ABSTRACT
Background
Because of low acceptance rates and limited capacity, complete diagnostic autopsies (CDAs) are seldom conducted in low- and middle-income countries (LMICs). There have been growing investments in less-invasive postmortem examination methodologies, including needle-based autopsy, known as minimally invasive autopsy or minimally invasive tissue sampling (MITS). MITS has been shown to be a feasible and informative alternative to CDA for cause of death investigation and mortality surveillance purposes.
Objective
The aim of this narrative review is to describe historical use and evolution of needle-based postmortem procedures as a tool to ascertain the cause of death, especially in LMICs.
Methods
Key word searches were conducted in PubMed and EBSCO in 2018 and 2019. Abstracts were reviewed against inclusion and exclusion criteria. Full publications were reviewed for those abstracts meeting inclusion criteria and a start set was established. A snowball search methodology was used and references for all publications meeting inclusion criteria were manually reviewed until saturation was reached.
Results
A total of 1,177 publications were initially screened. Following an iterative review of references, 79 publications were included in this review. Twenty-nine studies, published between 1955 and 2019, included MITS as part of postmortem examination. Of the publications included, 76% (60/79) have publication dates after 2010. More than 60% of all publications included addressed MITS in LMICs, and a total of nine publications compared MITS with CDA.
Conclusions
Although there is evidence of less-invasive postmortem sampling starting in the 1800s, more structured needle-based postmortem examination publications started to appear in the mid-twentieth century. Early studies were mostly conducted in high-income countries but starting in 2010 the number of publications began to increase, and a growing number of studies were conducted in LMICs. Initial studies in LMICs were disease-specific but since 2015 have evolved to include more expansive postmortem examination.
KEYWORDS: Percutaneous autopsy, needle-based autopsy, autopsy, less-invasive autopsy, minimally invasive autopsy
Background
Traditional pathological autopsy, also known as complete diagnostic autopsy (CDA), remains the gold standard methodology to investigate cause of death. However, since the beginning of the mid 1960s, declining rates of CDA have been well documented globally, and this approach to investigate cause of death remains infrequently performed in low- and middle-income countries (LMICs) [1,2]. The reason for the limited use of CDA in LMICs is multifactorial and includes social, cultural, religious, and structural factors such as limited human capacity and financial resources, an overall poor acceptability because of the disfiguring nature of the procedure, and the time required to carry it out, which can interfere with ceremonial and burial practices [3–5]. In high-income countries (HICs), the abundance of clinical records (and their easy accessibility) often allows adequate characterization of events preceding death, and thus, causes of the fatal outcome. However, this is not generally the case in LMICs, where the vast majority of premature deaths occur; in these settings, access to the health system is much more limited, resulting in a significant proportion of deaths occurring in the home. Alternative strategies such as verbal autopsy, a structured interview of the family members that is subsequently analyzed by clinicians or by analytical software, have been designed to ascertain causes of death but provide limited specificity and can give misleading results [6,7]. In these settings, clinical records of those deaths having reached the health system are valuable assets but when available typically have limited information and often entail a significant proportion of diagnostic errors, probably because of the scarcity of diagnostic tools available to clinicians [8]. Thus, the substantial shortcomings of verbal autopsy and clinical diagnosis, especially when not used in conjunction with other sources of information, significantly limit their ability to inform reliable cause-of-mortality data [5,7,9].
Accurate determination of causes of death is critical to guide effective preventive strategies and health systems planning. Because CDAs are not readily conducted in LMICs, alternative methods based on less-invasive postmortem approaches have been historically proposed to investigate cause of death. In this respect, minimally invasive tissue sampling (MITS), also known as minimally invasive autopsy (MIA), has been identified as a possible alternative to CDA to determine cause of death. MITS is a protocolized needle-based postmortem examination, designed as an acceptable proxy of the gold standard CDA, that can be performed by pathologists or pathology technicians with specialized training. MITS consists of inserting fine needles into the body and collecting small amounts of tissue and body fluids (e.g. blood, cerebrospinal fluid, effusions) from key and highly informative organs like the brain, lungs, liver, heart, and placenta (if applicable) [10,11]. These samples are then analyzed through standard and advanced histopathological, microbiological, and molecular biology methods, providing rich information on abnormalities present and likely responsible for the events leading to death.
The concept of minimally invasive postmortem study as a means to support cause of death determination dates back to the late 1800s in Baltimore, Maryland, where Dr. Howard Kelly described removing organs manually for autopsy through body orifices [12]. Some decades later in 1930, Décio Parreiras and Werneck Genofre used needle-based postmortem examination during a yellow fever outbreak in Brazil and hence developed the ‘Parreiras-Genofre Spindle’ used for targeted postmortem liver sampling, also known as ‘viscerotomy’ [13,14]. Over the last decade there have been growing investments in MITS-related studies. These investments have largely but not exclusively targeted the use of MITS for mortality surveillance purposes, particularly in children [15]. To better understand the growth and expansion of needle-based postmortem examination, or MITS, over time, we sought to identify key publications that have contributed to the evolution of MITS as a method to assist physicians and medical professionals in determining cause of death. Specifically, this review addressed the following questions:
How has postmortem tissue sampling evolved toward the current configuration of MITS to support cause of death investigation in LMICs, and what do we know of its potential acceptability and feasibility?
How has the MITS technique been validated against the CDA to assess whether it is an acceptable proxy for the gold standard CDA?
Methods
Database search and article selection
After an initial July 2018 search in COCHRANE for existing published reviews, a start set was established by searching English key words in PubMed and EBSCO. Non-English databases and publications were excluded. There were no date limitations set on the year of publication. Key words for the search included ‘minimally invasive tissue sampling’, ‘minimally invasive autopsy’, ‘percutaneous autopsy’, ‘needle-based postmortem examination’, and ‘needle-based autopsy’.
The term ‘minimally invasive autopsy’ has historically been used to cover a range of less-invasive autopsy methods including the use of advanced imaging techniques such as magnetic resonance imaging, computerized tomography, and ultrasonography, collectively referred to as ‘virtuopsy’ but which did not always include associated tissue sampling. For this review, we focused on postmortem examinations that were not full pathological autopsies and that were less-invasive or less comprehensive than CDAs and included targeted postmortem tissue sampling. Inclusion criteria consisted of MIA that included needle-based tissue sampling. Search results that included needle-based autopsy in conjunction with advanced imaging techniques were excluded as were publications that included MIA in the absence of needle-based autopsy. See Table 1 for inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Because of negative connotations with the word ‘autopsy,’ there was a judgment by researchers investigating the feasibility and acceptability of needle-based postmortem tissue sampling to exclude the word ‘autopsy’ in the procedure terminology because the word could convey an impression about the procedure that would interfere with acceptance. Hence the terminology was changed to MITS. The original language from the reference publications included both ‘MIA’ and ‘MITS’ but for the purposes of this review, the term ‘MITS’ is used to describe all needle-based autopsies, irrespective of whether MIA or MITS was used in the original text. Publications including tissue sampling in live patients were excluded as were publications where tissue sampling was used for the diagnosis of structural defects.
A snowball methodology was applied to identify publications for this review [16,17]. After an initial search in PubMed and EBSCO, all publication titles were manually reviewed by a single person (CP) against inclusion and exclusion criteria, and duplicates were removed. Abstracts for all publications initially identified were reviewed, and a definitive decision to include or exclude the publication was made after reading the full text, thus establishing a start set. From there, a backward and forward snowball search methodology was applied to establish yields two, three and four (Figure 1). In the backward approach, the titles and authors in the reference lists for all publications included as part of the start set were manually screened, and for those meeting provisional inclusion criteria the abstract was read. After reading the abstracts, the full text of those meeting the inclusion criteria was read and a final determination regarding inclusion was made. In the forward snowball approach PubMed was used to identify citations of publications included as part of the start set. Titles and authors were screened, and abstracts were read for those meeting provisional inclusion criteria. The full text was read before we made a final determination about inclusion. Both backward and forward iterative approaches were conducted until saturation was reached and no new references were identified. In addition to using PubMed alerts, updated searches of PubMed and EBSCO using the same initial search terms were conducted in February 2018 and October 2019 to identify new publications since the original July 2018 search (Figure 1).
Figure 1.
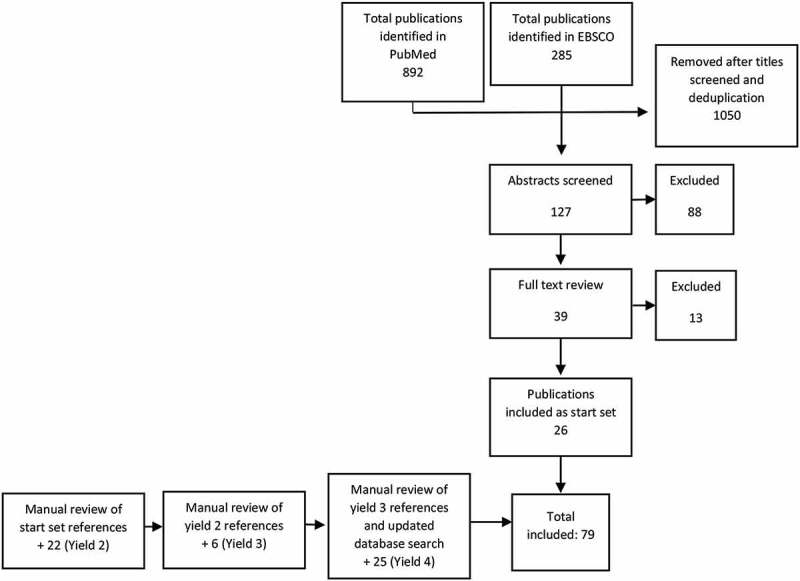
Flow diagram of publication screening and identification.
Definitions
We reviewed the country/countries in which each study was implemented and classified them as either HICs or LMICs based on the World Bank Country and Lending Group’s classification at the time of publication [18].
Unless otherwise specified by the study methods, for this review, stillbirth was defined as a death occurring in the period between 28 weeks of gestational age and birth and not showing any sign of life after delivery; neonatal deaths were defined as those occurring among babies born alive but deceased before 28 days of age; child deaths were defined as those occurring between 28 days and <15 years; and adult deaths were defined as those occurring after 15 years of age.
Results
A total of 79 journal articles with publication dates ranging from 1955 to 2019 were included in this review. The practice of needle-based postmortem examination to inform cause of death was documented in English-language literature as early as 1955. However, despite the concept of MITS being introduced over a half century ago, 75% of the publications identified were published within the last decade. Since 2010, the total number of MITS publications has been three times the number published prior to 2010.
The evolution of MITS in postmortem examination
Twenty-eight publications with publication dates ranging from 1969 to 2019 represented 19 distinct primary studies where MITS was used as a postmortem examination method to determine cause of death (Table 2) [10,11,19–44]. Three articles were published in the 1950s and 1960s evaluating the potential utility and feasibility of conducting needle autopsies [29,38,39]. The largest study of MITS in adults was conducted over two decades in New York and consisted of histological examination of samples taken by pathology residents from the liver, kidney, lung, and heart [39]. The authors found that the limited practice of the needle autopsy by pathologists in training reduced the efficiency of the needle-based postmortem examination but that with increased exposure and practice, the needle autopsy could serve as a suitable substitute for CDA. Although no publications were identified between the 1960s and 1980s, these early studies introduced the idea of needle-based postmortem examination.
Table 2.
Publications using MITS to ascertain cause of death.
| Total MITS Cases |
Tissues sampled |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Journal | Region of Study | Income Level | Population | Brain | Heart | Kidney | Liver | Lung | Other | |
| Terry [29] | 1955 | Journal of Clinical Pathology | North America | HIC | Adults | 24 | x | x | ||||
| West [38] | 1957 | American Journal of Medical Science | North America | HIC | Adults | 66 | x | x | Pancreas Prostate |
|||
| Wellman [39] | 1969 | American Journal of Clinical Pathology | North America | HIC | Adults | 394 | x | x | x | x | ||
| Underwood [40] | 1983 | BMJ | Europe | HIC | Adults | 5 | x | x | x | Spleen Prostate |
||
| Baumgart [41] | 1994 | Pathology | Europe | HIC | Adults | 16 | x | x | x | x | Muscle tissue Spleen Stomach Pancreas Bowel Aorta |
|
| Foroudi [42] | 1995 | Pathology | Australia | HIC | Adults | 21 | x | x | x | x | x | |
| Huston [43] | 1996 | Modern Pathology | North America | HIC | Adults | 20 | x | x | x | x | Breast Bone marrow Abdominal mass |
|
| Guerra [44] | 2001 | Pathology Research and Practice | Europe | HIC | Adults | 168 | x | x | x | x | x | Spleen Bone marrow |
| Chintu [19] | 2002 | Lancet | Africa | LMIC | Children | 264 | x | x | ||||
| El-Reshaid [20] | 2005 | Medical Principles and Practice | Middle East | HIC | Adults | 19 | x | x | x | x | ||
| Weustink [21] | 2009 | Radiology | Europe | HIC | Adults | 30 | x | x | ||||
| Garg [22] | 2009 | Fetal and Pediatric Pathology | SE Asia | LMIC | Stillbirths/ neonates |
25 | x | x | x | Spleen | ||
| Celiloglu [23] | 2013 | Turkish Pathology | Europe | HIC | Stillbirths/ neonates |
76 | x | x | x | x | x | Spleen |
| Cox [24] | 2014 | Journal of Acquired Immune Deficiency Syndrome | Africa | LMIC | Adults | 191* | x | x | x | x | x | Spleen |
| Cox [25] | 2014 | BMC Clinical Pathology | Africa | LMIC | Adults | 191* | x | x | x | x | x | Spleen |
| Castillo [11] | 2015 | PLoS One | Africa | LMIC | Adults | 30 Ϯ | x | x | x | x | x | Blood CSF Bone marrow Uterus |
| Castillo [27] | 2016 | PLoS Medicine | Africa | LMIC | Adults | 112 Ϯ | x | x | x | x | Spleen CSF Bone marrow Blood |
|
| Martínez [11] | 2016 | Diagnostic Microbiology and Infectious Disease | Africa | LMIC | Adults | 30 Ϯ | x | x | CSF Blood Uterus |
|||
| Karat [26] | 2016 | PLoS One | Africa | LMIC | Adults | 34§ | x | x | CSF Spleen |
|||
| Menendez [32] | 2017 | PLoS Medicine | Africa | LMIC | Stillbirths/ neonates |
59 | x | x | x | Blood CSF |
||
| Bassat Q [31] | 2017 | PLoS Medicine | Africa | LMIC | Children | 54 | x | x | x | x | x | Blood CSF Spleen Bone marrow CSF |
| Castillo [10] | 2017 | PLoS Medicine | Africa | LMIC | Adults | 57 Ϯ | x | x | x | x | x | Blood CSF Spleen Bone marrow Uterus |
| Karat [28] | 2017 | PLoS One | Africa | LMIC | Adults | 34§ | x | x | Blood CSF Spleen Naso/oro pharyngeal secretions |
|||
| Chawana [33] | 2019 | Clinical Infectious Diseases | Africa | LMIC | Children | 127 | x | x | x | Blood CSF Rectal swabs |
||
| Muhe [35] | 2019 | Lancet Global Health | Africa | LMIC | Stillbirths/ neonates |
126 | x | x | x | x | Spleen Intestines |
|
| Roberts [34] | 2019 | American Journal of Clinical Pathology | Africa | LMIC | Children | 64 | x | |||||
| Madhi [36] | 2019 | Clinical Infectious Diseases | Africa | LMIC | Stillbirths/ neonates |
129 | x | x | x | Blood CSF Placenta |
||
| Madhi [37] | 2019 | Clinical Infectious Diseases | Africa | LMIC | Stillbirths/ neonates |
153 | x | x | x | Blood CSF Stool |
||
* Represents same sample population from single study of 191 adults; Ϯ Represents same sample population from single study of 112 adults; § Represents same sample population from single study of 34 adults.
In 1983, the use of MITS in HICs resurfaced in a paper describing the advantages, feasibility, and limitations of needle-based postmortem examination [40]. This publication described the use of MITS in five case studies, and the authors concluded that two clear advantages of MITS were the speed at which samples could be obtained and the reduced risk of infection. The next study involving MITS was not published until more than 10 years later when in 1994 the first study demonstrating the use of MITS in HIV-positive populations was published [41]. Two additional studies were published in the mid1990s; one study suggested that MITS is a valuable alternative when CDA is not possible, and the second publication stated that when used in isolation, MITS is inferior, and suggested the use of radiology to improve its performance [42,43].
The early 2000s saw mixed opinions about the feasibility and acceptability of MITS in postmortem examination in the English-language literature in both HICs and LMICs. One study evaluated the feasibility of MITS in a predominantly Muslim culture and found it to be a more acceptable alternative to CDA [20]. However, another study in Zambia evaluating the acceptability of MITS in children found that offering MITS as a less-invasive alternative to CDA did not significantly increase consent [19]. Studies of MITS in children, neonates, and stillbirths conducted in the early 2000s consisted of comparing CDA with MITS and using MITS to confirm a specific condition, for instance, malaria (targeting the brain) or pneumonia (targeting the lungs) [19,22,45]. These studies not only expanded the use of MITS, they introduced new methods for obtaining tissue samples.
Between 2010 and 2015 an increasing number of studies aimed to validate the use of MITS against other methods of postmortem examination, including CDA and verbal autopsy [11,23–25]. During the same timeframe these studies were accompanied by other publications, including articles describing qualitative studies that examined the potential acceptability of MITS as part of postmortem examination, particularly in populations where CDA is rarely performed. Journal commentaries and editorials outlining the perceived utility and value of MITS in postmortem examinations and cause of death determination also began to emerge in the literature [7,46–51].
In 2016 publications began to arise from the pioneering CADMIA study. CADMIA assessed the acceptability of MITS and validated the MITS approach against the CDA in all age groups, including stillbirths in maternal deaths, in Mozambique and in Brazil [27,30–32,52,53]. The year 2019 saw a sharp rise in the quantity of MITS publications, including a study of children dying of respiratory illness in Kenya and a study of stillbirths and neonates in Ethiopia [34,35]. A large proportion of the 2019 increase in MITS publications is attributable to the October 2019 release of 13 articles describing MITS from the experience of the Child Health and Mortality Prevention Surveillance (CHAMPS) Network [2,15,33,36,37,54–62]. With promising results from the relatively few validation studies completed, the CHAMPS Network rapidly endorsed the use of MITS and is poised to both build on earlier validation studies and also improve on a number of aspects of MITS such as reducing the time and expense associated with performing MITS.
MITS in LMICs and HICs
In 28 publications documenting a total of 19 studies of MITS in postmortem examination and determination of cause of death published between 1955 and 2019, roughly half of the studies were conducted in HICs and half in LMICs. In early MITS studies, between 1955 and 2001, the scope of MITS was limited to HICs and adult populations [29,38–42,44]. Articles published between 1955 and 2010 continued to focus on HICs at a ratio of 4:1 compared to those published about LMICs (Figure 2). The first MITS study conducted in an LMIC was published in 2002 and notably was also the first study of MITS in children [19]. Although two additional studies of MITS in adults in HICs were published in 2005 and 2009, beginning in 2010 there was a significant shift from HICs to LMICs [20,21]. In 2011 the number of publications documenting MITS in postmortem examination in LMICs began to increase, and between 2011 and 2019 articles from LMICs outnumbered those from HICs by a ratio of 3:1 [11,24–28,30–32,34–37].
Figure 2.
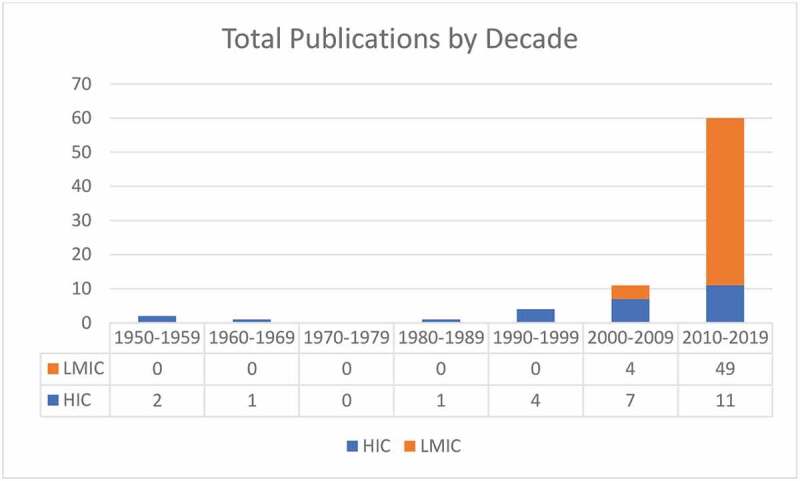
Number of MITS publications by decade focused on HICs and LMICs.
The number of qualitative studies aimed at understanding the facilitators and barriers to implementing MITS in a variety of cultures, religions, and populations, including health care providers, parents, families, and community leaders is relatively balanced in HICs versus LMICs. Of the seven qualitative studies with publication dates between 2011 and 2019, four were conducted in LMICs and three in HICs [3,49,50,52,58,59,63–65].
Based on the sample sizes and ages identified in the 28 primary studies using MITS in postmortem examination there have been roughly 40% more MITS carried out in LMICs than in HICs. There have been nearly twice as many MITS in adults as in stillbirths, neonates, and children combined; however, in LMICs, the majority of MITS conducted have been in children, neonates, and stillbirths (Figure 3) [10,17–33,35-42,64].
Figure 3.
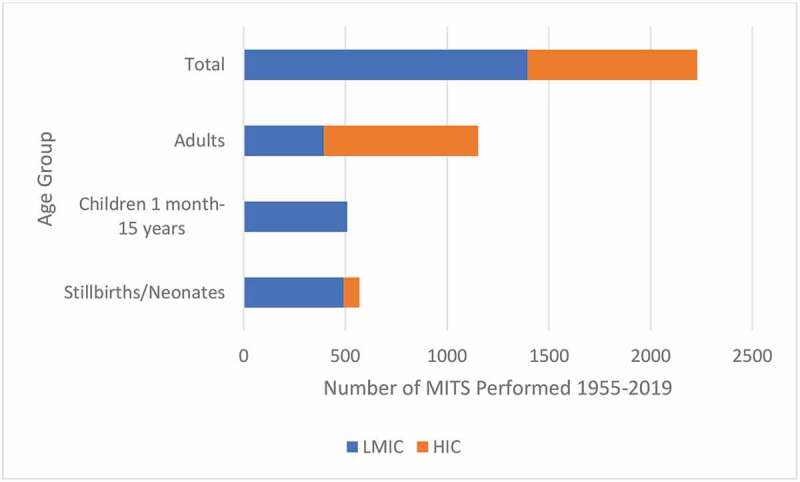
Number of MITS performed in LMICs and HICs by age group, 1955–2019.
MITS in specific disease investigations
A total of eight studies published between 1994 and 2019 document the use of MITS to investigate specific diseases or pathogens and to support determining cause of death in cases with multiple comorbidities or when clinical symptoms make it difficult to distinguish between potential causes of death [19,24–26,28,34,41,44,45,67–69].
The use of MITS in determining the role of specific diseases and pathogens in postmortem examination dates back to 1994 and 2001 when it was used in two studies to determine cause of death in HIV-positive adults in Europe [41,44]. Both studies highlighted the limitation of ascertaining cause of death through clinical diagnosis and in particular in HIV-positive adults who were likely to have multiple comorbidities [41,44]. Authors from both studies concluded that MITS is comparable with CDA in cause of death determination, may be invaluable in populations with limited resources, and may present fewer opportunities than CDA to infect technicians and pathologists in populations with high rates of infectious disease. During 2015 to 2017 researchers evaluated MITS against verbal autopsy in determining cause of death from HIV-associated tuberculosis in South Africa [28]. Researchers concluded that when used alone, verbal autopsy underestimated HIV-related mortality in patients with tuberculosis. In Malawi, MITS was found to be an effective technique for sampling brain tissue to confirm cerebral malaria in children who died with a clinical diagnosis of malaria [45]. In this study brain tissue was successfully sampled via a supraorbital approach, a novel approach, and was met with an apparent acceptability of the procedure [68].
MITS validation compared with CDA
A total of 13 articles published between 1957 and 2019 representing nine unique studies validating the use of MITS against CDA in determining cause of death were included in this review (Table 3).
Table 3.
Publications comparing MITS with CDA.
| First Author | Year | Journal | Specific Study Objective | Population Studied | Total MITS Conducted | Results |
|---|---|---|---|---|---|---|
| West [38] | 1957 | American Journal of Medical Science | Compare diagnosis made by MITS to CDA | Adults | 66 |
|
| Foroudi [42] | 1995 | Pathology | Compare results of MITS with CDA | Adults | 21 |
|
| Huston [43] | 1996 | Modern Pathology | Demonstrate the value and limitations of postmortem needle sampling in correlating tissue diagnosis compared with CDA | Adults | 20 |
|
| Cina [67] | 1999 | Military Medicine | Evaluate the role of percutaneous core biopsy of the liver in determining pathology in unexpected deaths related to hepatomegaly | Adults | 28 |
|
| Breeze [73] | 2008 | Virchows Archive | Determine the feasibility of percutaneous fetal organ biopsies: how frequently are adequate samples obtained; comparison of percutaneous biopsy with conventional block biopsy | Stillbirths | 30 |
|
| Garg [22] | 2009 | Fetal and Pediatric Pathology | Compare the specimen adequacy and histologic and laboratory findings on lung tissue collected using blind-needle biopsy MITS with those from CDA in a pediatric respiratory disease mortality study undertaken at a large public hospital in Kenya, assessing the contribution of molecular microbiologic testing |
Neonates | 25 |
|
| Van der Linden [74] | 2014 | PLoS One | Compare the RNA quality of samples obtained via MITS to samples obtained via CDA with fresh frozen samples as a reference | Adults | 24 |
|
| Castillo [27] | 2016 | PLoS Medicine | Compare cause of death in adults ascertained via MITS with cause of death after CDA | Adults | 112 |
|
| Castillo [10] | 2017 | PLoS Medicine | Compare cause of death in maternal cases ascertained via MITS with cause of death after CDA | Adults | 57 |
|
| Bassat [31] | 2017 | PLoS Medicine | Compare cause of death in children ascertained via MITS with cause of death after CDA | Children | 54 |
|
| Menendez [32] | 2017 | PLoS Medicine | Compare cause of death in stillbirths ascertained via MITS with cause of death after CDA | Stillbirths and neonates | 59 |
|
| Fernandes [75] | 2019 | Human Pathology | Determine the degree to which clinical information improves the diagnostic accuracy of the CDA and MIA | Adults | 112 |
|
| Maternal deaths | 57 | |||||
| Children | 54 | |||||
| Neonates | 41 | |||||
| Stillbirths | 18 |
The first study we identified comparing MITS and CDA was published in 1957 in North America [38]. The investigators found that in a study of 50 adult patients undergoing first MITS and then CDA, where the pathologists conducting MITS were blinded to the CDA results and clinical histories, the concordance in diagnosis between CDA and MITS was 48%, leaving the authors to conclude that CDA is preferable to MITS [38]. The next article comparing MITS and CDA was not published until 30 years later, in 1995, in Australia where 95% of CDA resulted in a cause of death determination compared with 43% of MITS cases [42]. The authors concluded that MITS was inferior to CDA, but the performance of MITS in determining cause of death might be improved using radiology. The 1990s saw two additional studies comparing MITS and CDA in adults. The first, published in 1996, found a correlation of 67% between MITS and CDA in cause of death determination and 80% correlation between MITS and CDA in additional major diagnoses in adults [43]. The authors concluded that although MITS should not replace CDA when CDA is feasible, MITS can be an efficient and satisfactory alternative, because it can be conducted in a relatively short period of time and in the hospital room prior to transferring to the morgue or funeral home [43]. The second, a study of adults published in 1999, found an 86% correlation in cause of death based on histological findings when comparing liver samples collected via MITS with those collected via CDA [67]. Furthermore, a study of HIV-positive adults in Uganda in 2014 found concordance rates between MITS and CDA in major diagnosis reaching 90%, leaving the authors to conclude that MITS is a viable alternative when CDA is not possible [24]. These studies highlight many of the advantages of MITS; MITS can be conducted in less time and more efficiently than CDA, may be a suitable alternative to families refusing CDA, and may be a safer alternative to CDA in cases of infectious pathogens.
Beginning in 2016, the CADMIA study in Mozambique and the Brazilian Amazon sampled the brain, heart, liver, spleen, kidneys, and lungs and found a high concordance rate in major diagnosis between CDA and MITS and like earlier studies, found that over time sampling technique improved and that where CDA is not possible, MITS is a valuable and robust alternative [10,11,27,30-32,70]. Concordance rates between CDA and MITS ranged from 68% in stillbirths to 89% in pediatric deaths with the highest concordance in deaths attributable to infectious diseases and malignant tumors [31,32]. Among other observations, the authors described that the lungs, liver, and brain tissue sampled using MITS have the greatest diagnostic yield, and the inclusion of clinical data in assigning a cause of death significantly improves the diagnostic capacity of MITS against CDA [8,11]. The CADMIA studies were also responsible for standardizing the MITS protocols for sample collection and histological processing and training of more than 60 project staff for the CHAMPS Network in addition to other MITS projects [11,56,71]. Most recently, in publications from 2019, researchers reported that 60% concordance in pathogen detection between MITS and CDA was observed in the MITS study of specimen adequacy and histologic and laboratory findings in 64 children in Kenya [34]. In Ethiopia, CDA and MITS were used in a mortality study of 125 stillbirth and neonatal deaths [35]. However, results describing the performance of MITS compared with CDA have not yet been published.
Discussion
This review documented that there were limited publications on the validation and use of MITS in disease-specific cause of death investigations in both HICs and LMICs between 1955 and 2010. MITS was initially studied more in HICs and was not introduced in LMICs until the early 2000s and was almost exclusively used in the context of disease-specific studies. However, 2010 marks the beginning of an increase in the total number MITS publications in both HICs and LMICs. The year 2010 also marks a distinct shift in publications predominantly describing MITS in HICs to an increasing number of publications describing MITS in LMICs. Since 2010, the studies using MITS in LMICs have also expanded the earlier scope from use in disease-specific contexts to include studies evaluating MITS to CDA and more broadly, using MITS in mortality surveillance.
However, it is worth noting that despite the rapid increase in MITS publications in the last decade, the total number of publications cannot, in this case, serve as a proxy for the number of MITS studies, and there remains relatively few studies evaluating MITS against CDA in LMICs. Since 2009, 19 MITS cause of death publications originated from eight unique projects. Seventy-five percent of those projects were funded by a single donor, the Bill & Melinda Gates Foundation. The significance of this data point is two-fold. First, the large investment in MITS suggests a high level of confidence and a deep belief in the value MITS can contribute to improved global mortality surveillance. Second, there remains significant opportunity to broaden the funding sources that support MITS research in mortality surveillance.
One initiative to further stimulate and facilitate the use of MITS to characterize causes of death is the MITS Surveillance Alliance, whose objective is to grow a network of researchers to support the use of MITS globally. To this end, the Alliance recently funded a number of small grants to support MITS feasibility studies in LMICs and the innovative use of MITS in other disease- and pathogen-specific mortality surveillance studies [72]. Such grants highlight both the global interest for MITS and the potential for MITS to refine disease-specific mortality across a broad range of pathogens.
As investments in MITS increase, so do the opportunities to expand and improve the utility of MITS and postmortem examination. For example, future investments in MITS that include funding to study the differences in MITS acceptability based on local customs and cultures and support community engagement will guide researchers in the design and implementation of future MITS studies in LMICs. Results from these qualitative studies will add to the body of literature on the utility of MITS in settings where barriers may prevent adoption and ultimately optimize its acceptance. The prospects of using artificial intelligence and machine learning to systematize and streamline MITS data analysis and interpretation, at both the individual and population level, has yet to be explored. Economic studies aimed at determining the relative cost-effectiveness and cost comparison between MITS and CDA will provide potential funders, researchers, and governments, particularly in LMICs, with valuable information when considering the feasibility of MITS in their respective settings. The use of MITS has been documented in HIV and tuberculosis, but there is still potential for additional targeted use of MITS in other pathogens and infectious disease-specific investigations. Further investment in MITS is needed to support its continued evolution and its role in postmortem examination.
In summary, although the concept of MITS and tissue-based postmortem examination dates back many decades, and the concept of MIA has been around since before the turn of the twentieth century, this review demonstrates a rapid increase in the number of MITS studies in the last decade. The results of this review support the conclusion that MITS is a feasible and comparable alternative method for postmortem examination compared with CDA. However, the literature also suggests that the potential value and impact of MITS in cause of death determination is still relatively unfulfilled. Continued investment, and importantly, multisectoral engagement, have the potential to accelerate MITS validation and adoption globally.
Acknowledgments
The authors would like to acknowledge Meera Viswanathan for valuable input and suggestions in the development of the review protocol and presentation of results. The authors would also like to thank Jaume Ordi for review and input into the manuscript.
Responsible Editor Peter Byass, Umeå University, Sweden
Funding Statement
The MITS Surveillance Alliance was funded by the Bill & Melinda Gates Foundation [Global Health grant number OPP1180554].
Author contributions
CP, NG, and QB were responsible for originating and designing the review. EM, QB, and NG provided extensive review of the test. All authors reviewed, contributed to, and approved the final draft of the review.
Disclosure statement
No potential conflict of interest was reported by the authors.
Paper context
Despite recent increases in the number of minimally invasive tissue sampling publications, this manuscript is the first to comprehensively document individual studies using needle-based postmortem examination in a single publication. This manuscript synthesizes these studies and gives readers deeper insight and a broader perspective into how and where minimally invasive tissue sampling has been performed, the different contexts in which it has been used, and how it has been validated against the complete diagnostic autopsy.
References
- [1].Jha P. Reliable direct measurement of causes of death in low- and middle-income countries. BMC Med. 2014. February 4;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raghunathan PL, Madhi SA, Breiman RF. Illuminating child mortality: discovering why children die. Clin Infect Dis. 2019. October 9;69:S257–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bunei M, Muturi P, Otiato F, et al. Factors influencing acceptance of post-mortem examination of children at a tertiary care hospital in Nairobi, Kenya. Ann Global Health. 2019. July 3;85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yawson AE, Tette E, Tettey Y. Through the lens of the clinician: autopsy services and utilization in a large teaching hospital in Ghana. BMC Res Notes. 2014. December 23;7:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bassat Q, Castillo P, Alonso PL, et al. Resuscitating the dying autopsy. PLoS Med. 2016. January 12;13:e1001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nichols EK, Byass P, Chandramohan D, et al. The WHO 2016 verbal autopsy instrument: an international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0. PLOS Med. 2018;15. DOI: 10.1371/journal.pmed.1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fligner CL, Murray J, Roberts DJ. Synergism of verbal autopsy and diagnostic pathology autopsy for improved accuracy of mortality data. Popul Health Metr. 2011. August 1;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ismail MR, Rakislova N, Munguambe K, et al. Contribution of the clinical information to the accuracy of the minimally invasive and the complete diagnostic autopsy. Hum Pathol. 2019. March 1;85:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Butler D. Verbal autopsy methods questioned. Nature. 2010;467:1015. [DOI] [PubMed] [Google Scholar]
- [10].Castillo P, Hurtado JC, Martínez MJ, et al. Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: an observational study. PLoS Med. 2017;14:e1002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castillo P, Ussene E, Ismail MR, et al. Pathological methods applied to the investigation of causes of death in developing countries: minimally invasive autopsy approach. Cappello F, editor. PLoS One. 2015. June 30;10:e0132057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wright JR. Sins of our fathers: two of the four doctors and their roles in the development of techniques to permit covert autopsies. Arch Pathol Lab Med. 2009;133:1969–1974. [DOI] [PubMed] [Google Scholar]
- [13].Bablet M. Sur l’organisation re´gionale d’une source de documentation pre´cieuse entre toutes aux colonies, la vis- cerotomie. Bull Soc Pathol Exot Filiales. 1945;38:122–126. [PubMed] [Google Scholar]
- [14].Benchimol JL Febre amarela: a doença e a vacina, uma história inacabada. Editora FIOCRUZ; 2001:470.
- [15].Dowell SF, Zaidi A, Heaton P. Why child health and mortality prevention surveillance? Clin Infect Dis. 2019. October 9;69:S260–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wohlin C Guidelines for snowballing in systematic literature studies and a replication in software engineering. EEASE ‘14: Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering; 2014. May; London, England. p. 1–10. [Google Scholar]
- [17].Reeves BC, Deeks JJ, Higgins JPT, et al. Including non-randomized studies on intevention effects. In: Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions. 6th ed. Chichester (UK); 2019. p. 595–620. [Google Scholar]
- [18].The World Bank . World bank country and lending groups – world bank data help desk [Internet]. World bank country and lending groups: historical classification by income in XLS format. 2019. [cited 2019 February 17]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- [19].Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: A descriptive necropsy study. Lancet. 2002;360:985–990. [DOI] [PubMed] [Google Scholar]
- [20].El-Reshaid W, El-Reshaid K, Madda J. Postmortem biopsies: the experience in Kuwait. Med Princ Pract. 2005;14:173–176. [DOI] [PubMed] [Google Scholar]
- [21].Weustink AC, Hunink MGM, van Dijke CF, et al. Minimally invasive autopsy: an alternative to conventional autopsy? Radiology. 2009. March;250:897–904. [DOI] [PubMed] [Google Scholar]
- [22].Garg S, Punia RPS, Basu S, et al. Comparision of needle autopsy with conventional autopsy in neonates. Fetal Pediatr Pathol. 2009;28:139–150. [DOI] [PubMed] [Google Scholar]
- [23].Celiloglu OS, Celiloglu C, Kurnaz E, et al. Diagnostic contribution of postmortem needle biopsies in neonates. Turkish Journal of Pathology. 2013;29:122. [DOI] [PubMed] [Google Scholar]
- [24].Cox JA, Lukande RL, Kalungi S, et al. Needle autopsy to establish the cause of death in HIV-infected hospitalized adults in Uganda: A comparison to complete autopsy. J Acquir Immune Defic Syndr. 2014;67:169–176. [DOI] [PubMed] [Google Scholar]
- [25].Cox JA, Lukande RL, Kalungi S, et al. Practice of percutaneous needle autopsy; a descriptive study reporting experiences from Uganda. BMC Clin Pathol. 2015. December 3;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karat AS, Omar T, Von Gottberg A, et al. Autopsy prevalence of tuberculosis and other potentially treatable infections among adults with advanced HIV enrolled in out-patient care in South Africa. PLoS One. 2016;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Castillo P, Martínez MJ, Ussene E, et al. Validity of a minimally invasive autopsy for cause of death determination in adults in Mozambique: an observational study. Byass P, editor. PLoS Med. 2016. November 22;13:e1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karat AS, Tlali M, Fielding KL, et al. Measuring mortality due to HIV-associated tuberculosis among adults in South Africa: comparing verbal autopsy, minimally-invasive autopsy, and research data. Isaakidis P, editor. PLoS One. 2017. March 23;12:e0174097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Terry R. Needle necropsy. J Clin Pathol. 1955;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martínez MJ, Massora S, Mandomando I, et al. Infectious cause of death determination using minimally invasive autopsies in developing countries. Diagn Microbiol Infect Dis. 2016. January;84:80–86. [DOI] [PubMed] [Google Scholar]
- [31].Bassat Q, Castillo P, Martínez MJ, et al. Validity of a minimally invasive autopsy tool for cause of death determination in pediatric deaths in Mozambique: an observational study. Byass P, editor. PLoS Med. 2017. June 20;14:e1002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Menendez C, Castillo P, Martínez MJ, et al. Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: an observational study. Byass P, editor. PLoS Med. 2017. June 20;14:e1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chawana R, Baillie V, Izu A, et al. Potential of minimally invasive tissue sampling for attributing specific causes of childhood deaths in South Africa: a pilot, epidemiological study. Clin Infect Dis. 2019. October 9;69:S361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roberts DJ, Njuguna HN, Fields B, et al. Comparison of minimally invasive tissue sampling with conventional autopsy to detect pulmonary pathology among respiratory deaths in a resource-limited setting. Am J Clin Pathol. 2019. June 22;152:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Muhe LM, McClure EM, Nigussie AK, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Heal. 2019. August;7:e1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Madhi SA, Pathirana J, Baillie V, et al. An observational pilot study evaluating the utility of minimally invasive tissue sampling to determine the cause of stillbirths in South African women. Clin Infect Dis. 2019. October 9;69:S342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Madhi SA, Pathirana J, Baillie V, et al. Unraveling specific causes of neonatal mortality using minimally invasive tissue sampling: an observational study. Clin Infect Dis. 2019. October 9;69:S351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].West M, Chomet B. An evaluation of needle necropsies. Am J Med Sci. 1957. November;234:554–60passim. [DOI] [PubMed] [Google Scholar]
- [39].Wellman K. The needle autopsy. Am J Clin Pathol. 1969;52:441–444. [DOI] [PubMed] [Google Scholar]
- [40].Underwood JCE, Slater N, Parsons MA. Hospital topics: the needle necropsy. BMJ. 1983;286. DOI: 10.1136/bmj.286.6378.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baumgart KW, Cook M, Quin J, et al. The limited (needle biopsy) autopsy and the acquired immunodeficiency syndrome. Pathology. 1994;26:141–143. [DOI] [PubMed] [Google Scholar]
- [42].Foroudi F, Cheung K, Duflou J. A comparison of the needle biopsy post mortem with the conventional autopsy. Pathology. 1995;27:79–81. [DOI] [PubMed] [Google Scholar]
- [43].Huston BM, Malouf NNAH, Huston BM, et al. Percutaneous needle autopsy needly sampling. Mod Pathol. 1996. December;9:1101–1107. [PubMed] [Google Scholar]
- [44].Guerra I, Ortiz E, Portu J, et al. Value of limited necropsy in HIV-positive patients. Pathol Res Pract. 2001;197:165–168. [DOI] [PubMed] [Google Scholar]
- [45].Milner DA Jr, Dzamalala CP, Liomba NG, et al. Sampling of supraorbital brain tissue after death: improving on the clinical diagnosis of cerebral malaria. J Infect Dis. 2005;191:805–808. [DOI] [PubMed] [Google Scholar]
- [46].Bassat Q, Ordi J, Vila J, et al. Development of a post-mortem procedure to reduce the uncertainty regarding causes of death in developing countries. Lancet Glob Heal. 2013;1:125–126. [DOI] [PubMed] [Google Scholar]
- [47].Kean S. Cause of death. Science. 2015;347:1410–1413. [DOI] [PubMed] [Google Scholar]
- [48].Gurley ES, Parveen S, Islam MS, et al. Family and community concerns about post-mortem needle biopsies in a Muslim society. BMC Med Ethics. 2011;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Breeze ACG, Statham H, Hackett GA, et al. Perinatal postmortems: what is important to parents and how do they decide? Birth. 2012. March;39:57–64. [DOI] [PubMed] [Google Scholar]
- [50].Kang X, Cos T, Guizani M, et al. Parental acceptance of minimally invasive fetal and neonatal autopsy compared with conventional autopsy. Prenat Diagn. 2014. November;34:1106–1110. [DOI] [PubMed] [Google Scholar]
- [51].Farag TH, Koplan JP, Breiman RF, et al. Precisely tracking childhood death. Am J Trop Med Hyg. 2017. July 12;97:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Maixenchs M, Anselmo R, Zielinski-Gutiérrez E, et al. Willingness to know the cause of death and hypothetical acceptability of the minimally invasive autopsy in six diverse African and Asian settings: a mixed methods socio-behavioural study. Byass P, editor. PLoS Med. 2016. November 22;13:e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Maixenchs M, Anselmo R, Sanz A, et al. Healthcare providers’ views and perceptions on post-mortem procedures for cause of death determination in Southern Mozambique. Heazell A, editor. PLoS One. 2018. July 6;13:e0200058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Blau DM, Caneer JP, Philipsborn RP, et al. Overview and development of the child health and mortality prevention surveillance determination of cause of death (decode) process and decode diagnosis standards. Clin Infect Dis. 2019. October 9;69:S333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].O’Mara Sage E, Munguambe KR, Blevins J, et al. Investigating the feasibility of child mortality surveillance with postmortem tissue sampling: generating constructs and variables to strengthen validity and reliability in qualitative research. Clin Infect Dis. 2019. October 9;69:S291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rakislova N, Fernandes F, Lovane L, et al. Standardization of minimally invasive tissue sampling specimen collection and pathology training for the child health and mortality prevention surveillance network. Clin Infect Dis. 2019. October 9;69:S302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Martines RB, Ritter JM, Gary J, et al. Pathology and telepathology methods in the child health and mortality prevention surveillance network. Clin Infect Dis. 2019. October 9;69:S322–32. [DOI] [PubMed] [Google Scholar]
- [58].Ngwenya N, Coplan D, Nzenze S, et al. Community acceptability of minimally invasive autopsy (MIA) in children under five years of age in Soweto, South Africa. Anthropol South Africa. 2017. June 2;40:108–121. [Google Scholar]
- [59].Blevins J, O’Mara Sage E, Kone A, et al. Using participatory workshops to assess alignment or tension in the community for minimally invasive tissue sampling prior to start of child mortality surveillance: lessons from 5 sites across the CHAMPS network. Clin Infect Dis. 2019. October 9;69:S280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Salzberg NT, Sivalogan K, Bassat Q, et al. Mortality surveillance methods to identify and characterize deaths in child health and mortality prevention surveillance network sites. Clin Infect Dis. 2019. October 9;69:S262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cunningham SA, Shaikh NI, Nhacolo A, et al. Health and demographic surveillance systems within the child health and mortality prevention surveillance network. Clin Infect Dis. 2019. October 9;69:S274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Diaz MH, Waller JL, Theodore MJ, et al. Development and Implementation of multiplex TaqMan array cards for specimen testing at child health and mortality prevention surveillance site laboratories. Clin Infect Dis. 2019. October 9;69:S311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lewis C, Riddington M, Hill M, et al. Availability of less invasive prenatal, perinatal and paediatric autopsy will improve uptake rates: a mixed methods study with bereaved parents. BJOG An Int J Obstet Gynaecol. 2019;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lewis C, Hill M, Arthurs OJ, et al. Health professionals’ and coroners’ views on less invasive perinatal and paediatric autopsy: a qualitative study. Arch Dis Child. 2018. February 8;103:archdischild-2017-314424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Maixenchs M, Anselmo R, Sanz A, et al. Healthcare providers’ views and perceptions on post-mortem procedures for cause of death determination in Southern Mozambique. PLoS One. 2018. July;13:e0200058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Karat A, Tlali M, Charalambous S, et al. Needle autopsies highlight challenges in defining HIV+ TB deaths using verbal autopsy. Top Antivir Med. 2015;23:373–374. [Google Scholar]
- [67].Cina SJ, Smialek JE. Postmortem percutaneous core biopsy of the liver. Mil Med. 1999. June;164:419–422. [PubMed] [Google Scholar]
- [68].Milner DA, Valim C, Luo R, et al. Supraorbital postmortem brain sampling for definitive quantitative confirmation of cerebral sequestration of plasmodium falciparum parasites. J Infect Dis. 2012;205:1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Njuguna HN, Zaki SR, Roberts DJ, et al. Determining the cause of death among children hospitalized with respiratory illness in Kenya: protocol for pediatric respiratory etiology surveillance study (PRESS). J Med Internet Res. 2019;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Palhares AEM, Ferreira L, Freire M, et al. Performance of the minimally invasive autopsy tool for cause of death determination in adult deaths from the Brazilian Amazon: an observational study. Virchows Arch. 2019;475:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hurtado C, Quintó J, Castillo P, et al. Postmortem interval and diagnostic performance of the autopsy methods. Sci Rep. 2018;8. DOI: 10.1038/s41598-018-34436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].MITS Surveillance Alliance . MITS surveillance alliance grantees [Internet]. [cited 2019. November 8]. Available from: https://mitsalliance.org/BecomeAnAllianceMember/Grantees
- [73].Breeze ACG, Jessop FA, Whitehead AL, et al. Feasibility of percutaneous organ biopsy as part of a minimally invasive perinatal autopsy. Virchows Arch. 2008;452:201–207. [DOI] [PubMed] [Google Scholar]
- [74].Van Der Linden A, Blokker BM, Kap M, et al. Post-mortem tissue biopsies obtained at minimally invasive autopsy: an RNA-quality analysis. Cappello F, editor. PLoS One. 2014. December 22;9:e115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fernandes F, Castillo P, Bassat Q, et al. Contribution of the clinical information to the accuracy of the minimally invasive and the complete diagnostic autopsy. Hum Pathol. 2019. March;1:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]


