Abstract
It is estimated that women with CKD are ten times more likely to develop preeclampsia than women without CKD, with preeclampsia affecting up to 40% of pregnancies in women with CKD. However, the shared phenotype of hypertension, proteinuria, and impaired excretory kidney function complicates the diagnosis of superimposed preeclampsia in women with CKD who have hypertension and/or proteinuria that predates pregnancy. This article outlines the diagnoses of preeclampsia and superimposed preeclampsia. It discusses the pathogenesis of preeclampsia, including abnormal placentation and angiogenic dysfunction. The clinical use of angiogenic markers as diagnostic adjuncts for women with suspected preeclampsia is described, and the limited data on the use of these markers in women with CKD are presented. The role of kidney biopsy in pregnancy is examined. The management of preeclampsia is outlined, including important advances and controversies in aspirin prophylaxis, BP treatment targets, and the timing of delivery.
Keywords: pre-eclampsia, chronic renal insufficiency, glomerulus, proteinuria, pregnancy, Placentation, blood pressure, Renal Insufficiency, Chronic, hypertension, Renal Elimination, Phenotype, Prednisolone, Biopsy
Definitions of Preeclampsia
Preeclampsia is a multisystem disorder that affects 3%–5% of pregnancies (1). It is a clinical diagnosis characterized by endothelial dysfunction after 20 weeks of gestation, leading to systemic features that are nonspecific in isolation. There is consensus that new-onset hypertension is an essential diagnostic feature (2,3). In the absence of other features, this is termed gestational hypertension. In the presence of additional endothelial dysfunction including proteinuria, maternal organ impairment, or uteroplacental dysfunction, a diagnosis of preeclampsia is made (Table 1). Hemolysis, elevated liver enzymes, and low platelet count syndrome is a severe variant of preeclampsia. However, traditional subclassification of “severe preeclampsia” is misleading because of the potential for rapid deterioration across the disease spectrum. The terminology “preeclampsia with or without severe features” is now preferentially used (2).
Table 1.
Diagnostic criteria for preeclampsia: Diagnosis requires one essential and one additional clinical feature for diagnosis
| Diagnostic Criteria | Preeclampsia | Preeclampsia in Women with CKD |
|---|---|---|
| Essential | >20 wk gestation | |
| New hypertension: systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg on two occasions | Hypertension: | |
| • In women without chronic hypertension: as for preeclampsia | ||
| • In women with chronic hypertension: no diagnostic threshold, de novo severe BP (systolic BP >160 mm Hg or diastolic BP >110 mm Hg) or an increase in treatment to maintain BP<160/110 mm Hg used in research cohorts (5,9) | ||
| Additional | Proteinuria: | Proteinuria: |
| • UPCR≥0.3 mg/mg (>30 mg/mmol) | • In women with nonproteinuric CKD: as for preeclampsia | |
| • >300 mg/24 h (not indicated if UPCR available) | • In women with proteinuric CKD: no diagnostic threshold, | |
| • Dipstick >2+ (if other methods unavailable) | >100% increase and UPCR≥0.3 mg/mg (>30 mg/mmol) used in research cohorts (5,9) | |
| • UACR>70 mg/g (>8 mg/mmol) (54,75) | ||
| Serum creatinine: | Serum creatinine: | |
| • Serum creatinine >1.0a to 1.1 mg/dlb,c | • In women with CKD and preserved excretory function: as for preeclampsia | |
| • Doubling of serum creatinine <1.1 mg/dlb,c | • In women with CKD and abnormal prepregnancy function: no consensus on diagnostic threshold for change in creatinine, >50% increase within 7 d used in research cohorts (5) | |
| Hematologic complications: | ||
| • Platelets <100b–150a×109/Lc | ||
| • Hemolysisc, disseminated intravascular coagulationc | ||
| Liver complications: | ||
| • AST or ALT >40 IU/La or double normal reference limitb,c | ||
| • Epigastric/right upper quadrant pain (not attributable to alternate diagnosis)c | ||
| Neurologic complications: | ||
| • Eclampsiac | ||
| • Altered mental statusc | ||
| • Blindness, persistent visual scotomatac | ||
| • Strokec | ||
| • Clonus | ||
| • New-onset headache not attributable to alternate diagnosisc | ||
| Respiratory complications: | ||
| • Pulmonary edema not attributable to another diagnosisc | ||
| Uteroplacental dysfunction: | ||
| • Fetal growth restriction, abnormal umbilical artery Doppler waveform, stillbirth | ||
Meta-analysis and contemporary cohort data show that women with CKD have ten times the risk of preeclampsia compared with women without CKD. Preeclampsia occurs in up to 40% of pregnancies in women with CKD, with an increment in risk with increasing prepregnancy CKD stage (4,5). However, epidemiologic data are limited in the absence of defined diagnostic criteria for women with prepregnancy hypertension and/or proteinuria. Physiologic adaptations of pregnancy include a fall in BP, with a nadir in the second trimester, before rising again toward term (6). There is also gestational increase in proteinuria because of physiologic hyperfiltration and change in glomerular pore size (7,8). Relative increases in BP and proteinuria during pregnancy are therefore insufficient in isolation for diagnosis (2). In a recent cohort study, standard diagnostic criteria for preeclampsia could not be used in 75% (45 of 60) of women with CKD (9). Modifications of diagnostic criteria for women with CKD are detailed in Table 1.
The Role of the Placenta
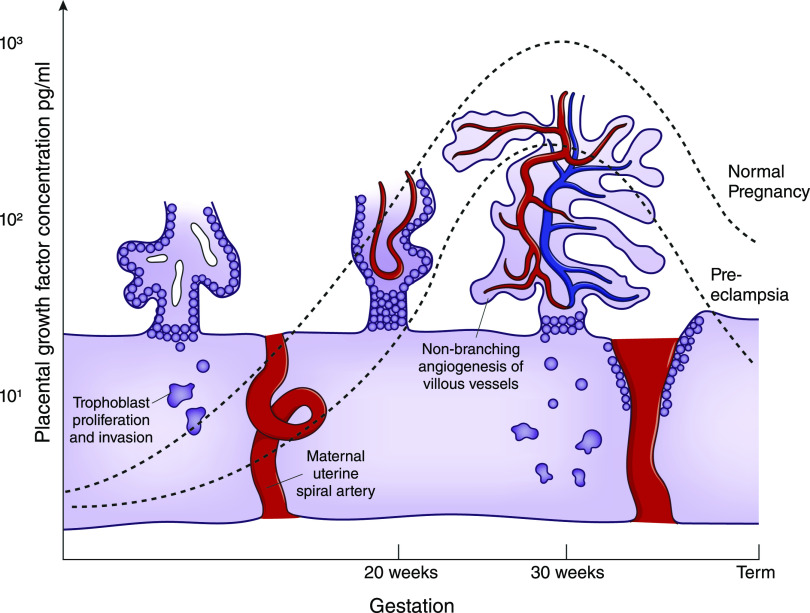
The placenta is a vascular organ containing 550 km of capillaries (10). A complex process, including stimulation by proangiogenic factors and regulation by antiangiogenic factors, regulates the vasculogenesis required for normal placental function. Placental growth factor (PlGF) stimulates endothelial cell activation and angiogenesis by binding to a membrane-anchored receptor, fms-like tyrosine kinase 1. PlGF concentrations rise with maturation of uteroplacental vessels in the second trimester, with peak concentrations at approximately 30 weeks of gestation (11) (Figure 1).
Figure 1.
Circulating placental growth factor (PlGF) concentrations gradually increase during pregnancy to reach a peak at approximately 30 weeks of gestation, coinciding with nonbranching angiogenesis of feto-placental vessels and maturation of the utero-placental circulation. PlGF concentrations (log scale) are on the basis of median values using the Triage PlGF test (Alere, San Diego) from Saffer et al. (11). Modified from reference 73, with permission.
PlGF also binds to a circulating receptor, soluble fms-like tyrosine kinase 1 (sFlt-1). sFlt-1 has antiangiogenic properties, reducing PlGF bioavailability, increasing endothelial sensitivity to inflammatory cytokines (12), and causing vasoconstriction via a reduction in nitric oxide (13). Placental sFlt-1 concentrations rise throughout normal pregnancy, and a decrease in PlGF production from the senescent placenta, in conjunction with binding by sFlt-1, is thought to trigger delivery at term. The gestational balance between angiogenic and antiangiogenic factors is fundamental to normal placental function and, by default, preeclampsia pathophysiology.
Preeclampsia and Angiogenesis
The pathogenesis of preeclampsia is a multistage process, initiated by disordered placental implantation. Small studies of placental bed biopsy specimens demonstrate defective trophoblast invasion and impaired spiral artery remodeling (14), hypothesized to be a result of a failure of maternal immune tolerance (15). Historically, the disease was considered to be silent until the clinical syndrome of preeclampsia developed in the latter half of pregnancy. However, new data demonstrate that impaired placentation leads to a dysfunctional uteroplacental circulation, which releases inflammatory cytokines and antiangiogenic factors into the maternal circulation (13). An abnormal balance between angiogenic (PlGF) and antiangiogenic (sFlt-1) factors is therefore quantifiable before the clinical syndrome manifests.
Women with preeclampsia have lower PlGF (16) and higher sFlt-1 concentrations (17) compared with pregnant women without preeclampsia. After evaluation through prospective cohort studies (18), and a randomized controlled trial (19), PlGF-based testing is now recommended as a diagnostic adjunct in women with suspected preeclampsia, with PlGF and sFlt-1 testing to be fast-tracked for clinical use in the United Kingdom in 2020 (20) (Table 2).
Table 2.
Angiogenic markers used in the prediction and diagnosis of preeclampsia in singleton pregnancies in general obstetric cohorts
A PlGF concentration <100 pg/ml before 35 weeks of gestation has been shown to rule out the need for delivery owing to preeclampsia within the next 2 weeks with 98% probability in a general obstetric population, with better predictive power than other clinical parameters, including BP, transaminitis, and dipstick proteinuria (18). Although this cohort included 287 women, of which 19 (7%) had CKD, median and interquartile ranges for serum creatinine were 0.58 mg/dl and 0.50–0.70 mg/dl, respectively. More recently, a randomized controlled trial of revealed versus concealed PlGF testing was shown to reduce the time to diagnosis and maternal adverse event rate, with no difference in perinatal outcomes (19), at a cost saving of £149 ($190) per patient compared with usual surveillance, including the test cost of £70 ($90) (21). Only 4% of women in this trial had CKD.
When PlGF was combined with sFlt-1 to generate a ratio of sFlt-1-to-PlGF, a value <38 had a negative predictive value of 99% for the development of preeclampsia within 1 week, and 95% for the next 4 weeks, in a cohort presenting with suspected disease (22). However, the proportion of women with CKD was not described, and only 1% (12 of 1050) had preexisting proteinuria. Similarly, CKD prevalence was not reported in the cohort used to establish the threshold of 85 for sFlt-1-to-PlGF (23).
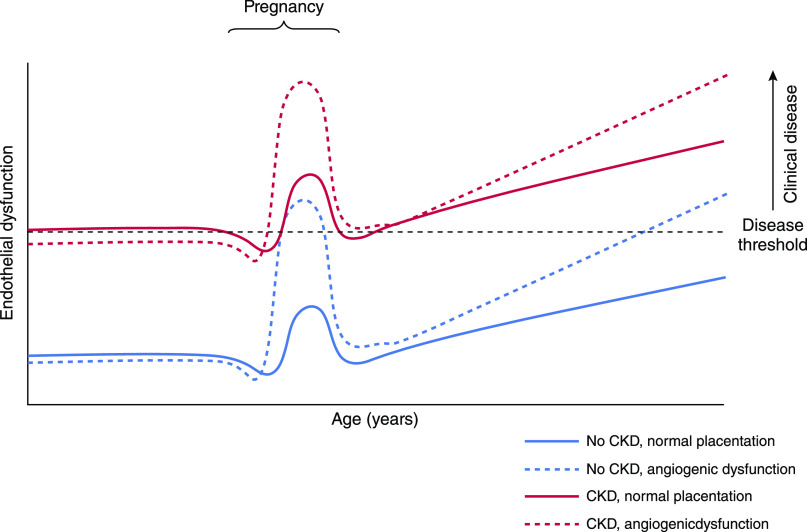
Preeclampsia encompasses a spectrum of disease including early and late forms, requiring delivery before or after 34 weeks of gestation, respectively. Early-onset preeclampsia, which typically has fetal involvement, is driven by placental pathology, evidenced by angiogenic imbalance. However, at later gestations, angiogenic dysregulation is less pronounced (24) and not exhibited by all women (25–27), with reduced accuracy of angiogenic markers (22). Thus, late preeclampsia is proposed to be owing to imbalance between placental perfusion and metabolic demand, rather than angiogenic dysfunction. This occurs in the absence of fetal growth restriction and is exacerbated by a predisposition to metabolic and cardiovascular disease (28). When there is preexisting endothelial dysfunction such as CKD, the physiologic stress-test of pregnancy may therefore be sufficient for development of preeclampsia in the absence of substantial angiogenic dysregulation (29) (Figure 2).
Figure 2.
Endothelial dysfunction in CKD means that the clinical syndrome of preeclampsia can manifest with less angiogenic dysregulation, compared with women without CKD. Physiologic changes in angiogenesis at the end of pregnancy trigger delivery, but do not manifest as clinical preeclampsia in women without CKD (blue line). Women with CKD can manifest preeclampsia without significant angiogenic dysfunction (red line) because endothelial dysfunction owing to CKD places them closer to the threshold for clinical disease. Women with and without CKD who have an abnormal placenta resulting in pathologic angiogenic dysfunction (dashed lines) can both develop pregnancy-related disease (preeclampsia or fetal growth restriction). Women without CKD who develop preeclampsia are more likely to have cardiovascular and kidney disease later in life. Modified from reference 74, with permission.
Preeclampsia and the Kidney
Pathologic Mechanisms
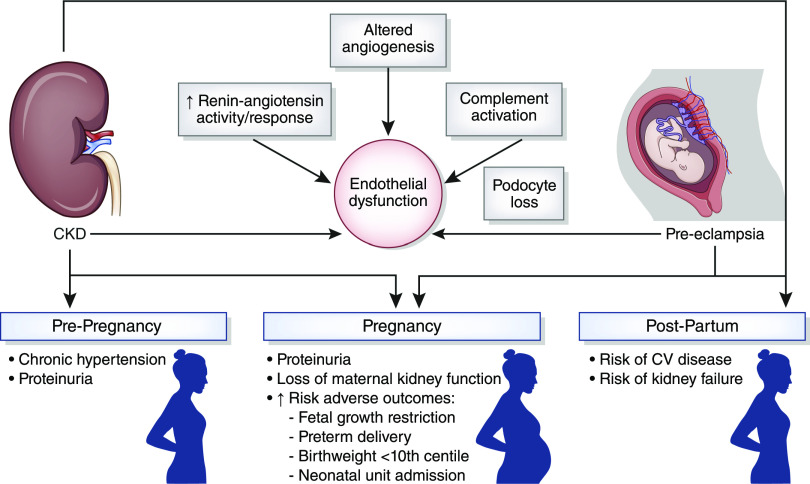
The relationship between preeclampsia and CKD is manifest by a shared phenotype of hypertension, proteinuria, impaired excretory kidney function, and increased cardiovascular risk (30,31); an increased incidence of preeclampsia in women with previous AKI (32) and underlying kidney disease (33); and an elevated lifetime risk of CKD in women with preeclampsia (34–37). Endothelial dysfunction is a common pathophysiologic mechanism underlying both preeclampsia and CKD. Putative pathologic mechanisms contributing to endothelial dysfunction include altered angiogenesis, renin-angiotensin system activation, complement activation, and podocyte loss (Figure 3).
Figure 3.
The relationship between CKD and preeclampsia includes a shared phenotype of endothelial dysfunction, chronic hypertension and proteinuria, adverse pregnancy outcomes, and an increased lifetime risk of cardiovascular and kidney disease. CKD increases the risk of adverse pregnancy outcomes, and preeclampsia is associated with an increased risk of CKD, including end-stage kidney failure. Common pathophysiological mechanisms include endothelial and angiogenic dysfunction, podocyte loss, complement activation, and RAS activation. CV, cardiovascular; RAS, renin-angiotensin system.
Affecting 3%–5% of pregnancies, preeclampsia is a highly prevalent cause of glomerular disease, diagnosed and managed in the majority of cases without a nephrologist. Although there are pathognomonic glomerular changes in preeclampsia, diagnosis is made clinically and kidney biopsy is not indicated.
When kidney biopsy has been performed for historical or research purposes, the predominant change is increased glomerular volume and endothelial cell swelling (endotheliosis), leading to narrowing and occlusion of glomerular capillaries. Although mild endotheliosis has been described in normal pregnancies, mesangial interposition distinguishes hypertensive women (38,39). Duplication of the glomerular basement membrane may be evident in severe disease. Arteriolar thrombosis is unusual and warrants exclusion of nonpreeclamptic thrombotic microangiopathy. Prominent podocytes with vacuolization and endocapillary foam cells are nonspecific in proteinuria, although foot process effacement is limited, suggesting an alternative mechanism for proteinuria in preeclampsia. Fibrin deposition may be prominent, with low-level Ig staining owing to nonimmune-mediated accumulation. Electron-dense deposits are not a feature of preeclampsia and warrant exclusion of an immune-mediated GN (30).
Kidney conditions presenting in pregnancy may mimic normal gestational physiology and preeclampsia (Table 3). Kidney biopsy may be indicated for the diagnosis of new or recurrent kidney disease. The risks of kidney biopsy are higher in pregnancy (7%) than outside of pregnancy (1%), with meta-analysis data showing peak bleeding risk at 23–26 weeks of gestation (40). Recent guidelines advocate kidney biopsy is considered in the first and early-second trimester, only when histologic diagnosis will change management (41). The timescale for postpartum resolution of preeclampsia is debated. Expert consensus recommends nephrology review for persistent proteinuria at 3–6 months to exclude underlying CKD. However, histologic changes in the kidney may still be evident at 18 months postpartum (42), with regression of proteinuria for up to 2 years (43).
Table 3.
Phenotype similarities and distinguishers between normal pregnancy, preeclampsia/HELLP, and kidney disease presenting in pregnancy
| Features | Normal Pregnancy | Preeclampsia/HELLP | Lupus Nephritis | Atypical HUS |
|---|---|---|---|---|
| Gestation | All | >20 wk | Any | Typically peri-/postpartum |
| Skin changes/rash | + | — | + | — |
| Hair loss | + | — | + | — |
| Edema | + | ++ | + | + |
| Hypertension (BP>140/90 mm Hg) | — | ++ | + | + |
| UPCR≥0.3 mg/mg (>30 mg/mmol) | — | + | + | + |
| Hematuria | Trace | — | + | –/+ |
| Anemia | + | + | + | –/+ |
| Thrombocytopenia (<100×109/L) | — | + | + | ++ |
| Hemolysis | — | + Improves 48–72 h postdelivery | Rare | ++ Persists >72 h after delivery |
| Serum creatinine | ⇓ in T1, ⇓⇓ in T2, ⇑ in T3 | ⇑ | ⇑ | ⇑⇑ |
| Transaminitis | No | Yes | No | No |
| ESR | ⇑ | — | ⇑ | ⇑ |
| dsDNA | — | — | ⇑ | — |
| Complement | Can ⇑ | — | ⇓ (within normal range) | ⇓ in 30%−50% |
HELLP, hemolysis, elevated liver enzymes, and low platelet count; HUS, haemolytic uraemic syndrome; +, part of phenotype; —, not part of phenotype; ++, common or substantially altered in phenotype; UPCR, urinary protein-to-creatinine ratio; –/+, occasionally seen in phenotype; ⇑, increase; T1, first trimester; ⇓, decrease; T3, third trimester; ⇑⇑, substantial increase; ESR, erythrocyte sedimentation rate; dsDNA, double-strand DNA.
Superimposed Preeclampsia
Diagnosis
There is no consensus in terminology for preeclampsia with underlying conditions. Superimposed preeclampsia is most commonly used to describe preeclampsia in women with chronic hypertension. Chronic hypertension is prevalent in pregnant women with CKD with rates in contemporary cohorts of 27%–40% (33), and is an independent risk factor for adverse pregnancy outcome (44). Similarly, proteinuria has been associated with accelerated loss of maternal kidney function in a small cohort of women with CKD (45). This heterogeneity within CKD is often not considered, with confounding by chronic hypertension and proteinuria accounted for in the minority of studies (4). This prevents an independent assessment of the risk associated with abnormal kidney function from risk due to chronic hypertension and/or proteinuria. Superimposed preeclampsia remains an inadequate term for women with CKD, including preeclampsia with abnormal kidney function, and/or chronic hypertension, and/or proteinuria, or any combination of features.
Evaluation of angiogenic profiling in CKD is warranted as a potential aid in distinguishing gestational physiology, de novo and recurrent CKD, and superimposed preeclampsia, yet published data remain limited. The sFlt-1-to-PlGF ratio distinguishes CKD (in the absence of preeclampsia) from preeclampsia (in the absence of CKD) (46), although this does not aid in the diagnosis of superimposed preeclampsia. In two cohorts with chronic GN, lower PlGF and higher sFlt-1 concentrations were demonstrated in superimposed preeclampsia compared with both proteinuria in the absence of hypertension, and CKD with a normal pregnancy course, although these studies included only five (47) and ten (48) women with superimposed preeclampsia. Bramham et al. (5) demonstrated diagnostic accuracy for angiogenic markers in superimposed preeclampsia in a mixed cohort of 165 women with chronic hypertension or CKD or both. The sensitivity, specificity, and positive and negative predictive values of PlGF concentrations below fifth percentile (100 pg/ml) for predicting delivery within 2 weeks were 63%, 75%, 56%, and 80%, respectively, in CKD (n=28), although these data included only eight women with superimposed preeclampsia.
PlGF is eliminated by the kidney (49), yet published data are insufficient to assess whether reduced kidney clearance affects the diagnostic and/or predictive capacity of PlGF and sFlt-1-to-PlGF ratios. In the cohorts of Masuyama et al. (47,48), less angiogenic dysfunction was seen with worsening kidney function, although numbers were small. In the larger cohort study by Bramham et al. (5), women with CKD had a median serum creatinine of 0.80 mg/dl (interquartile range, 0.57–1.33 mg/dl), thereby preventing assessment of diagnostic performance in advanced kidney dysfunction. Preexisting endothelial dysfunction may also mean that preeclampsia can present with less angiogenic dysfunction (i.e., at a higher PlGF concentration) in women with CKD (Figure 2).
Plasma hyaluronan and vascular cell adhesion molecule-1 concentrations are alternative markers of endothelial dysfunction, distinguishing superimposed preeclampsia (n=15) from pregnancy with CKD in the absence of preeclampsia (n=45) (9), and further validation is warranted. Despite mechanistic plausibility, biomarkers of complement, renin-angiotensin system, kidney injury, and cardiac function have failed to demonstrate diagnostic discrimination of superimposed preeclampsia in CKD (5,9).
Management of Preeclampsia in Women with CKD
Aspirin
Originally trialed because of a hypothesis that prostacyclin and thromboxane A2 metabolism were involved in pathogenesis, low-dose aspirin has been shown to reduce the risk of preeclampsia in systematic review and meta-analysis of large cohorts (50–53). Prophylactic use of low-dose aspirin in CKD is extrapolated from general obstetric cohort data.
The optimum gestation at which aspirin should be commenced is ambiguous, with meta-analysis data suggesting (53) and refuting (52) improved outcomes with commencement before 16 weeks of gestation. Current guidelines advise use from 12 weeks of gestation in women with CKD (54). The ethics of medication exposure during fetal organogenesis mean that safety data are limited for all drugs before 12 weeks of gestation. However, there is no evidence that aspirin is teratogenic and low-dose aspirin is not withheld before 12 weeks of gestation in women with an indication for treatment.
Optimal aspirin dose is also unclear, with 75–81 mg the most commonly prescribed in the United Kingdom and United States. Meta-analysis data demonstrate no benefit in prevention of preeclampsia at 60 mg, with most benefit evident at 100 mg (53), which is not available in the United Kingdom. A recent randomized controlled trial demonstrated a 62% reduction in preterm preeclampsia in women taking 150 mg aspirin from 11–14 weeks of gestation compared with placebo, with no increase in adverse events, including kidney injury (55). Despite absence of data comparing doses of 75–81 mg with 150 mg, this trial has been sufficient for many clinicians to switch to 150 mg for preeclampsia prophylaxis. It is noteworthy that in a secondary analysis, no reduction in preeclampsia was seen in women with chronic hypertension (56), although it is not clear whether this is a true phenomenon or reflects the challenge of diagnosing preeclampsia in women with chronic hypertension. The number of women with CKD was not reported, and the benefits and risks of 150 mg of aspirin in women with CKD remain unknown.
Management of BP
Historical guidance avoided strict BP control in pregnancy because of theoretical concerns that treatment compromised the fetal circulation. A randomized controlled trial of “tight” (target diastolic 85 mm Hg) with “less tight” (100 mm Hg) BP control in pregnancy was designed to address this. Although achieved BP differences between groups were less than intended (139/90 mm Hg versus 133/85 mm Hg), tighter control was associated with a reduction in the incidence of severe maternal hypertension (28% versus 41%) (57), with secondary analysis showing that severe hypertension was associated with an increased risk of adverse maternal and fetal outcomes in women with less-tight control (58). A recent systematic review including 59 trials and 4723 pregnant women with BPs between 140–169 and 90–109 mm Hg demonstrated that antihypertensive treatment halved the risk of severe maternal hypertension, with no adverse effects on maternal and fetal outcomes (59).
In 2019, guidance on hypertension in pregnancy was updated in both the United Kingdom and United States. In the United Kingdom, evidence of a reduction in severe maternal hypertension, in the absence of adverse fetal and neonatal consequences, was sufficient to reduce the recommended treatment threshold from 150/100 mm Hg to 140/90 mm Hg (60). In contrast, a reduction in severe maternal hypertension was insufficient to change United States guidance in the absence of a measurable benefit on maternal outcome. Treatment thresholds in the United States are 160/110 mm Hg for women with gestational hypertension or preeclampsia, and 150/100 mm Hg in women with end-organ damage—both higher than recommendations outside of pregnancy (61). There are insufficient data to determine the risks and benefits of specific BP targets for pregnant women with CKD. Recent expert consensus for women with CKD is the treatment of gestational BPs >140/90 mm Hg, aiming for a target of 135/85 mm Hg or below, with antihypertensive treatment withdrawn only if BPs are consistently <110/70 mm Hg (41).
The choice of antihypertensive agent in pregnancy is likely to be determined by licensing, availability, and clinician experience, with no high-level evidence to guide prescribing (54,62). Labetalol, nifedipine, and methyldopa are all considered safe for use in pregnancy. Fewer safety data are available for amlodipine and doxazosin, although no adverse fetal effects are reported (41). Angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists are contraindicated because of fetotoxicity in second and third trimesters.
Peripartum Management
Intravascular volume contraction with extracellular fluid overload complicates assessment of fluid status in preeclampsia. Capillary leak, reduced plasma oncotic pressure, and variable cardiac output contribute to a risk of pulmonary edema (63,64), with associated maternal morbidity and mortality. In the absence of other hemodynamic insults (e.g., hemorrhage, sepsis), fluid intake should be restricted to insensible losses (30 ml/h) plus anticipated urinary losses (0.5 ml/kg per hour), limiting intake to <85–100 ml/h to avoid the risk of pulmonary edema (2,54). There is no role for diuretics in the prevention of preeclampsia (65), although they may be required for symptomatic fluid overload.
Intravenous magnesium sulfate is indicated in the treatment of eclampsia, for eclampsia prophylaxis in preeclampsia with severe features and anticipated delivery within 24 hours, and for fetal neuroprotection when delivery occurs before 34 weeks of gestation (3,54). In women with reduced kidney function, a loading dose of 4 g can be given, but maintenance treatment, which is continued for 24 hours, should be reduced from 1 to 0.5 g/h, or withheld in severe kidney disease/injury. Although there is poor correlation between serum concentrations and clinical toxicity, monitoring is advocated in women with kidney dysfunction, and treatment stopped if concentrations are >3.7 mmol/L (9 mg/dl) (3).
Timing of delivery in preeclampsia balances the risks of maternal and fetal morbidity against those of prematurity. For preeclampsia presenting after 37 weeks of gestation, delivery is indicated. Before 34 weeks of gestation, it is usual to attempt expectant management to reduce neonatal morbidity associated with preterm delivery. Decision making between those gestations was aided by a recent trial in which women with preeclampsia at 34–37 weeks of gestation were randomized to expectant management or planned delivery. Planned delivery was associated with reduced maternal morbidity, with three quarters of expectantly managed women progressing to severe preeclampsia. Although neonatal admission was higher in those with planned delivery, this occurred without excess neonatal morbidity (66). Women with CKD, including those with kidney transplants, can have a vaginal delivery.
Postpartum Care
Preeclampsia is associated with an increased lifetime risk of kidney disease (34,35,37,67). However, data on the effect of superimposed preeclampsia on future kidney health in CKD are scarce, with heterogeneity of cohort size and kidney disease cause, variable definitions of superimposed preeclampsia, and the limitations of a binary diagnosis that does not consider severity of preeclampsia or CKD. Women with CKD and superimposed preeclampsia require close postpartum follow-up of BP and kidney function, with stabilization and optimization of kidney function before a future pregnancy. The therapeutic value of renin-angiotensin inhibition in the postpartum regression of proteinuria after preeclampsia is unknown. Safety data for angiotensin-converting enzyme inhibitor use in lactation are limited to isolated cases and small case series of women taking enalapril (n=5) (68) and captopril (n=11) (69).
Future Treatments
The pathogenic role of angiogenic dysfunction in preeclampsia has led to a focus on targeted therapies that either increase PlGF or block the action of s-Flt1. Animal models include therapeutic provision of vascular growth factors (70) and antagonism of sFlt-1 (71). sFlt-1 is positively charged and can be selectively removed by apheresis. This technique was described in 11 women with preterm preeclampsia, leading to a reduction in sFlt-1 concentrations, and an increase in gestation by 8 days after one treatment and 15 days after multiple treatments, compared with 3 days in untreated women (72). A proof-of-concept trial is underway examining safety, dosing, and efficacy of sFlt-1 apheresis (Clinicaltrials.gov identifier: NCT02923206).
Conclusions
Preeclampsia is a disease of placentation, which leads to a dysfunctional balance between angiogenic (PlGF) and antiangiogenic (sFlt-1) proteins that is measurable before clinical presentation. Quantification of PlGF concentrations or sFlt-1-to-PlGF ratio is now recommended as a diagnostic adjunct in the general obstetric population.
Preeclampsia and CKD have a shared phenotype and diagnosis is complicated by hypertension and proteinuria that predate pregnancy. Assessment of the diagnostic and predictive performance of angiogenic markers in women with CKD is limited. Impaired kidney clearance and preexisting endothelial dysfunction may mean that the angiogenic threshold at which preeclampsia manifests may be altered in CKD.
Management of preeclampsia includes aspirin prophylaxis, treatment of hypertension, careful fluid balance, intravenous magnesium, and iatrogenic delivery, with data extrapolated from general obstetric cohorts to women with CKD. There is increasing evidence that a lower threshold (140/90 mm Hg) for the treatment of hypertension in pregnancy reduces maternal complications without adverse effect on the neonate. Data on the therapeutic removal of sFlt-1 and other novel treatments are awaited.
Disclosures
K. Bramham has received consulting and lecture fees from Alexion. L. Lightstone has received lecture fees/honoraria from Alexion and Aurinia and consulting fees from Achillion, AstraZeneca, Aurinia, GSK, and Pfizer. All remaining authors have nothing to disclose.
Funding
L.C. Chappell is supported by National Institute for Health Research research professorship RP-2014-05-019. This work was also supported by the National Institute for Health Research Biomedical Research Centre at Imperial College Healthcare National Health Service Trust and Imperial College London. L. Lightstone received research grant funding from Roche.
Acknowledgments
The authors acknowledge the National Institute for Health Research (NIHR) Rare Diseases Translational Research Collaboration as well as the Biomedical Research Centre at Guy’s and St. Thomas’ National Health Service (NHS) Foundation Trust and King’s College London, for funding Dr. Wiles under the terms of a doctoral research fellowship.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ: Pre-eclampsia. Lancet 387: 999–1011, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S; International Society for the Study of Hypertension in Pregnancy (ISSHP): Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 72: 24–43, 2018 [DOI] [PubMed] [Google Scholar]
- 3.ACOG practice bulletin No. 202 summary: Gestational hypertension and preeclampsia. Obstet Gynecol 133: 211–214, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Zhang J-J, Ma X-X, Hao L, Liu L-J, Lv J-C, Zhang H: A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 10: 1964–1978, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramham K, Seed PT, Lightstone L, Nelson-Piercy C, Gill C, Webster P, Poston L, Chappell LC: Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int 89: 874–885, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Loerup L, Pullon RM, Birks J, Fleming S, Mackillop LH, Gerry S, Watkinson PJ: Trends of blood pressure and heart rate in normal pregnancies: A systematic review and meta-analysis. BMC Med 17: 167, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts M, Lindheimer MD, Davison JM: Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol 270: F338–F343, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Milne JEC, Lindheimer MD, Davison JM: Glomerular heteroporous membrane modeling in third trimester and postpartum before and during amino acid infusion. Am J Physiol Renal Physiol 282: F170–F175, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Wiles K, Bramham K, Seed PT, Kurlak LO, Mistry HD, Nelson-Piercy C, Lightstone L, Chappell LC: Diagnostic indicators of superimposed preeclampsia in women with CKD. Kidney Int Rep 4: 842–853, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton GJ, Jauniaux E: Sonographic, stereological and Doppler flow velocimetric assessments of placental maturity. Br J Obstet Gynaecol 102: 818–825, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Saffer C, Olson G, Boggess KA, Beyerlein R, Eubank C, Sibai BM; NORMALS Study Group: Determination of placental growth factor (PlGF) levels in healthy pregnant women without signs or symptoms of preeclampsia. Pregnancy Hypertens 3: 124–132, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS: Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc Res 89: 671–679, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton GJ, Redman CW, Roberts JM, Moffett A: Pre-eclampsia: Pathophysiology and clinical implications. BMJ 366: l2381, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Lyall F, Robson SC, Bulmer JN: Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 62: 1046–1054, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Redman CW, Sargent IL: Immunology of pre-eclampsia. Am J Reprod Immunol 63: 534–543, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, Capeau J, Uzan S, Rondeau E: Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem 50: 1702–1703, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, Redman CWG, Shennan AH: Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: A prospective multicenter study. Circulation 128: 2121–2131, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, Shennan AH, Chappell LC; PARROT trial group: Placental growth factor testing to assess women with suspected pre-eclampsia: A multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 393: 1807–1818, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence: PlGF-based testing to help diagnose suspected pre-eclampsia (Triage PlGF test, Elecsys immunoassay sFlt-1/PlGF ratio, DELFIA Xpress PlGF 1-2-3 test, and BRAHMS sFlt-1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio). Diagnostics guidance [DG23], 2016. Available at: https://www.nice.org.uk/guidance/dg23. Accessed January 23, 2020
- 21.Duhig KE, Seed PT, Myers JE, Bahl R, Bambridge G, Barnfield S, Ficquet J, Girling JC, Khalil A, Shennan AH, Chappell LC, Hunter RM: Placental growth factor testing for suspected pre-eclampsia: A cost-effectiveness analysis. BJOG 126: 1390–1398, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S: Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 374: 13–22, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, Sabria J, Markfeld-Erol F, Galindo A, Schoofs K, Denk B, Stepan H: New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 63: 346–352, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, Hassan SS, Kim CJ, Chaiworapongsa T: Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med 25: 498–507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ: Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 122: 478–487, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Rana S, Hacker MR, Modest AM, Salahuddin S, Lim KH, Verlohren S, Perschel FH, Karumanchi SA: Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 60: 451–458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore AG, Young H, Keller JM, Ojo LR, Yan J, Simas TA, Maynard SE: Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med 25: 2651–2657, 2012 [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin K, Zhang J, Lye SJ, Parker JD, Kingdom JC: Phenotypes of pregnant women who subsequently develop hypertension in pregnancy. J Am Heart Assoc 7: e009595, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts JM, Catov JM: Preeclampsia more than 1 disease: Or is it? Hypertension 51: 989–990, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Stillman IE, Karumanchi SA: The glomerular injury of preeclampsia. J Am Soc Nephrol 18: 2281–2284, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, Chappell L: Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: A CALIBER study. Circulation 140: 1050–1060, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Tangren JS, Powe CE, Ankers E, Ecker J, Bramham K, Hladunewich MA, Karumanchi SA, Thadhani R: Pregnancy outcomes after clinical recovery from AKI. J Am Soc Nephrol 28: 1566–1574, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccoli GB, Cabiddu G, Attini R, Vigotti FN, Maxia S, Lepori N, Tuveri M, Massidda M, Marchi C, Mura S, Coscia A, Biolcati M, Gaglioti P, Nichelatti M, Pibiri L, Chessa G, Pani A, Todros T: Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 26: 2011–2022, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Wang I-K, Muo C-H, Chang Y-C, Liang C-C, Chang C-T, Lin S-Y, Yen T-H, Chuang F-R, Chen P-C, Huang C-C, Wen C-P, Sung F-C, Morisky DE: Association between hypertensive disorders during pregnancy and end-stage renal disease: A population-based study. CMAJ 185: 207–213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kattah AG, Scantlebury DC, Agarwal S, Mielke MM, Rocca WA, Weaver AL, Vaughan LE, Miller VM, Weissgerber TL, White W, Garovic VD: Preeclampsia and ESRD: The role of shared risk factors. Am J Kidney Dis 69: 498–505, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA: Pre-eclampsia and risk of later kidney disease: Nationwide cohort study. BMJ 365: l1516, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strevens H, Wide-Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S, Willner J, Olsen S: Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG 110: 831–836, 2003 [PubMed] [Google Scholar]
- 39.Penning ME, Bloemenkamp KW, van der Zon T, Zandbergen M, Schutte JM, Bruijn JA, Bajema IM, Baelde HJ: Association of preeclampsia with podocyte turnover. Clin J Am Soc Nephrol 9: 1377–1385, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piccoli GB, Daidola G, Attini R, Parisi S, Fassio F, Naretto C, Deagostini MC, Castelluccia N, Ferraresi M, Roccatello D, Todros T: Kidney biopsy in pregnancy: Evidence for counselling? A systematic narrative review. BJOG 120: 412–427, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Wiles K, Chappell L, Clark K, Elman L, Hall M, Lightstone L, Mohamed G, Mukherjee D, Nelson-Piercy C, Webster P, Whybrow R, Bramham K: Clinical practice guideline on pregnancy and renal disease. BMC Nephrol 20: 401, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kincaid-Smith P: The renal lesion of preeclampsia revisited. Am J Kidney Dis 17: 144–148, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Berks D, Steegers EAP, Molas M, Visser W: Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 114: 1307–1314, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC: Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ 348: g2301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imbasciati E, Gregorini G, Cabiddu G, Gammaro L, Ambroso G, Del Giudice A, Ravani P: Pregnancy in CKD stages 3 to 5: Fetal and maternal outcomes. Am J Kidney Dis 49: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Rolfo A, Attini R, Tavassoli E, Neve FV, Nigra M, Cicilano M, Nuzzo AM, Giuffrida D, Biolcati M, Nichelatti M, Gaglioti P, Todros T, Piccoli GB: Is it possible to differentiate chronic kidney disease and preeclampsia by means of new and old biomarkers? A prospective study. Dis Markers 2015: 127083, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y: Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol 194: 551–556, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Masuyama H, Nobumoto E, Okimoto N, Inoue S, Segawa T, Hiramatsu Y: Superimposed preeclampsia in women with chronic kidney disease. Gynecol Obstet Invest 74: 274–281, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, Blink AL, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Urinary placental growth factor and risk of preeclampsia. JAMA 293: 77–85, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group: Antiplatelet agents for prevention of pre-eclampsia: A meta-analysis of individual patient data. Lancet 369: 1791–1798, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG: Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 160: 695–703, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Meher S, Duley L, Hunter K, Askie L: Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: An individual participant data meta-analysis. Am J Obstet Gynecol 216: 121–128.e2, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E: The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am J Obstet Gynecol 216: 110–120.e6, 2017 [DOI] [PubMed] [Google Scholar]
- 54.National Guideline Alliance (UK): Hypertension in Pregnancy: Diagnosis and Management, London, National Institute for Health and Care Excellence (UK), 2019 [PubMed] [Google Scholar]
- 55.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum-Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH: Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 377: 613–622, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, Leipold G, Akolekar R, Shearing S, De Stefani L, Jani JC, Plasencia W, Evangelinakis N, Gonzalez-Vanegas O, Persico N, Nicolaides KH: Aspirin for Evidence-Based Preeclampsia Prevention trial: Effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol 217: 585.e1–585.e5, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, Gruslin A, Helewa M, Hutton E, Lee SK, Lee T, Logan AG, Ganzevoort W, Welch R, Thornton JG, Moutquin J-M: Less-tight versus tight control of hypertension in pregnancy. N Engl J Med 372: 407–417, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Gafni A, Helewa M, Hutton E, Koren G, Lee SK, Logan AG, Ganzevoort W, Welch R, Thornton JG, Moutquin J-M; CHIPS Study Group: The CHIPS randomized controlled trial (control of hypertension in pregnancy study): Is severe hypertension just an elevated blood pressure? Hypertension 68: 1153–1159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abalos E, Duley L, Steyn DW, Gialdini C: Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 10: CD002252, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC; Guideline Committee: Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ 366: l5119, 2019 [DOI] [PubMed] [Google Scholar]
- 61.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy: Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol 122: 1122–1131, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC: Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: A systematic review and meta-analysis. J Am Heart Assoc 6: e05526, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown MA, Gallery ED: Volume homeostasis in normal pregnancy and pre-eclampsia: Physiology and clinical implications. Baillieres Clin Obstet Gynaecol 8: 287–310, 1994 [DOI] [PubMed] [Google Scholar]
- 64.Melchiorre K, Sutherland G, Sharma R, Nanni M, Thilaganathan B: Mid-gestational maternal cardiovascular profile in preterm and term pre-eclampsia: A prospective study. BJOG 120: 496–504, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Churchill D, Beevers GDG, Meher S, Rhodes C: Diuretics for preventing pre-eclampsia. Cochrane Database Syst Rev (1): CD004451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, Linsell L, Chiocchia V, Greenland M, Placzek A, Townend J, Marlow N, Sandall J, Shennan A; PHOENIX Study Group: Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): A randomised controlled trial. Lancet 394: 1181–1190, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khashan AS, Evans M, Kublickas M, McCarthy FP, Kenny LC, Stenvinkel P, Fitzgerald T, Kublickiene K: Preeclampsia and risk of end stage kidney disease: A Swedish nationwide cohort study. PLoS Med 16: e1002875, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Redman CW, Kelly JG, Cooper WD: The excretion of enalapril and enalaprilat in human breast milk. Eur J Clin Pharmacol 38: 99, 1990 [DOI] [PubMed] [Google Scholar]
- 69.Devlin RG, Fleiss PM: Captopril in human blood and breast milk. J Clin Pharmacol 21: 110–113, 1981 [DOI] [PubMed] [Google Scholar]
- 70.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP: Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves AC, Gröne HJ, Ahmed A, Weich HA: Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 14: 1857–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U, Kribs A, Danner M, Hamacher S, Mallmann P, Stepan H, Benzing T: Removal of soluble fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol 27: 903–913, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chau K, Hennessy A, Makris A: Placental growth factor and pre-eclampsia. J Hum Hypertens 31: 782–786, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sattar N, Greer IA: Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? BMJ 325: 157–160, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waugh J, Hooper R, Lamb E, Robson S, Shennan A, Milne F, Price C, Thangaratinam S, Berdunov V, Bingham J: Spot protein-creatinine ratio and spot albumin-creatinine ratio in the assessment of pre-eclampsia: A diagnostic accuracy study with decision-analytic model-based economic evaluation and acceptability analysis. Health Technol Assess 21: 1–90, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, Klein E, Lapaire O, Llurba E, Ramoni A, Vatish M, Wertaschnigg D, Galindo A: Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet Gynecol 45: 241–246, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]