ABSTRACT
Salivary proteins specific antibodies have been shown to be useful biomarkers of exposure to sand fly bites. This study aimed to investigate the level, duration, and dynamics of the human immune response against the SGL of Phlebotomus sergenti Parrot, 1917 (Diptera: Psychodidae), and to assess the immunoreactivity of human sera with SGL components in an endemic area of anthroponotic cutaneous leishmaniasis (ACL) in Iran. The study was carried out in 2-phase; longitudinal and cross-sectional. Sand flies were collected monthly from indoors and outdoors. In the longitudinal study, sera from healthy volunteers were collected monthly, and in the cross-sectional study, sera from healthy volunteers and patients with ACL lesion/s, were collected for immunoassay studies. The level of anti-P. sergenti saliva IgG was detected using the ELISA. Immunoreactivity of individual human sera with saliva components was also assessed by western blotting. Phlebotomus sergenti was the predominant sand fly species in the study area. The maximum and minimum percentages of IgG responses were seen in October (66%) and March (29%), respectively. Additionally, the cross-sectional study showed that 59.3% of the healthy volunteers and 80% of the patients were IgG positive. The antibody response against P. sergenti salivary gland was high during the sand fly active season and declined by the end of the activity of the vectors. Antibody response against the SGL components of P. sergenti was transient and individual-specific. Some individuals shared a strong reaction against certain individual antigens, which could be considered as vector exposure markers for further investigation.
List of abbreviations
ELISA: Enzyme-Linked Immunosorbent Assay; SDS PAGE: Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis; SGL: Salivary Gland Lysate; ACL: Anthroponotic Cutaneous Leishmaniasis; PBS: Phosphate Buffered Saline; BCA: Bicinchoninic Acid; PBS-T: Phosphate Buffered Saline Tween; FBS: Fetal Bovine Serum; HRP: Horseradish Peroxidase; TMB: 3,3′,5,5′-Tetramethylbenzidine; PVDF: Polyvinylidene Difluoride; SGA: Salivary Gland Antigens; OD: Optical Density; KDa: Kilodalton; VL: Visceral Leishmaniasis; CL: Cutaneous Leishmaniasis; SGs: Salivary glands
KEYWORDS: Salivary gland antigens, immune response, Phlebotomus sergenti, leishmaniasis, Iran
GRAPHICAL ABSTRACT
The antibody response was transient to P. sergenti salivary gland antigens, and some individuals shared a strong reaction toward some proteins of SGL.
1. Introduction
Leishmaniasis is a neglected parasitic disease that is transmitted through the bite of infected phlebotomine sand flies. It is estimated that one-tenth of the world’s population is at risk of contracting leishmaniasis [1]. It is endemic in more than 100 countries, and an estimated 0.9 to 1.3 million new cases and 20,000 to 30,000 deaths occur annually worldwide [2]. Cutaneous leishmaniasis due to Leishmania tropica Wright, 1903 (Kinetplastida: Trypanosomatidae) is endemic in the Middle East, including Iran [1]. Insect salivary proteins have been used as an alternative tool to determine human and animal exposure to vectors [3–5]. Previous research studies have demonstrated the potential vector-based vaccine properties of the salivary proteins of sand flies and the use of these proteins as vector exposure markers [6–8]. Additionally, in animal models, pre-exposure to saliva extracts of sand flies induced an immune response that prevented the effects of parasite infectivity [9,10]. It has been well demonstrated that humans and animals exposed to salivary gland proteins of sand flies, either naturally or experimentally, elicit specific antibodies against targeted proteins [3,11]. Salivary proteins of some blood-sucking insects such as mosquitos [12] tsetse flies [13], and triatomine bugs [14] have been used as biomarkers of exposure to insect bites. The level of salivary proteins-specific antibodies correlates with the intensity of exposure [15–17], and thus, these antibodies can be used as an epidemiological tool to measure the effectiveness of vector-control programs especially in the endemic foci of vector-borne diseases such as leishmaniasis. Furthermore, the intensity of anti-saliva antibodies correlates well with the risk of leishmaniasis, which means developing such tests could be valuable in predicting the risk zones of leishmaniasis. Previously, preventive measures against vectors in endemic areas were based on measuring the seasonal activity of the vectors and some other techniques such as evaluating vector landing behavior [18]. Recent studies have used simple, precise, and rapid techniques such as indirect enzyme-linked immunosorbent assay (ELISA) to measure humoral immune response against either whole saliva components or some immunodominant saliva proteins in human and other hosts to determine vector exposure [19–22].
In endemic areas, fluctuation in sand fly population during active seasons [23] may influence host anti-saliva antibody response in exposed inhabitants. The kinetics of anti-saliva antibodies in mice [15,24], dogs [16,17], humans [25,26], and rabbits [24] have been studied extensively.
This study aimed to investigate the level, duration, and dynamics of antibody response to the SGL of Phlebotomus sergenti among inhabitants in an ACL endemic area, and to assess the immunoreactivity of different individual human sera with SGL components.
2. Materials and methods
2.1. Study area
The study was conducted in Dehbakri, an endemic focus of ACL, 50 km from the city of Bam (29°03ʹ14.2” N, 57°54ʹ31.6” E) in Kerman province in Iran (Figure 1). L. tropica is the most common cause of ACL in the study area. The study was conducted between March 2015 and March 2016. The study area experienced a leishmaniasis outbreak in the past [27]. Dehbakri is a mountainous area (altitude 2052 m), with a very cold weather in the winter and moderate weather in the summer. It is visited by many people due to hot summers in the neighboring regions. The average maximum and minimum temperatures during the study period were 27°C and 5.7°C, respectively, and the average annual rain fall was 22.5 mm in 2015 (Kerman metrological organization).
Figure 1.
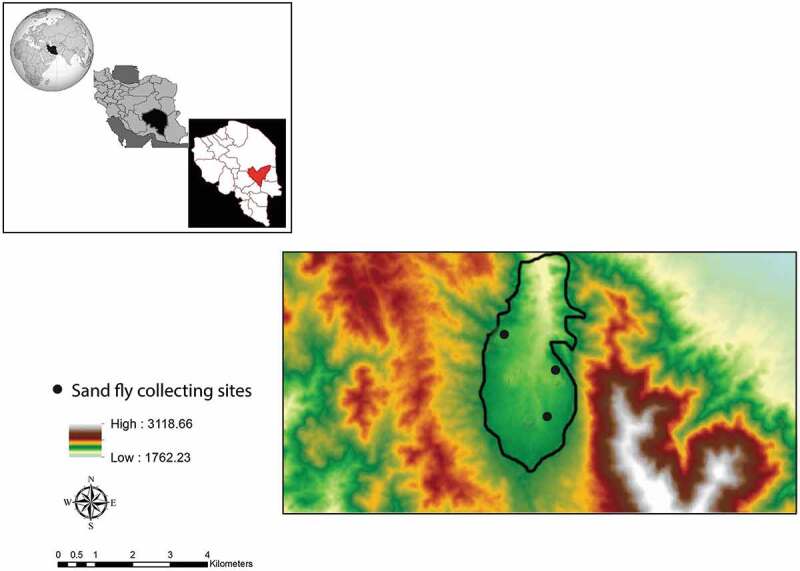
Map of studied area, and sand fly collecting sites, Dehbakri district, Bam County, Kerman province, Iran.
2.2. Sand fly collection
Sand flies were collected using two CDC miniature light trap and/or aspirating tubes from indoors (bedrooms, toilets and bathrooms) and outdoors habitats. The captured sand flies were kept in a cloth cage holding in a stainless-steel framework (35 × 35 × 35 cm), and were transferred to the Sand Fly Insectarium of the School of Public Health affiliated to Tehran University of Medical sciences in Tehran, Iran, for rearing. To determine monthly sand fly density, three fixed houses were selected in Dehbakri district. The sand flies were collected using 30 sticky traps (20 × 30 cm papers impregnated with castor oil), 15 for indoors (bedrooms, toilets and bathrooms), and 15 for outdoors habitats (outside of walls of houses facing open areas and near stables and/or warehouses), at each time point. A total of 480 sticky papers were used during the active season of the sand flies in the study area. Traps were set twice a month, with 15-days interval, from the beginning to the end of the active season of the sand flies in 2015. To determine the time of first emergence and diminishing of sand flies, two zero collections of sticky papers were performed both at the beginning and at the end of the active season. The sticky papers were set before sunset and collected before sunrise. Microscopic slides of the collected sand flies were prepared in Pauri’s medium, and the insects were identified based on the morphology of the male genitalia and spermathecae of the females using valid keys [28–30].
2.3. Sand fly salivary gland dissection
Sand flies were reared using the protocol of Killick-Kendrick and Killick-Kendrick [31] and Modi and Tesh [32] with minor modifications. An insect colony was established with about 350 (200 males, 150 females) P. sergenti captured with the aforementioned method in August 2015. First generations of sand flies were maintained on chicken, the preferred host in the field, and then switched to hamster, the conventional animal for maintaining sand fly colonies in the insectarium [33]. The sand flies were maintained at a mean temperature of 27–28°C, 70–80% relative humidity, and 14:10 (L:D) photoperiod. The immature stages were fed by larval food consisting of a mixture of rabbit feces and rabbit pellets prepared through a long fermentation process [34].
Four to 10-day-old female laboratory-reared P. sergenti (Generation fourth to sixth) were subjected to salivary glands (SGs) dissection. The SGs were dissected out using fine forceps (Dumont # 5) and needles (gauge 28) in cold phosphate-buffered saline (PBS; pH = 7.4) under a stereo microscope, and then transferred into 1.5 ml micro-tubes containing 20 µl PBS in groups of 20 glands. Finally, the SG tissues were kept at −20°C until further use.
To prepare salivary gland lysates, the glands were frozen in liquid nitrogen and thawed in boiling water (repeated 3 cycles), and then centrifuged at 18,000 g for 10 minutes. The supernatants were collected and used for the experiments. Protein concentration per gland was measured by BCA (Bicinchoninic acid assay) method using ‘Pierce® BCA Protein assay kit’, according to the manufacturer’s instructions.
2.4. Human sera collection
In the longitudinal study, to evaluate antibody response against SGL of P. sergenti, 100 healthy adults naturally exposed to sand fly bites in Dehbakri who had expressed their willingness to participate in the study and signed informed consent, were selected for the purposes of the study. Inclusion criteria were healthy adults without a documented history of CL and at the age range of 20–70 years old. Pregnant and nursing women were excluded from the study. At first, the purpose and the procedures of the study were explained to the potential volunteers. Blood samples were then collected from each individual monthly from April 2015 to March 2016. In addition, a cross-sectional study was conducted to measure the incidence of the antibody-positive response among the study population. Sera of 123 healthy individuals and 10 patients with progressive L. tropica lesion(s) were collected once in November 2016 in the same area. The patients received free treatment until the complete healing of the lesion(s), and also the aforementioned inclusion and exclusion criteria were considered for selecting volunteers in the cross-sectional study. As a negative control group, 20 newborn serum samples were collected from bio-bank of the Children’s Medical Center of TUMS. All the collected and prepared sera were kept at a − 20°C freezer until further use.
2.5. ELISA
The level of anti-P. sergenti saliva IgG antibodies were measured using the ELISA technique. ELISA tests were done in triplicates. Briefly, microtiter plates (Maxisorb; Nunc, Roskilde, Denmark) were coated with 50 µl of SGL (0.18 µg protein per well) in a carbonate-bicarbonate buffer (0.01 M NaHCO3, Na2CO3, pH 9.6) and incubated at 4°C overnight. The wells were then washed three times using 0.05% Tween 20 PBS buffer, and the free binding sites were blocked by incubation with 100 µl of 5% bovine serum albumin (BSA) and 10% fetal bovine serum (FBS) in 0.05% PBS-T for 2 hours at 37°C. The wells were washed again, and 50 µl of the sera diluted in 5% BSA/PBS-T at 1:50 was added to each well, and the plates were incubated again at 37°C for 90 min. Subsequently, the plates were washed and 50 µl of HRP-conjugated anti-Human IgG (Sigma) diluted in PBS-T at 1:16,000 was added to each well, and incubated at 37°C for 90 min. Then, the plates were washed three times, and 50 µl of the substrate (3,3ʹ,5,5ʹ-Tetramethylbenzidine. TMB (Sigma)) was added to each well. The chromatic reaction was allowed to develop for 10 minutes at room temperature. Finally, 25 µl of 10% H2SO4 (stopping solution) was added to each well, and the optical density (OD) was read using the BioTek ELISA reader (ELx808) at 450 nm wavelength. Sera from experimentally exposed volunteers and naïve sera from newborns were used as positive and negative controls, respectively. Furthermore, two wells without layer one (coating) and two wells without layer two (human sera) were included in all test plates as negative controls. The cutoff points were calculated by adding two standard deviations to the mean optical densities of sera from naïve newborn samples as negative controls.
2.6. Western blotting
Initially, proteins of SGL were separated on 10% polyacrylamide gels using ‘Mini-PROTEAN® Tetra Cell’ (Bio-Rad), under reducing conditions. Ten homogenized female P. sergenti salivary glands were loaded into one lane to be separated by electrophoresis. Separated proteins were electro-transferred onto a polyvinylidene difluoride (PVDF) membrane using ‘Mini-PROTEAN® Tetra Cell’ (Bio-Rad). To visualize the protein bands, with the aim of cutting into individual strips, the PVDF membranes were stained by ‘Ponceau S’. After precise cutting, strips were washed using PBS, and then blocked with 5% BSA and 10% FBS in PBS-T at 4 ◦C overnight. The strips were subsequently washed three times (15 min each) with PBS-T on a shaker. ELISA positive-anti-Human-IgG sera were diluted at 1:100 in PBS-T containing 2.5% BSA, and added individually to the various strips. The strips were then incubated with the diluted sera at room temperature (RT) for 90 min on a shaker. Subsequently, the strips were washed six times in PBS-T, and then incubated with HRP-conjugated goat anti-human IgG (1:64,000 diluted in 0.05% PBS-T) at RT for 90 min. The procedure was followed by another washing step six times each for 15 minutes in PBS-T. Finally, the chromatic reaction was developed with TMB liquid substrate system for membranes (Sigma). Sera from naïve newborn samples were used as negative controls.
2.7. Statistical analysis
Data obtained by ELISA were subjected to One-way ANOVA to assess statistical significance for each person between different months of the year. Mean and standard deviation of ODs were calculated per month. The mean ODs of sera collected in April was compared with every other month by t-test. For all tests, the p value <0.05 was considered statistically significant. Statistical analyses were performed using STATA 16 software. All figures were generated using GraphPad prism 8.
3. Results
3.1. Entomological survey
A total of 671 sand flies (281 from indoors and 390 from outdoors) were collected during the sand fly active season from April to November 2015. Seven sand fly species belonging to the genus Phlebotomus and genus Sergentomyia were identified. These species included P. sergenti, P. papatasi, Phlebotomus major, Sergentomyia baghdadis, Sergentomyia sintoni, Sergentomyia theodori, and Sergentomyia dentata (Table 1). The density of each species per m2 is shown in Table 1.
Table 1.
Species composition and number of sand flies collected in indoor and outdoor places from April to November 2016, Dehbakri district, Bam county, Kerman province.
| Indoors |
Outdoors |
|||||
|---|---|---|---|---|---|---|
| Species | No. | % | No. | % | Total | specimens/m2 |
| P. sergenti | 207 | 45 | 249 | 55 | 456 | 15.8 |
| P. papatasi | 7 | 41 | 10 | 59 | 17 | 0.59 |
| P. major | 0 | 0 | 1 | 100 | 1 | 0.03 |
| S. baghdadis | 28 | 29 | 67 | 71 | 95 | 3.9 |
| S. sintoni | 14 | 37 | 24 | 63 | 38 | 1.3 |
| S. thodori | 13 | 52 | 12 | 48 | 25 | 0.8 |
| S. dentata | 12 | 31 | 27 | 69 | 39 | 1.3 |
| Total | 281 | 42 | 390 | 58 | 671 | 23.2 |
The seasonal activity of P. sergenti started in the first half of April and ended in the second half of November in both indoor or outdoor habitats. As shown in Figure 2, three peaks of activities were seen in May, July, and September in indoor and outdoor habitats.
Figure 2.
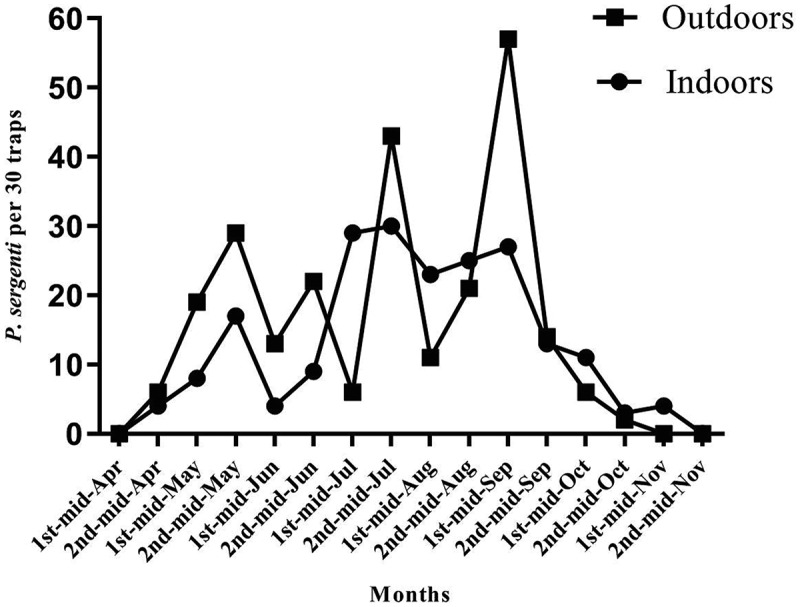
Seasonal activity of Phlebotomus sergenti in indoors and outdoors, Dehbakri district, Bam County, Kerman Province, Iran, 2015. Full circle and full rectangular representing the number of captured P. sergenti in indoors and outdoors per 30 traps respectively.
Table 1 shows species composition and number of sand flies collected in indoor and outdoor places from April to November 2016 in Dehbakri in Bam county, Kerman province.
3.2. Evaluation of human IgG response induced against SGL of P. sergenti
During the longitudinal study from April 2015 to March 2016, 749 sera were collected from volunteers, of which 358 were anti-P. sergenti SGL IgG positive (47.8%). As shown in Table 2, the maximum and minimum percentages of individuals with positive IgG response were observed in October (66%) and March (29%). The percentage of anti-SGL IgG-positive individuals fluctuated from April 2015 to February 2016, and reached the highest level in October. The percentage of IgG-positive individuals started to decrease from February, reaching the lowest level in March 2016. The highest and the lowest mean anti-IgG antibody responses in the individual volunteers were recorded in November and March, respectively, as shown in Figure 3. In addition, the results of the cross-sectional seroprevalence study from samples taken in November 2016 show that 59.3% of the inhabitants (73/123) were anti-SGL IgG positive. Among the 10 patients with active CL lesion, 8 (80%) were anti-P. sergenti SGL IgG positive.
Table 2.
The number of sera collected from native volunteers and their positivity from April 2015 to March 2016, Dehbakri district, Bam county, Kerman province.
| April | May | June | July | August | September | October | November | December | January | February | March | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Positive | 18 | 17 | 44 | 47 | 41 | 21 | 37 | 31 | 31 | 36 | 19 | 16 |
| Total | 58 | 42 | 100 | 82 | 72 | 45 | 56 | 53 | 53 | 77 | 52 | 55 |
| % | 31 | 40.4 | 44 | 57.3 | 56.9 | 46.6 | 66 | 58.4 | 58.4 | 46.7 | 33.6 | 29 |
Figure 3.
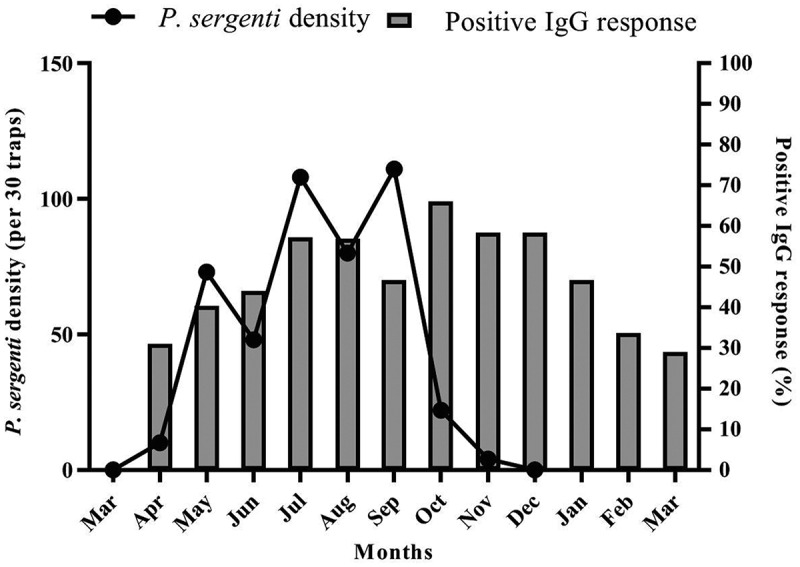
The percentage of IgG-positive individuals and Phlebotomus sergenti monthly activity, Dehbakri district, Bam County, Kerman Province, Iran, 2015–16. Gray bars are showing the percentage of positive sera, ODs above the cutoff point. Cutoff points were determined as the addition of two standard deviations to the mean optical densities of negative controls. The ELISA test were conducted triplicate and the mean of three wells represented the result.
3.3. The IgG dynamics and Phlebotomus sergenti density
With the onset of seasonal activity of P. sergenti in April, 31% of the population under the study were detected IgG positive. One month later, the seasonal activity of the vector reached a slight peak, and there was a peak in the percentage of antibody response in July. The monthly activity of sand flies showed three prominent peaks in May, July and September, whereas IgG antibody response was highest (66%) in October and then started to decrease and reached the lowest level in March (22%) (Figure 3).
Figure 4 presents the mean monthly anti-IgG response of the IgG-positive volunteers and the monthly activity of P. sergenti. Vector density reached the first slight peak in May, and on the other hand, antibody response reached a slight peak in July. Furthermore, there were three prominent peaks of vector density in May, July, and September, whereas antibody response was highest in November. The mean ODs of individuals in April were compared with the mean ODs of every other month. We observed a statistically significant difference between the mean ODs of individuals in April and November (P˂0.05). After September, the vector density started to decline and reached its minimum in December. On the other hand, the antibody response started to decrease with 2-months delay, and reached the lowest level in March.
Figure 4.
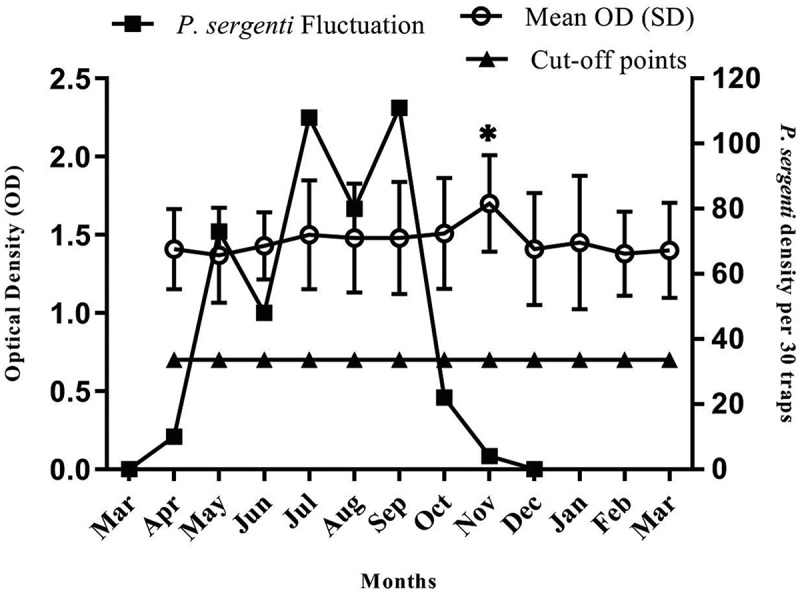
The monthly IgG dynamics in individual volunteers and Phlebotomus sergenti monthly activity, Dehbakri district, Bam County, Kerman Province, Iran, 2015–16. Cutoff points were determined as the addition of two standard deviations to the mean optical densities of negative controls. The ELISA test were conducted triplicate and data are presented as means ± standard errors. Asterisk represents significant change in the mean compared to April sampling.
3.4. Western blotting
Among the individual human sera assessed with ELISA, 10 sera with high ODs were randomly selected for dot blotting. Figure 5 demonstrates the immunoreactivity of the human sera collected from inhabitants of Dehbakri district with SGL components of P. sergenti. As shown in Figure 5, the immunoreactivity between antibodies and SGL antigens was individual-specific. The maximum antigenic bands were eight in strip one and five to seven bands in the other strips. There were six antigenic proteins with an approximate molecular weight of 60, 48, 24, 38, 28, and 15 kDa in SGL of P. sergenti. All the individual sera from the volunteers reacted with these antigenic proteins with the same intensity. In the blood sample collected from volunteer No. 1, one antigenic band with a molecular mass of 35 kDa and in the sample from volunteer No. 6, one antigenic band with a molecular mass of around 30 kDa were observed, but these bands were not detectable in any other samples. Newborn sera, used as negative controls, did not recognize any salivary protein from P. sergenti.
Figure 5.
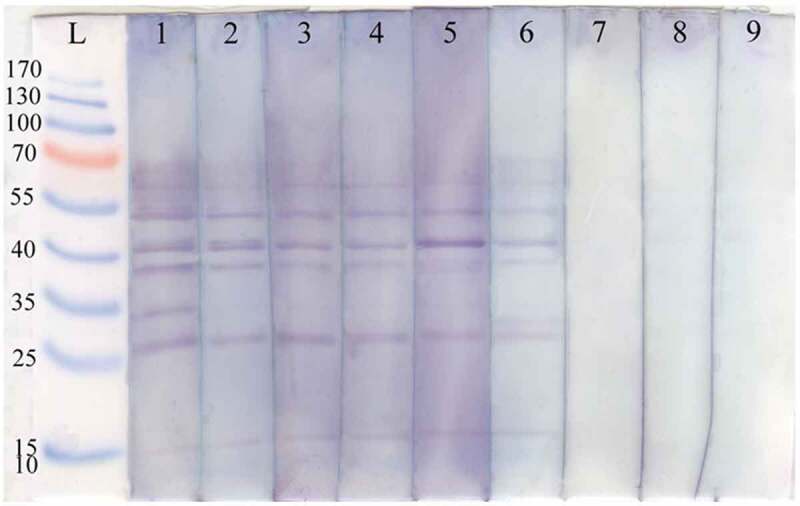
Immunoblots of Phlebotomus sergenti SGL with sera of human volunteers collected from Dehbakri district, Bam County, Kerman Province, Iran. L: PageRuler™ Prestained Protein Ladder (Fermentas), lane1-6: SGL with sera collected from human volunteers live in Dehbakri district, lane 7–9: SGL are from sera of 10–20-days-old naïve infants.
4. Discussion
Salivary proteins of insects have been used as a new and alternative tool investigating vector exposure in animals and humans [3,5]. The human immune response against the salivary proteins of sand flies could be used as a potential epidemiological indicator of vector exposure in leishmaniasis-endemic areas [26,35]. The present study demonstrated that anti-SGL antibodies are induced by the bites of sand flies in ACL endemic areas and that the antibody response fluctuates during the active season of sand flies. Some individual proteins are strongly recognized by all human sera with the same intensity, suggesting their potential use as biomarkers of exposure.
In the present study, P. sergenti was the predominant species and accounted for the majority of collected sand flies both in indoor and outdoor resting places. Phlebotomus sergenti is a predominantly mountainous species in Iran with a wide distribution range, and is considered as the main vector of ACL in Iran [36,37]. Some populations of the species are domestic and endophilic, and exist mostly in indoor places. A similar result was obtained in a previous study carried out in the city of Bam in which P. sergenti accounted for the majority (77.25%) of collected sand flies [38]. In the present study, monthly activity of sand flies started in April, but in another study conducted in the same area, it started with a 1-month delay in May and ended in November. This difference might be due to the climate changes in different years. Also, in line with our results, the predominant species was P. sergenti with 68.91% abundance in another study [39].
In our study, the IgG antibody against SGL of P. sergenti was assessed in 100 healthy volunteers for a period of 12 months. It is worth mentioning that blood sample collection was not possible from all the volunteers on every visit during the 12-month period due to various reasons, thus the percentage of the samples which were positive for anti- SGL IgG antibodies were calculated among the target population. To the best of our knowledge, this is the first study that has evaluated the IgG antibody response against SGL of P. sergenti and its kinetics in an endemic area of ACL. The percentage of antibody-positive individuals started to increase from April and reached the highest level (66%) in October and then started to decrease from October to March. It has been shown that the sand fly population fluctuates seasonally in the field [23], which may cause changes in anti-saliva antibody response in corresponding hosts. In our study, changes in anti-SGL antibody levels correlated well with the seasonal activity of P. sergenti. In other words, the antibody level was higher during the sand flies’ active season and declined in the season with no sand fly activity which is well corroborated by the findings of previous studies [22,40–42]. The key point is that by the end of the P. sergenti activity season, still, about 58% of the volunteers were IgG positive before it decreased afterward. Also, in the 4th month following the end of P. sergenti activity, about 29% of the volunteers were still showed antibody response. In the case of mean IgG dynamics among positive individuals, a similar finding was observed, and 4 months after P. sergenti’s last activity, the mean anti-saliva IgG titer level remained above cutoff. The inhabitants of endemic areas are exposed to SGL antigens through sand fly bites and receive booster exposure to SGL in every sand fly season. Several studies have reported that inhabitants of endemic areas are mostly positive for whole anti-SGL antibodies [21,43], but some individuals are positive only for specific antibodies like IgG1, IgG4, and IgE [25]. In our recent study, we demonstrated that long-term exposure of individuals to uninfected, laboratory-reared P. sergenti elicited significant anti-P. sergenti antibody response, which declined slightly by the end of the exposure. However, short-term exposure did not provoke any response [44]. In agreement with our study, antibody response in dogs exposed naturally to Lutzomyia longipalpis bites influenced by seasonal transmission and sand fly density; the response rate decreased after the wet season synchronically with the decline in sand fly numbers [22,40]. A similar scenario has been reported in laboratory exposed dogs, as antibody response rate decreased to about half the initial peak a few weeks after the last exposure, however persisted at low level for at least 19 weeks [16,17]. Similar to our study, a field study conducted in India showed a significant logarithmic correlation between antibody responses against P. argentipes saliva in the sera of inhabitant of endemic areas of VL and the geometric average number of P. argentipes collected in indoors [26]. In another study, it was shown that anti-Lu. longipalpis SGL IgG antibody level peaked in 5–6 weeks after exposure in dogs and remained significantly elevated until week 23 [17].
The results of our cross-sectional study show that 59.3% of the randomly selected volunteers of the endemic area were antibody positive against SGL of P. sergenti in December. A similar study that was carried out in Turkey showed that 45.2% (lower than our study) of the inhabitants of an ACL endemic foci were IgG positive against P. sergenti SGL [21]. The present study reaffirms that the prevalence of individuals who are positive for anti-sand fly saliva antibodies are affected by the time of sampling and seasonal activity of the vector. Out of the 10 patients with active lesion(s) of ACL, 8 were IgG positive. In previous studies, patients with L. tropica lesion(s) had significantly higher anti-P. sergenti IgG levels compared with healthy individuals, which reflects increased exposure to vector bites, and thus a higher probability of getting an infection [21,35].
In agreement with this finding, it was reported that a high percentage of inhabitants living in endemic areas of ACL were anti-SGL antibody positive. Most of the samples were positive against P. sergenti (45.2%) and P. papatasi (40.2%) [21].
We identified six antigenic proteins, with molecular weight of 35–60 kDa, which might correspond to yellow-related proteins [45]. In the present study, all individual human sera collected from the volunteers reacted with the same intensity to all the six antigenic proteins, indicating that they could be used as P. sergenti exposure markers. The antibody response against SGL of P. sergenti was individual-specific, which may be due to the genetic diversity caused different protein expression profiles. Consistent with this finding, some other studies indicated that the antigenicity of SGL is host-species specific [3,21,46]. Similarly, the pattern of antigens recognized by anti-P. perniciosus antibodies of hares and rabbits had several differences [3].
On the other hand, provoked antibody response against sand fly SGL was shown to be species specific [47–49]. Antibody responses in mice exposed individually to P. papatasi, P. sergenti, or Lu. longipalpis were specific to the respective vector [48]. On the other hand, anti-sand fly saliva antibodies showed the composition of the local sand fly fauna as well as individual species and thus could be used as a biomarker of exposure to the sand fly vector specially in the epidemiological studies [35].
Phlebotomus papatasi was the second predominant sand fly found in the study area. Although a slight cross-reactivity between P. sergenti and P. papatasi has been shown by a previous study [21], we did not check the cross-activity to P. papatasi salivary antigens due to unavailability of its SGL. However, cross-reactivity might not be a concern in this scenario given the low density of P. papatasi. Immunoreactivity of mouse sera repeatedly bitten by P. papatasi, P. sergenti, and Lu. longipalpis showed four to six antigenic bands. In the same study, immunoreactivity of sera from indigenous people in endemic foci of ACL with P. papatasi, and P.sergenti was evaluated as well. Similar to the present study, sera of the individuals who were antibody positive against P. sergenti SGL showed 1–6 antigenic bands with molecular weight of 20–70 kDa. In the case of P. papatasi, all the tested sera strongly reacted to a 30 kDa protein, though, similar to our study, reaction to the SGL proteins of P. sergenti was individual-specific. Some individuals showed a strong reaction to SGL of P. sergenti and a weak reaction to the SGL of P. papatasi and vice versa [21]. In another study, immunoreactivity of indigenous people in a visceral leishmaniasis region with Lu. longipalpis’ SGL was evaluated. The authors reported six antigenic bands, with three bands showing more intensive reaction [43].
Apart from species-specificity and host-specificity, saliva contents or expression patterns of some transcripts of wild population of P. papatasi might be affected in certain biological or environmental condition [46,47].
5. Conclusion
In this study, we demonstrated that antibody response against P. sergenti salivary gland antigens are transient, and the level of antibodies in some individuals declines dramatically by the end of the vector activity. The immunogenicity of SGL components is individual-specific. However, some individuals shared a strong reaction against some individual proteins, which could be considered as potential candidate(s) for further studies as vector exposure markers.
Funding Statement
This work was supported by the Tehran University of Medical sciences [95-02-27-31419].
Ethics approval and consent to participate
The consent forms were filled out by all-volunteer participants and the experiments were approved by the Ethical Committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.VCR.REC.1395.253).
Disclosure statement
The authors declare that they have no conflicts of interest.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].WHO | Leishmaniasis . 2014. [cited 2019 December25]. https://www.who.int/leishmaniasis/en/
- [3].Martín-Martín I, Molina R, Rohoušová I, et al. High levels of anti-Phlebotomus perniciosus saliva antibodies in different vertebrate hosts from the re-emerging leishmaniosis focus in Madrid, Spain. Vet Parasitol. 2014;202(3–4):207–216. [DOI] [PubMed] [Google Scholar]
- [4].Drahota J, Martin-Martin I, Sumova P, et al. Recombinant antigens from phlebotomus perniciosus saliva as markers of canine exposure to visceral leishmaniases vector. PLoS Negl Trop Dis. 2014;8(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Doucoure S, Mouchet F, Cournil A, et al. Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: a new biomarker of exposure to dengue vector bites. Am J Trop Med Hyg. 2012;87(3):504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Oliveira F, de Carvalho AM, de Oliveira CI.. Sand-fly saliva-Leishmania-man: the trigger trio. Front Immunol. 2013;4:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abdeladhim M, Kamhawi S, Valenzuela JG.. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect Genet Evol. 2014;28:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Risueño J, Spitzová T, Bernal LJ, et al. Longitudinal monitoring of anti-saliva antibodies as markers of repellent efficacy against Phlebotomus perniciosus and Phlebotomus papatasi in dogs. Med Vet Entomol. 2019;33(1):99–109. [DOI] [PubMed] [Google Scholar]
- [9].Belkaid Y, Mendez S, Lira R, et al. A natural model of leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165(2):969–977. [DOI] [PubMed] [Google Scholar]
- [10].Rohoušová I, Volf P.. Sand fly saliva: effects on host immune response and Leishmania transmission. Folia Parasitol (Praha). 2006;53(3):161–171. [PubMed] [Google Scholar]
- [11].Marzouki S, Ben AM, Boussoffara T, et al. Characterization of the antibody response to the saliva of Phlebotomus papatasi in people living in endemic areas of cutaneous leishmaniasis. Am J Trop Med Hyg. 2011;84(5):653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rizzo C, Ronca R, Fiorentino G, et al. Humoral response to the Anopheles gambiae salivary protein gSG6: a serological indicator of exposure to Afrotropical malaria vectors. PLoS One. 2011;6(3):e17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Caljon G, Hussain S, Vermeiren L, et al. Description of a nanobody-based competitive immunoassay to detect tsetse fly exposure. PLoS Negl Trop Dis. 2015;9(2):e0003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dorňáková V, Salazar-Sanchez R, Borrini-Mayori K, et al. Characterization of guinea pig antibody responses to salivary proteins of Triatoma infestans for the development of a triatomine exposure marker. PLoS Negl Trop Dis. 2014;8(4):e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vlkova M, Rohousova I, Hostomska J, et al. Kinetics of antibody response in BALB/c and C57BL/6 mice bitten by Phlebotomus papatasi. PLoS Negl Trop Dis. 2012;6(7):e1719. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [16].Vlkova M, Rohousova I, Drahota J, et al. Canine antibody response to Phlebotomus perniciosus bites negatively correlates with the risk of Leishmania infantum transmission. PLoS Negl Trop Dis. 2011;5(10):e1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hostomska J, Rohousova I, Volfova V, et al. Kinetics of canine antibody response to saliva of the sand fly lutzomyia longipalpis. Vector-Borne Zoonotic Dis. 2008;8(4):443–450. [DOI] [PubMed] [Google Scholar]
- [18].Billingsley PF, Baird J, Mitchell JA, et al. Immune interactions between mosquitoes and their hosts. Parasite Immunol. 2006;28(4):143–153. [DOI] [PubMed] [Google Scholar]
- [19].Lestinova T, Rohousova I, Sima M, et al. Insights into the sand fly saliva: blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl Trop Dis. 2017;11(7):e0005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gomes RB, Brodskyn C, de Oliveira CI, et al. Seroconversion against lutzomyia longipalpis saliva concurrent with the development of anti–leishmania chagasi Delayed-Type Hypersensitivity. J Infect Dis. 2002;186(10):1530–1534. [DOI] [PubMed] [Google Scholar]
- [21].Rohousova I, Ozensoy S, Ozbel Y, et al. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology. 2005;130(5):493–499.. [DOI] [PubMed] [Google Scholar]
- [22].Kostalova T, Lestinova T, Sumova P, et al. Canine antibodies against salivary recombinant proteins of Phlebotomus perniciosus: a longitudinal study in an endemic focus of canine leishmaniasis. PLoS Negl Trop Dis. 2015;9(6):e0003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maroli M, Feliciangeli MD, Bichaud L, et al. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27(2):123–147. [DOI] [PubMed] [Google Scholar]
- [24].Martín-Martín I, Molina R, Jiménez M. Kinetics of anti-Phlebotomus perniciosus saliva antibodies in experimentally bitten mice and rabbits. PLoS One. 2015;10(11):e0140722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vinhas V, Andrade BB, Paes F, et al. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. Eur J Immunol. 2007;37(11):3111–3121. [DOI] [PubMed] [Google Scholar]
- [26].Clements MF, Gidwani K, Kumar R, et al. Measurement of recent exposure to Phlebotomus argentipes, the vector of indian visceral leishmaniasis, by using human antibody responses to sand fly saliva. Am J Trop Med Hyg. 2010;82(5):801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pouresmaeiliyan S, Sharifi I, Aflatoniyan MR, et al. A new focus of anthroponotic cutaneous leishmaniasis in Dehbakry region of Bam district, southeastern Iran 2008. J Kerman Univ Med Sci. 2010;16:15–24. [Google Scholar]
- [28].Gordon RM. Insects of Medical Importance. Adien press: British Museum, Natural History, London; 1956. DOI: 10.1136/bmj.2.5001.1103-b. [DOI] [Google Scholar]
- [29].Theodor O, Mesghali A. On the phlebotominae of Iran. J Med Entomol. 1964;1(3):285–300. [DOI] [PubMed] [Google Scholar]
- [30].Sayedi Rashti MA, Nadim A. The genus Phlebotomus (Diptera: psychodidae: phlebotominae) of the countries of the Eastern Mediterranean Region. Iran J Public Heal. 1992;21:11–50. [Google Scholar]
- [31].Killick-Kendrick M, Killick-Kendrick R. The initial establishment of sandfly colonies. Parassitologia. 1991;33(Suppl):315–320. [PubMed] [Google Scholar]
- [32].Modi GB, Tesh RB. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: psychodidae) in the laboratory. J Med Entomol. 1983;20(5):568–569. [DOI] [PubMed] [Google Scholar]
- [33].Veysi A, Yaghoobi-Ershadi MR, Rassi Y, et al. Rearing and biology of Phlebotomus sergenti, the main vector of anthroponotic cutaneous leishmaniasis in Iran. J Arthropod Borne Dis. 2017;11:504-514. [PMC free article] [PubMed] [Google Scholar]
- [34].Fatemi M, Yaghoobi-Ershadi MR, Mohebali M, et al. Assessing the ovarian accessory glands to determine the parity of Phlebotomus papatasi, vector of zoonotic cutaneous leishmaniasis, under laboratory condition. J Arthropod Borne Dis. 2017;11:161-165. [PMC free article] [PubMed] [Google Scholar]
- [35].Rohoušová I, Talmi-Frank D, Vlková M, et al. Serological evaluation of cutaneous Leishmania tropica infection in Northern Israel. Am J Trop Med Hyg. 2018;98(1):139–141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yaghoobi-Ershadi MR. Phlebotomine sand flies (Diptera: psychodidae) in Iran and their role on Leishmania transmission. J Arthropod Borne Dis. 2012;6(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- [37].Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AL, et al. Epidemiological study in a new focus of cutaneous leishmaniasis in the Islamic Republic of Iran. East Mediterr Heal J. 2003;9:816–826. [PubMed] [Google Scholar]
- [38].Aghasi M, Sharifi I. Survey of the Fauna and monthly activity of the sandfly as the vectors of the cutaneous Leishmaniasis in the city of Bam. J Kerman Univ Med Sci. 2003;10:85–91. [Google Scholar]
- [39].Aghaei Afshar A. Epidemiological chains of cutaneous leishmaniasis and impact/outcome indicators following residual spraying with deltamethrin in new foci of Dehbakri. Bam county, Kerman province, 2011;PhD Thesis:103. Tehran University of Medical Sciences. [Google Scholar]
- [40].Quinnell RJ, Soremekun S, Bates PA, et al. Antibody response to sand fly saliva is a marker of transmission intensity but not disease progression in dogs naturally infected with Leishmania infantum. Parasit Vectors. 2018;11(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Akhavan AA. Immune response of great gerbil against Phlebotomus papatasi saliva. Saarbrücken, Ger: L Lambert Acad Publ; 2011. [Google Scholar]
- [42].Velez R, Spitzova T, Domenech E, et al. Seasonal dynamics of canine antibody response to Phlebotomus perniciosus saliva in an endemic area of Leishmania infantum. Parasit Vectors. 2018;11(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Barral A, Honda E, Caldas A, et al. Human immune response to sand fly salivary gland antigens: A useful epidemiological marker? Am J Trop Med Hyg. 2000;62(6):740–745. [DOI] [PubMed] [Google Scholar]
- [44].Veysi A, Mahmoudi AR, Yaghoobi-Ershadi MR, et al. Salivary gland antigens of laboratory-bred Phlebotomus sergenti and their immunogenicity in human volunteers in laboratory condition. Asian Pac J Trop Med. 2020;13(1):17. [Google Scholar]
- [45].Rohousova I, Subrahmanyam S, Volfova V, et al. Salivary gland transcriptomes and proteomes of Phlebotomus tobbi and Phlebotomus sergenti, vectors of leishmaniasis. PLoS Negl Trop Dis. 2012;6(5):e1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Martín-Martín I, Molina R, Jiménez M. An insight into the Phlebotomus perniciosus saliva by a proteomic approach. Acta Trop. 2012;123(1):22–30. [DOI] [PubMed] [Google Scholar]
- [47].Volf P, Rohoušova I. Species-specific antigens in salivary glands of phlebotomine sandflies. Parasitology. 2001;122(1):37–41. [DOI] [PubMed] [Google Scholar]
- [48].Thiakaki M, Rohousova I, Volfova V, et al. Sand fly specificity of saliva-mediated protective immunity in Leishmania amazonensis-BALB/c mouse model. Microbes Infect. 2005;7(4):760–766. [DOI] [PubMed] [Google Scholar]
- [49].De Moura TR, Oliveira F, Novais FO, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis. 2007;1(2):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


