Abstract
Context: Pseudomeningocele is a relatively uncommon postoperative complication of spine surgery. Although the condition tends to be asymptomatic and self-limiting, it may cause radicular pain and neurological defect due to herniation of the nerve root or the spinal cord. Its pathophysiology remains unclear. Only few cases with intraoperative photos have been reported.
Finding: We present a case of pseudomeningocele with nerve root entrapment after percutaneous endoscopic lumbar discectomy (PELD). A 52-year-old man had undergone PELD for sciatic pain and showed good postoperative recovery. Unfortunately, he was readmitted for progressive right leg pain at six weeks after the surgery. After the failure of conservative therapy, he received PELD again to explore the surgical site. Intraoperatively, a pseudomeningocele-containing nerve root, herniating through a small defect in the dural sac, was identified. During the dissection process, the pseudomeningocele was broken, which led to entrapment of the nerve root. Thereafter, the microsurgical technique was adopted to relocate the nerve root into the thecae sac and to repair the dural tear by non-resorbable suture.
Conclusion: To our knowledge, this case report is the first documented instance of identification of a pseudomeningocele under an endoscope, and provides insights into the transformation of a pseudomeningocele into a cerebrospinal fluid fistula with nerve root entrapment. For neurological deficit caused by pseudomeningocele following PELD, operative revision by the microsurgery technique is the appropriate strategy.
Keywords: Pseudomeningocele, Dural tears, Percutaneous endoscopic lumbar discectomy, Postoperative, Complications
Introduction
Pseudomeningocele and cerebral spinal fluid (CSF) fistula, characterized by extradural CSF collection, are relatively uncommon complications for spine surgery. They are caused by similar mechanisms and represented a continuum.1 A majority of them are asymptomatic and self-limiting conditions; however, in some cases, these may lead to neurologic defect owing to spinal cord compression or nerve entrapment.2,3 Percutaneous endoscopic lumbar discectomy (PELD), as a typical technique of minimally invasive spine surgery, is the use of neural foramen to access the herniated discs, following by limited tissue disruption and reduced perioperative morbidity. Compared with open conventional procedures, it provides superior illumination and visibility by an endoscope. On the other hand, pseudomeningocele and CSF fistula caused by dural tear are much more difficult to detect under continuous irrigation.4,5
Here, we report a patient who developed pseudomeningocele with nerve root entrapment which identified by endoscope intraoperatively, and completely recovered after repair of the dural defect. To our knowledge, the fine pictures of pseudomeningocele and the process of transformation of a pseudomeningocele into CSF fistula with nerve root herniation have never been discussed.
Case report
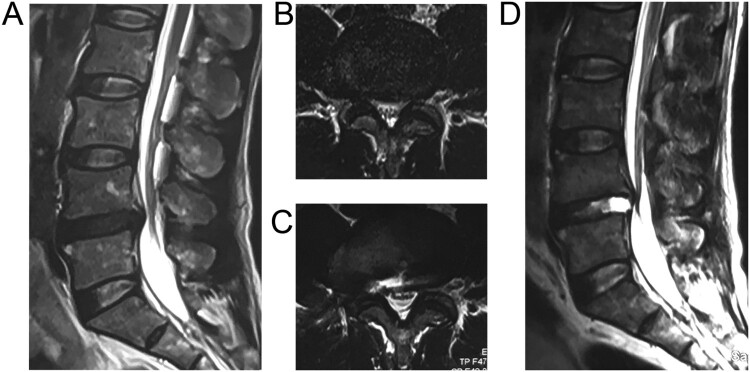
A 52-year-old man presented with complaints of progressive, severe sciatic pain. The symptoms were aggravated by coughing and straining, and relieved by bed rest. He also complained of numbness and tingling sensation in the right great toe. There was no previous history of surgery or traumatic injury to the spine. Spinal MRI showed a large median and right paramedian disc herniation between the L4 and L5 vertebrae (Fig. 1(A,B)). He underwent transforaminal PELD at the L4-L5. All procedures were performed according to the standard transforaminal endoscopic selective discectomy technique.4 An 18-gauge spinal needle was inserted into the disc through the foraminal approach guided by the fluoroscopic image. The gravity flow system was used to irrigate, and the infusion bag was suspended from 1.5 m above the patients’ back level. The selective fragmentectomy and decompression was then conducted using endoscopic forceps under clear endoscopic visualization. Dural tear or CSF leakage was not observed during the procedure. The patient had a good postoperative recovery and showed improvement in right leg pain. Unfortunately, he was readmitted for progressive right leg pain after six weeks, which radiated from the right buttock to the foot. No headache or swelling on the back was presented. On physical examination, the straight leg raise test was positive only on the right side, with a 30-degree angle raise. Postoperative magnetic resonance imaging (MRI) confirmed the removal of the herniated disc, accompany with the para-spinal fluid collection (Fig. 1(C,D)).
Figure 1.
Sagittal (A) and axial (B) MRI revealed paramedian-median disc herniation at L4-L5 level. Sagittal (C) and axial (D) of Post-operative MRI showing para-spinal fluid collection after resection of the herniated tissue by the first PELD.
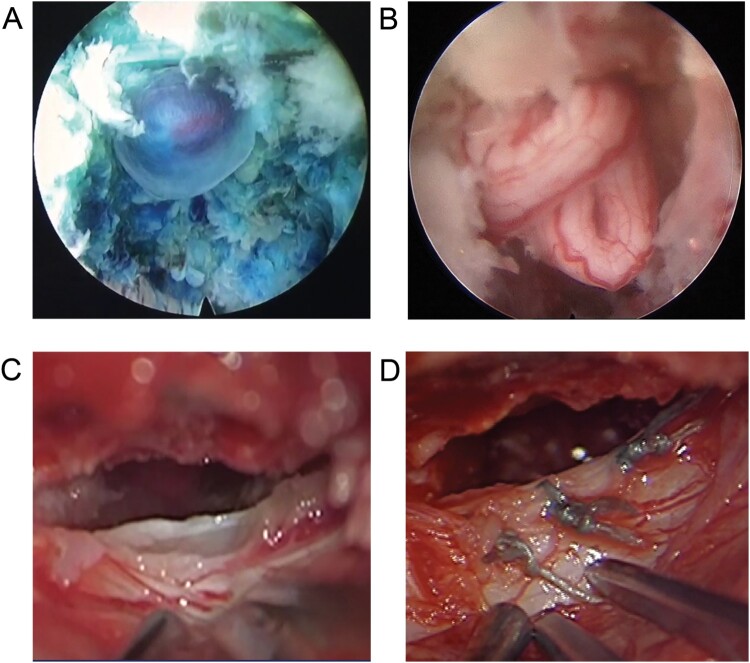
As the patient’s symptoms were not relieved by conservative therapy and epidural injection, Surgery was considered. He underwent L4-L5-PELD to re-explore the surgical site for potential recurrence of disc herniation. Intraoperatively, a pseudomeningocele-containing nerve root, herniating through a small defect in the ventrolateral dural sac was identified (Fig. 2(A)). Initially, the surgeon attempted to directly pull them back into the intradural space with gelfoam, as the arachnoid bleb was considered to be intact. However, the arachnoid bleb was broken, which led to nerve root entrapment (Fig. 2(B)). Consequently, a laminectomy for L4 was performed. After fluid evacuation, a dural defect, circa 1.5 cm, at the L4-L5 interlaminar region with the entrapped nerve root was identified under a microscope (Fig. 2(C)). The nerve root then was dissected and relocated into the thecae sac by the microsurgical technique. Finally, the dural tear was closed by non-resorbable suture (Fig. 2(D)).
Figure 2.
Intraoperative photograph under endoscope showing pseudomeningocele (A) and nerve roots entrapped through the dural defect (B). The microscope figure revealing dural defect (C) and the appearance (D) after repair using non-resorbable suture.
After the operation, the patient’s symptom disappeared, without neurological deficit or headache. Five days later, the patient was discharged. One year after surgery, the neurological condition of the patient is excellent.
Discussion
Few case reports of pseudomeningocele with different clinical presentations have been reported.6 The precise incidence of pseudomeningocele and CSF fistula is not known, as many patients tend to be asymptomatic. The estimated prevalence ranges from 0.068% to 2%, and may be as high as 17.4%.7–10 Both CSF fistula and pseudomeningocele develop through a similar mechanism. When the dura is breached but the arachnoid remains intact, the arachnoid may herniate through the dura and the arachnoid-lined sac becomes the pseudomeningocele. However, if both the dura and arachnoid are torn, it would result in CSF fistula. As far as we known, the pathophysiological process of pseudomeningocele developing into a CSF fistula has never been observed under the endoscope.11 This patient is the first reported case of pseudomeningocele with nerve root herniation, confirmed by intraoperative endoscope image.
Because of the various pathological symptom, pseudomeningocele and CSF fistula are difficult to detect early and to distinguish from abscess, seroma, persistent or recurrent symptoms after discectomy, yet MRI is useful to evaluate a suspected case. Among the pseudomeningocele patients, low back pain, headache and gradually enlarging lump under the skin are common symptoms, which may occur several weeks or months after lumbar surgery. Occasionally, nerve roots and spinal cord may herniate into the sac and induce radicular pain. However, the headache and lump were inapparent for this patient, as relatively intact bone and soft tissue preserved by PELD confined the expansion of pseudomeningocele and CSF fistula. On MRI, pseudomeningocele typically appears as a para-spinal CSF collection which exhibits low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. These collections, usually located adjacent to the thecae sac and the laminectomy site, tend to be irregular, lobulated, or oblong.12 However, MRI imaging does not provide adequate visualization to determine nerve root entrapment. Delayed computerized tomography myelography and CISS imaging may be helpful to detect the dural tear and nerve root entrapment.13
In this case, the improper operation of spinal needle is a probable reason for pseudomeningocele, even though most iatrogenic dural tears take place during the discectomy step. The surgeons had meticulously analyzed the surgical video record and didn’t detect any obvious problem under endoscopic in the first operation. Then, we presume that the dura sac may be breached by the adjustment of spinal needle. According to the X-ray image of the localized step, the spinal needle once entered the upper-medial aspect of the foramina, which may pierce the dural membrane. This secondary tiny dural tear is difficult to be observed, because the positive pressure irrigation may keep the nerve tissue in the thecae sac. Over time, the intraspinal pressure may gradually enlarge the dural tear and cause syndromes related to pseudomeningocele in the postoperative period. To avoid this complication, the surgeon must have careful preoperative planning and thorough understanding of 3-dimension anatomy for the transforaminal approach. Skilled puncturing technique and proper trajectory guided by the fluoroscopic image are required. The needle tip should be kept lateral to the medial margin of pedicle during the localized step. Aberrant placement or dissection of the spinal needle in the intervertebral foramen and spinal canal may cause unintended injuries.
Management of pseudomeningocele depends on many factors, especially the size and clinical symptoms.14 Small asymptomatic pseudomeningocele may only need to be monitored, as most of these gradually resolve spontaneously. In addition, large expanded pseudomeningocele may require intervention. In any case, conservative methods, including focal compression or bed rest with appropriate positioning, should be suggested as initial treatment. If conservative treatment failed, more aggressive intervention could be considered, including drainage, epidural blood patch and operative revision. Drainage for 3–5 days has a success rate of over 90% in the treatment of CSF leakage, but is associated with a risk of infection. Epidural blood patch has been suggested for pseudomeningocele and postural puncture headache, as clot formation and inflammation help to seal the tiny fistulas.15 If herniation of nerve tissue is suspected, operative revision should be performed as soon as possible.
The aim of surgical treatment is to dissect the tethering nerve roots and to repair the dural defect. Endoscopic surgery could be used to explore the surgical site, distinguish etiology and provide uncomplex treatment. For this case, endoscopic surgery provides a helpful visual angle to observe the pseudomeningocele at the ventrolateral of the dural sac which would be probably broken in the traditional surgery. However, untethering and suturing are much more difficult to complete under an endoscope, the microsurgical technique is the preferred approach for entrapped nerve root. Any entrapped nerve root should be freed and relocated into the dura. Either Gelfoam or muscle alone placed over the dural breach is ineffective in stopping the leak. Use of non-absorbable suture to close the dural defect is recommended. In addition, artificial dura, tissue glue, free fat grafts and myofascial grafts have also been used to enhance the dura.16
Conclusion
To the best of our knowledge, this is the first case report of pseudomeningocele developing after PELD. This case demonstrates a pseudomeningocele with herniated nerve root under endoscope. For neurological deficit caused by pseudomeningocele following PELD, operative revision by the microsurgery technique is the appropriate strategy.
Disclaimer statements
Contributors None.
Funding details None.
Declaration of interest None.
Financial support This study did not benefit from funding sources. All authors declare absence of financial support.
Conflict of interest The authors report no conflicts of interest.
Ethics approval None.
References
- 1.Daniel C, Charles L, Branch JR.. Spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus 2003;15(6):1–5. [DOI] [PubMed] [Google Scholar]
- 2.Ijiri K, Hida K, Yano S, Komiya S, and Iwasaki Y.. Traumatic spinal-cord herniation associated with pseudomeningocele after lower-thoracic nerve-root avulsion. Spinal Cord 2009;47(11):829–31. doi: 10.1038/sc.2009.38 [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Ikuma H, Nakanishi K, Sugimoto Y, Misawa H, Takigawa T, et al. Spinal cord herniation into pseudomeningocele after traumatic nerve root avulsion: case report and review of the literature. Eur Spine J 2008;17(2):263–6. doi: 10.1007/s00586-007-0537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei S, Tao W, Zhu H, and Li Y.. Three-dimensional intraoperative imaging with O-arm to establish a working trajectory in percutaneous endoscopic lumbar discectomy. Videosurgery Other Miniinvasive Tech 2016;10(4):555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sairyo K, Matsuura T, Higashino K, Sakai T, Takata Y, Goda Y, et al. Surgery related complications in percutaneous endoscopic lumbar discectomy under local anesthesia. JMI 2014;61(3–4):264–9. [DOI] [PubMed] [Google Scholar]
- 6.Oterdoom DLM, Groen RJM, and Coppes MH.. Cauda equina entrapment in a pseudomeningocele after lumbar Schwannoma extirpation. European Spine J 2010;19(2):158–61. doi: 10.1007/s00586-009-1219-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson HS and Fincher EF.. Extradural arachnoidal cysts of traumatic origin. J Neurosurg 1947;4(6):530–8. doi: 10.3171/jns.1947.4.6.0530 [DOI] [PubMed] [Google Scholar]
- 8.Ahn Y, Lee HY, Lee SH, and Lee JH.. Dural tears in percutaneous endoscopic lumbar discectomy. Eur Spine J 2011;20(1):58–64. doi: 10.1007/s00586-010-1493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teplick JG, Peyster RG, Teplick SK, Goodman LR, and Haskin ME.. CT identification of postlaminectomy pseudomeningocele. AJR 1983;140(6):1203–6. doi: 10.2214/ajr.140.6.1203 [DOI] [PubMed] [Google Scholar]
- 10.Khazim R, Dannawi Z, Spacey K, Khazim M, Lennon S, Reda A, et al. Incidence and treatment of delayed symptoms of CSF leak following lumbar spinal surgery. Eur Spine J 2015;24(9):2069–76. doi: 10.1007/s00586-015-3830-4 [DOI] [PubMed] [Google Scholar]
- 11.Asha MJ, George KJ, and Choksey M.. Pseudomeningocele presenting with Cauda equina syndrome: is a ‘ball-valve’ theory the answer? Brit J Neurosurg 2011;25(6):766–8. doi: 10.3109/02688697.2011.578768 [DOI] [PubMed] [Google Scholar]
- 12.Radcliff K, Morrison WB, Kepler C, Moore J, Sidhu GS, Gendelberg D, et al. Distinguishing Pseudomeningocele, epidural hematoma, and postoperative infection on postoperative MRI. Clin Spine Surg 2016;29(9):471–4. doi: 10.1097/BSD.0b013e31828f9203 [DOI] [PubMed] [Google Scholar]
- 13.Hans FJ, Reinges MH, and Krings T.. Lumbar nerve root avulsion following trauma: balanced fast field-echo MRI. Neuroradiology 2004;46(2):144–7. doi: 10.1007/s00234-003-1139-1 [DOI] [PubMed] [Google Scholar]
- 14.Tu A, Tamburrini G, and Steinbok P.. Management of postoperative pseudomeningoceles: an international survey study. Child Nerv Syst 2014;30(11):1791–801. doi: 10.1007/s00381-014-2501-9 [DOI] [PubMed] [Google Scholar]
- 15.Cornman-Homonoff J, Schweitzer A, and Chazen JL.. CT-guided epidural blood patch for treatment of CSF leak and pseudomeningocele following tethered cord release in a 3-year-old. Clin Imag 2016;40(6):1191–4. doi: 10.1016/j.clinimag.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Weng YJ, Cheng CC, Li YY, Huang TJ, and Hsu RWW.. Management of giant pseudomeningoceles after spinal surgery. BMC Musculoskelet Disord 2010;11(1):53. doi: 10.1186/1471-2474-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]