Trial Group : Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 378: 603–614, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Parikh CR, Liu C, Mor MK, Palevsky PM, Kaufman JS, Philbrook HT, Weisbord SD: Kidney biomarkers of injury and repair as predictors of contrast-associated AKI: A substudy of the PRESERVE trial. Am J Kidney Dis 75: 187–194, 2019. 31547939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisbord SD, Gallagher M, Kaufman J, Cass A, Parikh CR, Chertow GM, Shunk KA, McCullough PA, Fine MJ, Mor MK, Lew RA, Huang GD, Conner TA, Brophy MT, Lee J, Soliva S, Palevsky PM: Prevention of contrast-induced AKI: A review of published trials and the design of the prevention of serious adverse events following angiography (PRESERVE) trial. Clin J Am Soc Nephrol 8: 1618–1631, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang WR, Parikh CR: Biomarkers of acute and chronic kidney disease. Annu Rev Physiol 81: 309–333, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Mansour SG, Verma G, Pata RW, Martin TG, Perazella MA, Parikh CR: Kidney injury and repair biomarkers in marathon runners [published correction appears in Am J Kidney Dis 70: 452, 2017]. Am J Kidney Dis 70: 252–261, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR: Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG; TRIBE-AKI Consortium : Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8: 1079–1088, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisbord SD, Palevsky PM, Kaufman JS, Wu H, Androsenko M, Ferguson RE, Parikh CR, Bhatt DL, Gallagher M; PRESERVE Trial Investigators : Contrast-associated acute kidney Injury and serious adverse outcomes following angiography. J Am Coll Cardiol 75: 1311–1320, 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.