Graphical abstract
Abstract
The coronavirus disease 2019 (COVID-19) is a public health emergency of international concern. The rising number of cases of this highly transmissible infection has stressed the urgent need to find a potent drug. Although repurposing of known drugs currently provides an accelerated route to approval, there is no satisfactory treatment. Polyphenols, a major class of bioactive compounds in nature, are known for their antiviral activity and pleiotropic effects. The aim of this review is to assess the effects of polyphenols on COVID-19 drug targets as well as to provide a perspective on the possibility to use polyphenols in the development of natural approaches against this viral disease.
Current Opinion in Food Science 2020, 32:149–155
This review comes from a themed issue on Functional foods and nutrition
Edited by Andreas Schieber
For a complete overview see the Issue and the Editorial
Available online 9th September 2020
https://doi.org/10.1016/j.cofs.2020.08.004
2214-7993/© 2020 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
The worldwide outbreak of highly transmissible fatal pneumonia referred to as Coronavirus Disease-2019 (COVID-19) is caused by a zoonotic pathogenic virus called Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). SARS-CoV-2 and SARS-CoV belong to the β-coronaviruses lineage B and are similar to the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) that had emerged worldwide in 2002 and 2012 [1]. Coronaviruses are enveloped, non-segmented, positive‐sense single‐stranded RNA viruses whose genomes range from 26 to 32 kilobases, the largest known viral RNA genome [2•]. Located at the 5’ end of the SARS-CoV-2 genome, open reading frames ORF1a and ORF1b encode for polyproteins that are consequently processed by proteolytic cleavage into non-structural proteins such as RNA-dependent RNA polymerase (RdRp), papain-like protease (PLpro) and 3-chymotrypsin-like protease (3CLpro) [3,4]. ORFs located at the 3’ end of the viral genome encodes for structural proteins including spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins [3]. The viral surface proteins (S, E and M) are embedded in a lipid bilayer, while the nucleocapsid protein coats the single-stranded positive-sense viral RNA [4]. SARS-CoV-2 utilizes the extensively glycosylated S protein that protrudes from the viral surface to bind to angiotensin-converting enzyme 2 (ACE2) and mediate host-cell entry [5]. After binding the host-cell receptor, host proteases such as the serine protease TMPRSS2 cleave the viral S protein to release the spike fusion peptide [4,5,6•].
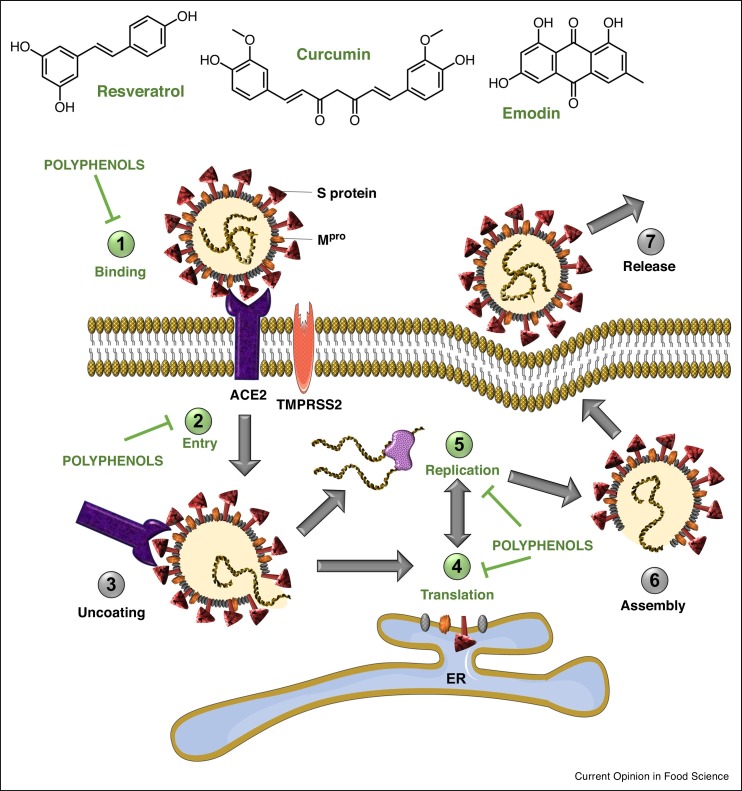
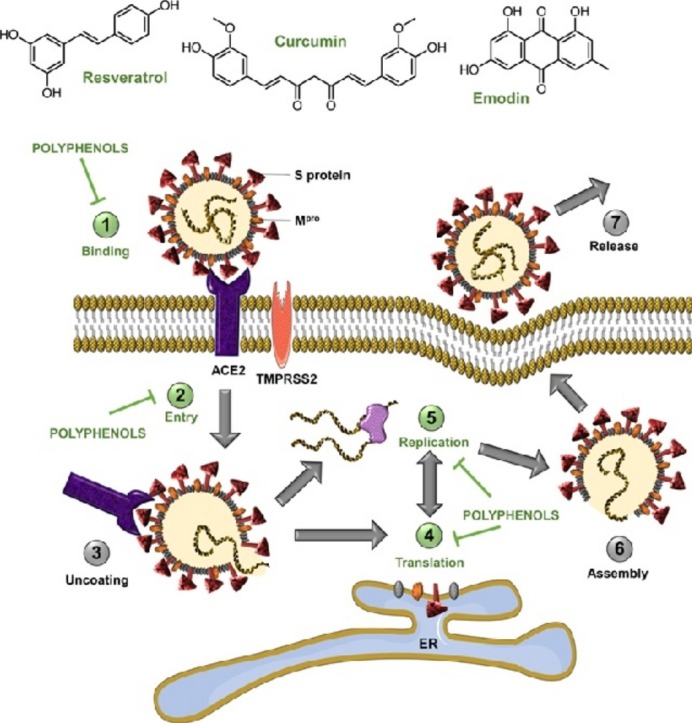
Therapies against coronavirus can be categorized into two groups: drugs targeting the virus and drugs acting on human cells or the immune system. The key SARS-CoV-2 targets comprise three non-structural proteins (3CLpro, PLpro and RdRp) and a structural protein (S protein), which are responsible for replication, transcription and host cell recognition [7]. However, therapies such as vaccines and monoclonal antibodies may lose their efficiency if the virus mutates and changes its antigenicity. Therefore, drugs targeting host-cell viral receptors (ACE2) and improving the immune response have strong potential. Polyphenols have a broad antiviral activity against a diverse group of viruses such as influenza A virus (H1N1), hepatitis B and C viruses (HBV/HCV), herpes simplex virus 1 (HSV-1), human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) [8]. The present mini-review aims to report in silico and in vitro evidence of the potential of polyphenols as anti-SARS-CoV-2 agents. Putative mechanisms of action by which these natural compounds exert their potential activity against SARS-CoV-2 are presented in Figure 1 . We also summarize research approaches that may accelerate the discovery of anti-SARS-CoV-2 polyphenols. We have reviewed literature spanning from 2000 to 2020 and 53% of the cited references were published in the past two years.
Figure 1.
Effect of polyphenols on different steps of the SARS-CoV-2 life cycle. Polyphenols potentially inhibit binding of SARS-CoV-2 spike protein to host-cell receptor ACE2, prevent viral entry into the host cell, and inhibit viral RNA replication and protein processing.
Polyphenols inhibiting SARS-CoV-2 fusion/entry
Polyphenols binding to S protein
The S protein is a large membrane glycoprotein that belongs to a group of class I viral fusion glycoproteins that also includes HIV glycoprotein 160 (Env), influenza haemagglutinin (HA) and Ebola virus glycoprotein [9]. The peripheral amino (S1) subunit can independently bind cellular receptors while the carboxy (S2) terminus is embedded into the viral envelope and is required to mediate fusion of viral and cellular membranes [10]. In coronaviruses, the S protein is the sole viral membrane protein responsible for cell entry. It defines viral tropism by its receptor specificity and membrane fusion activity during virus entry into cells [11]. Drugs targeting SARS-CoV-2 spike protein impede spike-mediated membrane fusion and prevent virus entry into the host cells. These therapeutic agents include vaccines, antibodies, small interfering RNAs, peptides, and non-peptidic small molecules such as polyphenols [9].
Luteolin and quercetin inhibited SARS-CoV infection by preventing virus entry into Vero E6 cells with EC50 values of 10 μM and 83 μM, respectively [12•]. In the same study, luteolin was found to bind with high affinity to SARS-CoV S protein, suggesting an antiviral mechanism of action involving interference with the function of the S protein. A literature-based discovery approach [13] revealed that emodin, an anthraquinone-type polyphenol found in rhubarb roots (Rheum officinale) interfered with the S protein-ACE2 interaction in a cell-free competition assay with an IC50 of 200 μM [14]. The same study also revealed that emodin reduced the infection of Vero E6 cells expressing ACE2 by an S protein-pseudo typed retrovirus. Although the mechanism of action is still unclear, these results suggest competition at the S protein receptor binding domain (RBD). Following the findings of a host-virus interactome network analysis of various viruses including SARS-CoV [15••], emodin has emerged as one of 16 most repurposable agents for COVID-19 with least expected adverse effects and highest target specificity [16].
Molecular docking and dynamic simulation studies predict polyphenols from plants such as Citrus and Curcuma species to have a potential inhibitory effect on SARS-CoV-2 infection by interacting with the S protein RBD. A study has shown stronger interactions of polyphenols from Curcuma spp. (curcumin and derivatives) and Citrus spp. (tangeretin, hesperetin, hesperidin) to the S protein (PDB: 6LXT) than nafamostat [8], the reference antiviral [17]. Hesperidin was predicted to target the binding interface between S protein and ACE2 by positioning on the middle shallow pit of the surface of the S protein RBD [18•]. Naringenin, found in a variety of herbs and fruits, had a stronger binding energy with the spike glycoprotein (PDB: 6VSB) than remdesivir [19], an antiviral temporarily approved by the FDA in the treatment of COVID-19 [20]. Epigallocatechin gallate, abundant in tea, as well as herbacetin from Rhodiola spp. (golden root) and other flavonoids also interacted strongly with S protein RBD (PDB: 6VXX) in silico [21].
Polyphenols targeting ACE2
ACE2 is a type I transmembrane metallocarboxypeptidase found in many tissues such as the lungs, heart, blood vessels, kidneys, liver and epithelial cells [22]. ACE2 is a pivotal enzyme in the physiological renin-angiotensin system, as it hydrolyzes vasoconstricting angiotensin II to generate vasodilating angiotensin (1-7) [23]. Being SARS-CoV-2’s point of entry into the host cells, ACE2 has gained attention as a potential drug target. Screening for ligands of ACE2 with a binding affinity strong enough to inhibit virus entry has unveiled polyphenols as promising candidates. A molecular docking study using a computational model of the SARS-CoV-2 spike protein interacting with human ACE2 receptor found that eriodictyol, a flavanone found in yerba santa (Eriodictyon californicum) had one of the greatest binding affinity for the human ACE2 receptor portion of the interface amongst 77 candidates [24]. Another computational study showed that flavonoids curcumin and catechin establish hydrogen bonds, carbon-hydrogen bonds and π–σ interactions with ACE2, resulting in binding affinities of −7.8 kcal/mol and −8.9 kcal/mol respectively [25]. Although in silico experiments predict promising results, more in vitro and in vivo studies are needed to evaluate whether polyphenols binding to ACE2 impacts viral entry.
Growing evidence suggests that controlling ACE2 expression might help modulate COVID-19 symptoms. In fact, SARS-CoV infection was found to downregulate ACE2 receptor [26]. Moreover, mice with inactivated or knocked-out ACE2 developed more severe acute lung injury following SARS-CoV infection than wild-type mice and these symptoms were reversed after administration of recombinant ACE2 [27•]. Similarly, cell-based assays have shown that SARS-CoV and SARS-CoV-2 viral entry and infection were blocked by soluble forms of ACE2 [22,28], indicating that recombinant ACE2 might act as a decoy receptor for the S protein. The soluble recombinant human ACE2, APN01 developed by the Austrian biotech company Apeiron Biologics [29] is currently undergoing phase II clinical trials for the treatment of COVID-19.
It was recently suggested that dietary intake of resveratrol, a polyphenol found at high concentrations in the skin of red wine grapes (Vitis vinifera) could modulate SARS-CoV-2 disease severity by regulating ACE2 expression and function [30•]. Rodents fed a high-fat diet supplemented with resveratrol have shown upregulated ACE2 expression [31] and increased ACE2 protein levels [32,33] compared to rodents fed a high-fat diet alone. Several publications have also reported that curcumin targeted the renin-angiotensin system by regulating angiotensin II levels in mice [34,35]. These results suggest a potential for polyphenols to modulate the severity of COVID-19 symptoms through modulation of ACE2 abundance.
Therefore, polyphenols might (i) reduce SARS-CoV-2 viral infection by binding to the ACE2 receptor, preventing the viral entry, and (ii) modulate the severity of lung injury associated with COVID-19 by regulating ACE2 expression. However, it is important to note that, given ACE2 pivotal role in physiopathological processes, targeting the enzyme still needs careful evaluation to ensure the benefit-risk balance is favorable.
Polyphenols disrupting SARS-CoV-2 replication
Polyphenols inhibiting SARS-CoV-2 viral proteases
Protease inhibitors have been developed to stop the spread of viruses that cause diseases such as HIV-AIDS, MERS, and SARS [36,37]. Thus, drugs inhibiting viral proteases are also suggested to be the good candidates to hinder SARS-CoV-2’s life cycle. Replication of coronaviruses requires correct proteolytic processing of the replicase polyproteins by viral proteases leading to the release of non-structural and structural proteins [38,39]. SARS-CoV-2 polyproteins are processed by a main protease, 3CLpro (also known as Mpro), and by papain-like proteases, PLpro [39]. These proteases are involved in the replication and transcription of the SARS-CoV-2, especially 3CLpro, which plays a vital role in polyprotein processing and virus maturation [4,7]. Hence, 3CLpro is one of SARS-CoV-2 best characterized drug targets, and studies have shown that development of antiviral agents targeting 3CLpro could provide an effective first line of defense against coronaviruses infections [39, 40, 41, 42]. Natural compounds inhibitors of SARS-CoV proteases include diarylheptanoids [43,44•], terpenoids [7,45], cinnamic amides [46], flavonoids [47, 48, 49, 50] and coumarins [47].
Inhibition of 3CLpro was shown in silico and in vitro with epigallocatechin gallate (IC50 = 73 μM), gallocatechin gallate (IC50 = 47 μM) and quercetin (IC50 = 73 μM) [50,51]. Structure-activity relationship analysis of seven polyphenols revealed that flavonoids and isoflavonoids lacking an OH group at 5’-position of the B ring decreased 3CLpro inhibitory activity [50]. Screening by molecular docking of 33 molecules including natural products, antivirals, antifungals and antiprotozoal agents revealed that rutin (a citrus flavonoid) could bind to the active site of the SARS-CoV-2 3CLpro (PDB: 6Y84) with the highest affinity among the molecules screened [44•]. Other citrus flavonoids such as tangeretin and naringenin and polyphenols from Curcuma spp. were also reported to bind strongly to SARS-CoV-2 3CLpro substrate binding domain, while interacting with the S protein and ACE2 in silico, predicting stronger antiviral potential of these polyphenols compared to lopinavir and nafamostat [8].
Several polyphenols were also found to have a synergistic effect on 3CLpro and PLpro. In cell-free and cell-based assays, chalcones isolated from Angelica keiskei exhibited competitive inhibition of the SARS-CoV serine protease 3CLpro, whereas noncompetitive inhibition was observed with the SARS-CoV cysteine protease PLpro [47]. Dietary flavonoids such as kaempferol and isoliquiritigenin, as well as polyphenols from Broussonetia papyrifera also synergistically inhibited 3CLpro and PLpro in vitro [49].
Polyphenols inhibiting SARS-CoV-2 RdRp
SARS-CoV-2 RdRp is a key target in the development of therapies against COVID-19. One of the antivirals temporarily approved by the FDA for the treatment of COVID-19, remdesivir is an analogue of adenosine and acts as a false substrate for RdRp [20,52]. Remdesivir terminates RNA synthesis once it gets incorporated into the viral RNA at position I, successfully inhibiting RdRp [52].
Potential inhibition of SARS-CoV-2 RdRp by polyphenols emerged from evidence that resveratrol significantly inhibited MERS-CoV replication in vitro by inhibition of RNA expression and nucleocapsid protein expression [53•]. Such evidence suggests that resveratrol may also be effective against SARS-CoV-2 infection [54]. However, resveratrol has limited bioavailability; thus, nanoparticle formulations and intranasal administration have been proposed to improve its efficacy in the treatment of COVID-19 [55]. Fenoterol, a polyphenolic β2-adrenergic receptor agonist, as well as the naturally occurring flavone, baicalin from Scutellaria baicalensis and several xanthones from Swerti apseudochinensis were identified as potential SARS-CoV-2 RdRp inhibitors by computational methods [18•]. Another in silico study recently reported that epigallocatechin gallate, myricetin, quercetagetin and other polyphenols exhibited high binding affinity towards the RdRp of both SARS-CoV and SARS-CoV-2 [56].
Polyphenols suppressing the host inflammatory response
The host response to SARS-CoV-2 ranges from minimal to severe respiratory failure with multiple organ failure [16]. In some patients, SARS-CoV-2 induces excessive non-effective host immune responses, referred to as the cytokine storm, that are associated with severe lung pathology, leading to death. The cytokine storm is characterized by increased plasma concentrations of interleukins, granulocyte-colony stimulating factor, interferon-γ-inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, and tumor necrosis factor α [57]. The effects of cytokine storm have been attributed to IL-6 cytokine [16] and viral activation of the NLRP3 inflammasome, which causes increased production of inflammatory cytokines [54]. Although several IL-6 inhibitors (e.g. sarilumab, siltuximab and tocilizumab) are in various stages of COVID-19 clinical testing, in the absence of sufficient clinical data, the National Institutes of Health (NIH) does not presently support a recommendation for or against the use of IL-6 inhibitors for the treatment of COVID-19 [58].
Developing effective regulators of the immune response would inhibit the cytokine-driven hyperinflammatory syndrome for the management of COVID-19. As such, indomethacin has been proposed as an adjunct to COVID-19 antiviral therapy, since it combines anti-inflammatory properties and antiviral activity against SARS-CoV-2 [55]. Similarly, polyphenols, whose immunomodulatory properties are well documented [59,60,61••], could have a beneficial effect against SARS-CoV-2-induced cytokine storm. A non-extensive list of polyphenols that reduced pro-inflammatory cytokines in vitro and in vivo includes curcumin, resveratrol, epigallocatechin gallate, emodin, naringenin, apigenin and kaempferol [61••,62•]. In our own research, oral treatment of high-fat fed mice with the hop flavonoid xanthohumol lowered plasma IL-6 levels by about 80% compared to control mice [63].
Systematic exploration of polyphenols as antiviral agents
Our review of the literature demonstrates that polyphenols have been investigated for their potential against SARS-CoV viruses in molecular modeling studies, cell-free polyphenol–protein interaction studies, and in cell-based virus infection studies. Convincing evidence suggests polyphenols such as epigallocatechin gallate, resveratrol and curcumin are prime candidates for preclinical and clinical studies. As a note of caution, in silico and in vitro approaches used for screening do not validate the efficacy of the tested polyphenols against the human viral disease. A potential modulation of COVID-19 severity by polyphenols regulating ACE2 in vivo has been suggested [30•,64] but there are very few studies investigating the antiviral effect of polyphenols against SARS-CoV-2 in vivo. Pudilan Xiaoyan Oral Liquid (PDL), a traditional Chinese medicine containing four herbs and more than 180 ingredients exhibited potent anti-SARS-CoV-2 activity in infected hACE2 mice [65•]. It was also reported that a nebulized formula of quercetin and N-acetylcysteine greatly alleviated SARS-CoV-2 respiratory symptoms in a patient treated with hydroxychloroquine and antibiotics [66•]. This confirms the importance of further clinical studies to evaluate the potential of polyphenol-based nutraceuticals as adjuvant or main therapy for COVID-19.
High-throughput screening approaches can accelerate the in vitro discovery of lead candidates, the limitation being the availability of polyphenol libraries. Screening of polyphenol-rich plant extracts is an alternative, widely used approach, but it has the disadvantage that extracts contain a multitude of natural products with inherent problems of not being able to readily identify active principles and the potential for pharmacological antagonism. These problems can be overcome by combining classical bioassay-guided fractionation with machine learning approaches to reveal the identity of bioactive natural products in extracts without the need for purification to homogeneity. In our opinion, the latter combination approach holds promise to accelerate discovery because many antiviral in vitro assays can be performed without handling live viruses and because identification of polyphenols (and other natural products) has become easier over the past several years thanks to advances in plant metabolomics and the ever growing natural product databases such as Phenol-Explorer [67], KnapSack [68], and the Global Natural Product Social Networking (GNPS) database [69].
Conclusions and perspectives
COVID-19 is a new disease with significant morbidity and mortality for which there is no satisfactory treatment available as of August 2020. The foregoing review of the literature demonstrates that polyphenols have not yet been widely considered and systematically investigated for potential antiviral effects against SARS-CoV-2. This area of research is at the proverbial infancy stage and certainly has the potential to deliver valuable antiviral therapeutics or anti-inflammatory agents in reducing SARS-CoV-2 morbidity and mortality. Many naturally occurring polyphenols are inexpensive to produce and have low risk for development of toxicity, making these compounds good candidates for preventive treatment to decrease viral infectivity and to dampen the risk of a virus-induced inflammatory storm. At the molecular level, polyphenols hold promise as inhibitors of viral proteases involved in viral replication due to their general affinity to proteins via hydrogen bonding and their low risk of toxic effects. The same may hold true for binding of polyphenols to S protein, although pre-clinical and clinical studies are required to strengthen existing evidence. Another point that should be taken into account is the proper formulation for these polyphenol-based nutraceuticals. To counteract low bioavailability concerns and increase concentrations of active polyphenols in the respiratory tract, the primary site of infection, using aerosol delivery systems, such as nebulizers and inhalers should be considered [66•,70].
Conflicts of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This study was supported by National Institutes of Health grant # R01AT010271, the Linus Pauling Institute, and the Oregon State University College of Pharmacy.
References
- 1.Bermingham A., Chand M., Brown C., Aarons E., Tong C., Langrish C., Hoschler K., Brown K., Galiano M., Myers R. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 2•.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides an update on coronaviruses infections, highlights the importance of immune responses during infection and improve the understanding of the coronavirus-induced inflammatory response.
- 3.Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19:100682. doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol. 2020:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that SARS-CoV-2 uses ACE2 as receptor for host-cell entry and the S protein needs the serine protease TMPRSS2 for priming.
- 7.Murugan N.A., Pandian C.J., Jeyakanthan J. Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials. J Biomol. 2020:1–12. doi: 10.1080/07391102.2020.1777901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utomo R.Y., Meiyanto E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints. 2020 doi: 10.20944/preprints202003.0214.v1. 2020030214. [DOI] [Google Scholar]
- 9.Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV — a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified small molecules that bind to SARS S protein using a two-step screening method combining frontal affinity chromatography-mass spectrometry (FAC/MS) and pseudo typed virus infection assay.
- 13.Kostoff R.N. Literature-related discovery: potential treatments and preventatives for SARS. Technol Forecast Soc Change. 2011;78:1164–1173. doi: 10.1016/j.techfore.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study presents the findings of a host-virus interactome network analysis, an integrative antiviral drug repurposing methodology quantifying the interplay between virus-host interactome and drug targets.
- 16.Omolo C.A., Soni N., Fasiku V.O., Mackraj I., Govender T. Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur J Pharmacol. 2020 doi: 10.1016/j.ejphar.2020.173348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J.-i, Matsuda Z. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60:6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study performed target-based virtual ligand screening of a total of 21 targets against several compound databases to provide lead compounds for further in vitro and in vivo studies of SARS-CoV-2.
- 19.Ubani A., Agwom F., Morenikeji O.R., Shehu N.Y., Luka P., Umera E.A., Umar U., Omale S., Nnadi E., Aguiyi J.C. Molecular docking analysis of some phytochemicals on two SARS-CoV-2 targets. BioRxiv. 2020 doi: 10.1101/2020.03.31.017657. 03.31.017657. [DOI] [Google Scholar]
- 20.Hendaus M.A. Remdesivir in the treatment of coronavirus disease 2019 (COVID-19): a simplified summary. J Biomol. 2020:1–6. doi: 10.1080/07391102.2020.1767691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tallei T.E., Tumilaar S.G., Niode N.J., Fatimawali F., Kepel B.J., Idroes R., Effendi Y. Potential of plant bioactive compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) glycoprotein inhibitors: a molecular docking study. Preprints. 2020 doi: 10.20944/preprints202004.0102.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., et al. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 24.Micholas S., Jeremy C.S. Repurposing therapeutics for COVID-19: supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. Chemrxiv. 2020 doi: 10.26434/chemrxiv.11871402.v4. [DOI] [Google Scholar]
- 25.Jena A.B., Kanungo N., Nayak V., Chainy G., Dandapat J. Catechin and Curcumin interact with corona (2019-nCoV/SARS-CoV2) viral S protein and ACE2 of human cell membrane: insights from computational study and implication for intervention. Preprint from Research Square. 2020 doi: 10.21203/rs.3.rs-22057/v1. [DOI] [Google Scholar]
- 26.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports that ACE2 and the angiotensin II type 2 receptor protect mice from severe acute lung injury induced by acid aspiration or sepsis.
- 28.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci. 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 29.Biologics A. 2009. Safety and Tolerability Study of APN01 (Recombinant Human Angiotensin Converting Enzyme 2) ClinicalTrials.gov identifier NCT00886353. [Google Scholar]
- 30•.Horne J.R., Vohl M.-C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am J Physiol Endocrinol Metab. 2020;318:E830–E833. doi: 10.1152/ajpendo.00150.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review analyzes literature on the interactions between dietary fat, resveratrol and ACE2 gene variations and their impact on SARS-CoV-2 illness severity.
- 31.Kim E.N., Kim M.Y., Lim J.H., Kim Y., Shin S.J., Park C.W., Kim Y.-S., Chang Y.S., Yoon H.E., Choi B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis. 2018;270:123–131. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira Andrade J.M., Paraíso A.F., Garcia Z.M., Ferreira A.V.M., Sinisterra R.D.M., Sousa F.B., Guimarães A.L.S., de Paula A.M.B., Campagnole-Santos M.J., dos Santos R.A., et al. Cross talk between angiotensin-(1–7)/Mas axis and sirtuins in adipose tissue and metabolism of high-fat feed mice. Peptides. 2014;55:158–165. doi: 10.1016/j.peptides.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Tiao M.-M., Lin Y.-J., Yu H.-R., Sheen J.-M., Lin I.C., Lai Y.-J., Tain Y.-L., Huang L.-T., Tsai C.-C. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis. 2018;17:178. doi: 10.1186/s12944-018-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H.L., Kim W.K., Ha A.W. Effects of phytochemicals on blood pressure and neuroprotection mediated via brain renin-angiotensin system. Nutrients. 2019;11:2761. doi: 10.3390/nu11112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H.-Y., Yang M., Li Z., Meng Z. Curcumin inhibits angiotensin II-induced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-γ activity and reducing oxidative stress. Int J Mol Med. 2017;39:1307–1316. doi: 10.3892/ijmm.2017.2924. [DOI] [PubMed] [Google Scholar]
- 36.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh A.K., Osswald H.L., Prato G. Recent progress in the development of HIV-1 protease inhibitors for the treatment of HIV/AIDS. J Med Chem. 2016;59:5172–5208. doi: 10.1021/acs.jmedchem.5b01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren Z., Yan L., Zhang N., Guo Y., Yang C., Lou Z., Rao Z. The newly emerged SARS-like coronavirus HCoV-EMC also has an “Achilles' heel”: current effective inhibitor targeting a 3C-like protease. Protein Cell. 2013;4:248. doi: 10.1007/s13238-013-2841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai W., Zhang B., Jiang X.-M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adem S., Eyupoglu V., Sarfraz I., Rasul A., Ali M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: An in silico strategy unveils a hope against CORONA. Preprints. 2020 doi: 10.20944/preprints202003.0333.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 42.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg Med Chem. 2020;30:127377. doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J.-Y., Jeong H.J., Kim J.H., Kim Y.M., Park S.-J., Kim D., Park K.H., Lee W.S., Ryu Y.B. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol Pharm Bull. 2012 doi: 10.1248/bpb.b12-00623. b12-00623. [DOI] [PubMed] [Google Scholar]
- 44•.Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J Biomol. 2020:1–11. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study screened a total of 33 molecules using a blind molecular docking approach to identify possible inhibitors of the SARS-CoV-2 main protease.
- 45.Park J.-Y., Kim J.H., Kim Y.M., Jeong H.J., Kim D.W., Park K.H., Kwon H.-J., Park S.-J., Lee W.S., Ryu Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg Med Chem. 2012;20:5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y.H., Kim D.W., Curtis-Long M.J., Yuk H.J., Wang Y., Zhuang N., Lee K.H., Jeon K.S., Park K.H. Papain-like protease (PLpro) inhibitory effects of cinnamic amides from Tribulus terrestris fruits. Biol Pharm Bull. 2014;37:1021–1028. doi: 10.1248/bpb.b14-00026. [DOI] [PubMed] [Google Scholar]
- 47.Park J.-Y., Ko J.-A., Kim D.W., Kim Y.M., Kwon H.-J., Jeong H.J., Kim C.Y., Park K.H., Lee W.S., Ryu Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzyme Inhib Med Chem. 2016;31:23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 48.Kim D.W., Seo K.H., Curtis-Long M.J., Oh K.Y., Oh J.-W., Cho J.K., Lee K.H., Park K.H. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzyme Inhib Med Chem. 2014;29:59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 49.Park J.-Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Ryu Y.B., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32:504–512. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen T.T.H., Woo H.-J., Kang H.-K., Nguyen V.D., Kim Y.-M., Kim D.-W., Ahn S.-A., Xia Y., Kim D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghosh R., Chakraborty A., Biswas A., Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors – an in silico docking and molecular dynamics simulation study. J Biomol. 2020:1–13. doi: 10.1080/07391102.2020.1779818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Lin S.-C., Ho C.-T., Chuo W.-H., Li S., Wang T.T., Lin C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first to demonstrate that resveratrol is a potent anti-MERS agent in vitro.
- 54.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marinella M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh S., Sonawane A., Sadhukhan S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA‐dependent RNA polymerase (RdRp) inhibition: an in-silico analysis. J Biomol. 2020:1–16. doi: 10.1080/07391102.2020.1796810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395:e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atal S., Fatima Z. IL-6 Inhibitors in the treatment of serious COVID-19: a promising therapy? Pharm Med. 2020:1–9. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yahfoufi N., Alsadi N., Jambi M., Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10:1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding S., Jiang H., Fang J. Regulation of immune function by polyphenols. J Immunol. 2018;2018:1264074. doi: 10.1155/2018/1264074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Gupta S.C., Tyagi A.K., Deshmukh-Taskar P., Hinojosa M., Prasad S., Aggarwal B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]; This review extensively describes various plant-derived polyphenols that can suppress TNFα-activated inflammatory pathways.
- 62•.Lim H., Min D.S., Park H., Kim H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol Appl Pharmacol. 2018;355:93–102. doi: 10.1016/j.taap.2018.06.022. [DOI] [PubMed] [Google Scholar]; This study demonstrated the structure-activity profiles of flavonoids in NLRP3 inflammasome activation and mechanisms of cellular action for the first time.
- 63.Miranda C.L., Elias V.D., Hay J.J., Choi J., Reed R.L., Stevens J.F. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch Biochem Biophys. 2016;599:22–30. doi: 10.1016/j.abb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manoharan Y., Haridas V., Vasanthakumar K.C., Muthu S., Thavoorullah F.F., Shetty P. Curcumin: a wonder drug as a preventive measure for COVID19 management. Ind J Clin Biochem. 2020;35:373–375. doi: 10.1007/s12291-020-00902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Deng W., Xu Y., Kong Q., Xue J., Yu P., Liu J., Lv Q., Li F., Wei Q., Bao L. Therapeutic efficacy of Pudilan Xiaoyan Oral Liquid (PDL) for COVID-19 in vitro and in vivo. Signal Transduct Target Ther. 2020;5:66. doi: 10.1038/s41392-020-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses hACE2 transgenic mice to test the effect of a traditional Chinese medicine preparation composed of four plant extracts on COVID-19.
- 66•.Schettig R., Sears T., Klein M., Tan-Lim R., Matthias Jr R., Aussems C., Hummel M., Sears R., Poteet Z., Warren D. COVID-19 patient with multifocal pneumonia and respiratory difficulty resolved quickly: possible antiviral and anti-inflammatory benefits of Quercinex (Nebulized Quercetin-NAC) as adjuvant. Ther Adv Infect Dis. 2020;10:45–55. [Google Scholar]; This report presents a clinical case involving the use of a nebulized formula of quercetin and N-acetylcysteine to improve unresolved respiratory symptoms in a patient with SARS-CoV-2-induced multifocal pneumonia.
- 67.Rothwell J.A., Urpi‐Sarda M., Boto‐Ordoñez M., Llorach R., Farran‐Codina A., Barupal D.K., Neveu V., Manach C., Andres‐Lacueva C., Scalbert A. Systematic analysis of the polyphenol metabolome using the Phenol‐Explorer database. Mol Nutr Food Res. 2016;60:203–211. doi: 10.1002/mnfr.201500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shinbo Y., Nakamura Y., Altaf-Ul-Amin M., Asahi H., Kurokawa K., Arita M., Saito K., Ohta D., Shibata D., Kanaya S. Plant Metabolomics. Springer; Berlin, Heidelberg: 2006. KNApSAcK: a comprehensive species-metabolite relationship database; pp. 165–181. [Google Scholar]
- 69.Wang M., Carver J.J., Phelan V.V., Sanchez L.M., Garg N., Peng Y., Nguyen D.D., Watrous J., Kapono C.A., Luzzatto-Knaan T. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williamson G., Kerimi A. Testing of natural products in clinical trials targeting the SARS-CoV-2 (Covid-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction. Biochem Pharmacol. 2020;178:114123. doi: 10.1016/j.bcp.2020.114123. [DOI] [PMC free article] [PubMed] [Google Scholar]