Abstract
Objective: To describe and compare (1) classification of obesity using clinical proxies of body composition that are easily accessible in the outpatient clinic setting, (2) cardiometabolic risk using existing screening tools and staging systems, and (3) the presence of metabolic syndrome (MetS) using four commonly-used definitions in adults with spinal cord injury (SCI).
Design: Retrospective chart review
Setting: Outpatient Veterans Affairs (VA) SCI Annual Evaluation Clinic
Participants: Patients who attended an annual evaluation appointment with demographic, anthropometric, and biochemical data documented in their medical records as part of routine medical care.
Outcome measures: Obesity classification (body mass index, waist circumference, ideal body weight percentage), cardiometabolic risk scores (Framingham Risk Score, Cardiometabolic Disease Staging System, Edmonton Obesity Staging System), and MetS classification (using four commonly-used definitions) were described and compared.
Results: Of the 155 veterans included in this analysis, 93% were considered “at risk” by at least one of the measurements studied. However, there was considerable variation between the different screening tools. The κ-agreement between various definitions of MetS ranged from fair to moderate.
Conclusion: Screening tools that were developed for the non-SCI population produced variable assessments of risk when applied to veterans with SCI. Due to the fair to moderate inter-rater agreement between MetS definitions, it is unknown which definition is superior to identify MetS in the SCI population. An SCI-specific screening tool is needed to accurately classify obesity, cardiometabolic risk, and MetS in order to provide timely education and intervention.
Keywords: Spinal cord injuries, Obesity, Veterans, Retrospective studies, Metabolic syndrome
Introduction
Current literature suggests an increased prevalence of obesity, cardiometabolic risk, and metabolic syndrome (MetS) among individuals with spinal cord injuries (SCI), potentially due to decreased physical activity, lower metabolism, and decreased muscle mass.1–8 However, evidence-based screening tools designed specifically for the SCI population to identify and classify those at risk currently do not exist.9,10
Obesity, defined by excess body fat accumulation, is associated with diabetes, all-cause mortality, and cardiovascular disease (CVD)-mortality.11,12 Identification of obesity can lead to appropriate interventions and improved health outcomes.13 Methods used to assess or estimate body fat percentage include air displacement plethysmography, bioelectrical impedance analysis (BIA), and imaging methods such as computerized tomography (CT), magnetic resonance imaging (MRI), and dual-energy X-ray absorptiometry (DXA).10 Although many of these methods are used in SCI-related research, they are often not available in an outpatient clinical setting.3,10,14–20
When accurate body composition measurement methods are not accessible in the outpatient clinical setting, clinical proxies of body composition are often used to classify obesity. Body mass index (BMI) is an anthropometric measure of weight adjusted for height (kg/m2) often used to classify obesity.21 However, since BMI underestimates adiposity in individuals with SCI, it is not sensitive for detecting obesity status and is a poor predictor of CVD in this population.1,3,19,22–25 Adjusted BMI classification schemes have been proposed for the SCI population to account for the changes in body composition after injury; however, they have not yet been validated in a large population.10,24,26 Similarly, it has been proposed that waist circumference (WC) may be an indicator of obesity-related comorbidities, but there are currently no SCI-specific cut-off points that have been validated.27,28 In place of BMI, it has been suggested to calculate and adjust ideal body weight (IBW) for individuals with SCI, but these approaches are dated, not validated, and are not accompanied by a classification scheme.29,30 A standard classification of obesity in the SCI population remains unknown.10
In addition to solely relying on obesity classification, risk clustering has been used to determine cardiometabolic or CVD risk.23,30–34 Based on the concept of risk clustering, several screening tools and staging systems have been developed to assist providers with clinical decision making using easily-accessible data. Among these screening tools are the Framingham Coronary Heart Disease Risk Score (FRS),35,36 Cardiometabolic Disease Staging System (CMDS),37,38 and Edmonton Obesity Staging System (EOSS).39,40
The FRS is one of the most widely-used CVD risk prediction scores.41 It involves a sex-specific scoring system to identify 10-year CVD risk.35 Although it has been applied in the SCI population,28,42 it has not been validated in a large cohort in this population. The CMDS was developed to evaluate the stage and severity of the cardiometabolic disease to predict the risk of diabetes, CVD mortality, and all-cause mortality. This 5-stage system was validated using two large national cohorts of the non-SCI population,38 but to our knowledge has not been studied or validated in the SCI population. The EOSS is a 5-stage system that incorporates morbidity and functional limitations associated with excess weight to predict all-cause mortality.40 While the EOSS has been studied in the non-SCI population,43–47 it has not been studied nor validated in the SCI population, to our knowledge.
Additionally, MetS classification is used to define the risk of CVD and diabetes by identifying a cluster of traits.11 Common algorithms for classifying MetS have been established by the World Health Organization (WHO);48 National Heart, Lung, and Blood Institute and American Heart Association (NHLBI/AHA);49 National Cholesterol Education Program (NCEP);50 and the International Diabetes Federation (IDF).51 Previous studies investigating the prevalence of MetS in SCI populations have yielded equivocal results and have demonstrated the limitations of the existing definitions due to the physiologic changes associated with SCI.8,9,52–56
These tools and classification schemes were developed in the non-injured population, and it is unknown whether they can be extrapolated to consistently assess risk among those with SCI. Therefore, the objectives of this study were to describe and compare (1) classification of obesity using clinical proxies of body composition that are easily accessible in the outpatient clinic setting, (2) cardiometabolic risk using existing screening tools and staging systems, and (3) the presence of MetS using four commonly-used definitions.
Methods
A retrospective chart review was conducted of veterans who attended an annual evaluation appointment in the outpatient clinic of the Spinal Cord Injuries/Disorders unit at the Edward Hines, Jr. Veterans Affairs (VA) Hospital in Hines, Illinois from January 1, 2017 through December 31, 2017. Inclusion criteria included age 18–89 years and a diagnosis of an SCI. Patients with a diagnosis of multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), or a non-SCI spinal cord disorder were excluded. Pregnant females, patients with limb amputations, and patients who had experienced the SCI ≤ 6 weeks prior to the appointment were excluded due to expected changes in body composition in addition to SCI-related changes.
Demographic, anthropometric, and biochemical data were obtained and documented in the computerized patient record system (CPRS) by trained nursing staff during a single visit as part of routine medical care for an annual evaluation. As part of standard care in the clinic, patients were instructed to fast before the appointment, standard venipuncture procedures were followed, and blood was analyzed in a CLIA-certified laboratory. As part of routine care, WC was obtained by trained clinic staff using a flexible measuring tape while the patient was either standing, sitting, or in the supine position, depending on the patient’s level of injury and practicality of positioning. The patient records were retrospectively reviewed by a single author in January 2018 after the appointments were completed.
As described below, these data were then used to determine obesity classification, cardiometabolic risk scores, MetS classification, and additional descriptive frequencies.
Obesity classification
BMI was calculated for each patient. The prevalence of obesity was determined using the cut-off point of ≥30 kg/m2 according to the WHO 21 definition, as well as proposed SCI-specific cut-off points of ≥25.3 kg/m2 per Ayas et al.26 and >22 kg/m2 per Laughton et al.24
The WC cut-off point of ≥102 cm27 and the SCI-specific cut-off of ≥ 94 cm per Ravensbergen et al.28 were used as proxy indicators of excess visceral adiposity and risk for obesity-related comorbidities.
IBW was calculated for each patient using the Hamwi57,58 method based on height in inches and weight in pounds. For men, Hamwi IBW is calculated as 106 pounds plus 6 pounds for every inch over 60 in. of height. For example, if a patient was 67 in. tall, the following equation would be used to calculate IBW:
IBW = 106 + [6 × (67 – 60)] = 148 pounds
Using recommendations specific to the SCI population from the Academy of Nutrition and Dietetics Evidence Analysis Library,29 this estimation was further adjusted by subtracting 10–15% of body weight or 15–20 pounds for individuals with tetraplegia or by subtracting 5–10% of body weight or 10–15 pounds for individuals with paraplegia. For example, if a veteran with paraplegia was 67 in. tall and had a calculated IBW of 148 pounds per the Hamwi method, two additional adjusted IBW measurements were calculated:
IBW adjusted by percentage low range: 148 − (148 × 0.1) = 133.2 pounds
IBW adjusted by percentage high range: 148 − (148 × 0.05) = 140.6 pounds
IBW adjusted by pounds low range: 148 − 15 = 133 pounds
IBW adjusted by pounds high range: 148 − 10 = 138 pounds
This individual would have an adjusted IBW range of 133.2–140.6 pounds (adjusted by percentage of body weight) or 133–138 pounds (adjusted by pounds).
Each individual’s percent IBW (%IBW) was calculated for each estimation by dividing actual body weight by calculated IBW.
Cardiometabolic risk scores
Demographic, anthropometric, and biochemical data were entered into existing screening tools (FRS,35 CMDS,38 EOSS40) to determine cardiometabolic risk. A FRS >20% was considered high risk of developing CVD. A CMDS or EOSS stage >0 was considered at risk. Full criteria for each of the screening tools can be found in Supplemental Materials.
Metabolic syndrome classification
MetS was calculated using criteria as defined by IDF,51 NCEP,50 NHLBI/AHA,49 and WHO48 (Supplemental Materials).
Additional descriptive frequencies
As part of routine care in the outpatient annual evaluation clinic, nursing staff documented and asked each veteran, “How would you rate your overall health?” Each veteran was given the option to reply with “excellent,” “good,” “fair,” or “poor.” These responses were collected in this chart review. Medications were also recorded from the active medications section of the electronic health record. Medications were categorized as “yes” if there was the presence of at least one active medication in a given category or “no” if the medication was not listed as active for each of the following categories: hypertensive medications, diabetes medications, or lipid medications.
Statistical analysis
Descriptive statistics were used to summarize demographic, anthropometric, and biochemical data. To compare the agreement between MetS definitions, inter-rater reliability was measured for each combination of the four MetS definitions using Kappa (κ) values and categorized using divisions published by Landis et al. (κ < 0.00: poor, 0.00–0.20: slight, 0.21–0.40: fair, 0.41–0.60: moderate, 0.61–0.80: substantial, 0.81–1.00: almost perfect).59 Simple linear regression was used to determine the difference between the adjusted IBW calculated by subtracting pounds versus percentage of body weight. Visualization method was used to determine the difference of adjustment in the category of SCI by examining regression plots graphically. Analyses were performed using IBM® SPSS® Statistics Version 25. All tests were two-tailed, and P < 0.05 was considered statistically significant.
Results
Table 1 displays each of the screening tools and measurements used in this study, along with the strengths and limitations of each. Of the 197 charts screened, 167 met inclusion criteria. Three additional patients were excluded due to insufficient data available in the chart. Due to the small number of females (n = 9), the authors decided to only include males in the final analyses. Therefore, a total of 155 patients were included for final analyses. Demographic characteristics of the included patients are displayed in Table 2. Anthropometric and biochemical data were obtained for all patients (n = 155), with the exception of WC (n = 74) and glycated hemoglobin (n = 59). Additional anthropometric and biochemical data are shown in Table 3. A total of 144 out of the 155 veterans (93%) were considered “at risk” or obese by at least one of the measurements or screening tools studied.
Table 1. Strengths and limitations of existing obesity, cardiometabolic risk, and metabolic syndrome classification tools.
| Screening tool or classification measurement | Strengths or rationale | Limitations specific to SCI population |
|---|---|---|
| Obesity Classification | ||
| BMI ≥ 30 (WHO)21 |
|
|
| Adjusted BMI ≥ 25.3 (Ayas)10,26 |
|
|
| Adjusted BMI > 22 (Laughton)24 |
|
|
| IBW Equation (Hamwi)57,58 |
|
|
| Adjusted IBW29,30 |
|
|
| Waist Circumference (WC) 27 > 40ʺ or 102 cm (men) WC > 35ʺ or 88 cm (women) |
|
|
| Adjusted WC ≥ 94 cm (Ravensbergen)28 |
|
|
| Metabolic Syndrome (MetS) Classification | ||
| International Diabetes Federation (IDF)51 |
|
|
| National Cholesterol Education Program (NCEP)50 |
|
|
| National Heart, Lung, and Blood Institute and American Heart Association (NHLBI/AHA)49 |
|
|
| World Health Organization (WHO)48 |
|
|
| Cardiometabolic Risk Screening Tools | ||
| Framingham Risk Score (FRS)35 |
|
|
| Cardiometabolic Disease Staging System (CMDS)38 |
|
|
| Edmonton Obesity Staging System (EOSS)40 |
|
|
| SCI-Specific Screening Tool Needed | ||
| Proposed SCI-Specific Screening Tool |
Combining the strengths of the aforementioned screening tools, an SCI-specific tool should be/include:
|
After evaluating the limitations of the aforementioned screening tools, an SCI-specific tool should take into account:
|
BMI, body mass index; BP, blood pressure; CRP, C-Reactive Protein; CVD, Cardiovascular Disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL, high-density lipoprotein; IBW, ideal body weight; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; SBP, systolic blood pressure; SCI, spinal cord injury; T2DM, type 2 diabetes mellitus; TG, triglycerides; WC, waist circumference; WHO, World Health Organization; WNL, within normal limits.
Table 2. Demographic and injury characteristics.
| Total (n = 155) | |
|---|---|
| Age (years), mean ± SD (min–max) | 61.1 ± 13.7 (24–85) |
| Race/ethnicity, n (%) | |
| White | 88 (56.8%) |
| Black/African American | 53 (34.2%) |
| Asian | 2 (1.3%) |
| Pacific Islander or Native Hawaiian | 3 (1.9%) |
| Other or unknown | 9 (5.7%) |
| Type of injury and AIS score, n (%) | |
| Paraplegia | 60 (38.7%) |
| ASIA A, n | 29 |
| ASIA B, n | 5 |
| ASIA C, n | 8 |
| ASIA D, n | 16 |
| ASIA E, n | 2 |
| Tetraplegia | 92 (59.4%) |
| ASIA A, n | 16 |
| ASIA B, n | 7 |
| ASIA C, n | 9 |
| ASIA D, n | 59 |
| ASIA E, n | 1 |
| Unknown | 3 (1.9%) |
| Age at Injury (years), mean ± SD (min–max) | 42.5 ± 18.3 (17–79) |
| Years since Injury, mean ± SD (min–max) | 18.7 ± 14.3 (0–59) |
AIS, American Spinal Injury Association (ASIA) Impairment Scale.
Table 3. Anthropometric and biochemical characteristics.
| Total (n = 155) | Paraplegia | Tetraplegia | |||
|---|---|---|---|---|---|
| Incomplete (n = 30) |
Complete (n = 30) |
Incomplete (n = 75) |
Complete (n = 17) |
||
| Height (cm), Mean (SD) | 178.0 (10.4) | 178.5 (7.0) | 180.3 (5.7) | 176.8 (13.2) | 179.4 (6.3) |
| Weight (kg), Mean (SD) | 90.1 (24.0) | 92.5 (27.1) | 89.4 (18.0) | 91.3 (25.6) | 81.7 (22.4) |
| BMI (kg/m2), Mean (SD) | 28.3 (7.1) | 29.0 (8.1) | 27.9 (5.8) | 28.8 (7.5) | 25.3 (5.9) |
| WC (cm), Mean (SD) | 111.7 (18.8) (n = 74) |
110.0 (22.6) (n = 17) |
109.7 (17.0) (n = 14) |
112.9 (18.1) (n = 39) |
115.3 (18.8) (n = 4) |
| WC method used (n, %) | |||||
| Sitting | 28 (18.1%) | 7 (23.3%) | 7 (23.3%) | 11 (14.7%) | 3 (17.6%) |
| Standing | 15 (9.7%) | 4 (13.3%) | 0 | 11 (14.7%) | 0 |
| Supine | 12 (7.7%) | 3 (10.0%) | 3 (10.0%) | 5 (6.7%) | 1 (5.9%) |
| Unknown | 100 (64.5%) | 16 (53.3%) | 20 (66.7%) | 48 (64%) | 13 (76.5%) |
| SBP (mmHg), Mean (SD) | 121.3 (15.7) | 123.8 (15.8) | 120.0 (13.7) | 122.6 (16.4) | 113.2 (14.9) |
| DBP (mmHg), Mean (SD) | 68.3 (9.4) | 70.1 (68.0) | 67.3 (8.4) | 66.1(9.2) | 67.3 (11.4) |
| TG (mg/dL), Mean (SD) | 132.5 (82.8) | 140.4 (80.5) | 149.1 (94.4) | 114.7 (62.2) | 154.9 (118.6) |
| HDL (mg/dL), Mean (SD) | 47.9 (15.1) | 50.2 (16.2) | 43.5 (11.9) | 50.0 (16.3) | 43.1 (11.8) |
| LDL (mg/dL), Mean (SD) | 79.1 (32.0) | 86.4 (41.2) | 86.3 (29.3) | 70.0 (27.0) | 90.2 (30.3) |
| TChol (mg/dL), Mean (SD) | 152.0 (35.2) | 163.9 (41.1) | 158.5 (31.1) | 141.7 (30.9) | 162.1 (40.0) |
| FBG (mg/dL), Mean (SD) | 102.9 (31.2) | 105.7 (29.4) | 95.6 (24.1) | 104.4 (31.6) | 100.1 (38.5) |
| A1C (%), Mean (SD) | 6.4 (1.0) (n = 59) |
6.6 (1.0) (n = 10) |
6.1 (1.4) (n = 10) |
6.4 (1.2) (n = 32) |
6.2 (1.8) (n = 5) |
| eGFR (mL/min/1.73 m2), Mean (SD) | 111.3 (51.0) | 98.3 (34.1) (n = 29) |
125.2 (36.7) | 100.0 (42.1) | 164.6 (87.9) |
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TChol, total cholesterol; FBG, fasting blood glucose; A1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate.
Obesity classification
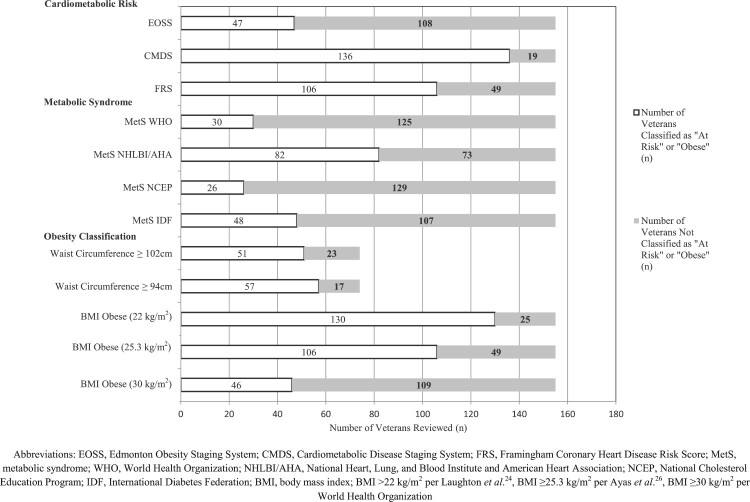
The mean (SD) BMI was 28.3 (7.1) kg/m2. Figure 1 demonstrates that the proportion of veterans who were classified as obese using BMI cut-off points of 30, 25.3, and 22 kg/m2 was 30, 68, and 84%, respectively. Obesity classification per BMI organized by level of injury can be found in Fig. 2.
Figure 1.
Proportion of Veterans Screened as “At Risk,” Proportion of veterans screened as “at risk” according to cardiometabolic risk screening tools, presence of metabolic syndrome per WHO, NHLBI/AHA, NCEP, and IDF criteria, and classified as obese using various waist circumference and BMI cut-off points.
Figure 2.
Obesity Classification per BMI Cut-off by Level of Injury. Percentage of veterans classified as obese using BMI cut-off points per World Health Organization and SCI-Specific per Laughton et al. and Ayas et al. categorized by classification of injury (paraplegia versus tetraplegia) and American Spinal Injury Association (ASIA) Impairment Scale (AIS).
The mean (SD) WC was 111.7 cm (18.8). Sixty-nine percent and 77% of the 74 veterans with available WC measurements were classified as having a high WC per the cut-offs of 102 and 94 cm, respectively (Fig. 1).
Regression of %IBW adjusted by pounds and that adjusted by percent showed that both adjustments are very similar. The R2 values for regressions at both ranges (low and high) were higher than 0.99. Regression coefficient for low range was 0.993 (P < 0.000) and that for high range was 1.06 (P < 0.000).
Cardiometabolic risk scores
The cardiometabolic risk screening tools produced varying results; 30, 88, and 68% of veterans were considered “at risk” according to the EOSS, CMDS, and FRS, respectively. The proportion of patients who screened “at risk” by each tool is displayed in Fig. 1.
Metabolic syndrome classification
The prevalence of MetS was 31% (IDF), 17% (NCEP), 53% (NHLBI/AHA), and 19% (WHO). The κ-agreement between the various definitions of MetS ranged from fair to moderate, as displayed in Table 4. The highest prevalence of MetS was per the NHLBI/AHA definition throughout all categories of level of injury and American Spinal Injury Association (ASIA) classification. The distribution of the presence of MetS by ASIA classification is displayed in Fig. 3.
Table 4. Comparison of metabolic syndrome definitions using kappa values.
| Combination | κ-value | Agreement-strength | P value |
|---|---|---|---|
| MetS (IDF) and MetS (NCEP) | 0.516 | Moderate | <0.001 |
| MetS (IDF) and MetS (NHLBI/AHA) | 0.47 | Moderate | <0.001 |
| MetS (IDF) and MetS (WHO) | 0.461 | Moderate | <0.001 |
| MetS (NCEP) and MetS (NHLBI/AHA) | 0.304 | Fair | <0.001 |
| MetS (NCEP) and MetS (WHO) | 0.521 | Moderate | <0.001 |
| MetS (NHLBI/AHA) and MetS (WHO) | 0.327 | Fair | <0.001 |
MetS, metabolic syndrome; IDF, International Diabetes Federation; NCEP, National Cholesterol Education Program; NHLBI/AHA, National Heart, Lung, and Blood Institute and American Heart Association; WHO, World Health Organization.
Figure 3.
Metabolic Syndrome by Level of Injury. Percentage of total sample in each classification with presence of MetS per IDF, NCEP, NHLBI/AHA, and WHO criteria. For example, of the veterans with ASIA A paraplegia (n = 29), the criteria for MetS as defined by the IDF identified presence of MetS in n = 9 (31%).
Additional descriptive frequencies
Out of the veterans who rated their overall health as “good” or “excellent” (n = 106), 62% were taking at least one medication for hypertension, diabetes mellitus, and/or hyperlipidemia. Of those same 106 veterans, 28%, 65%, and 82% were classified as obese per BMI cut-off of ≥30, ≥25.3, or >22 kg/m2, respectively.
Discussion
The primary aim of this study was to describe and compare the classification of obesity, cardiometabolic risk, and MetS using existing screening tools that are typically available in a clinical setting among veterans with SCI. Frequencies of those screened as “at risk” or obese differed considerably among the various tools. Physiologic changes unique to SCI may confound commonly-used risk assessment tools, therefore potentially limiting the validity and/or reliability of these tools in the SCI population.
Various parameters included within the different screening tools may be affected by physiological changes specific to SCI. For example, fasting blood glucose levels may be affected by SCI-related complications and may not correlate with glucose tolerance in the SCI population.7,60,61 Individuals with SCI also experience blood pressure fluctuations due to neurological changes.62–64 WC may be affected by atrophy of abdominal muscles and increases in visceral adiposity in the SCI population, leading current WC cut-off points to underestimate central adiposity and consequent cardiometabolic risk.17,27
Similarly, BMI of 30 kg/m2 or greater is considered “obese” for the non-injured population.21 Although two different SCI-specific BMI cut-off points have been proposed, these have only been validated in small, select populations.10,24,26 Two different methods of adjusting the traditional Hamwi IBW calculation29 have been suggested for patients with SCI, but the proposed adjustments have not been validated. Nevertheless, results of this study indicate that the two different equations for adjusting IBW produce similar results. Since the intercepts were low and insignificant for both equations, it can be concluded that both adjustments are similar. Therefore, it can be inferred that no one equation is superior to the other and clinicians should continue to use clinical judgment in determining which equation to use until more research is available. Considering both adjusted IBW together with SCI-specific BMI cut-offs may be a prudent approach to the clinical assessment of weight status.
Many of the aforementioned criteria are used as parameters to assess cardiometabolic and CVD risk in screening tools and classification systems developed for the non-SCI population such as the FRS, CMDS, EOSS, and MetS classification. This study found only fair to moderate agreement between the different definitions of MetS in this sample of veterans with SCI, so it is not known which of the four definitions is most accurate.
Inconsistent results among previous studies examining the prevalence of MetS in SCI populations illustrate the complexity of identifying MetS in this population, and this warrants caution in applying standard definitions of MetS to patients with SCI. Results from Finnie et al. (n = 56) revealed a prevalence of 3.6% (WHO), 12.3% (NCEP), 15.8% (NHLBI/AHA), and 19.3% (IDF) compared to our results (n = 155) of 19% (WHO), 17% (NCEP), 53% (NHLBI/AHA), and 31% (IDF).9 Gater et al. (n = 473) found a higher prevalence of 57% (IDF) when using a surrogate obesity marker to account for the SCI differences, compared to 22.9% (IDF) when using the standard BMI measurement.8 Maruyama et al. (n = 44) found a higher prevalence of 43% (NCEP) when adjusting the WC for the Japanese population.56 These differences may be due to different populations, sample sizes, and criteria used within a standard definition. For example, as WC was not available, Gater et al. used BMI to determine central obesity while we incorporated WC if the measure was available.8
Of note, while the criteria for the presence of MetS per the IDF requires central obesity as defined by ethnicity-specific WC cut-offs, the 2006 IDF Consensus Statement indicates that if BMI is >30 kg/m2 central obesity can be assumed and waist circumference does not need to be measured.51 Since WC is not always measured in clinical practice, BMI > 30 kg/m2 is typically used alone to meet this criterion. Of the 74 patients who had a WC measured in our cohort, 34 (46%) had a WC ≥ 94 cm, but also BMI <30. This means that, if WC was not available, these patients would have automatically screened negative for MetS per IDF using BMI alone even though their WC indicates they actually were at risk. Taken together, the evidence suggests that current definitions of MetS that were developed in the non-injured population cannot be extrapolated to individuals with SCI because they may likely underestimate true risk.9 The differences in prevalence of MetS within our own sample according to different definitions and also compared to other studies shows the inconsistencies that may be found in research and clinical practice and may lead to different interpretations of true risk.
The results of this study also demonstrated that patients may not be aware of their health risks. Although >90% of this sample screened as “at risk” by the screening tools investigated, 68% rated their overall health as “good” or “excellent.” Of those 106 patients who rated their health as “good” or “excellent,” 62% were on at least one medication for hypertension, diabetes, or hyperlipidemia. Previous research has pointed out that healthcare providers report challenges caring for people with SCI, and patients have expressed perceptions that their providers do not acknowledge or appreciate the complexity of their conditions.65–68 Lack of agreement between health risk screening tools can add to further confusion, especially if the same patient visits multiple providers who use different tools that come to discrepant outcomes.
There are limitations of this study that should be noted. The first is the potential lack of generalizability to other patient groups. Since this cohort only included male veterans from the Chicago, Illinois area, findings may not be generalizable to women with SCI or those from other regions. Since each veteran is encouraged to schedule an annual evaluation once a year, only one year of appointments was reviewed. However, this could have limited the sample size as it would not capture patients who did not consistently attend one annual evaluation per calendar year. Inherent limitations of retrospective medical record reviews must be acknowledged, including a lack of data that could have added valuable insight such as physical activity levels, additional medications (e.g. drugs that may impact inflammation), C-reactive protein levels (which have been suggested to be associated with CVD risk),9,53 or presence of undernutrition.
Similarly, since active prescriptions of medications were recorded as a dichotomous value of “yes” or “no” during chart review, the interactions of specific medications were not analyzed. It also does not consider if medications were updated or if patients were taking the medications as prescribed. Due to the retrospective nature of this study involving variables that were collected by clinic staff as part of routine care, it is unknown whether the anthropometric characteristics, clinical measures, and blood draws were obtained consistently or in the same manner as stated in the criteria for MetS. However, the intention of this study was to describe and compare the realistic use of these measures in the clinical setting, which adds to the strengths of this study.
Although robust measurements of body composition such as MRI, DXA, CT, or air displacement plethysmography are more accurate at measuring visceral adipose tissue and potentially better predictors of cardiometabolic health in the SCI population, they are not routinely available in non-research settings. A strength of this study was the focus on measures that can be easily and routinely obtained in a fast-paced outpatient clinic for the SCI patient. Furthermore, this study compares the variation in outcomes from a wide variety of existing screening tools and compiles the strengths and limitations specific to individuals with SCI for each tool or measure investigated (Table 1). This table can be used to guide the development of an SCI-specific screening tool by adjusting the limitations to better fit the SCI-specific conditions while building upon the strengths of the existing tools. An evidence-based SCI-specific screening tool can address the unique physiologic changes after SCI, allow for consistent measurement in cardiometabolic and/or CVD risk, help guide communication and informed decision-making, assist providers with caring for this vulnerable population, and assure the individual that his/her SCI-specific conditions are being addressed.
Conclusion
The current tools that were developed for the non-injured population to classify obesity, cardiometabolic risk, and MetS produce varying results in the SCI population and may not represent true risk given the unique physiologic changes after injury. Future research on this topic should focus on developing an SCI-specific screening tool in order to more accurately identify risk and provide timely education and intervention. This SCI-specific tool can incorporate strengths of the existing tools, such as including measures that are easily attainable in the clinic, while building upon the limitations in the SCI population such as addressing physiologic changes after SCI (Table 1). It would also be beneficial to investigate current practices and barriers for assessing risk by surveying professionals who work with the SCI population before developing an SCI-specific screening tool. In the interim, the interdisciplinary team should consider treating all patients with SCI as at elevated risk, regardless of screening tool assessments.
Supplementary Material
Acknowledgements
This research was supported by non-financial resources (staff and data) from the Department of Veterans Affairs at the Edward Hines, Jr. VA Hospital and the University of Alabama. We wish to acknowledge the Department of Veterans Affairs Spinal Cord Injury, Research, and Nutrition and Food Services at the Edward Hines, Jr. VA Hospital in Hines, Illinois and the Department of Human Nutrition and Hospitality Management and Department of Information Systems, Statistics, Management Science at the University of Alabama, Tuscaloosa, Alabama.
Disclaimer statements
Contributors None.
Funding This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest Authors have no conflict of interests to declare.
Ethics approval
This study was approved by the Institutional Review Board (IRB) at the Edward Hines, Jr. VA Hospital and University of Alabama.
References
- 1.Cao Y, Krause JS, DiPiro N.. Risk factors for mortality after spinal cord injury in the USA. Spinal Cord. 2013;51(5):413–8. [DOI] [PubMed] [Google Scholar]
- 2.DiMarco AF, Dawson NV.. Risk factors for mortality in spinal cord injury. J Spinal Cord Med. 2014;37(6):670–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR.. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37(6):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatchett PE, Mulroy SJ, Eberly VJ, Haubert LL, Requejo PS.. Body mass index changes over 3 years and effect of obesity on community mobility for persons with chronic spinal cord injury. J Spinal Cord Med. 2016;39(4):421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitland Schladen M, Groah SL.. State of the science on cardiometabolic risk after spinal cord injury: recap of the 2013 Asia pre-conference on cardiometabolic disease. Top Spinal Cord Inj Rehabil. 2014;20(2):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchholz AC, McGillivray CF, Pencharz PB.. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77(2):371–8. [DOI] [PubMed] [Google Scholar]
- 7.Bauman WA, Spungen AM.. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266–77. [DOI] [PubMed] [Google Scholar]
- 8.Gater DR, Jr., Farkas GJ, Berg AS, Castillo C.. Prevalence of metabolic syndrome in veterans with spinal cord injury. J Spinal Cord Med. 2018:1–8. doi: 10.1080/10790268.2017.1423266. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnie AK, Buchholz AC, Martin Ginis KA, Group SSR.. Current coronary heart disease risk assessment tools may underestimate risk in community-dwelling persons with chronic spinal cord injury. Spinal Cord. 2008;46(9):608–15. [DOI] [PubMed] [Google Scholar]
- 10.Silveira SL, Ledoux TA, Robinson-Whelen S, Stough R, Nosek MA.. Methods for classifying obesity in spinal cord injury: a review. Spinal Cord. 2017;55(9):812–7. [DOI] [PubMed] [Google Scholar]
- 11.Martin KA, Mani MV, Mani A.. New targets to treat obesity and the metabolic syndrome. Eur J Pharmacol. 2015;763(Pt A):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly T, Yang W, Chen CS, Reynolds K, He J.. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32(9):1431–7. [DOI] [PubMed] [Google Scholar]
- 13.Rinehart CS, Oliver JS.. A clinical Protocol for the assessment of obesity: addressing an Epidemic. Nurs Clin North Am. 2015;50(3):605–11. [DOI] [PubMed] [Google Scholar]
- 14.Farkas GJ, Gorgey AS, Dolbow DR, Berg AS, Gater DR.. The influence of level of spinal cord injury on adipose tissue and its relationship to inflammatory adipokines and cardiometabolic profiles. J Spinal Cord Med. 2018;41(4):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumrell RM, Nightingale TE, McCauley LS, Gorgey AS, Pelletier C.. Anthropometric cutoffs and associations with visceral adiposity and metabolic biomarkers after spinal cord injury. PLoS One. 2018;13(8):e0203049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghatas MP, Lester RM, Khan MR, Gorgey AS.. Semi-automated segmentation of magnetic resonance images for thigh skeletal muscle and fat using threshold technique after spinal cord injury. Neural Regen Res. 2018;13(10):1787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin KC, O’Brien LC, Gorgey AS.. Quantification of trunk and android lean mass using dual energy x-ray absorptiometry compared to magnetic resonance imaging after spinal cord injury. J Spinal Cord Med. 2018:1–9. doi: 10.1080/10790268.2018.1438879. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wade RC, Lester RM, Gorgey AS.. Validation of anthropometric muscle Cross-Sectional area equation after spinal cord injury. Int J Sports Med. 2018;39(5):366–73. [DOI] [PubMed] [Google Scholar]
- 19.Eriks-Hoogland I, Hilfiker R, Baumberger M, Balk S, Stucki G, Perret C.. Clinical assessment of obesity in persons with spinal cord injury: validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord Med. 2011;34(4):416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han SH, Lee BS, Choi HS, Kang M-S, Kim BR, Han Z-A, et al. Comparison of Fat mass percentage and body mass index in Koreans With spinal cord injury according to the severity and Duration of Motor Paralysis. Ann Rehabil Med. 2015;39(3):384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obesity: preventing and managing the global epidemic. report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 22.Alschuler KN, Gibbons LE, Rosenberg DE, Ehde DM, Verrall AM, Bamer AM, et al. Body mass index and waist circumference in persons aging with muscular dystrophy, multiple sclerosis, post-polio syndrome, and spinal cord injury. Disabil Health J. 2012;5(3):177–84. [DOI] [PubMed] [Google Scholar]
- 23.Bauman WA, Spungen AM.. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46(7):466–76. [DOI] [PubMed] [Google Scholar]
- 24.Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE, Group SSR.. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47(10):757–62. [DOI] [PubMed] [Google Scholar]
- 25.Cragg JJ, Ravensbergen HJ, Borisoff JF, Claydon VE.. Optimal scaling of weight and waist circumference to height for adiposity and cardiovascular disease risk in individuals with spinal cord injury. Spinal Cord. 2015;53(1):64–8. [DOI] [PubMed] [Google Scholar]
- 26.Ayas NT, Epstein LJ, Lieberman SL, Tun CG, Larkin EK, Brown R, et al. Predictors of loud snoring in persons with spinal cord injury. J Spinal Cord Med. 2001;24(1):30–4. [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a Consensus Statement from Shaping America's health: Association for weight Management and obesity Prevention; NAASO, the obesity Society; the American Society for Nutrition; and the American diabetes Association. Obesity (Silver Spring). 2007;15(5):1061–7. [DOI] [PubMed] [Google Scholar]
- 28.Ravensbergen HR, Lear SA, Claydon VE.. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma. 2014;31(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Academy of Nutrition and Dietetics Evidence Analysis Library . “SCI: Assessment of Body Composition: Estimation of Ideal Body Weight” Accessed 18 May 2018: https://wwwandealorg/templatecfm?template=guide_summary&key=2305#supportevidence
- 30.Peiffer SC, Blust P, Leyson JF.. Nutritional assessment of the spinal cord injured patient. J Am Diet Assoc. 1981;78(5):501–5. [PubMed] [Google Scholar]
- 31.Libin A, Tinsley EA, Nash MS, Mendez A, Burns P, Elrod M, et al. Cardiometabolic risk clustering in spinal cord injury: results of exploratory factor analysis. Top Spinal Cord Inj Rehabil. 2013;19(3):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nash MS, Mendez AJ.. A guideline-driven assessment of need for cardiovascular disease risk intervention in persons with chronic paraplegia. Arch Phys Med Rehabil. 2007;88(6):751–57. [DOI] [PubMed] [Google Scholar]
- 33.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R.. Increased cardiovascular disease risk in Swedish persons with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med. 2010;42(5):489–92. [DOI] [PubMed] [Google Scholar]
- 34.Kip KE, Marroquin OC, Kelley DE, Mendez A, Burns P, Elrod M, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the women's Ischemia syndrome evaluation (WISE) study. Circulation. 2004;109(6):706–13. [DOI] [PubMed] [Google Scholar]
- 35.D'Agostino RB, Sr., Vasan RS, Pencina MJ., Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 36.Gander J, Sui X, Hazlett LJ, Cai B, Hebert JR, Blair SN.. Factors related to coronary heart disease risk among men: validation of the Framingham risk score. Prev Chronic Dis. 2014;11:E140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo F, Garvey WT.. Development of a Weighted cardiometabolic Disease Staging (CMDS) System for the prediction of Future diabetes. J Clin Endocrinol Metab. 2015;100(10):3871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo F, Moellering DR, Garvey WT.. The progression of cardiometabolic disease: validation of a new cardiometabolic disease staging system applicable to obesity. Obesity (Silver Spring). 2014;22(1):110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuk JL, Ardern CI, Church TS, Sharma AM, Padwal R, Sui X, et al. Edmonton obesity Staging System: association with weight history and mortality risk. Appl Physiol Nutr Metab. 2011;36(4):570–76. [DOI] [PubMed] [Google Scholar]
- 40.Sharma AM, Kushner RF.. A proposed clinical staging system for obesity. Int J Obes (Lond). 2009;33(3):289–95. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121(15):1768–77. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman JA, Hammond FM, Barringer TA, Norton HJ, Goff DC, Bockenek WL, et al. Comparison of coronary artery calcification scores and National Cholesterol Education program guidelines for coronary heart disease risk assessment and treatment paradigms in individuals with chronic traumatic spinal cord injury. J Spinal Cord Med. 2011;34(2):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vigna LM, Brunani A, Gori F, Mussino F, Tomaino SCM, Agnelli GM, et al. Edmonton obesity Staging System (EOSS) and work Ability in the evaluation of Workers affected by obesity: A Preliminary report. J Occup Environ Med. 2018;60(8):732–6. [DOI] [PubMed] [Google Scholar]
- 44.Grammatikopoulou MG, Chourdakis M, Gkiouras K, Roumeli P, Poulimeneas D, Apostolidou E, et al. Edmonton obesity staging system among pediatric patients: a validation and obesogenic risk factor analysis. J Endocrinol Invest. 2018;41(8):947–57. [DOI] [PubMed] [Google Scholar]
- 45.Chiappetta S, Stier C, Squillante S, Theodoridou S, Weiner RA.. The importance of the Edmonton obesity Staging System in predicting postoperative outcome and 30-day mortality after metabolic surgery. Surg Obes Relat Dis. 2016;12(10):1847–55. [DOI] [PubMed] [Google Scholar]
- 46.Hadjiyannakis S, Buchholz A, Chanoine JP, Jetha MM, Gaboury L, Hamilton J, et al. The Edmonton obesity Staging System for Pediatrics: A proposed clinical staging system for paediatric obesity. Paediatr Child Health. 2016;21(1):21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill RS, Karmali S, Sharma AM.. The potential role of the Edmonton obesity staging system in determining indications for bariatric surgery. Obes Surg. 2011;21(12):1947–9. [DOI] [PubMed] [Google Scholar]
- 48.Alberti KG, Zimmet PZ.. Definition, diagnosis and classification of diabetes mellitus and its complications. part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. [DOI] [PubMed] [Google Scholar]
- 49.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/national heart, lung, and blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. [DOI] [PubMed] [Google Scholar]
- 50.Expert Panel on Detection E, Treatment of high blood Cholesterol in A. Executive Summary of The Third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, evaluation, And Treatment of high blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 51.Alberti KG, Zimmet P, Shaw J.. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International diabetes Federation. Diabet Med. 2006;23(5):469–80. [DOI] [PubMed] [Google Scholar]
- 52.de Groot S, Adriaansen JJ, Tepper M, Snoek GJ, van der Woude LH, Post MW.. Metabolic syndrome in people with a long-standing spinal cord injury: associations with physical activity and capacity. Appl Physiol Nutr Metab. 2016;41(11):1190–6. [DOI] [PubMed] [Google Scholar]
- 53.Lee MY, Myers J, Hayes A, Madan S, Froelicher VF, Perkash I, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med. 2005;28(1):20–5. [DOI] [PubMed] [Google Scholar]
- 54.Li C, DiPiro ND, Cao Y, Szlachcic Y, Krause J.. The association between metabolic syndrome and pressure ulcers among individuals living with spinal cord injury. Spinal Cord. 2016;54(11):967–72. [DOI] [PubMed] [Google Scholar]
- 55.Liang H, Chen D, Wang Y, Rimmer JH, Braunschweig CL.. Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil. 2007;88(9):1198–204. [DOI] [PubMed] [Google Scholar]
- 56.Maruyama Y, Mizuguchi M, Yaginuma T, Kusaka M, Yoshida H, Yokoyama K, et al. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord. 2008;46(7):494–9. [DOI] [PubMed] [Google Scholar]
- 57.Hamwi G. Therapy: changing dietary concept. In: Danowski TS, ed. Diabetes mellitus: diagnosis and Treatment. New York, NY: American Diabetes Association. 1964:73–8. [Google Scholar]
- 58.Shah B, Sucher K, Hollenbeck CB.. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract. 2006;21(3):312–9. [DOI] [PubMed] [Google Scholar]
- 59.Landis JR, Koch GG.. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 60.Jung IY, Kim HR, Chun SM, Leigh JH, Shin HI.. Severe spasticity in lower extremities is associated with reduced adiposity and lower fasting plasma glucose level in persons with spinal cord injury. Spinal Cord. 2017;55(4):378–82. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan SD, Nash MS, Tefara E, Tinsley E, Groah S.. Relationship between Gonadal Function and cardiometabolic risk in Young Men With Chronic spinal cord injury. PM R. 2018;10(4):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen KJ, Leslie SW.. Autonomic Dysreflexia. Treasure Island (FL: ): StatPearls; 2018. [PubMed] [Google Scholar]
- 63.Hubli M, Krassioukov AV.. Ambulatory blood pressure monitoring in spinal cord injury: clinical practicability. J Neurotrauma. 2014;31(9):789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.West CR, Mills P, Krassioukov AV.. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord. 2012;50(7):484–92. [DOI] [PubMed] [Google Scholar]
- 65.McColl MA, Gupta S, Smith K, McColl A.. Promoting long-term health among people with spinal cord injury: What's New? Int J Environ Res Public Health. 2017;14(12):1–11. doi: 10.3390/ijerph14121520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bunning K, Gona JK, Newton CR, Hartley S.. Caregiver perceptions of children who have complex communication needs following a home-based intervention using augmentative and alternative communication in rural Kenya: an intervention note. Augment Altern Commun. 2014;30(4):344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McColl MA, Forster D, Shortt SE, et al. Physician experiences providing primary care to people with disabilities. Healthc Policy. 2008;4(1):e129–47. [PMC free article] [PubMed] [Google Scholar]
- 68.Hagen EM, Grimstad KE, Bovim L, Gronning M.. Patients with traumatic spinal cord injuries and their satisfaction with their general practitioner. Spinal Cord. 2012;50(7):527–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.