Abstract
In the fight against the outbreak of COVID-19 in China, we treated some asymptomatic infected individuals. This study aimed to detect pathogens in biological and environmental samples of these asymptomatic infected individuals and analyse their association. Using a cross-sectional study design, we collected biological and environmental samples from 19 patients treated in the isolation ward of Nanjing No.2 Hospital. Biological samples included saliva, pharyngeal swabs, blood, anal swabs, and exhaled breath condensate. Swab samples from the ward environment included inside masks, outside masks, palm swabs, bedside handrails, bedside tables, cell phone screens, toilet cell phone shelves, toilet pads and toilet lids. We also obtained some samples from public areas. We used RT-PCR to detect pathogens and colloidal gold to detect antibodies. As results, 19 asymptomatic infected individuals participated in the survey, with 8 positives for pathogens and 11 positives only for antibodies. Three positive samples were detected from among 96 environmental samples, respectively, from a cell phone surface, a cell phone shelf and a bedside handrail. No positive samples were detected in the exhaled breath condensate in this work. All patients identified pathogens in the environment had positive anal swabs. There was a statistical association between positive anal swabs and positive environmental samples. The association of positive samples from the surrounding of asymptomatically infected patients with positive anal swabs suggested that patients might secrete the virus for a more extended period.
Keywords: Asymptomatic infected individuals, Aerosols, Environmental samples, Cell phone screen, Exhaled breath condensate
Graphical abstract
1. Introduction
The Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) (Gorbalenya et al., 2020) emerged in China, and the associated disease was termed COVID-19 (Lai et al., 2020a). China has adopted extremely stringent quarantine measures, which have been proven to be effective in controlling the outbreak (Zhang et al., 2020). One study estimated that without these quarantine controls, the number of cases of COVID-19 would have increased 67-fold (Lai et al., 2020b). After more than three months of strict isolation, the lockdown was eased in Wuhan, Hubei Province. The population resumed free movement and regular activity. However, we found some asymptomatic infected individuals from Wuhan or in the quarantine population entering China. The SARS-CoV-2 infection has been reported to present as an asymptomatic carrier state (Lai et al., 2020a). However, we know very little about the characteristics of viral transmission in people with asymptomatic infections (Xu et al., 2020). This study is to examine the aspects of these asymptomatic infected individuals and the possible pathways by which they excrete the SARS-CoV-2 from bodies. The study will provide some reference information for similar problems that will be faced by countries outside China in the future.
According to the WHO, the SARS-CoV-2 is primarily transmitted from person to person through respiratory droplets and other exposure routes (Chan et al., 2020; Li et al., 2020a). In the analysis of 75,465 cases of COVID-19 in China, no airborne transmission was reported (WHO, 2020). However, a new study indicates that SARS-CoV-2 could exist in the air in poorly ventilated buses for at least 30 min (Luo and Hai, 2020). For COVID-19 pneumonia patients, researchers have detected the pathogen in their ward environment, especially the toilet environment (Ong et al., 2020). A study reported that SARS-CoV-2 was found in mask swabs (Li et al., 2020b) and the air (Guo et al., 2020). Viruses were widely distributed on floors, computer mouses, trash cans, and sickbed handrails and were detected in air ≈4 m from patients (Guo et al., 2020). The samples of sputum and faeces remained positive for SARS-CoV-2 according to RT-qPCR for up to 39 and 13 days, respectively, after the obtained pharyngeal samples tested negative (Chen et al., 2020). Mobile phones represented a pathway for microbial transmission (Olsen et al., 2020); however, there have been no reports of detection of SARS-CoV-2 on mobile phones.
The concentration of SARS-CoV-2 RNA in aerosols detected in isolation wards and ventilated patient rooms was remarkably low. Still, it was elevated in the patient toilet areas (Liu et al., 2020b), which indicated that SARS-CoV-2 might have the potential to be transmitted via aerosols. However, there is still no direct evidence of SARS-CoV-2 detection in exhaled breath. Exhaled breath condensate (EBC) is a condensed form of small droplets of the lung lining fluid that is usually emitted and contains a variety of components from small ions to proteins and organelles and can even contain viruses, fungi and bacteria (McDevitt et al., 2013). EBC is supposed to be a potential specimen for diagnosing COVID-19 (Khoubnasabjafari et al., 2020). If SARS-CoV-2 is detected in EBC, this could suggest that there is a strong possibility of aerosol transmission of the virus, but there have been no reports confirming this suppose.
The asymptomatic infected individuals in this study were either carriers of the virus or IgM antibody-positive individuals detected by screening who had no clinical symptoms such as cough or fever or only mild symptoms with no progression to pneumonia. For asymptomatic infections, the characteristics of the environmental distribution of SARS-CoV-2 virus in isolation wards have not been reported in the literature, and this study addresses this issue by conducting related research in the isolation ward. This study is of considerable significance to understand and protect the polluted environment around asymptomatic patients.
2. Materials and method
2.1. Patients and design
We conducted biological and environmental sampling for all asymptomatic COVID-19 patients admitted to Nanjing No. 2 Hospital on April 13 and April 17, 2020, respectively. A total of 19 asymptomatic infected patients in the isolation ward participated in this study. Biological samples included pharyngeal swabs, anal swabs, serum and EBC. Environmental samples come from the isolation wards, ward bathrooms and the public areas of the hospital. The specimen in the patient rooms were from the bedside table, the bedside handrails, the mobile phone screen, the palm of the patient's hand, the double sides of the mask, and the fog mask. The toilet collection sites were the cell phone shelves and toilet pads. Samples in the public areas were from a trash can lid (inside) and the wall behind it, as well as the nurses' station floor. This study was a part of the Jiangsu CDC epidemiological studies of the COVID-19 outbreak; the ethics committee of Jiangsu CDC approved this study.
2.2. Biological and environmental sample collection
During the period of admission, under the condition of strictly protection, pharyngeal swabs, blood and anal swabs of patients were collected one day before environmental sample collection from the isolation ward for COVID-19 nucleic acid detection. Saliva samples of 2–3 ml were from 10 hospitalized patients who were IgM positivity. After rinsing the patient's mouth, the patient spits into a sterile wide-mouth plastic container with a lid.
We used a swabbing method to collect the virus on the surface of the object with a sampling area of 5 × 5 cm. Patients wear a mask for at least 1 h and cough vigorously for 10 times before sampling. After flattening the surface of the masks, we dipped a cotton swab into the virus preservation solution, which was repeatedly applied to the mask surface; the handle of the swab was broken, and the sampling end of the swab was placed into the virus sampling tube, which was kept at a low temperature in an insulated refrigerator. Environmental samples were transferred to the pathogenic microbiology laboratory of the CDC of Jiangsu Province for testing, and biological samples were examined by the Laboratory of the No.2 Hospital of Nanjing City.
2.3. EBC collection
We used equipment from Beijing Dinglan Technology for sample collection. After the cooling module cooled to sub-zero temperatures, the patient inhaled through the nose and exhaled deeply through a 20 cm-long 2.5 cm-thick diameter straw into the condensing module. After cooling, the EBC accumulated in a 2 ml freezer tube. The sampling time was 10 min. The volume of EBC collected was approximately 1–1.5 ml.
2.4. Sample testing
The detection method included nucleic acid extraction (NP968, Tianlong Science & Technology, Xi-An, China) and real-time quantitative RT-PCR amplification (ABI QuantStudio Dx). The virus detection kit was from Shanghai Wuseshi Medical Research Co. Ltd. The RT-PCR amplification system was prepared according to the instructions of the detection kit, and RT-PCR was performed in duplicate for each sample. We tested all samples for the open reading frame (ORF) 1ab and nucleoprotein (N) genes of SARS-CoV-2 by quantitative real-time PCR. We used the colloidal gold method to detect antibodies (IgM, IgG) in serum (INNOVITA, Beijing, China). All samples tested in duplicate, and positive samples were retested at least once.
2.5. Statistics
We analysed the data with R software (Version 3.6.2, R Core Team, Vienna, Austria.). Positivity rates were compared using Fisher's exact probability calculation method.
3. Results
3.1. Patient characteristics and biological sample results
As shown in Table 1 , we sampled 19 patients with asymptomatic SARS-CoV-2 infection. Pathogens were positive in 8 patients. Among them, all patients were pharyngeal swabs positive, and another 4 were anal swabs positive at the same time. Antibodies were positive in the other 11 patients. Of the 11 patients admitted for IgM positivity (no pathogen positivity), 5 were also IgG-positive.
Table 1.
Characteristics of patients and results of sample testing.
| Items | N |
|---|---|
| Patients | 19 |
| Age (mean (min–max)) | 35 (13–58) |
| Gender (M/F) | 12/7 |
| Days in the hospital before sampling (days) | 6.4 ± 5.8 |
| Environmental samples (+) | 17 ± 1.7 |
| Environmental samples (−) | 4.4 ± 3.7 |
| Biological samples | |
| Nucleic acid positive (positive/N) | 8/19 |
| Only pharyngeal swab (+) | 4 |
| Only anal swab (+) | 0 |
| Both (+) | 4 |
| Antibody positive (+) (positive/N) | 11/19 |
| Only IgM (+) | 5 |
| Only IgG (+) | 1 |
| Positive IgM + IgG | 5 |
| EBC sample (positive/N) | 0/19 |
| Saliva (positive/N) | 0/10 |
| Environmental samples (positive/N) | |
| Hand palm | 0/9 |
| Mobile phone screen | 1/9 |
| Mobile phone shelf at toilet | 1/9 |
| Bedside handrail | 1/9 |
| Mask (inside) | 0/19 |
| Mask (outside) | 0/9 |
| Bedside table (top) | 0/9 |
| Toilet lid and pad | 0/18 |
| Public area samples (postive/N) | |
| Trash can lid (inside) | 0/2 |
| Walls behind the trash can | 0/2 |
| Floors of nurse's station | 0/2 |
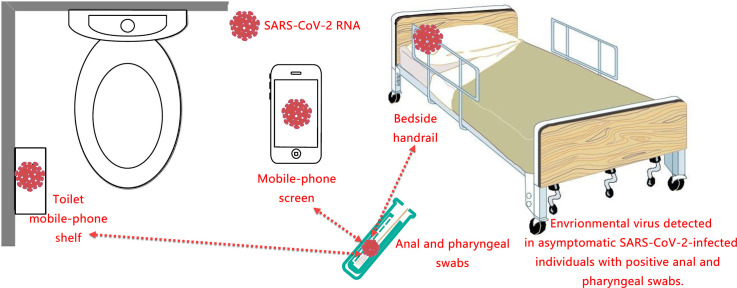
We identified 3 positive environmental samples (nucleoprotein gene positivity only), and two of them were associated with cell phones. One swab collected from cell phone screen was nucleic acid positive (RT-PCR, Ct value = 35), and the other sample was from a toilet cell phone shelf (RT-PCR, Ct value = 37). We also got a positive swab from the right side of the bedside handrail of a patient (RT-PCR, Ct value = 36). Palm swab samples were obtained only from patients who were positive for the pathogen and showed no positive. All other samples from masks, toilets or the working areas of the medical staff were negative.
3.2. EBC-related sample results
Saliva samples were from 10 patients who were IgM-positive, and none was positive. Each EBC sample was higher than 1 ml in volume; however, we did not find any EBC-positive examples among the 19 samples. Ten swab samples from the mask covering the outlet of the condensate collection device were also negative.
3.3. Factors associated with environmental sample positivity
Of all 96 environmental samples, a total of 3 samples with nucleic acid RNA were detected, all from patients with positive anal and pharyngeal swabs. Only one patient with a positive anal swab (positive pharyngeal swabs) did not have a SARS-CoV-2 -positive environmental sample. For patients who were IgM-positive only, no positive surroundings samples were detected. As shown in Table 2 , χ2 tests (Fisher's Exact Tests) showed that patients with positive anal swabs had a significantly higher chance of being associated with a SARS-CoV-2-positive environmental sample. Besides, patients with positive surroundings samples had been in the hospital for an average of 17 ± 1.7 days before sampling, which was significantly higher than other patients without positive environmental samples (4.4 ± 3.7, P < 0.001).
Table 2.
Associations between virus detection in environmental and biological samples.
| Environmental samplea (+) | Environmental sample (−) | P value (Fisher's Exact Test) |
|
|---|---|---|---|
| Anal swabb (+) | 3 | 1 | 0.004⁎ |
| Anal swab (−) | 0 | 15 | |
| Pharyngeal swab (+) | 3 | 5 | 0.06 |
| Pharyngeal swab (−) | 0 | 11 |
P < 0.01.
Samples from the ward environment were obtained from inside masks, outside masks, palm swabs, bedside handrails, bedside tables, cell phone screens, toilet cell phone shelves, toilet pads and toilet lids.
Both biological and environmental samples were collected on the same day.
4. Discussion
A previous study detected the SARS-CoV-2 on the surfaces of bedside handrails in intensive care units (6/14, 42.9%) at Huoshenshan Hospital in Wuhan, China. They reported that the rate of positivity was relatively high for the surfaces of objects that were frequently touched by patients or medical staff (Guo et al., 2020). Our results showed that the SARS-CoV-2 was on the handrails of beds of asymptomatically infected patients in the general ward, which indicated that asymptomatically infected patients, like symptomatic patients, could excrete the virus.
A 2016 report stated that users touched their mobile phones on average for 3 h and up to 2617 times per day (Olsen et al., 2020). In fact, for our young patients in the isolation ward, loneliness and anxiety kept them glued to their mobile phones, with WeChat voice calls and touch screens being a staple of their ward life. Therefore, it is not at all shocking to detect the pathogen on the phone screen. The present study reported the first detection of the SARS-CoV-2 virus on the screen of a cell phone of an asymptomatic infected individual. This result also confirmed the concerns of Olsen et al. (2020) about the spread of viruses from mobile phones. We were already aware of the possibility of cell phones in the transmission of SARS-Cov-2, and disinfection of cell phone screens has been a daily practice of our patients. The probable reason for detecting only one positive sample was that some patients had just finished cleaning their phone screen shortly before sampling. The SARS-CoV-2 nucleic acid also polluted another patient's toilet phone shelf. The virus on the shelf could have come from a cell phone, or it could have come from faeces, as the patient's throat swab and anal swab were both positive.
EBC was suggested as a potential specimen type for diagnosing COVID-19 (Khoubnasabjafari et al., 2020). Since the virus spreads via respiratory droplets, the success rate of the use of bronchoalveolar lavage fluid (BLF) for SARS-CoV-2 detection was better than that of sputum, nasal and pharyngeal swabs (Liu et al., 2020a; Wang et al., 2020; Zhu et al., 2020). EBC was considered a more appropriate specimen type for monitoring the virus using RT-PCR due to its similarities with BLF. However, no studies reported the detection of SARS-CoV-2 in the EBC. In this study, the EBC specimens from the 19 patients were all negative, while 8 of these patients had positive pharyngeal or anal swabs. These results at least suggested that EBC was not a particularly sensitive specimen type for testing for the SARS-CoV-2. Also, for asymptomatic infected individuals in this study, there was no evidence to support the transmission of the SARS-CoV-2 virus via exhaled aerosols.
Positive IgM antibody against the SARS-CoV-2 is considered a biomarker of early infection and is, therefore, one of the current criteria for quarantine treatment in China. The testing for 11 IgM-positive asymptomatically infected individuals in this study did not reveal any positive results from the biological or environmental samples, and these confined individuals were discharged from the hospital after their IgM results became negative. This result suggested that these individuals with IgM positivity (no pathogen positivity), with or without IgG positivity, might not excrete the virus.
The most exciting result of this study was that the detection of positive environmental samples was associated with positive anal swabs. This result strongly suggested the role of faeces in the environmental transmission of the virus. A previous study has isolated live viruses in the faeces of confirmed COVID-19 patients (WHO, 2020). The positive anal swab result usually indicates that the individual has been infected over a long period, an average of 17 days in this study (Table 1). Such a finding suggested that a patient could excrete the virus for a longer time. It was noteworthy that these anal swab-positive individuals also had positive pharyngeal swabs. Therefore, environmental SARS-CoV-2 might also come from the respiratory tract.
This study provided a preliminary characterization of the environmental excretion of SARS-CoV-2 by asymptomatic individuals. The strength of this study was that we measured both the biological and environmental samples from asymptomatic SARS-CoV-2-infected individuals. Besides, this study performed a series of tests associated with EBC specimens, including saliva, EBC and breath mask swabbing. The main limitation was that we used a cross-sectional survey design in which the course of their disease influenced the results of each subject. Other restrictions included the small sample size, and that virus was not quantified and isolated.
In summary, the present study, by examining the excretion pathways of viruses in 19 asymptomatically infected COVID-19 patients, found the presence of viruses on a patients' cell phone, bedside handrail and toilet cell phone shelf, suggesting that an asymptomatically infected individual can transmit the virus in the environment. We did not find viruses in EBC, preliminarily suggesting that exhaled aerosol transmission might not be the main route of infection by asymptomatic individuals. None of the IgM-positive patients had a positive nucleic acid result, implying that IgM-positive individuals at a later stage of the epidemic were less likely to secrete the virus. Therefore, there is no need for the public and the media to panic for the discovery of individual asymptomatic infections. The statistical association between positive anal swabs and environmental samples also suggested that anal swabs might be an essential indicator of a patient's ability to transmit the virus. Therefore, frequent hand washing is still a meaningful way to prevent and control the spread of infection.
CRediT authorship contribution statement
Ying Huang: Conceptualization, Investigation, Resources, Writing - original draft. Zhen Ding: Methodology, Investigation, Writing - original draft, Funding acquisition. Qi Chen: Investigation. Ling Wu: Investigation, Methodology, Resources. Lili Guo: Investigation, Resources. Chunhua Zhao: Resources. Li Sha: Investigation, Resources. Hong Sun: Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Cui lunbiao and his fellows in the lab of Jiangsu CDC for their support in virus detection. This study was supported by the Jiangsu Provincial Commission of Health and Family Planning (CXTDB2017012, QNRC2016551), the National Natural Science Foundation of China (NSFC91743205), and the Jiangsu Social Development Project (BE2018745).
Editor: Jianmin Chen
References
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Gao G., Xu Y., Pu L., Wang Q., Wang L., et al. SARS-CoV-2–positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann. Intern. Med. 2020;172:832–834. doi: 10.7326/M20-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.D., Zhong-Yi W., Shou-Feng Z., Xiao L., Lin L., Chao L., et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. J. 2020;26 doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoubnasabjafari M., Jouyban-Gharamaleki V., Ghanbari R., Jouyban A. Exhaled breath condensate as a potential specimen for diagnosing COVID-19. Bioanalysis. 2020 doi: 10.4155/bio-2020-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J. Microbiol. Immunol. Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S., Ruktanonchai N.W., Zhou L., Prosper O., Luo W., Floyd J.R., et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020 doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.H., Fan Y.Z., Jiang L., Wang H.B. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol. Infect. 2020;148:e154. doi: 10.1017/S0950268820001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Han H., Liu F., Lv Z., Wu K., Liu Y., et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Luo K.W., Hai Z. A COVID-19 patient travelled in buses infected 13 people. ShiyongYufangYixue. 2020;3 [Google Scholar]
- McDevitt J.J., Koutrakis P., Ferguson S.T., Wolfson J.M., Fabian M.P., Martins M., et al. Development and performance evaluation of an exhaled-breath bioaerosol collector for influenza virus. Aerosol Sci. Technol. 2013;47:444–451. doi: 10.1080/02786826.2012.762973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M., Campos M., Lohning A., Jones P., Legget J., Bannach-Brown A., et al. Mobile phones represent a pathway for microbial transmission: a scoping review. Travel Med. Infect. Dis. 2020:101704. doi: 10.1016/j.tmaid.2020.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. (March 27, 2020) [Google Scholar]
- Xu T., Huang R., Zhu L., Wang J., Cheng J., Zhang B., et al. Epidemiological and clinical features of asymptomatic patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92:1884–1889. doi: 10.1002/jmv.25944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Litvinova M, Liang Y, Wang Y, Wang W, Zhao S, et al.. Changes in Contact Patterns Shape the Dynamics of the COVID-19 Outbreak in China. 2020. eabb8001. [DOI] [PMC free article] [PubMed]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]