ABSTRACT
Exosomes are 30 to 100 nm extracellular vesicles that are secreted by many cell types. Initially viewed as cellular garbage with no biological functions, exosomes are now recognized for their therapeutic potential and used in regenerative medicine. Cell-derived exosomes are released into almost all biological fluids, making them abundant and accessible vesicles for a variety of diseases. These naturally occurring nanoparticles have a wide range of applications including drug delivery and regenerative medicine. Exosomes sourced from a specific tissue have been proven to provide greater therapeutic effects to their native tissue, expanding exosome sources beyond traditional cell lines such as mesenchymal stem cells. However, standardizing production and passing regulations remain obstacles, due to variations in methods and quantification techniques across studies. Additionally, obtaining pure exosomes at sufficient quantities remains difficult due to the heterogeneity of exosomes. In this review, we will underline the uses of exosomes as a therapy and their roles in lung regenerative medicine, as well as current challenges in exosome therapies.
KEYWORDS: Exosome, regenerative medicine, lung spheroid cell, stem cell
Introduction
Exosomes are a type of extracellular vesicle (EV) whose diameter ranges from 30 to 100 nm and are characterized by a cup-shaped morphology. They originate from multivesicular bodies (MVBs) and are released into the extracellular space following plasma membrane fusion. Exosomes carry cargo composed of proteins, lipids, and nucleic acids to recipient cells, which take up the cargo and initiate a response [1]. These EVs can target cells through ligand-receptor mediated interactions, membrane infusion, and endocytosis. Exosomes and their components have the ability to influence the tissue microenvironment to mediate cellular communication through local and distal alterations of extracellular matrix [2,3]. Exosomes can be classified through immunolabeling, by identifying exosomal surface proteins that include tetraspanins (CD9, CD63, and CD81), integrins (ITG), cell adhesion molecules (CAM), and growth factor receptors [4,5]. Variations in proteins and receptors on the exosome surface allow for targeting to recipient cells, enabling exosomes to be utilized as a tissue-specific delivery vehicle [6]. Although exosome characterization has been further elucidated, as scientific articles continue to be published regarding extracellular vesicles as a whole, the Minimal Information for Studies of Extracellular Vesicles (MISEV) provides guidelines to ensure standardized characterization, isolation, and purification of particular EVs [7]. With consideration of the MISEV, exosome production and characterization can be more replicable, resulting in more reproducible data across studies.
Exosomes as drug delivery vehicles
Drug systems that use exosome nanotechnology as a delivery mechanism are increasing in popularity since exosomes can be used to treat a wide range of diseases with increased therapeutic efficacy while remaining minimally invasive. A naturally occurring nanoparticle, exosomes are detected in the majority of organs and body fluids, making them a native delivery vesicle for targeted drug delivery with non-toxic and non-immunogenic characteristics [8]. The parent cell of the exosome contributes to the exosome’s natural ability to target and carry out a tissue-specific response by controlling the RNAs, proteins, and lipids contained within the exosome [9]. To optimize targeting efficiency and retention, exosomes can be engineered by manipulation of specific cargo loading and surface decorating or “cloaking”, such as in the fabrication of artificial chimeric exosomes [10–13]. Bio-engineered exosomes can allow for in vitro and in vivo traceability, cellular response initiation, and increased cellular uptake through surface charge modifications [13,14]. However, these manipulations reduce clinical translatability, by altering the exosome’s natural state. Three main strategies have been developed for exosome drug delivery: isolating naïve exosomes and loading into drug-incorporated cells, loading drugs into parental cells which then release exosomes, and transfecting parental cells with DNA-encoding drugs which then release exosomes [15]. These strategies have clinical applications but remain difficult to scale up to human patients, due to time-intensive and costly production [15]. Currently, there are only two ongoing Phase I clinical trials using exosomes as drug delivery vehicles (Table 1). Under the M.D. Anderson Cancer Center, mesenchymal stromal cell-derived exosomes loaded with small interference RNA against KrasG12D, coined “iExosomes”, are being used to treat participants with pancreatic cancer with a KrasG12D mutation (https://clinicaltrials.gov, NCT 03608631). Under the James Graham Brown Cancer Center, the effects of curcumin-conjugated plant exosomes are being evaluated in patients undergoing surgery for newly diagnosed colon cancer (https://clinicaltrials.gov, NCT 01294072). It is evident that exosomal cargo loading and surface decorating are effective therapeutic strategies with clinical applications. As exosome sourcing is refined, their targeting and retentive abilities will be optimized, making exosomes a more feasible nanotechnology.
Table 1.
Clinical applications of exosomes in drug delivery and regenerative medicine.
| Drug delivery | Regenerative medicine | |
|---|---|---|
| Definitions | In which exosomal cargo is modified to contain a specific compound, including but not limited to a particular protein, miRNA, or pharmaceutical drug, for treatment | In which exosomes are used as naturally produced for treatment |
| Number of Papers | 360 | 338 |
| Number of Clinical Trials | 2 | 13 |
| Pros | Highly specific organotropic behaviour [6,15] Biocompatible [6] Can be engineered to increase drug load or improve stability and targeting ability [6,12,15] Stable during cryopreservation [16] Naturally crosses cell membranes and the BBB [30,54] Avoid macrophage uptake, resulting in long blood circulation [54] Large surface area to volume ratio allows exosomes to carry a significant amount of surface cargo [4] Can carry hydrophobic and hydrophilic drugs [15] |
Highly specific organotrophic behaviour [40,48,54, B. 18,26] Nonimmunogenic [54] Can be selected to carry specific proteins, receptors, or nucleic acids for gene therapy via cell culture microenvironment [9,16,35,64, B. 26] Stable during cryopreservation [16] Naturally crosses cell membranes and the BBB [63] Regulate tissue homoeostasis [36,54] Initiates tissue repair and regeneration [2,17,34,36,40,48] Released by most cell types, enabling tissue-specific signalling [48,54] Long-lasting paracrine effects [39,40] |
| Cons | Difficult to produce enough yield to be effective in
humans [6] All isolation techniques have cons, including time, monetary cost, forming aggregates, introducing contaminants [6,54] Systemic administration has not reflected targeting potential [6] |
Difficult to produce enough yield to be effective in
humans [10,64] All isolation techniques have cons, including time, monetary cost, forming aggregates, introducing contaminants [16,63,66] No single way to manufacture exosomes of a specific potency [10,16] |
| Representative Companies | Anjarium Biosciences ArunA Biomedical Codiak Biosciences Evox Therapeutics Exogenus Therapeutics |
Aegle Therapeutics BreStem Therapeutics Capricor Therapeutics Kimera Exosomes ReNeuron Tavec Pharma Xollent Biotech |
Exosomes for regenerative medicine
Exosomes are already a feasible therapy for tissue regeneration across all major organs. Selectivity based on parent cell type and exosomal cargo loading increases therapeutic outcomes and specificity while minimizing adverse effects. Exosomes have demonstrated regenerative properties by reducing inflammation and apoptosis while promoting proliferation and angiogenesis. Mesenchymal stem cells (MSCs) and MSC-derived exosomes are popular in regenerative medicine, due to their multipotency and self-renewing properties. This multipotency provides therapeutic benefits to many organ and tissue types. For example, exosomes are able to combat cardiovascular disease, promote bone remodelling, and regress liver fibrosis, despite vast differences across disease models [16–18]. Exosomes have also shown promising regenerative effects in a wide variety of models, such as myocardial infarction, kidney injury, and neurological injury. Injection of MSC-exosomes has been shown to reduce infarct size in a rat myocardial infarction model, while restoring long-term cardiac function [19]. Human cardiosphere-derived exosomes have shown greater global heart function than media controls in an acute MI mouse model [20]. Hypoxic cardiac progenitor cell-derived exosomes improve cardiac function, fibrosis, angiogenesis, and hypertrophy in a rat model of ischaemia reperfusion [21,22]. Exosomal microRNA miR-21-5p helps regulate apoptosis and angiogenesis and improves heart function in a mouse model of myocardial infarction [23]. Exosome-mediated delivery of Sonic hedgehog protein improves therapeutic efficacy of human CD34+ stem cells in a mouse model of acute myocardial infarction [24]. Engineered hydrogel patches loaded with induced pluripotent stem cell-derived cardiomyocyte EVs offer a slowly released cell-free therapeutic that reduces infarct size and cell hypertrophy in a rat model of acute myocardial infarction [25]. Delivery of MSC-exosomes overexpressing miR-let7 c targets and reverses kidney fibrosis in a murine unilateral ureteral obstruction model [B. 26]. Exosomes secreted from hypoxic rat renal proximal tubular cells are protective against renal tubular cell injury [27]. Additionally, human kidney tubular cells and their secreted exosomes prevent renal injury in a rat model of bilateral renal ischaemia [28]. Adipose-derived mesenchymal stem cells with overexpressed miR-181-5p provide targeted therapy to hepatic stellate cells and attenuate liver injury in the mouse model of liver fibrosis [29]. Modified exosomes can effectively deliver miR-124, bypassing the blood-brain barrier and inducing neurogenesis following ischaemic injury in a murine photothrombosis model [30,31]. Human neural stem cell-derived EVs have a neuroprotective effect, leading to significant improvements at the tissue and function level of both mouse models of thromboembolic stroke and pig models of ischaemic stroke [32,33]. It is evident that exosome-mediated tissue regeneration has potential, as exosomes and their secreted factors have both shown improvement in tissue regeneration. MSC-secreted bioactive molecules have also been shown to exert paracrine effects on surrounding cells in the tissue microenvironment, providing therapeutic benefits [34,35]. This indicates that both MSC-exosomes and their secreted factors contribute to tissue regeneration. Exosome secretome can be manipulated to improve its therapeutic effects through cellular pre-conditioning [35]. Identification of the optimal combination of cargo-loading and culture components will allow for more specialized and effective exosome targeting and regeneration, increasing clinical translatability. Currently, there are numerous clinical trials using exosomes for regenerative medicine, ranging from cutaneous wound healing to macular hole regeneration (Table 1). Under Kumamoto University, serum-derived exosomes are being used to accelerate cutaneous wound healing in patients with intractable cutaneous ulcers (https://clinicaltrials.gov, NCT 02565264). Under Tianjin Medical University, mesenchymal stem cell-derived exosomes are being used to promote healing of large and refractory macular holes (MHs) in patients diagnosed with large and long-standing idiopathic MHs (https://clinicaltrials.gov, NCT 03437759). Exosome use for regenerative medicine is a feasible therapeutic, offering practical applications for a wide range of diseases.
Lung regenerative medicine
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are among the most popular stem cell types in research, due to their differential potential and ability to maintain tissue homoeostasis [36]. MSCs can be obtained from multiple sources, such as bone marrow, adipose tissue, peripheral blood, placenta, and umbilical cord. The use of MSCs in clinical trials continues to expand each year, with over 900 studies listed in ClinicalTrails.gov and over 50 for the use of MSCs for lung diseases specifically (https://www.clinicaltrials.gov). The trials focus on a range of lung diseases including acute lung injury, idiopathic pulmonary fibrosis, acute respiratory distress syndrome, and bronchopulmonary dysplasia, demonstrating that MSCs can be used to treat a wide spectrum of diseases, even within a particular organ. Yi et al. found that overexpressing miR-30b-3p in MSC-derived exosomes helped relieve inflammation and repair alveolar epithelial cells in a mouse model of acute lung injury [37]. Systemic administration of MSCs to two patients with acute respiratory distress syndrome alleviated respiratory, haemodynamic, and multiorgan failure [38]. Intranasal delivery of MSCs helped attenuate lung injury in a neonatal rat model of bronchopulmonary dysplasia [39]. MSCs and MSC-derived exosomes are clearly effective biological therapeutics for lung regenerative medicine. Small-scale clinical studies show promising results for MSCs in their early stages, giving hope for future acellular exosome clinical applications. Transitioning from stem cell therapies to their acellular exosomes will help overcome current obstacles of low cellular engraftment and stability in the lung, making MSC-derived exosomes an attractive alternative [40]. Despite these advances over the past several years, challenges such as batch consistency and purity remain when transitioning from rodent models of lung disease to large-scale clinical trials [41]. Nevertheless, MSC-exosomes offer an alternative to cellular therapies that may provide similar regenerative abilities, devoid of adverse effects following cellular administration.
MSC-exosomes carry a unique and diverse group of nucleic acids, proteins, and lipids that have yet to be identified to reside in one or multiple types of exosomes (Figure 2) [36]. This array of cargo elicits a diverse range of cellular responses to provide therapeutic benefits for various diseases but may require higher specificity to provide superior tissue regeneration and homoeostasis for organs like the lung [36]. Although MSCs and MSC-exosomes are popular biological agents in lung regenerative medicine, elucidating their cargo and developing consistent batches would help unveil key targets for specific lung diseases.
Figure 2.
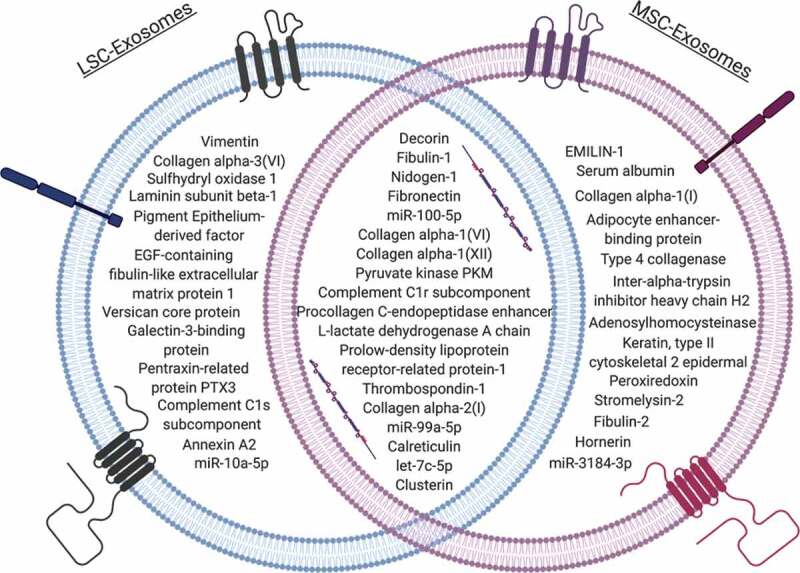
Comparison of exosomal cargo between lung spheroid cell- and mesenchymal cell-derived exosomes [49].
Lung progenitor and spheroid cells
Lung progenitor cells (LPCs) prove to be popular cell types for lineage tracing and differentiation studies, regulatory studies in cancer models, and lung regenerative medicine [42–44]. As opposed to MSCs, lung progenitor cells are a more intrinsic stem cell type and better facilitate lung cell regeneration after injury, offering greater potential targeting and retention abilities [45]. Our lab has developed a heterogeneous lung cell line from healthy human whole-lung and transbronchial biopsies, that consists of a lung progenitor cell core supported by a surrounding layer of stromal cells [40]. The lung progenitor cell core, positive for alveolar type II cells (ProSPC+) and secretory cells (CCSP+), is formed when cells outgrow from lung tissue explants and aggregate into three-dimensional lung spheroids when seeded onto ultra-low-attachment flasks [45]. Supported by stromal-like cells, positive for CD90 and CD105, the progenitor core significantly increases stemness when assuming a 3D structure [40]. This three-dimensional culturing technique may also enhance stemness by more closely mimicking an in vivo stem cell niche. These spheroids can reactivate the plasticity of differentiated lung cells upon disease or injury and have greater therapeutic targeting to the lung than traditional adherent mesenchymal stem cells [45–47]. When plated on an adherent surface, lung progenitor cells can dissociate from their three-dimensional spheroid shape to a monolayer of cells we termed “lung spheroid cells” (LSCs) (Figure 1). LSCs maintain a similar phenotype to lung progenitor cells but may differentiate into mature lung cells, indicated by an increase in Epcam+ expression [40]. LPC and LSC expansion and conditioning can provide novel exosomes and exosome-secretome that may elicit a greater regenerative effect to the lungs than traditionally used MSCs. Such intrinsically sourced cell therapy can elucidate therapeutic targets and pathways that current cell models cannot achieve.
Figure 1.
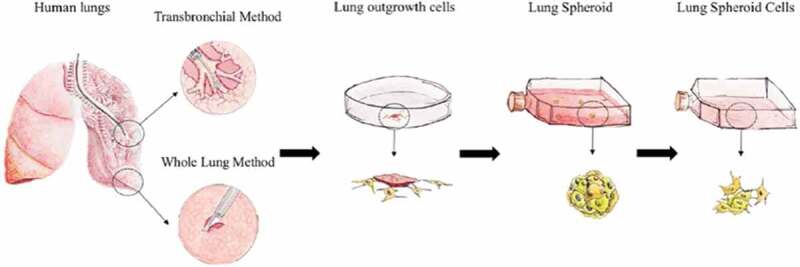
Schematic of the derivation of lung spheroid cells (LSCs) from whole lung and minimally invasive transbronchial lung biopsies [From Respiratory Research 40].
The effect of adult human stem cells, such as our LSCs, is superior to multipotent stem cells for the treatment of lung-specific diseases by providing a more specialized tissue microenvironment [40]. Although a heterogeneous lung cell population, allogeneic LSCs can alleviate inflammation, minimize immunological response, and slow fibrotic development through paracrine mechanisms and direction regeneration in both mouse and rat models of bleomycin-induced pulmonary fibrosis, in a superior fashion to MSCs (Figure 2) [40,48]. LSCs are retained for several weeks in the lungs of mouse and rat models of pulmonary fibrosis, providing prolonged cellular therapy to target diseased tissues [40,48]. Additionally, major organs including the lung heart, liver, kidneys, and spleen are devoid of tumour formation in both mouse and rat models [40,48]. Mice who received LSCs had less fibrotic thickening and tissue infiltration than mice who received adipose-derived MSCs [45]. Additionally, administration of LSC-derived exosomes yields therapeutic effects in a pulmonary fibrosis mouse model, providing a cell-free alternative [49]. LSC-derived exosomes decrease fibrosis and attenuate the alveolar epithelium and vasculature, making exosomes and their unique array of cargo a potential target for cell-free therapy [49]. This intrinsic source of stem cells has the ability to treat a variety of pulmonary diseases and is not limited to fibrosis models, making LSCs and LSC-derived exosomes noteworthy cellular therapies in regenerative medicine. Similar strategies using cardiosphere derivation and cardiosphere-derived exosomes are also implemented in models of ischaemic heart failure and myocardial infarction. Allogeneic cardiosphere-derived cells (CDCs) extracted and isolated from human myocardium have promising preclinical and clinical results, triggering native cardiomyocyte proliferation and recruiting endogenous progenitor cells [50]. CDCs have been utilized in several clinical trials, including the CADUCEUS and DYNAMIC trials [51,52]. A decrease in intramyocardial scarring and minimization of adverse remodelling has also been demonstrated in a pig model of acute and convalescent myocardial infarction treated with CDC-exosomes, demonstrating clinical potential for a cell-free alternative [53].
3D culturing of intrinsically sourced stem cells into spheroids is a promising cellular therapy for a wide variety of diseases beyond the heart and lungs. Studies should expand beyond traditional mesenchymal cell sources, to elicit greater therapeutic effects and enhance cellular targeting and retention.
Challenges in exosome therapy
Exosome production, expansion, and delivery remain prominent challenges in regenerative medicine. As purification and production continue to improve, exosomes in regenerative medicine will become more targeted and tissue-specific. However, further research needs to be conducted to fully understand the complete contents of exosomes, methods of tissue targeting, impact on targeted tissues, and long-term safety effects [54]. Overall, standardizing the production of exosomes will improve consistency, dosage, and potency in the translational medicine community.
Isolation and purification of exosomes
Isolation and purification of exosomes remains imperfect and difficult to produce in large scales for clinical applications. Although considered the current optimal method for exosome isolation, ultracentrifugation-based isolation techniques suffer from contamination and exosome loss due to the heterogeneous nature of exosomes [55]. Despite these drawbacks, ultracentrifugation remains a popular isolation technique because it is easy and relatively cheap. Two common types of ultracentrifugation are differential and density gradient ultracentrifugation. In differential ultracentrifugation, a series of centrifugation cycles isolate sample components by size, with forces ranging around ~100,000 x g. These cycles are used to separate exosomes from unwanted components, such as apoptotic cells and cell debris. In density gradient ultracentrifugation, samples are separated through centrifugation in a density gradient medium. This creates defined solute zones of specific densities [55]. However, due to the heterogeneous nature of exosomes, these isolation techniques cannot perfectly differentiate exosomes from all other unwanted components; overlaps in cell size and density across exosome samples hinders current isolation techniques. Immunoaffinity capture-based techniques have also been developed to isolate exosomes using exosome-specific surface markers, such as CD63 [10]. This technique provides highly specific and pure isolation, but is costly and is hindered by the lack of exosome-specific surface markers. A possible solution to improve isolation and purification of exosomes is to combine ultracentrifugation with immunoaffinity capture techniques, using surface markers that specifically target exosomes from the sample undergoing such isolation and purification. An additional solution involves implementing higher resolution density gradients when undergoing ultracentrifugation. Higher resolution density gradients are necessary to produce more pure exosome fractions and gain a better understanding of exosome composition [56]. Jeppesen et al. employed a combination isolation technique by using density gradient fractionation to separate extracellular vesicles from non-vesicular material, along with direct immunoaffinity capture to specifically isolate exosomes [56]. This combination successfully separated exosomes for other heterogenous sample matter, allowing for more accurate and specific identification and analysis of exosomal properties. Chemical-based isolation techniques isolate highly purified exosome populations and are available as commercial kits, but risk composition alteration and chemical retention in exosome samples [57]. Commercial kits for polymer precipitation have also been developed to overcome these disadvantages, by using solutions of superhydrophilic polymers for isolation. However, these kits are expensive and not suitable for large sample sizes. Additionally, EV type cannot be differentiated, making biomarker elucidation difficult [57]. Microfluidic devices have gained popularity for EV isolation, by trapping vesicles of defined diameter into nanofilters [58]. Although this allows for isolation customization, complex fabrication of the device along with low recovery rates makes microfluidic devices hard to translate to clinical settings [58]. Because of the heterogeneous nature of exosomes, optimal isolation strategies vary with each sample. Although many exosomes types contain similar surface markers (CD9, CD81, and CD63) that can be targeted for affinity-based purification, chemical alterations and low yields may outweigh the benefits of such pure isolation. A combination of isolation techniques optimized for particular exosome samples will allow for proper sample selection while maintaining clinically relevant yields. Overall, as the characteristics and surface phenotypes of tissue-specific exosomes become more defined, isolation and purification techniques will improve and become more clinically translatable.
Large-scale production and immortalization
Translation to large-scale production of exosomes for clinical trials remains difficult, as traditional cell culture is inefficient in terms of time and cost. Production will be optimized once the ideal exosomal microenvironment is identified in an in vitro system [59]. Currently, 2D culture techniques remain common means of production, but they are labour-intensive and result in low exosome yield. Transitioning to 3D culturing techniques increases efficiency, but still does not meet the demands for clinical application, which requires significantly more cells for treatment than traditional animal models. Bioreactors and microcarriers provide large-scale expansion methods, by maximizing surface area for stem cell and exosome growth [60]. Exosome yields can be increased ~40-fold through bioreactor culture, in comparison to conventional cell culture [61]. Although requiring more media and more frequent passaging, these larger-scale methods maximize surface area and allow for efficient cell expansion, all without comprising cellular function. However, exosome isolation in large-scale applications remains impractical, with structural damage and batch inconsistencies hindering clinical translation. Although solutions such as higher resolution density gradients would help produce purer batches, they are not a feasible approach for large-scale production and GMP standards, due to discrepancies in successful isolation and retained quality [62]. Systems such as tangential flow filtration (TFF) have been developed to combat such limitations, assuring more efficient exosome yields and decreased vesicular damage [63]. By directing liquid suspension flow tangentially, as opposed to dead-end, filter clogging by larger particles is minimized and particles are separated more efficiently. This redirection yields higher concentrations of isolated exosomes from the same sample, making TFF desirable for large-scale production. Cell line immortalization can also produce higher yields and ensure infinite supply. Because some stem cell lines, such as our LSCs, have only a finite number of cell divisions before they senesce, discovery of immortalization agents for these specific cell types will allow infinite cell division and safer expansion. Chen et al. implemented this strategy by immortalizing MSCs through transfection of lentivirus carrying the MYC gene [64]. This transfection was a practical strategy that not only ensured an infinite supply of cells and exosomes but also increased proliferative rates [64]. Although immortalization generates large genomic and proteomic outputs, gene expression is manipulated and may no longer represent its native stem characteristics [65]. Stem cell marker expression must be validated upon each passage, to ensure cell characteristics and functions do not deviate among generations. Large-scale exosome production will be optimized through a combination of advanced culturing and isolation techniques. Because of the heterogeneous nature of cell types and their secreted exosomes, no one combination of large-scale production and exosome isolation is optimal for all cell types. An individual evaluation of the risk factors associated with the translation to large-scale production and cellular immortalization must be taken before achieving a clinically relevant product.
Dosage and potency
Standardization of exosome dosage and potency needs to be implemented but is difficult due to the heterogeneous nature of exosomes across various progenitor cells, variations in animal models, and differences in cellular characteristics and evaluation. Therefore, extrapolating exosome dosage from animal models to humans also requires careful consideration. Inconsistencies in dosage methods and potency quantifications make it difficult to reference and exchange data across studies and clinical trials [66]. Since exosome characteristics also vary among cell types, standards may be more effectively formed when considering the tissue being targeted and the mechanisms by which the exosomes reach the target tissue. An in-depth evaluation of the pharmacokinetics and pharmacodynamics of exosomes across various tissue sources will help elucidate optimal dosage and catalyse regulatory approval. The biodistribution and cellular uptake of exosomes need to be identified, to further investigate exosomal half-life, off-target organ accumulation, and surface modification to minimize non-target cell interactions [67]. Standardization will allow for more comparable results and will increase consistency across batches [68].
Regulatory hurdles
FDA approval processes pose as obstacles in translating exosomes to clinical trials. Many commercial companies have emerged, producing products and therapies that utilize exosomes, with Aegle Therapeutics being a noteworthy company to receive IND clearance from the FDA (Table 2). However, the heterogenetic nature of exosomes between batches, cell sources, and purification techniques complicates the approval of such biological products by impeding reproducibility. With the FDA’s definition of biological products continuing to be revised, it is difficult to interpret and fit within these standards. In addition, extensive testing on exosome stability, sterility, quality, and potency must be measured and maintained during processing and shipping conditions. The effects of such processing are not well known and may result in exosome content or composition variations during each processing step. As guidelines for exosome therapeutics are developed specifically to aid in manufacturing and validation during each processing step, exosomes will become easier biological products to approve.
Table 2.
List of commercial companies that use exosomes for therapies.
| Company name | Product/therapy description | URL |
|---|---|---|
| Aegle Therapeutics | Phase 1/2a clinical trial of MSC-derived exosomes to treat severe dermatological disorders. | https://www.aegletherapeutics.com/index.html |
| Anjarium Biosciences | Hybridosome™ platform for vesicle modification to provide additional specificity and biodistribution. | http://www.anjarium.com |
| ArunA Biomedical | Neural exosomes to treat various central nervous system and neurodegenerative disorders. | https://arunabio.com |
| BioVision Incorporated | Production of exosome quantification, isolation, and
DNA/RNA extraction kits. Production of disease-specific exosomal marker antibodies. |
https://www.biovision.com |
| Capricor Therapeutics | Exosomal technology for the treatment of severe, rare,
and inflammatory disorders at a clinical level. Exosomes isolated from chromosphere-derived cells. |
http://capricor.com |
| Codiak Biosciences | Engineered exosomes for precise
targeting. Industrial quality and scale of exosomes for clinical applications. |
http://www.codiakbio.com |
| Evox Therapeutics | Targeting technology, manufacturing, and purification
methods for transformational therapeutics. Molecular engineering, drug loading, and targeting strategies. |
https://www.evoxtherapeutics.com |
| Exogenus | Exosome-based drug development for the treatment of skin and autoimmune diseases. | http://www.exogenus-t.com |
| Exopharm | Development of Ligand-based Exosome Affinity Purification (LEAP) technology to isolate exosomes using affinity chromatography. | https://exopharm.com |
| Exosome Diagnostics | Detection, diagnosis, treatment, and monitoring of
cancer and other diseases. Serum/plasma and urine kits for biomarker discovery, liquid biopsy, clinical diagnostic development, and targeted therapies. |
http://www.exosomedx.com |
| Exosome Sciences | Exosome-based biomarkers to diagnose Alzheimer’s,
Chronic Traumatic Encephalopathy, and other neurological
disorders. Development of the TauSome™ biomarker used in blood testing to identify Chronic Traumatic Encephalopathy. |
https://www.exosomesciences.com |
| Exosomics | Biofluid tests for cancer screening and liquid biopsy
using tumour exosomes. Development of tumour-derived DNA/RNA from exosomes and cancer-derived exosome standards. |
https://www.exosomics.it |
| Kimera Labs | Development of purified placental MSC exosomes for research and therapeutic purposes, including wound healing and immunological manipulation. | https://kimeralabs.com |
| ReNeuron | Pre-clinical development of permanent stem cell line, CTX, -derived exosomes. | http://www.reneuron.com |
| Tavec Pharma | Pre-clinical development of injectable miRNA-loaded exosomes to reduce cholangiocarcinoma tumours. | http://tavecpharma.com |
Exosome-based biomarker development
Currently, there are FDA-approved diagnostic tests that utilize exosome collections from liquid biopsies, such as blood and urine [69]. In such minimally invasive diagnostic tests, unique exosomal surface antigens and cargo are targeted and serve as precise biomarkers for disease diagnosis [70]. Disease evolution can also be evaluated through exosome concentration, proving exosomal-based liquid biopsy to be a powerful tool for cancer metastasis or disease progression [70,71]. However, improvements need to be made to increase sensitivity and diagnostic accuracy in other disease models to expand FDA approval outward from liquid biopsies [72]. From a clinical standpoint, Exosome Diagnostics offers cutting-edge technology that utilizes patient-derived exosomes for biomarker discovery, RNA analysis, mutation detection, protein detection, and signal enhancement through their approved productions: Exolution RNATM, Exolution PlusTM, and EDDETM (Table 2). From a research standpoint, atherosclerosis diagnosis through circulating microRNAs in blood is a promising diagnostic tool, but still targets eight microRNAs, whose expression levels vary as atherosclerosis manifests [73]. Although early detection would significantly improve patient outcome, since clinical signs may take years to develop, identifying a single or combination of microRNAs that are sensitive and precise at the right concentration would be crucial for FDA approval [73]. Translating exosomes to a clinical setting is feasible, but refining biomarker selection for diagnosis is imperative for FDA approval.
Exosome therapeutics
Exosomes pose as a cell-free alternative to cellular therapies but continue to face challenges when translating to clinical studies. Although the immunogenicity, safety, and efficacy of exosomes from xenogeneic and allogenic sources have not been fully characterized, numerous preclinical studies have made such evaluations in cellular therapies. With studies showing disease improvement in both allogeneic and autologous treatments, it is promising that pre-clinical studies using exosomes from such cellular sources would better overcome regulatory hurdles when translated to a clinical setting [48,74,75]. Since some exosome studies have shown unaffected differentially expressed genes related to inflammation or toxicity, as well as no visual signs of toxicity after repeated administration, exosome therapies show promise [76,77]. However, the efficacy of such treatments is more difficult to validate, as disease outcomes following exosome therapeutics may vary between patients due to product variations. Federal regulation of exosome therapies will help improve consistency by implementing standardized data collection, product-tracing information, dosage levels, and potency quantification. These regulations would help facilitate data exchange across studies and disease models. Exosome therapy has great potential and FDA approval will catalyse clinical trials for translational medicine.
Conclusion
Exosomes have great potential to treat a wide variety of diseases. Secreted by many cell types and into almost all biological fluids, exosomes harness the great potential for biological therapies. Drug delivery and regenerative medicine are just some of the many applications. Through bio-engineering techniques such as cargo loading and surface modifications, exosomes can be enhanced to deliver superior therapeutic components at greater concentrations to targeted cell types. Exosomes in regenerative medicine continue to improve across all major organs and tissues, as parent cell selection enhances cargo specificity, void of adverse immune responses or rejection seen in cellular therapies. Intrinsic sources of exosomes further facilitate these enhancements, expanding sourcing from traditional multipotent cell lines to cells derived from the target tissue. Nevertheless, new manufacturing techniques need to be developed and regulated to perfect exosome production, expansion, and delivery. Isolation and purification will be improved as separation is perfected through an optimal combination of techniques. Large-scale production of exosomes will meet demands as culturing techniques transition from standard 2D practices, to 3D culturing techniques and bioreactors. Immortalization of stem cell lines will also ensure infinite expansion, but at the cost of cell manipulation. Standardization of exosome dosage and potency across studies and trials will improve data exchange and consistency with help through federal approval and regulation. Even with production optimizing, exosomes will continue to face challenges in clinical translation, with federal guidelines and requirements continuing to change and become more stringent. As the heterogeneous nature of exosomes is further elucidated, exosomes will become more reproducible and easier to translate. Exosomes currently harness the great potential for biological therapies and will continue to improve as current challenges are met.
Disclosure of interest
K.C. is an equity holder of BreStem Therapeutics. BreStem provides no funding to the present study.
References
- [1].Simons M, Raposo G.. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–11. [DOI] [PubMed] [Google Scholar]
- [2].Hutcheson JD, Aikawa E. Extracellular vesicles in cardiovascular homeostasis and disease. Curr Opin Cardiol. 2018;33(3):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou L, Lv T, Zhang Q, et al. The biology, function and clinical implications of exosomes in lung cancer. Cancer Lett. 2017;407:84–92. [DOI] [PubMed] [Google Scholar]
- [4].Buzás EI, Tóth EÁ, Sódar BW, et al. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 2018;40(5):453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lotvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10(4):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kahroba H, Hejazi MS, Samadi N. Exosomes: from carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell Mol Life Sci. 2019;76(9):1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kao C-Y, Papoutsakis ET. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr Opin Biotechnol. 2019;60:89–98. [DOI] [PubMed] [Google Scholar]
- [10].Kim D, Nishida H, An SY, et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113(1):170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lutz H, Hu S, Dinh P-U, et al. Cells and cell derivatives as drug carriers for targeted delivery. Med Drug Discovery. 2020;100014 DOI: 10.1016/j.medidd.2020.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shen Z, Nieh M-P, Li Y. Decorating nanoparticle surface for targeted drug delivery: opportunities and challenges. Polymers. 2016;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang K-L, Wang Y-J, Sun J, et al. Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem Sci. 2018;10(5):1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].García-Manrique P, Matos M, Gutiérrez G, et al. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles. 2018;7(1):1422676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bellin G, Gardin C, Ferroni L, et al. Exosome in cardiovascular diseases: a complex world full of hope. Cells. 2019;8(2):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen L, Brenner DA, Kisseleva T. Combatting fibrosis: exosome-based therapies in the regression of liver fibrosis. Hepatol Commun. 2019;3(2):180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xie Y, Chen Y, Zhang L, et al. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med. 2017;21(5):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang Z, Yang J, Yan W, et al. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc. 2016;5(1):e002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ibrahim AG-E, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2(5):606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Agarwal U, George A, Bhutani S, et al. Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell–derived exosomes from pediatric patients. Circ Res. 2017;120(4):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116(2):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qiao L, Hu S, Liu S, et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest. 2019;129(6):2237–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mackie AR, Klyachko E, Thorne T, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111(3):312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu B, Lee BW, Nakanishi K, et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018;2(5):293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang B, Yao K, Huuskes BM, et al. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24(7):1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang W, Zhou X, Yao Q, et al. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017;313(4):F906–F913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dominguez JM 2nd, Dominguez JH, Xie D, et al. Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PloS One. 2018;13(8):e0202550–e0202550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Qu Y, Zhang Q, Cai X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cho KHT, Xu B, Blenkiron C, et al. Emerging roles of miRNAs in brain development and perinatal brain injury. Front Physiol. 2019;10:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang J, Zhang X, Chen X, et al. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Webb RL, Kaiser EE, Jurgielewicz BJ, et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49(5):1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Webb RL, Kaiser EE, Scoville SL, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9(5):530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. [DOI] [PubMed] [Google Scholar]
- [35].Ferreira JR, Teixeira GQ, Santos SG, et al. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lai RC, Yeo RWY, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- [37].Yi X, Wei X, Lv H, et al. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383(2):111454. [DOI] [PubMed] [Google Scholar]
- [38].Simonson OE, Mougiakakos D, Heldring N, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4(10):1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moreira A, Winter C, Joy J, et al. Intranasal delivery of human umbilical cord Wharton’s jelly mesenchymal stromal cells restores lung alveolarization and vascularization in experimental bronchopulmonary dysplasia. Stem Cells Transl Med. 2019. DOI: 10.1002/sctm.18-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dinh P-UC, Cores J, Hensley MT, et al. Derivation of therapeutic lung spheroid cells from minimally invasive transbronchial pulmonary biopsies. Respir Res. 2017;18(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Poltavtseva RA, Poltavtsev AV, Lutsenko GV, et al. Myths, reality and future of mesenchymal stem cell therapy. Cell Tissue Res. 2019;375(3):563–574. [DOI] [PubMed] [Google Scholar]
- [42].Liu K, Tang M, Liu Q, et al. Bi-directional differentiation of single bronchioalveolar stem cells during lung repair. Cell Discov. 2020;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zacharias WJ, Frank DB, Zepp JA, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555(7695):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhou B, Flodby P, Luo J, et al. Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. J Clin Invest. 2018;128(3):970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Henry E, Cores J, Hensley MT, et al. Adult lung spheroid cells contain progenitor cells and mediate regeneration in rodents with bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2015;4(11):1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hoshino A, Costa-Silva B, Shen T-L, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schilders KAA, Eenjes E, van Riet S, et al. Regeneration of the lung: lung stem cells and the development of lung mimicking devices. Respir Res. 2016;17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cores J, Hensley MT, Kinlaw K, et al. Safety and efficacy of allogeneic lung spheroid cells in a mismatched rat model of pulmonary fibrosis. Stem Cells Transl Med. 2017;6(10):1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dinh P-UC, Paudel D, Brochu H, et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11(1):1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ashur C, Frishman WH. Cardiosphere-derived cells and ischemic heart failure. Cardiol Rev. 2018;26(1):8–21. [DOI] [PubMed] [Google Scholar]
- [51].Chakravarty T, Henry TD, Kittleson M, et al. Allogeneic cardiosphere-derived cells for the treatment of heart failure with reduced ejection fraction: results of the dilated cardiomyopathy iNtervention with allogeneic myocardially-regenerative cells (DYNAMIC) trial. EuroIntervention. 2019. DOI: 10.4244/EIJ-D-19-00035 [DOI] [PubMed] [Google Scholar]
- [52].Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38(3):201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li P, Kaslan M, Lee SH, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177(2):428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Carnino JM, Lee H, Jin Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: a review and comparison of different methods. Respir Res. 2019;20(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yang F, Liao X, Tian Y, et al. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J. 2017;12(4). DOI: 10.1002/biot.201600699 [DOI] [PubMed] [Google Scholar]
- [59].Patel DB, Santoro M, Born LJ, et al. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. 2018;36(8):2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Phan J, Kumar P, Hao D, et al. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7(1):1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Watson DC, Bayik D, Srivatsan A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mastrolia I, Foppiani EM, Murgia A, et al. Challenges in clinical development of mesenchymal stromal/stem cells: concise review. Stem Cells Transl Med. 2019;8(11):1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Busatto S, Vilanilam G, Ticer T, et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. 2018;7(12):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen TS, Arslan F, Yin Y, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Carter M, Shieh JC. Chapter 13 - Cell Culture Techniques (M. Carter & J. C. B. T.-G. to R. T In: Shieh N.editor). 2010. DOI: 10.1016/B978-0-12-374849-2.00013-6. [DOI] [Google Scholar]
- [66].Willis GR, Kourembanas S, Mitsialis SA. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med. 2017;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Morishita M, Takahashi Y, Nishikawa M, et al. Pharmacokinetics of exosomes-an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J Pharm Sci. 2017;106(9):2265–2269. [DOI] [PubMed] [Google Scholar]
- [68].Saludas L, Pascual-Gil S, Roli F, et al. Heart tissue repair and cardioprotection using drug delivery systems. Maturitas. 2018;110:1–9. [DOI] [PubMed] [Google Scholar]
- [69].De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172–186. [DOI] [PubMed] [Google Scholar]
- [70].He C, Zheng S, Luo Y, et al. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mege D, Panicot-Dubois L, Ouaissi M, et al. The origin and concentration of circulating microparticles differ according to cancer type and evolution: A prospective single-center study. Int J Cancer. 2016;138(4):939–948. [DOI] [PubMed] [Google Scholar]
- [72].Santoni G, Morelli MB, Amantini C, et al. Urinary markers in bladder cancer: an update. Front Oncol. 2018;8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lu M, Yuan S, Li S, et al. The exosome-derived biomarker in atherosclerosis and its clinical application. J Cardiovasc Transl Res. 2019;12(1):68–74. [DOI] [PubMed] [Google Scholar]
- [74].Colbath AC, Dow SW, Hopkins LS, et al. Allogeneic vs. autologous intra-articular mesenchymal stem cell injection within normal horses: clinical and cytological comparisons suggest safety. Equine Vet J. 2020;52(1):144–151. [DOI] [PubMed] [Google Scholar]
- [75].Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, et al. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: proof of concept in rats. J Transl Med. 2015;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Saleh AF, Lázaro-Ibáñez E, Forsgard -MA-M, et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11(14):6990–7001. [DOI] [PubMed] [Google Scholar]
- [77].Zhu X, Badawi M, Pomeroy S, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6(1):1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]


