Abstract
Recently the use of bioactive α-glucosidase inhibitors for the treatment of diabetes have been proven to be the most efficient remedy for controlling postprandial hyperglycemia and its detrimental physiological complications, especially in type 2 diabetes. The carbohydrate hydrolysing enzyme, α-glucosidase, is generally competitively inhibited by the α-glucosidase inhibitors and results in the delayed glucose absorption in small intestine, ultimately controlling the postprandial hyperglycemia. Here we have reviewed the most recent updates in the bioactive α-glucosidase inhibitors category. This review provides an overview of the α-glucosidase inhibitory potentials and efficiency of controlling postprandial hyperglycemia of various bioactive compounds such as flavonoids, phenolic compound, polysaccharide, betulinic acid, tannins, anthocyanins, steroids, polyol, polyphenols, galangin, procyanidins, hydroxyl-α-sanshool, hydroxyl-β-sanshool, erythritol, ganomycin, caffeoylquinic acid, resin glycosides, saponins, avicularin, oleanolic acids, urasolic acid, ethanolic extracts etc., from various dietary and non-dietary naturally occurring sources.
Keywords: α-glucosidase inhibitors, Postprandial hyperglycemia, Diabetes, ROS
Nomenclature
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- AGEs
advanced glycation end products
- AGIs
α-glucosidase inhibitors
- AMSTAR
A Methodological Tool to Assess Systematic Reviews
- Arg
Arginine
- Asn
Asparagine
- Asp
Aspertic Acid
- BPD
B-type procyanidin dimer
- CD
Circular Dichroism
- CP
Cyclocarya paliurus
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- FDA
Food and Drug Administration
- FRAP
Ferric Reducing Antioxidant Power
- GLP1
Glucagon like Peptide 1
- Gln
Glutamine
- Glu
Gultamic Acid
- Glut 4
Glucose Transporter Type-4
- GSH
Glutathione
- HAS
hydroxyl-α-sanshool
- HBS
hydroxyl-β-sanshool
- His
Histidine
- HMG-CoA reductase
3-hydroxy-3-methyl glutaryl coenzyme A reductase
- IC50
Half maximal Inhibitory Concentration
- IDF
International Diabetes Federation
- Ile
Isoleucine
- Lys
Lysine
- Met
Methionine
- ORAC
Oxygen Radical Absorbance Capacity
- PCOS
Polycystic Ovarian Syndrome
- Phe
Phenylalanine
- PKC
Protein Kinase C
- pNPG
p-Nitrophenol-αD-Glucopyranoside
- PPG
postprandial glucose
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PTP1
Protein Tyrosine Phosphatase 1B
- ROS
Reactive Oxygen Species
- Ser
Serine
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- Trp
Tryptophan
- Tyr
Tyrosine
- UCP1
Uncoupling Protein 1
- Val
Valine
- WHO
World Health Organization
1. Introduction
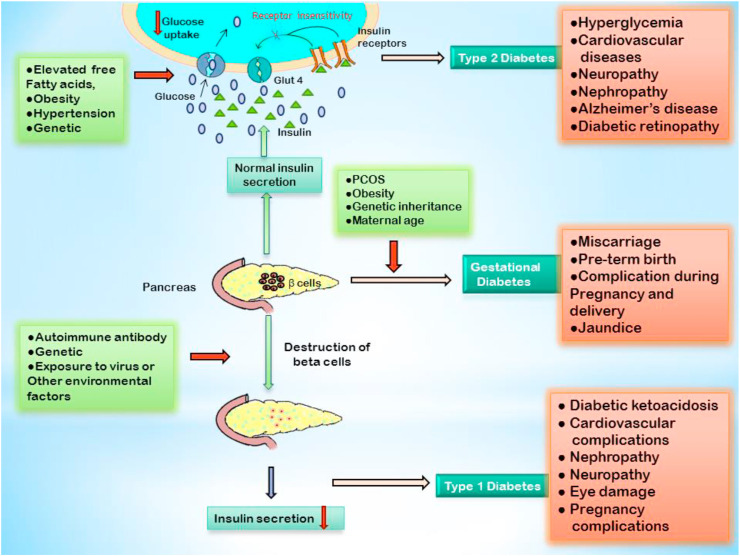
Diabetes is a group of metabolic disorders corresponding with prolonged high blood sugar level. According to worldwide survey by the World Health Organization (WHO) (2016), 422 million people are globally effected by diabetes mellitus and it will be the 7th leading cause of death by 2030. Currently, 72.9 million people in India are the victims of diabetes mellitus, according to the International Diabetes Federation (IDF), 2017 (Cho et al., 2018). Depending on the mechanism by which it occurs, diabetes can be of three types: Type 1 diabetes (T1D), Type 2 diabetes (T2D) and Gestational diabetes. T1D effects about 5–10% of the total diabetic patients and is caused due to autoimmune mediated selective destruction of β cells of pancreas that produce insulin, leading to absolute insulin deficiency, hyperglycemia, oxidative stress, inflammation and other metabolic complications (Li et al., 2014; Rashid et al., 2017). The prevalence of T2D is about 90% among the global diabetic patients and will reach 592 million by the end of 2035 (Zimmet et al., 2001). Insulin resistance due to insulin receptor (IR) insensitivity, chronic hyperglycemia, low grade inflammation, dyslipidemia are the features of T2D (Esser et al., 2015; Zimmet et al., 2001). As the name suggests, gestational diabetes is diagnosed in pregnant women, featuring adverse clinical condition in mother and offspring (Association, 2013) [Fig. 1 ]. Hyperglycemia is the most critical criteria of all types of diabetes and its consistency leads to various complications such as cardiovascular disorders, kidney failure, neuropathy, lipid metabolism disorders, etc. So, controlling the blood glucose level in diabetic patients is most vital (Bello et al., 2014; Jiao et al., 2018). Various bioactive molecules have been reported to ameliorate different pathophysiological conditions (Basak et al., 2017; Das et al., 2009; Ghosh et al., 2017; Manna et al., 2008, 2009; 2010, 2012; Sarkar et al., 2016; Sinha et al., 2007). Accordingly, in recent updates on the treatment of diabetes mellitus, α-glucosidase inhibitors (AGIs) from various plant sources are trending for their ability to inhibit α-glucosidase activity leading to reduction of hydrolytic cleavage of non-reducing ends of dietary oligosaccharides and diminished release of α-glucose (Kumar et al., 2011), that retard carbohydrate digestion and absorption of glucose in small intestine. This mechanism of action plays an important role in controlling postprandial hyperglycemia, which is one of the modern therapeutic approach towards stabilizing blood glucose level in diabetic patients especially in T2D (Ghani, 2015). Anti-diabetic drugs having α-glucosidase inhibiting properties such as acarbose, voglibose, miglitol and emiglitate are now commercially available for controlling postprandial hyperglycemia. Nevertheless, regular consumption of these drugs leads to various side effects such as diarrhoea, vomiting, flatulence, severe stomach pain, allergic reactions, etc., (Krentz and Bailey, 2005; Patil et al., 2015). So, in spite of these commercially available efficient AGIs, researchers are still engaged in the discovery of new bioactive AGIs with high inhibitory potential and least side effects. Here, in this overview, we have focused on the most recent discoveries of AGIs from naturally occurring dietary and non-dietary sources. Recent updates include flavonoids, phenolic compounds, polysaccharides, betulinic acid, tannins, anthocyanins, steroids, polyols, oleanolic acids, urasolic acid, ethanolic extracts, etc., from various natural sources such as potatoes, berries, persimmon, guava, red cabbage, beans, mushrooms, medicinal plants, etc (Booth, 2001).
Fig. 1.
Schematic diagram of different types of diabetes, their cause and consequences. [Glut 4, Glucose Transporter Type-4; PCOS, Polycystic Ovarian Syndrome].
2. Review methodology
The data for this overview have been obtained, analysed and summarized based on the following principles.
2.1. Search strategy and selection criteria
In this review, the literature survey mainly focussed on scientific research papers published in the last five years. The search strategy involved the use of keywords like α-glucosidase inhibitors, postprandial hyperglycemia and α-glucosidase inhibitors, diabetic complications and α-glucosidase inhibitors, reactive oxygen species and α-glucosidase inhibitors, enzyme kinetics of α-glucosidase inhibitors, plant sources of α-glucosidase inhibitors etc. The databases used in course of the search were Google Scholar and National Centre for Biotechnology Information (NCBI). In vitro studies taken under consideration, were obtained from reports related to ganomycin from lingzhi mushrooms, polysaccharides from guava juice etc. Clinical significance of studies like those involving oleanolic acid and ursolic acid from Queen's crepe-myrtle have been incorporated.
2.2. Data extraction and analysis
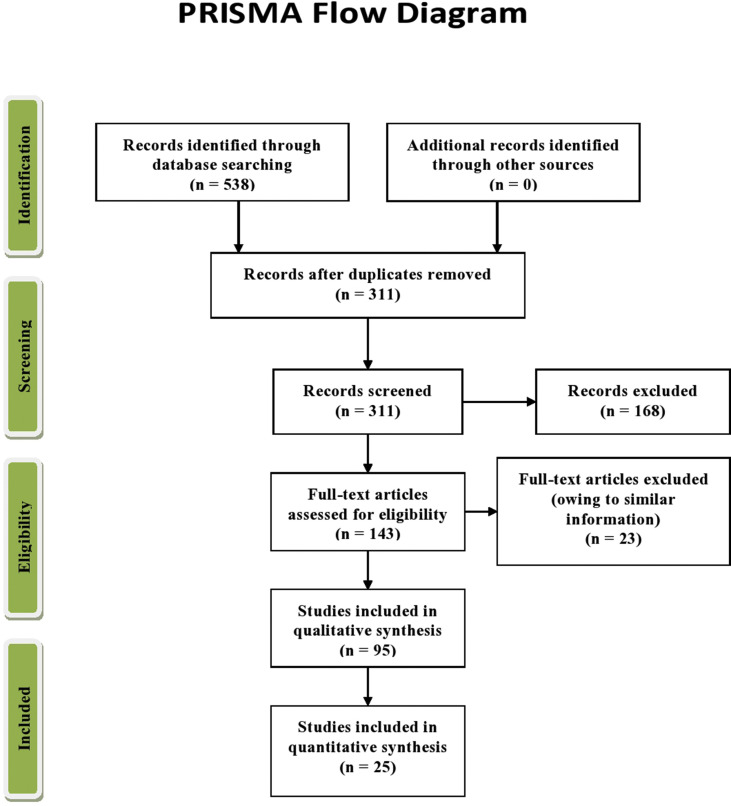
Since an overview is partially synonymous to a systematic review, therefore the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist and PRISMA flow diagram were consulted for improvement of the review in terms of data extraction and analysis [Fig. 2 ] (Liberati et al., 2009). The PRISMA checklist has been included as Supplementary material I.
Fig. 2.
PRISMA flow diagram.
2.3. Risk-of-bias assessment
Based on A Methodological Tool to Assess Systematic Reviews (AMSTAR) tool, this review has been categorized as a moderate quality review in terms of risk-of-bias assessment (Shea et al., 2017). The AMSTAR report has been included as Supplementary material II.
3. Result of literature study and analyses
The key findings of this review have been summarized in Table 1 .
Table 1.
Key findings of this review.
| Type of α-glucosidase inhibitor | Source | Nature | Observation |
|---|---|---|---|
| Polysaccharide | Guava | Guava juice | Antioxidant |
| Anthocyanin | Red cabbage, Potatoes, Black current | Extract | Antioxidant |
| Betulinic acid | Chinese date tree | Pure form | Hypoglycemic |
| Tannin | Persimmon | Extract | Hypoglycemic |
| Phenols | Berries, Millets | Extract | Antioxidant |
| Erythritol | Grapes | Pure form | Competitive inhibition |
| Quercetin | Tea leaves | Extract | Competitive inhibition |
| Galangin | Rhizome of herbs | Extract | Competitive inhibition |
| Procyanidins | Apples | In-silico analysis | Competitive inhibition |
| Sanshools | Shinchuan pepper | Pure form | Competitive inhibition |
| Flavonoids | Princess tree, Asparagus | Extract | Competitive inhibition |
| Fucoxanthine | Sea weed | Extract | Mixed type inhibition |
| Ganomycin derivatives | Lingzhi mushroom | Synthesized | Cholesterol biosynthesis |
| Oleanolic and Ursolic acid | Queen's crepe-myrtlu | Pure form | Hypoglycemic |
| Cholesterol derivatives | Thai medicinal plants | Extract | Antioxidants |
| Stigmasterol | Mimosa pudica | Pure form | Metal chelator |
| Resin glycosides | Morning glory | In-silico analysis | Competitive inhibition |
4. Role of α-glucosidase inhibitors in controlling postprandial hyperglycemia
Hyperglycemia is a critical condition in both T1D and T2D patients and is the main contributing factor behind oxidative stress and its deleterious consequences. Hyperglycemia can induce ROS (reactive oxygen species) generation and accumulation via various metabolic pathways (Vanessa Fiorentino et al., 2013). In diabetic condition, as glucose uptake in insulin dependent tissues (fat and muscle) is minimized, uptake of glucose is elevated in insulin independent tissues (King and Loeken, 2004). This excessive intracellular glucose is converted to the polyalcohol sorbitol, resulting in decrease of NADPH/NADP + ratio and glutathione (GSH) concentration. In addition, hyperglycemia leads to activation of PKC (protein kinase C) isoforms, induction of hexokinase pathway and over production of advanced glycation end products (AGEs). All these effects of hyperglycemia are responsible for diminishing antioxidant agents and overproduction and accumulation of reactive oxygen species, which ultimately leads to oxidative stress (King and Loeken, 2004; Vanessa Fiorentino et al., 2013). The detrimental consequences of this oxidative stress condition are long term damage and pathophysiological conditions of various organs like kidney, heart, liver, testis, spleen etc. (Chowdhury et al., 2016; Ghosh et al., 2018; Pal et al., 2014; Rashid and Sil, 2015),. For this reason, researchers are trying to control hyperglycemic condition in order to reduce various diabetic complications. The most eminent hyperglycemia controlling agents are the AGIs, which slow down α-glucosidase activity and efficiently diminish postprandial hyperglycemia. According to the guidelines of IDF, AGIs in combination with insulin, metformin and sulfonylureas is the best treatment option for uncontrolled hyperglycemia in diabetic patients (Lozano et al., 2016; Ghosh et al., 2018).
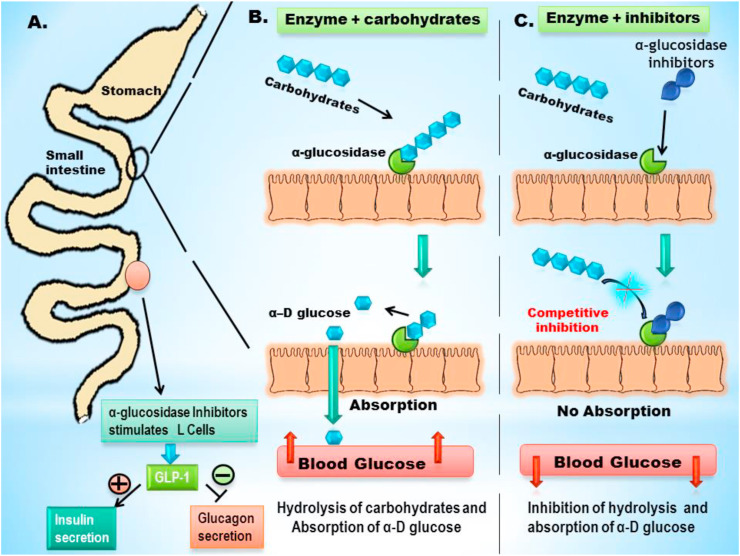
Almost all AGIs structurally resemble disaccharides or oligosaccharides and can bind to the active site of α-glucosidase, forming complexes with stronger affinity than that of carbohydrate-α-glucosidase complex. This results in the competitive inhibition of α-glucosidase activity and diminishes carbohydrate hydrolysis and glucose absorption in the brush boarder site of small intestine [Fig. 3 ].
Fig. 3.
Role of α-glucosidase inhibitors in controlling postprandial hyperglycemia; (A) schematic diagram of small intestine; stimulation of L cells by α-glucosidase inhibitors and secretion of glucagon like peptide-1 (GLP-1) from lower part of small intestine; (B) hydrolysis of carbohydrates by α-glucosidase along the brush border of small intestine, release of α-D glucose and its absorption leading to elevation of blood glucose level; (C) Competitive inhibition of α-glucosidase by its inhibitors and restrict the hydrolysis of carbohydrates and absorption of α-D glucose, leading to decrease in postprandial elevation of blood glucose level.
A study on the inhibitory effects of Eucomis humilis “Baker bulb”, commonly called dwarf pineapple flower, on carbohydrate metabolizing enzymes, sheds light on the enzyme kinetics of its α-glucosidase inhibitory activity. It has been reported that ethanolic extract of E. humilis exhibits highest percentage of α-glucosidase inhibition compared to aqueous and hydro-ethanolic extracts. Ethanolic extract of E. humilis also exhibited the lowest half maximal inhibitory concentration (IC50) for α-glucosidase, thereby indicating strong α-glucosidase inhibition. To analyse the mode of inhibition of α-glucosidase by the ethanolic extract of E. humilis, the kinetics of inhibition was studied using Lineweaver-Burk plots. The result revealed that α-glucosidase was inhibited by the ethanolic extract of E. humilis through the competitive route. This indicated that the active ingredient of the extract resembled the normal substrate of α-glucosidase structurally and could bind to the active site of the enzyme instead of the normal substrate (Kazeem et al., 2017). Thus, α-glucosidase inhibitors function through competitive inhibition.
Most of the carbohydrates that are not hydrolysed are subsequently broken down in lower parts of small intestine and result in delayed glucose absorption after meal (Mehta et al., 1998; Patil et al., 2015). This mechanism of action of AGIs reduces the postprandial hyperglycemia, which is an efficient remedy against various diabetic complications. Another striking characteristic of AGIs is that it can assist in the stimulation of glucagon like peptide (GLP1) (an incretin hormone) secretion, that helps lowering the postprandial hyperglycemia by triggering insulin secretion and inhibiting glucagon secretion (Drucker and Nauck, 2006). GLP1 is secreted from intestinal L cells, on sensing food intake. AGIs delay polysaccharide digestion that results in increased local carbohydrate concentration in the lower gut. Since, lower gut has sufficient amount of GLP1 secreting cells, belated carbohydrate absorption helps to stimulate GLP1 secretion properly. Thus, AGI helps in GLP1 secretion, which in turn stimulates insulin secretion (Patil et al., 2015).
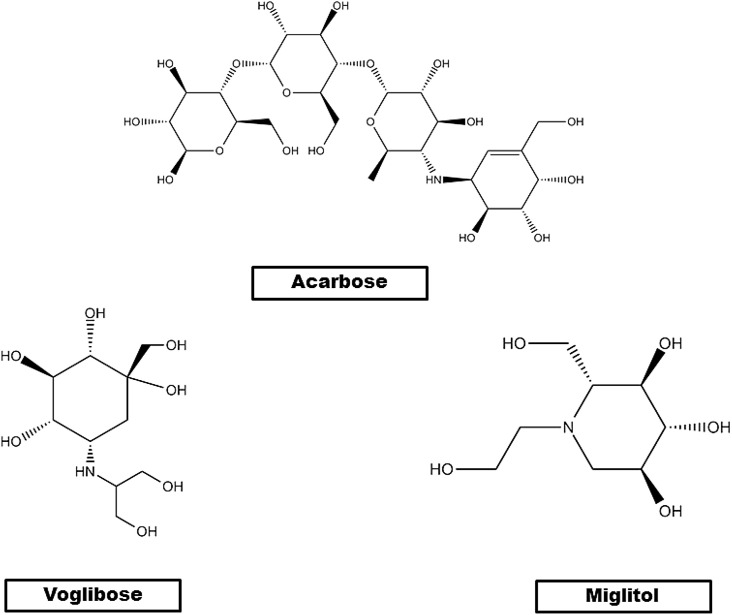
The most featured AGIs are acarbose, voglibose, and miglitol [Fig. 4 ]. Acarbose, first obtained from various Actinomycetes, is a nitrogen-containing pseudo-tetrasaccharide (Wehmeier and Piepersberg, 2004). It was the first drug in AGI category to be approved by Food and Drug Administration (FDA) with the commercial name ‘Precrose’ in USA. Acarbose acts locally on the small intestinal brush border cells (GODA et al., 1982; Pyner et al., 2017), delaying release of glucose from polysaccharides by competitively binding with α-glucosidase and lowering PPG level (Drucker and Nauck, 2006; Ketema and Kibret, 2015). The second traditional AGI, Voglibose, is a valiolamine derivative and is a research product of Takeda Chemical Industries of Japan (Dimitriadis et al., 1985; Madar and Omursky, 1991; Patil et al., 2015). Voglibose hinders uptake and metabolism of polysaccharides by reversibly inhibiting carbohydrate digestive enzymes. Since, voglibose does not inhibit pancreatic α-amylase and lactase, it makes voglibose more selective than acarbose as a disaccharide inhibitor (Baron, 1998; Kalra, 2014). Voglibose also enhances the release of glycogen like peptide 1 (GLP1) (Wehmeier and Piepersberg, 2004). Miglitol, a derivative of nojirimycin, the first pseudo-monosaccharide α-glucosidase inhibitor, was approved by FDA in 1996. Miglitol is almost fully absorbed in the small intestine and lowers postprandial glucose (PPG) (Yee and Fong, 1996). Recent findings by Sugimoto et al. shows that miglitol upregulates the expression of uncoupling protein 1 (UCP1) present in brown fat. Thus, miglitol increases energy expenditure in diet induced obese mice through β3-adrenergic receptor-cAMP-protein kinase A pathway (GODA et al., 1982; Pyner et al., 2017). This finding can be correlated with postprandial energy expenditure in T2D diabetes regarding diet therapy (Coniff et al., 1995).
Fig. 4.
Chemical structures of commercially available α-glucosidase inhibitors.
In order to overcome the side effects of the traditional AGIs, discoveries of new bioactive inhibitors continue. In this review, we have categorized the natural sources and mechanistic details of recently updated bioactive α-glucosidase inhibitors into dietary and non-dietary sources.
5. Dietary sources and their bioactive α-glucosidase inhibitors
5.1. Anthocyanin and polyphenols in red cabbage
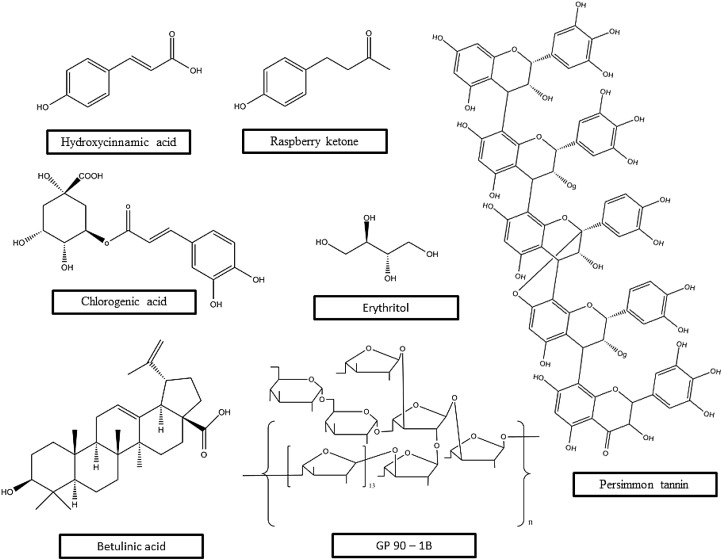
Red cabbage (Brassica oleracea capitatarubra) is a popular vegetable consumed all over the world, mainly cultivated in North America, Japan, China and Europe. It is composed of high amount of phenolic components having a large proportion of anthocyanins (Wu et al., 2006) and is well known for its anti-diabetic effects (Kataya and Hamza, 2008). Podsedek et al. found that the polyphenol levels differ in different varieties. Koda variety was found to have maximum levels of polyphenols, whereas Kissendrup variety has maximum level of anthocyanin (Podsedek et al., 2017). The IC50 value of Koda and Kissendrup in context of α-glucosidase inhibition was found to be 3.87 and 4.97 mg of dry weight per ml respectively, which are several folds lower than acarbose (0.5 mg/mL). Podsedek et al. found 18–21 types of anthocyanin in red cabbage which was in accordance with other studies, although the content may vary depending on vegetation period of the plants (Wiczkowski et al., 2014). Mizgier et al. found 21 hydroxycinnamic acid derivatives from red cabbage which were made up of residues of antioxidant molecules like ferulic acid and p-coumaric acid [Fig. 5 ]. The antioxidant property of red cabbage extract was confirmed by ferric reducing antioxidant power (FRAP) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method (Mizgier et al., 2016). Ethanolic extract of red cabbage (1 g/kg body weight, daily) was found to improve weight loss of diabetic rats (Kataya and Hamza, 2008). 4-methylumbelliferone (4-MUG) based α-glucosidase inhibition assay established red cabbage extracts as AGIs (Podsedek et al., 2017).
Fig. 5.
Chemical structures of some bioactive α-glucosidase inhibitors from dietary sources.
5.2. Phenolic compounds in berries
Berries are rich in polyphenols (100–300 mg/100 g), anthocyanins, ellagic acid derivatives etc., and are well-known α-glucosidase inhibitors (Edirisinghe and Burton-Freeman, 2016; Yin et al., 2012) (Boath et al., 2012; McDougall et al., 2005). Studies showed that different variants of raspberry phenolic extract have antioxidant property and acts as an inhibitor to starch digestive enzymes (Zhang et al., 2010). Recently raspberry ketone, one of the well-known key compounds present in raspberry responsible for its aroma, was characterized as an AGI having an IC50 value of 6.17 mM [Fig. 5]. In silico protein ligand docking simulation revealed that several key residues (ASP68, TYR71, HIS111, PHE157, PHE158, PHE177, GLN181, ASP214, THR215, ASP349, ASP408, and ARG439) of isomaltase (PDB: 3AJ7) interacts with the (–OH) group of raspberry ketone. Interestingly, kinetic analysis showed that the compound binds reversibly and non-competitively with α-glucosidase (Xiong et al., 2018). Phenolic compound rich ethyl acetate soluble extract of mulberry fruit (Morus alba L.) showed re-alteration of antioxidant enzyme activity like catalase, superoxide dismutase, glutathione dismutase as well as lowering of fasting blood glucose level in streptozotocin induced diabetes rats (Wang et al., 2013).
5.3. Tannins in persimmon
Persimmons are edible fruits of several species of trees under genus Diospyros and are mostly native to tropical regions with few distributed in temperate regions (Germplasm Resources Information Network, 2016). Tannins are polyphenolic biomolecules and can be extracted by widely variable procedures from various plant sources. The extraction and purification of persimmon tannins are done from astringent persimmon (Li et al., 2010). In a recent study, the inhibitory effect of persimmon tannin on α-glucosidase and its role in decreasing postprandial blood glucose level has been clearly manifested in rat model [Fig. 5]. Li et al., experimentally showed that IC50 of persimmon tannin and acarbose (positive control) on α-glucosidase were 0.2391 and 0.2445 mg/ml, respectively, pointing out a similar potential of tannin extracted from persimmon on inhibiting α-glucosidase in comparison to the positive control. Moreover, persimmon tannin has a strong binding potential with starch granules, resulting in reduction of starch digestibility and hence decreases postprandial hike of blood glucose level (Li et al., 2018).
5.4. Anthocyanin and polyphenols in potatoes
Potatoes are major cheap food crop cultivated and consumed all over the world. Although there are no reports regarding potatoes preventing diabetes, some studies suggests that polyphenols present in potato tubers may act as AGIs (Kalita et al., 2018). Total content of polyphenols and anthocyanins were found be greater in red and purple potato tubers than white and yellow tubers. Mass spectrometric analysis revealed that the main constituent of potato extract is chlorogenic acid and its different isomers namely petunidin-3-coumaroylrutinoside-5-glucoside, Peonidin-3-coumaroyllrutinoside-5-glucoside, malvidin-3-coumaroylrutinoside-5-glucoside, cyanidin-3-coumaroylrutinoside-5-glucoside, Pelarogonidin-3-caffeoylrutinoside-5-glucoside, Pelarogondin-3-feruloylrutinoside-5-glucoside (Kalita et al., 2018) [Fig. 5]. The methanolic extract showed radical scavenging property in DPPH, ABTS, ORAC assays and the total phenolic content was found to be strongly correlated with radical scavenging property with different verity (Kalita and Jayanty, 2014). The mode of α-glucosidase inhibition by the extracts from potatoes was found to be both non-competitive and mixed type and the IC50 value ranges between 42.42 and 78.65 μg/mL, lower than acarbose (15.65 μg/mL) (positive control) but causes least side effects (Kalita et al., 2018).
5.5. Erythritol
Erythritol, a sugar alcohol (C4 polyol), occurs naturally in grapes, pears, watermelon, mushrooms and in some fermented items such as sake, wine, etc. (Shindou et al., 1988; Wen et al., 2018) [Fig. 5]. Recently, erythritol is considered as a substitute for sucrose for diabetic and overweight individuals and is also approved as safe food additives in many countries due to its zero-caloric value and is rapidly absorbed in proximal intestine (Rzechonek et al., 2018). In a study, the beneficiary role of erythritol in diabetic rat was assessed and showed that it has long term anti-hyperglycemia potentials and can reduce the kidney damage caused due to oxidative stress led by hyperglycemia (Yokozawa et al., 2002). In a recent study, it was demonstrated that erythritol can control postprandial hyperglycemia by inhibiting α-glucosidase. Erythritol strongly inhibit α-glucosidase with IC50 value of 6.43 mg/mL (52.7 mM). Enzyme kinetics study reveals that erythritol exhibit competitive inhibition by binding to the active site of α-glucosidase. The (-OH) group on C1 atom of erythritol forms hydrogen bonds with Asp69 and Arg446 in active site of α-glucosidase via H113 water molecules and another (-OH) group on C4 atom form identical bonds with Asp215, Arg213, and Asp352 via H132 water molecules, clearly suggesting the occupancy of erythritol in the active site of α-glucosidase (Wen et al., 2018). So, erythritol can be considered as a potential α-glucosidase inhibitor and it can overcome the drawbacks of the traditional α-glucosidase inhibitors due to its negligible side effects and caloric value.
5.6. Betulinic acid
Betulinic acid (BA) is a naturally occurring pentacyclic triterpenoid found in various food sources such as winged bean (Psophocarpus tetragonolobus), persimmon, chinese date tree (Ziziphus mauritiana), etc., and in many flowering plants (Ali-Seyed et al., 2016; Ding et al., 2018b) [Fig. 5]. It has been reported that BA possess a remarkable anti-diabetic potential and it can reduce blood glucose concentration by inhibiting α-glucosidase activity and regulating various signaling pathways in diabetic mice (Vinayagam et al., 2017). In enzyme kinetics study, it was revealed that BA binds to α-glucosidase by competing with pNPG (p-nitrophenol-αD-glucopyranoside), a substrate used for the assay of α-glucosidase activity. Moreover, BA can also efficiently bind with α-glucosidase-pNPG complex to form a tertiary complex. These results indicate that α-glucosidase inhibition by BA is a mixed competitive inhibition. The inhibition by BA is a reversible process and interaction between BA and α-glucosidase involves non-covalent bonds (Ding et al., 2018b; Han et al., 2017). Estimated IC50 value of BA was (1.06 ± 0.02) × 10−5 mol L−1 and that of acarbose (positive control) was (1.76 ± 0.03) × 10−4 mol L−1, indicating that α-glucosidase inhibition by BA is 17 times lower than positive control. In spite of this result, BA would be more preferable than acarbose as it can overcome the side effects caused due to chronic medication involving acarbose or other synthetic anti-diabetic drugs (Ding et al., 2018b).
5.7. Polysaccharides in guava
Guavas (Psidium guajava) are the common tropical fruits, native to Mexico, Central America, northern & southern America and have extended throughout many tropical and subtropical regions (Compendium, 2017). In some studies, it has been demonstrated that in the treatment of diabetes mellitus, guava juice can be used as an adjuvant (Zhang et al., 2016). Extracts from guava can revive loss of body weight and have hypoglycemic effect in streptozotocin (STZ) induced diabetic rats (Huang et al., 2011). Polysaccharides that are water soluble have been reported to have anti-hyperglycemic properties (Hu et al., 2013). Zhang et al. have isolated GP90-1B (further purification of GP90) and P90 as water soluble polysaccharides from guava and have experimentally demonstrated their α-glucosidase inhibitory and antioxidant properties [Fig. 5]. GP90 and P90 showed a dose-dependent inhibitory effect on α-glucosidase with EC50 2.27 μg/mL and 0.18 mg/mL respectively, compared to positive control (acarbose) EC50 of 3.13 mg/mL. This suggests that P90 and GP90 have 17 and 1379 times higher inhibitory effect than positive control respectively (Zhang et al., 2016). In a recent study, Jiao et al. (2018), extracted a novel heteropolysaccharide, GP70-3 from guava and manifested its outstanding inhibitory effect on α-glucosidase. GP70-3 exhibited α-glucosidase inhibition in vitro, with IC50 value of 2.539 μM and that of acarbose was 4.744, indicating that GP70-3 has 1867 times higher inhibition activity then positive control (Jiao et al., 2018). Besides this remarkable inhibitory effect, both of these polysaccharides have 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities, especially GP90, having much higher activity than P90 and ascorbic acid (positive control) (Zhang et al., 2016). Hence, from these results, it is evident that polysaccharides in guava are responsible for controlling postprandial hyperglycemia and reducing oxidative stress.
Other than guava, other sources of polysaccharide α-glucosidase inhibitors are Abel fruit hull, fruit pulp pf Annona squamosa etc (Ren et al., 2017; Zhang et al., 2015). They can provide new avenues to anti-diabetic research.
5.8. Procyanidins from apples, grape seeds and cocoa beans
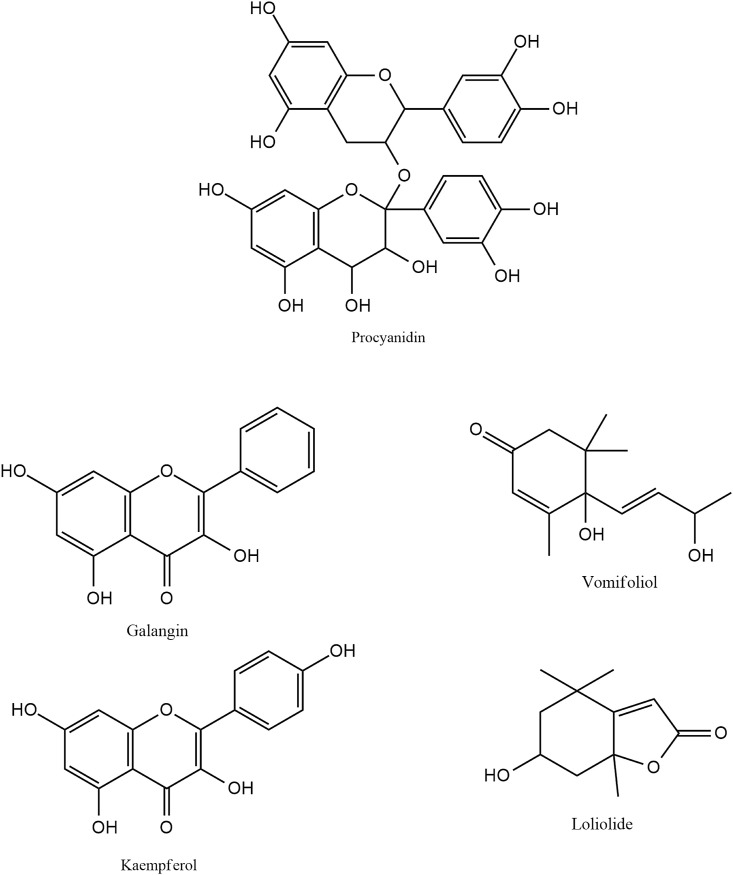
Procyanidins, obtained from apples, grape seeds, cocoa beans inhibit α-glucosidase activity [Fig. 6 ]. In silico analyses reveal that B-type procyanidin dimer (BPD) binds to the active site of α-glucosidase through both hydrophobic interactions and hydrogen bonding (Dai et al., 2019).
Fig. 6.
Chemical structures of some other bioactive α-glucosidase inhibitors from dietary sources.
5.9. Galangin from rhizome of Alpinia galanga
Galangin, a flavonol obtained from the rhizome of the edible herb Alpinia galanga, reversibly inhibits α-glucosidase activity [Fig. 6]. It does so via a monophasic kinetic process. It provokes a conformational change of α-glucosidase by generating an α-glucosidase-galgangin complex. Galangin interacts with the amino acid residues located within the active site of α-glucosidase enzyme, thereby preventing the entry of the actual substrate. This decreases the catalytic efficiency of the enzyme (Zeng et al., 2019).
5.10. Leaves and twigs of Sesbania grandiflora
The leaves and twigs of Sesbania grandiflora, an edible medicinal plant are found to be rich in flavonoids and terpenes such as vomifoliol, loliolide, kaempferol and quercetin which exhibit α-glucosidase inhibitory activity [Fig. 6] (Thissera et al., 2020).
5.11. α-glucosidase inhibitors in tea leaves
Extracts of Cyclocarya paliurus (CP) tea leaves inhibit α-glucosidase activity at an IC50 value of 31.5 ± 1.05 μg/mL which is very much lower than the IC50 value of 296.6 ± 1.06 μg/mL, i.e., of acarbose, the positive control (Ning et al., 2019). The active components of the extract with α-glucosidase inhibitory activity were quercetin-3-O-glucuronide, quercetin, kaempferol-3-O-rhamnoside, kaempferol, genistein and asiatic acid [Fig. 6]. Studies related to molecular docking have revealed that these components can occupy the active sites of α-glucosidase more easily than acarbose (Ning et al., 2019).
5.12. Phenolic antioxidants of selective breeding cultivations of foxtail and little millet
In a particular study, the major phenolic antioxidants in the soluble fraction of little millets have been found to be ferulic acid, sinapic acid and caffeic acid. However, ferulic and p-coumaric acids were abundant in the bound fractions. The phenolic antioxidants from little millets showed higher inhibitory potential against α-glucosidase than foxtail millet counterparts. Thus, millets can be used for the treatment of diabetes (Pradeep and Sreerama, 2018).
5.13. Anthocyanins from blackcurrant, blueberry and blue honeysuckle fruits
The anthocyanins obtained from the extracts of blackcurrant, blueberry and blue honeysuckle fruits are glycosidic anthocyanins. They are converted to anthocyanidins during acid hydrolysis and act as α-glucosidase inhibitors. They are mixed-type inhibitors which establish hydrogen bonds more efficiently to α-glucosidase than α-glucosidase-substrate complex (Zhang et al., 2019).
5.14. Hydroxyl-α-sanshool and hydroxyl-β-sanshool from sinchuan pepper
Sichuan pepper, a common ingredient for food seasoning, bears hydroxyl-α-sanshool (HAS) and hydroxyl-β-sanshool (HBS) as active components. It has been reported that both HAS and HBS inhibit α-glucosidase activity (IC50 value of 9.5 and 18.6 μg/mL) more strongly than that acarbose, the positive control (IC50 value of 241 μg/mL) (Li et al., 2020).
5.15. α-glucosidase inhibitors from edible seaweed
Fucoxanthin from extracts of edible brown seaweed Undaria pinnatifidae inhibits α-glucosidase activity through mixed type of inhibition (Zaharudin et al., 2019).
6. Non-dietary sources and their bioactive α-glucosidase inhibitors
6.1. Ganomycin from an active ingredient of lingzhi mushroom
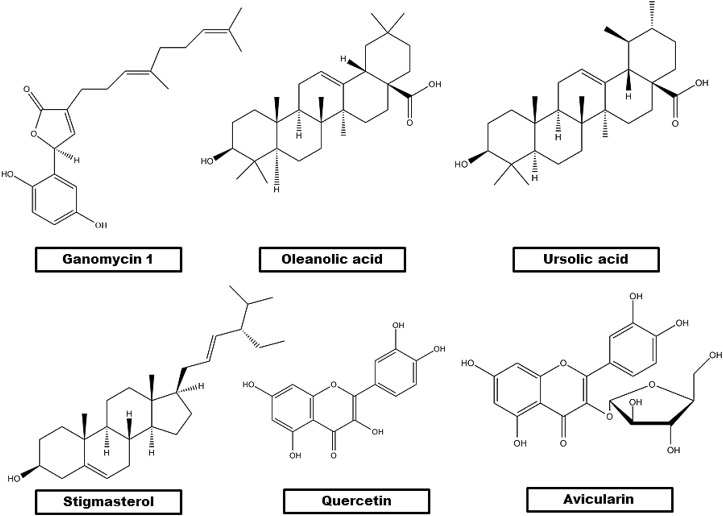
Polypore lingzhi mushroom (Ganoderma sp.) is an important source of Ganomycin I [Fig. 7 ]. It acts as a dual inhibitor of α-glucosidase and HMG-CoA reductase. 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMG-CoA reductase) catalyses the conversion of HMG-CoA to mevalonate, thereby augmenting cholesterol biosynthesis (Friesen and Rodwell, 2004). According to Liu et al. ganomycin I has been found to exhibit anti-diabetic effects on KK-Ay mice (Wang et al., 2017). The para-dihydroxyl benzene moiety in its induces chemical instability. To overcome this disadvantage, 14 ganomycin I derivatives were synthesized and screened for their dual inhibitory effect on α-glucosidase and HMG-CoA reductase activity in vitro. Of the 14 derivatives, (R, E)-5-(4-(tert-butyl)phenyl)-3-(4,8-dimethylnona-3,7-dien-1-yl)furan-2(5H)-one was found to exhibit substantial stability and potent dual inhibitory activity. Further in vivo studies revealed that gut microbiota augmented the therapeutic effects of this compound (Wang et al., 2018).
Fig. 7.
Chemical structures of bioactive α-glucosidase inhibitors from non-dietary sources.
6.2. Oleanolic acid and ursolic acid from Queen's crepe-myrtle
Six pentacyclic triterpenes isolated from Lagerstroemia speciosa (commonly called Queen's crepe-myrtle) leaves exhibited α-glucosidase activity as follows: corosolic acid > maslinic acid > oleanolic acid> 23- hydroxyursolic acid > arjunolic acid > asiatic acid (Hou et al., 2009). Of these, oleanolic acid and ursolic acid are isomers which vary in the position of a methyl residue connected to C-19 or C-20 position in their E ring (Sheng and Sun, 2011). The study focussed on oleanolic acid and ursolic acid as they can inhibit the increase of blood sugar level and diabetic complications (Castellano et al., 2013).
Spectrophotometric study of change in absorbance due to interaction of oleanolic acid and ursolic acid with α-glucosidase revealed that the relative activity of α-glucosidase gradually decreased with increase in the concentrations of oleanolic acid and ursolic acid in a dose-dependent manner [Fig. 7]. In comparison to ursolic acid, oleanolic acid was found to exhibit a higher inhibitory activity on α-glucosidase. Three-dimensional fluorescence spectroscopic studies showed that in comparison to ursolic acid, oleanolic acid exerted a greater inhibitory effect on α-glucosidase conformation. Adoption of circular dichroism (CD) technique revealed the increase in the α-helical and random coil contents conforming to the fact that the structure of α-glucosidase tends to be more condensed in the presence of oleanolic acid and ursolic acid, thereby inducing a decline in its stability and the α-glucosidase catalytic activity (Ding et al., 2018a). Studies have also shown that the binding of oleanolic acid to α-glucosidase induces its conformational change to facilitate the binding of ursolic acid thereby resulting in synergistic inhibitory effect on α-glucosidase activity. Oleanolic acid mainly interacts with amino acid residues Trp14, Ser295, Ala289, His258, Lys12, Tyr292, Lys262, Val265, Ile271 and Glu270 of α-glucosidase. On the other hand, ursolic acid interacts with amino acid residues such as Trp465, Glu405, Lys410, Asn411, Val407, Ser179, Arg180, Gln67, Gln66 and Met69. Molecular docking simulation experiments have revealed that hydrogen bond contributes immensely to the binding of oleanolic acid and ursolic acid to α-glucosidase (Ding et al., 2018a).
6.3. Stigmasterol, quercetin and avicularin from Mimosa pudica
In a particular study, α-glucosidase inhibitors from Mimosa pudica were isolated through a bioassay mediated fractionation approach. Repeated silica gel and sephadex LH 20 column chromatography of bioactive fractions resulted in the identification of stigmasterol, quercetin and avicularin whose IC50 values as compared to acarbose (351.02 ± 1.46 μg/mL) were found to be as 91.08 ± 1.54, 75.16 ± 0.92 and 481.7 ± 0.703 μg/mL respectively (Tasnuva et al., 2017) [Fig. 7]. Stigmasterol or Wulzen anti-stiffness factor is 3.8 fold more potent than acarbose. It acts as a metal chelator and lipid peroxide scavenger (Torres-Piedra et al., 2010). Quercetin is 4.6 times more potent than the acarbose. It protects the pancreas from oxidative stress (Li et al., 2009). Avicularin, a quercetin derivative, also showed potent inhibitory effect against α-glucosidase enzyme. Presence of a sugar moiety attached to the quercetin skeleton significantly reduces the scavenging power of this molecule (Kumar and Pandey, 2013).
6.4. Flavonoids from princess tree
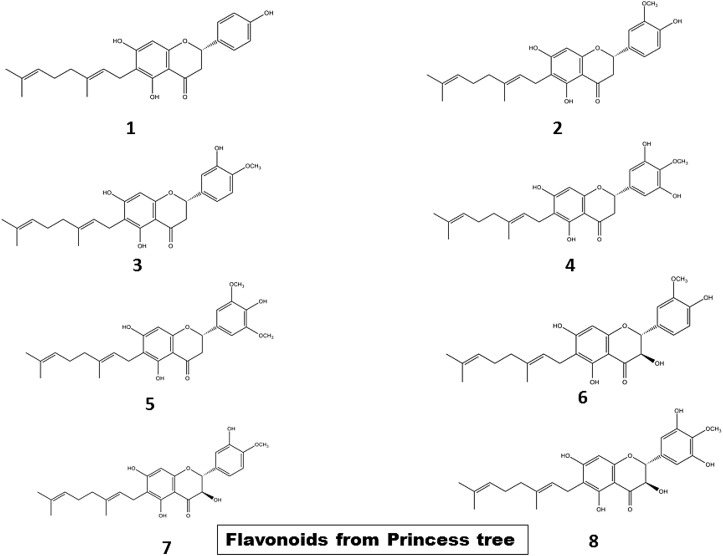
Paulownia tomentosa (princess tree) of the Paulowniaceae family, a deciduous tree widely spread in Korea, Japan and China, harbours a large pool of metabolites of which geranylated flavonoids are the major bioactive members (Hanáková et al., 2015; Schneiderová and Šmejkal, 2015; Šmejkal et al., 2007). Various studies have revealed the antioxidant effects of these compounds (Lee et al., 2014). Spectroscopic analyses have shown that these flavonoids are characterized by the presence of a geranyl group at their C-6 position. 8 such compounds isolated from methanolic extracts from princess tree include flavanones like mimulone, 30-O-methyldiplacone, 40-O-methyldiplacone, 6-geranyl-30, 5, 50,7-tetrahydroxy-40-methoxyflavanone, 30-O-Methyl-50 -O-methyldiplacone and dihydroflavonols like 30-O-methyldiplacol, 40-O-methyldiplacol, and 6-geranyl-3,30,5,50,7-pentahydroxy-40-methoxyflavane (Asai et al., 2008) [Fig. 8 ]. These components have been found to exhibit mixed type inhibition against PTP1B with IC50 value of 1.9–8.2 μM and noncompetitive inhibition towards α-glucosidase with IC50 value of 2.2–78.9 μM. Mimulone is most effective against PTP1B with IC50 value of 1.9 μM while 6-geranyl-3,30,5,50,7-pentahydroxy-40-methoxyflavane is a potent inhibitor of α-glucosidase with IC50 value of 2.2 μM. Inhibition of α-glucosidase decreases postprandial hyperglycaemia and the same time, protein tyrosine phosphatase 1B (PTP1B) downregulates insulin signaling by catalyzing the dephosphorylation of the activated insulin receptor (Johnson et al., 2002). PTP1B is a non-transmembrane phosphatase, belonging to the PTPs enzymes family, is highly expressed in the tissues targeted by insulin such as muscle, liver etc. (Dwek et al., 2002). Thus, such flavonoids antagonize hyperglycaemia and significantly augment insulin sensitization (Song et al., 2017).
Fig. 8.
Chemical structures of flavonoids (1–8) from princess tree (Song et al., 2017).
6.5. Thai folk medicinal formulations
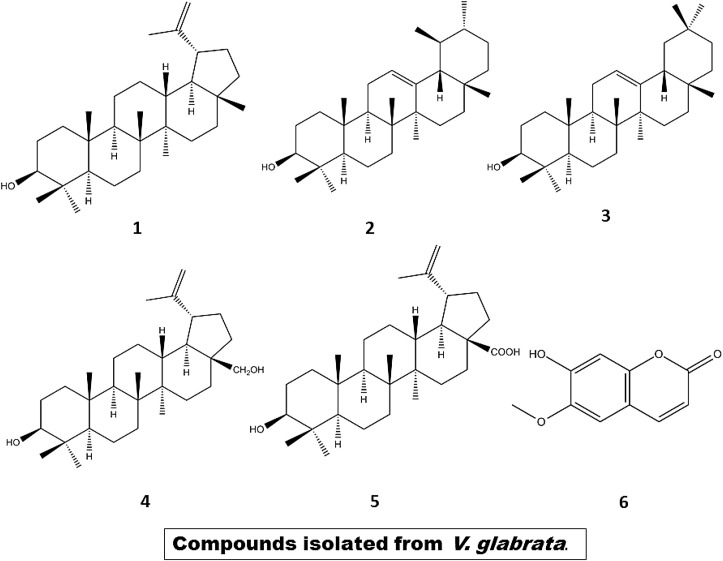
Thai traditional medicinal system involves polyherbal preparations for the treatment of diabetes. In a particular study inspired from Thai Mor Porn's folk medicinal recipe, 15 medicinal plants i.e., Vitex glabrata, Acanthus ebracteatus, Zea mays, Mimosa pudica, Pandanus odorus, Diospyros rhodocalyx, Abutilon polyandrum, Phyllanthus amarus, Senna siamea, Terminalia catappa, Rhinacanthus nasutus, Salacia chinensis, Acarbose, Legerstroemia speciosa and Senna alata were selectively screened for their α-glucosidase activity. The study showed the ethanolic extracts from V. glabrata (popularly called “Khai-nao” in Thai) to exhibit the highest α-glucosidase inhibitory activity (Somtimuang et al., 2018). The genus Vitex of the family Verbenaceae consists of about 250 tropical species most of which have been traditionally used for various treatments. For example, V. cannabifolia is used as an analgesic, V. agnus as a diuretic and V. trifolia against fever and inflammation (Somtimuang et al., 2018). Chromatographic analyses of bark and leaf extracts of Vitex glabrata has revealed the presence of ecdysteroids, 11α,20-dihydroxyecdysone, 7-dehydrocholesterol, pterosterone and 20-hydroxyecdysone, khainaoside A,- B and - C [Fig. 9 ] (Chouhan et al., 2012; Sridevi et al., 2012). Khai-nao has been used in Thai folk medicine as an antipyretic, antidiarrheal and anthelmintic, for treatment of gastrointestinal disorders and promotion of lactation (Chouhan et al., 2012). The ethanolic extracts of its leaves have been reported to exhibit anti-inflammatory and antioxidant properties (Sridevi et al., 2012).
Fig. 9.
Chemical structures of bioactive α-glucosidase inhibitors isolated from stem of V. glabrata (Somtimuang et al., 2018).
6.6. Flavonoids and caffeoylquinic acid from artemisia
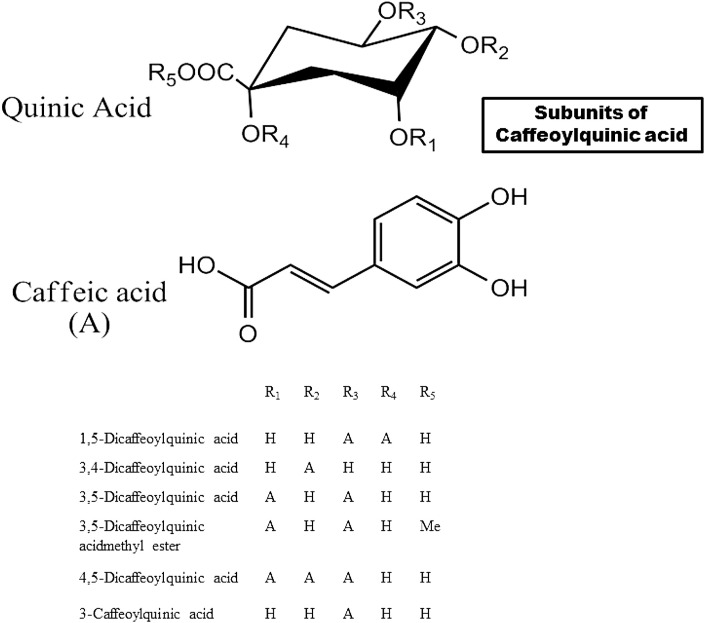
Artemisia is a miscellaneous genus of plants having more than 400 species. Presence of various bioactive compounds including flavonoids (Ferreira et al., 2010) and caffeoylquinic acids (Carnat et al., 2000) makes Artemisia plants a promising hub of naturally occurring therapeutic agent of diabetes [Fig. 10 ]. Several recent researches profoundly studied about the identification of bioactive agents present in this genus and their role as an alpha glucosidase inhibitor. Artemisia is used as traditional herbal medicine for years for the treatment of diabetes (Islam et al., 2013). Oral ingestion of alcoholic extract of Artemisia dracunculus and Artemisia pallens was found to act as anti-hyperglycemic agent in diabetic mice (Ribnicky et al., 2006; Subramoniam et al., 1996). Islam et al. studied the α-glucosidase inhibitory activity of 12 species of this genus and found that extracts from Artemisia capillaris are the most potent inhibitor. Further isolation of the anti-hyperglycemic compounds discovered several phenolic compounds like derivatives of caffeoylquinic acids such as 1,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid methyl ester, 4,5-dicaffeoylquinic acid, 3-caffeoylquinic acid; flavonoids like quercetin, cirsilineol, isorhamnetin, hyperoside and coumarins like esculetin, umbeliferone, daphnetin, 6-methoxy artemicapin C, scopoletin (Islam et al., 2013). Olennikov et al. studied 12 Siberian Artemisia species and found that all the plant extract have alpha glucosidase inhibitor property (IC50 = 214.42–754.12 μg/mL; acarbose IC50 = 1209.59 μg/mL), with caffeoylquinic acid derivatives being the major inhibitors (Olennikov et al., 2018).
Fig. 10.
Chemical structure of the subunits of caffeoylquinic acids from Artemisia.
6.7. Resin glycosides from morning glory
Morning glory is a mainly tropical plant belonging to Ipomoea genus. The main bioactive compounds in this genus were found to be resin glycosides (Pereda-Miranda et al., 2010). Resin derivatives are jalapinolic acid (11S-hydroxyhexadecanoic acid) with glycosylation derivatives. Out of 27 tested resin glycosidases, only four namely purginoside II, pescapreins V, pescapreins I and purgin III showed α-glucosodase inhibitory effect with IC50 values of 1067, 724, 626 and 330 mM, respectively. These result clearly suggests that purgin III exhibit almost similar inhibitory effect on α-glucosodase in comparison to positive control (acarbose; IC50 value = 332.99 mM). Structure analysis by molecular docking revealed that the residues, HIS279 and GLN322 of glucosidase are responsible for the interaction with these resin glycosidas which is similar to acarbose (Rosas-Ramírez et al., 2018).
6.8. Flavonoids, tannins, saponins from Asparagus racemosus leaf extracts
Root extracts of the Asparagus racemosus exhibit α-glucosidase inhibitory activity lower than acarbose. The major active components of the extract are flavonoids, tannins and saponins (Vadivelan et al., 2019).
6.9. Flavonoids, glycosides and tannins from the woody acer tree
Extracts from the leaves of the woody Acer palmatum and A. truncatum tree bear flavonoids, glycosides and tannins which exhibit α-glucosidase inhibitory activity (Zhang et al., 2019).
7. α-glucosidase inhibitors against COVID-19
Diabetic people with viral infection face an elevated risk of diabetic ketoacidosis, a condition experienced in people with T1D. Diabetic ketoacidosis impairs fluid intake and electrolyte levels. This may lead to sepsis. Sepsis and septic shock are important COVID-19 infection related complications (Apicella et al., 2020).
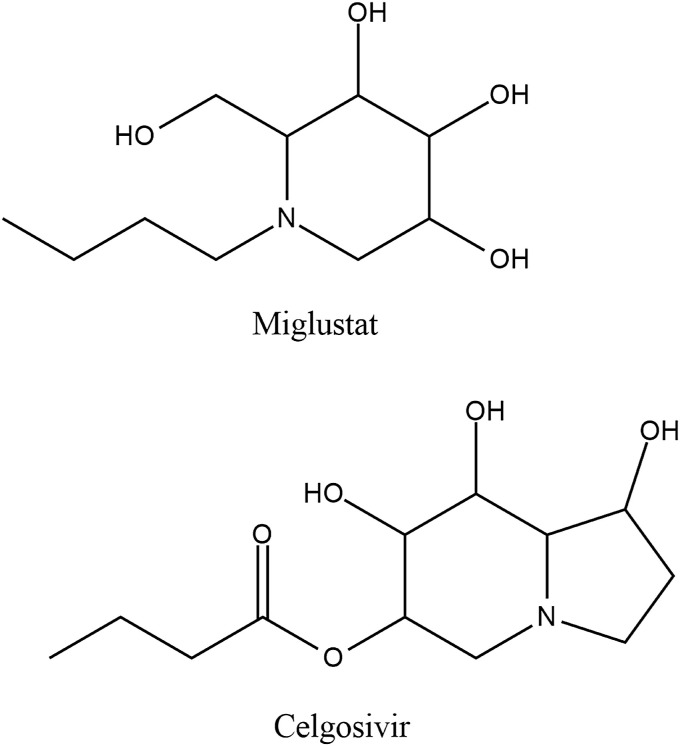
Protein folding patterns of the spike protein (S) of the coronavirus involves the N-glycosylation and calnexin pathway. Therefore, it is hypothesized that inhibition of α-glucosidase I & II can disrupt SARS-CoV2 replication as observed in SARS-CoV1. Miglustat and celgosivir are considered potent candidates for this purpose [Fig. 11 ] (Ritchie et al., 2010; Fukushi et al., 2012; Williams et al., 2020). However, since α-glucosidase inhibitors are not recommended in patients with diabetic ketoacidosis, the use of miglustat and celgosivir may pose risk of side-effects (Derosa and Maffioli, 2012).
Fig. 11.
Chemical structures of miglustat and celgosivir.
8. Conclusion
Bioactive α-glucosidase inhibitors can potentially inhibit the breakdown of disaccharides and oligosaccharides and release of α-D glucose, resulting in delayed absorption of glucose in the small intestine and efficiently diminishes the postprandial hyperglcemia and its deleterious physiological disorders. Recently isolated AGIs from various dietary sources such as polysaccharides (GP 90, GP 70-3) from guava, possess higher inhibitory effect than positive control (acarbose), whereas betulinic acid, tannins from persimmon, anthocyanins, polyphenols from berries and potatoes, galangin from rhizome of Alpinia galang, anthocyanins from blackcurrant, blueberry and blue honeysuckle fruits, procyanidins from apples, grape seeds and cocoa beans, hydroxyl-α-sanshool and hydroxyl-β-sanshool from Sinchuan pepper, active components from leaves and twigs of Sesbania grandiflora, edible seaweed, millets & tea leaves and erythritol have more or less similar inhibitory effect on α-glucosidase as compared to positive control. From non-dietary sources, oleanolic acid & ursolic acid, ganomycin, flavonoids, stigmasterol, quercetin, caffeoylquinic acid, resin glycosides, tannins, saponins and avicularin have proven to have more or less similar potential as that of acarbose in context of α-glucosidase inhibition. As the above said compounds are in the stage of preliminary examination, further investigation and clinical trials must be carried out. In summary, we could postulate that these compounds may be considered as potent AGIs in medication for ameliorating diabetic complications in future due to their immense efficiency in controlling postprandial hyperglycemia and their least side effects.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fct.2020.111738.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
PRISMA checklist for data extraction and analysis.
AMSTAR report for risk-of-bias assessment.
References
- Ali-Seyed M., Jantan I., Vijayaraghavan K., Bukhari S.N.A. Betulinic acid: recent advances in chemical modifications, effective delivery, and molecular mechanisms of a promising anticancer therapy. Chem. Biol. Drug Des. 2016;87:517–536. doi: 10.1111/cbdd.12682. [DOI] [PubMed] [Google Scholar]
- Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T., Hara N., Kobayashi S., Kohshima S., Fujimoto Y. Geranylated flavanones from the secretion on the surface of the immature fruits of Paulownia tomentosa. Phytochemistry. 2008;69:1234–1241. doi: 10.1016/j.phytochem.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Association, A.D Standards of medical care in diabetes—2013. Diabetes Care. 2013;36:S11. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A.D. Postprandial hyperglycaemia and α-glucosidase inhibitors. Diabetes Res. Clin. Pract. 1998;40:S51–S55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Basak P., Sadhukhan P., Sarkar P., Sil P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Tox. Rep. 2017;4:306–318. doi: 10.1016/j.toxrep.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello N.A., Pfeffer M.A., Skali H., McGill J.B., Rossert J., Olson K.A., Weinrauch L., Cooper M.E., de Zeeuw D., Rossing P. 2014. Retinopathy and clinical outcomes in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia. BMJ Open Diabetes Res. Care. 2, e000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boath A.S., Stewart D., McDougall G.J. Berry components inhibit α-glucosidase in vitro: synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012;135:929–936. doi: 10.1016/j.foodchem.2012.06.065. [DOI] [PubMed] [Google Scholar]
- Booth A. Systematic reviews of health information services and systems. Health Info. Libr. J. 2001;18:60–63. doi: 10.1046/j.1365-2532.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Carnat A., Heitz A., Fraisse D., Carnat A.-P., Lamaison J.-L. Major dicaffeoylquinic acids from Artemisia vulgaris. Fitoterapia. 2000;71:587–589. doi: 10.1016/s0367-326x(00)00163-5. [DOI] [PubMed] [Google Scholar]
- Castellano J.M., Guinda A., Delgado T., Rada M., Cayuela J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 2013;62:1791–1799. doi: 10.2337/db12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Chouhan H.S., Sridevi K., Singh N.K., Singh S.K. Anti-inflammatory activity of ethanol extract of Vitex glabrata leaves. Pak. J. Pharm. Sci. 2012;25:131–134. [PubMed] [Google Scholar]
- Chowdhury S., Ghosh S., Rashid K., Sil P.C. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem. Toxicol. 2016;97:187–198. doi: 10.1016/j.fct.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Compendium I.S. Website. 2017. http://www.cabi.org Accessed February.
- Coniff R.F., Shapiro J.A., Robbins D., Kleinfield R., Seaton T.B., Beisswenger P., McGill J.B. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM: a placebo-controlled dose-comparison study. Diabetes Care. 1995;18:817–824. doi: 10.2337/diacare.18.6.817. [DOI] [PubMed] [Google Scholar]
- Dai T., Chen J., McClements D.J., Li T., Liu C. Investigation the interaction between procyanidin dimer and α-glucosidase: spectroscopic analyses and molecular docking simulation. Int. J. Biol. Macromol. 2019;130:315–322. doi: 10.1016/j.ijbiomac.2019.02.105. [DOI] [PubMed] [Google Scholar]
- Das J., Ghosh J., Manna P., Sinha M., Sil P.C. Arsenic-induced oxidative cerebral disorders: protection by taurine. Drug Chem. Toxicol. 2009;32:93–102. doi: 10.1080/01480540802564171. [DOI] [PubMed] [Google Scholar]
- Derosa G., Maffioli P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci.: AMS. 2012;8:899. doi: 10.5114/aoms.2012.31621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis G.D., Tessari P., Go V.L., Gerich J.E. α-Glucosidase inhibition improves postprandial hyperglycemia and decreases insulin requirements in insulin-dependent diabetes mellitus. Metab. Clin. Exp. 1985;34:261–265. doi: 10.1016/0026-0495(85)90010-1. [DOI] [PubMed] [Google Scholar]
- Ding H., Hu X., Xu X., Zhang G., Gong D. Inhibitory mechanism of two allosteric inhibitors, oleanolic acid and ursolic acid on α-glucosidase. Int. J. Biol. Macromol. 2018;107:1844–1855. doi: 10.1016/j.ijbiomac.2017.10.040. [DOI] [PubMed] [Google Scholar]
- Ding H., Wu X., Pan J., Hu X., Gong D., Zhang G. New insights into the inhibition mechanism of betulinic acid on α-glucosidase. J. Agric. Food Chem. 2018;66:7065–7075. doi: 10.1021/acs.jafc.8b02992. [DOI] [PubMed] [Google Scholar]
- Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Dwek R.A., Butters T.D., Platt F.M., Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discov. 2002;1:65. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- Edirisinghe I., Burton-Freeman B. Anti-diabetic actions of Berry polyphenols–Review on proposed mechanisms of action. J. Berry Res. 2016;6:237–250. [Google Scholar]
- Esser N., Paquot N., Scheen A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs. 2015;24:283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- Ferreira J.F., Luthria D.L., Sasaki T., Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen J.A., Rodwell V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004;5:248. doi: 10.1186/gb-2004-5-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi M., Yoshinaka Y., Matsuoka Y., Hatakeyama S., Ishizaka Y., Kirikae T., Sasazuki T., Miyoshi-Akiyama T. Monitoring of S protein maturation in the endoplasmic reticulum by calnexin is important for the infectivity of severe acute respiratory syndrome coronavirus. J. Virol. 2012;86:11745–11753. doi: 10.1128/JVI.01250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: finding needle in the haystack. Eur. J. Med. Chem. 2015;103:133–162. doi: 10.1016/j.ejmech.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Basak P., Dutta S., Chowdhury S., Sil P.C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem. Toxicol. 2017;103:41–55. doi: 10.1016/j.fct.2017.02.028. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Chowdhury S., Sarkar P., Sil P.C. Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem. Toxicol. 2018;118:272–286. doi: 10.1016/j.fct.2018.05.029. [DOI] [PubMed] [Google Scholar]
- Goda T., Yamada K., Sugiyama M., Moriuchi S., Hosoya N. Effect of sucrose and acarbose feeding on the development of streptozotocin-induced diabetes in the rat. J. Nutr. Sci. Vitaminol. 1982;28:41–56. doi: 10.3177/jnsv.28.41. [DOI] [PubMed] [Google Scholar]
- Han L., Fang C., Zhu R., Peng Q., Li D., Wang M. Inhibitory effect of phloretin on α-glucosidase: kinetics, interaction mechanism and molecular docking. Int. J. Biol. Macromol. 2017;95:520–527. doi: 10.1016/j.ijbiomac.2016.11.089. [DOI] [PubMed] [Google Scholar]
- Hanáková Z., Hošek J., Babula P., Dall'Acqua S., Václavík J.í., Šmejkal K. C-geranylated flavanones from Paulownia tomentosa fruits as potential anti-inflammatory compounds acting via inhibition of TNF-α production. J. Nat. Prod. 2015;78:850–863. doi: 10.1021/acs.jnatprod.5b00005. [DOI] [PubMed] [Google Scholar]
- Hou W., Li Y., Zhang Q., Wei X., Peng A., Chen L., Wei Y. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother Res. 2009;23:614–618. doi: 10.1002/ptr.2661. [DOI] [PubMed] [Google Scholar]
- Hu J., Pang W., Bai S., Zheng Z., Wu X. Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla. BMC Complement. Altern. Med. Plus. 2013;13:267. doi: 10.1186/1472-6882-13-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.S., Yin M.C., Chiu L.C. Antihyperglycemic and antioxidative potential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011;49:2189–2195. doi: 10.1016/j.fct.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Islam M.N., Jung H.A., Sohn H.S., Kim H.M., Choi J.S. Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch Pharm. Res. (Seoul) 2013;36:542–552. doi: 10.1007/s12272-013-0069-7. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Hua D., Huang D., Zhang Q., Yan C. Characterization of a new heteropolysaccharide from green guava and its application as an α-glucosidase inhibitor for the treatment of type II diabetes. Food Funct. 2018;9:3997–4007. doi: 10.1039/c8fo00790j. [DOI] [PubMed] [Google Scholar]
- Johnson T.O., Ermolieff J., Jirousek M.R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 2002;1:696. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- Kalita D., Holm D.G., LaBarbera D.V., Petrash J.M., Jayanty S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PloS One. 2018;13 doi: 10.1371/journal.pone.0191025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita D., Jayanty S.S. Comparison of polyphenol content and antioxidant capacity of colored potato tubers, pomegranate and blueberries. J. Food Process. Technol. 2014;5:1. [Google Scholar]
- Kalra S. Alpha glucosidase inhibitors. JPMA. Journal Pak. Med. Assoc. 2014;64:474–476. [PubMed] [Google Scholar]
- Kataya H.A., Hamza A.A. Red cabbage (Brassica oleracea) ameliorates diabetic nephropathy in rats. Evid. Based Complementary Altern. Med. Plus. 2008;5:281–287. doi: 10.1093/ecam/nem029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazeem M.I., Ashafa A.O.T. Kinetics of inhibition of carbohydrate-metabolizing enzymes and mitigation of oxidative stress by Eucomis humilis Baker bulb. Beni-Suef Univ. J. Basic Appl. Sci. 2017;6:57–63. [Google Scholar]
- Ketema E.B., Kibret K.T. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch. Publ. Health. 2015;73:43. doi: 10.1186/s13690-015-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G.L., Loeken M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- Krentz A.J., Bailey C.J. Oral antidiabetic agents. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- Kumar S., Narwal S., Kumar V., Prakash O. α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharm. Rev. 2011;5:19. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Pandey A.K. 2013. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Ryu Y.B., Youn H.-S., Cho J.K., Kim Y.M., Park J.-Y., Lee W.S., Park K.H., Eom S.H. Structural basis of sialidase in complex with geranylated flavonoids as potent natural inhibitors. Acta Crystallogr. D (Dallas, 1978) 2014;70:1357–1365. doi: 10.1107/S1399004714002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Leverence R., Trombley J.D., Xu S., Yang J., Tian Y., Reed J.D., Hagerman A.E. High molecular weight persimmon (Diospyros kaki L.) proanthocyanidin: a highly galloylated, A-linked tannin with an unusual flavonol terminal unit, myricetin. J. Agric. Food Chem. 2010;58:9033–9042. doi: 10.1021/jf102552b. [DOI] [PubMed] [Google Scholar]
- Li K., Yao F., Du J., Deng X., Li C. Persimmon tannin decreased the glycemic response through decreasing the digestibility of starch and inhibiting α-amylase, α-glucosidase, and intestinal glucose uptake. J. Agric. Food Chem. 2018;66:1629–1637. doi: 10.1021/acs.jafc.7b05833. [DOI] [PubMed] [Google Scholar]
- Li M., Song L.J., Qin X.Y. Advances in the cellular immunological pathogenesis of type 1 diabetes. J. Cell Mol. Med. 2014;18:749–758. doi: 10.1111/jcmm.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wu Y., Liu Y., Tang Y., Che Z., Wu T. Chemical profiles and screening of potential α-glucosidase inhibitors from Sichuan pepper using ultra-filtration combined with UHPLC-Q-TOF. Ind. Crop. Prod. 2020;143:111874. [Google Scholar]
- Li Y.Q., Zhou F.C., Gao F., Bian J.S., Shan F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009;57:11463–11468. doi: 10.1021/jf903083h. [DOI] [PubMed] [Google Scholar]
- Lozano-Ortega G., Goring S., Bennett H.A., Bergenheim K., Sternhufvud C., Mukherjee J. Network meta-analysis of treatments for type 2 diabetes mellitus following failure with metformin plus sulfonylurea. Curr. Med. Res. Opin. 2016;32:807–816. doi: 10.1185/03007995.2015.1135110. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Madar Z., Omursky Z. Inhibition of intestinal α-glucosidase activity and postprandial hyperglycemia by α-glucosidase inhibitors in fa/fa rats. Nutr. Res. 1991;11:1035–1046. [Google Scholar]
- Manna P., Sinha M., Sil P.C. Cadmium induced testicular pathophysiology: prophylactic role of taurine. Reprod. Toxicol. 2008;26:282–291. doi: 10.1016/j.reprotox.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Manna P., Sinha M., Sil P.C. Prophylactic role of arjunolic acid in response to streptozotocin mediated diabetic renal injury: activation of polyol pathway and oxidative stress responsive signaling cascades. Chem. Biol. Interact. 2009;181:297–308. doi: 10.1016/j.cbi.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Manna P., Ghosh J., Das J., Sil P.C. Streptozotocin induced activation of oxidative stress responsive splenic cell signaling pathways: protective role of arjunolic acid. Toxicol. Appl. Pharmacol. 2010;244:114–129. doi: 10.1016/j.taap.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Manna P., Ghosh M., Ghosh J., Das J., Sil P.C. Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: role of IκBα/NF-κB, MAPKs and mitochondrial signal. Nanotoxicology. 2012;6:1–21. doi: 10.3109/17435390.2011.552124. [DOI] [PubMed] [Google Scholar]
- McDougall G.J., Shpiro F., Dobson P., Smith P., Blake A., Stewart D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005;53:2760–2766. doi: 10.1021/jf0489926. [DOI] [PubMed] [Google Scholar]
- Mehta A., Zitzmann N., Rudd P.M., Block T.M., Dwek R.A. α-Glucosidase inhibitors as potential broad based anti-viral agents. FEBS Lett. 1998;430:17–22. doi: 10.1016/s0014-5793(98)00525-0. [DOI] [PubMed] [Google Scholar]
- Mizgier P., Kucharska A.Z., Sokół-Łętowska A., Kolniak-Ostek J., Kidoń M., Fecka I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Food. 2016;21:133–146. [Google Scholar]
- Ning Z.W., Zhai L.X., Huang T., Peng J., Hu D., Xiao H.T., Wen B., Lin C.Y., Zhao L., Bian Z.X. Identification of α-glucosidase inhibitors from cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct. 2019;10:1893–1902. doi: 10.1039/c8fo01845f. [DOI] [PubMed] [Google Scholar]
- Olennikov D.N., Chirikova N.K., Kashchenko N.I., Nikolaev V.M., Kim S.-W., Vennos C. Bioactive phenolics of the genus Artemisia (asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacology. 2018;9:756. doi: 10.3389/fphar.2018.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P.B., Sinha K., Sil P.C. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PloS One. 2014;9 doi: 10.1371/journal.pone.0107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P., Mandal S., Tomar S.K., Anand S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015;54:863–880. doi: 10.1007/s00394-015-0974-2. [DOI] [PubMed] [Google Scholar]
- Pereda-Miranda R., Rosas-Ramírez D., Castaneda-Gomez J. Resin glycosides from the morning glory family. Progress in the chemistry of organic natural products. J. Am. Chem. Soc. Springer. 2010;92:77–153. doi: 10.1007/978-3-211-99661-4_2. [DOI] [PubMed] [Google Scholar]
- Podsedek A., Majewska I., Kucharska A.Z. Inhibitory potential of red cabbage against digestive enzymes linked to obesity and type 2 diabetes. J. Agric. Food Chem. 2017;65:7192–7199. doi: 10.1021/acs.jafc.7b02499. [DOI] [PubMed] [Google Scholar]
- Pradeep P.M., Sreerama Y.N. Phenolic antioxidants of foxtail and little millet cultivars and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2018;247:46–55. doi: 10.1016/j.foodchem.2017.11.103. [DOI] [PubMed] [Google Scholar]
- Pyner A., Nyambe-Silavwe H., Williamson G. Inhibition of human and rat sucrase and maltase activities to assess antiglycemic potential: optimization of the assay using acarbose and polyphenols. J. Agric. Food Chem. 2017;65:8643–8651. doi: 10.1021/acs.jafc.7b03678. [DOI] [PubMed] [Google Scholar]
- Rashid K., Chowdhury S., Ghosh S., Sil P.C. Curcumin attenuates oxidative stress induced NFκB mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochem. Pharmacol. 2017;143:140–155. doi: 10.1016/j.bcp.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Rashid K., Sil P.C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim. Biophy. Mol. Basis Dis. 2015;1852:70–82. doi: 10.1016/j.bbadis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Ren Y.Y., Zhu Z.Y., Sun H.Q., Chen L.J. Structural characterization and inhibition on α-glucosidase activity of acidic polysaccharide from Annona squamosa. Carbohydr. Polym. 2017;174:1–12. doi: 10.1016/j.carbpol.2017.05.092. [DOI] [PubMed] [Google Scholar]
- Ribnicky D., Poulev A., Watford M., Cefalu W., Raskin I. Antihyperglycemic activity of Tarralin™, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13:550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Ritchie G., Harvey D.J., Feldmann F., Stroeher U., Feldmann H., Royle L., Dwek R.A., Rudd P.M. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology. 2010;399(2):257–269. doi: 10.1016/j.virol.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ramírez D., Escandón-Rivera S., Pereda-Miranda R. Morning glory resin glycosides as α-glucosidase inhibitors: in vitro and in silico analysis. Phytochemistry. 2018;148:39–47. doi: 10.1016/j.phytochem.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Rzechonek D.A., Dobrowolski A., Rymowicz W., Mirończuk A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018;38:620–633. doi: 10.1080/07388551.2017.1380598. [DOI] [PubMed] [Google Scholar]
- Sarkar A., Ghosh S., Chowdhury S., Pandey B., Sil P.C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. BBA-General Subjects. 2016;1860:2065–2075. doi: 10.1016/j.bbagen.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Schneiderová K., Šmejkal K. Phytochemical profile of paulownia tomentosa (thunb) Steud. Phytochem. Rev. 2015;14:799–833. doi: 10.1007/s11101-014-9376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H., Sun H. Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011;28:543–593. doi: 10.1039/c0np00059k. [DOI] [PubMed] [Google Scholar]
- Shindou T., Sasaki Y., Miki H., Eguchi T., Hagiwara K., Ichikawa T. Determination of erythritol in fermented foods by high performance liquid chromatography. J. Food Hyg. Saf. 1988;29:419–422. [Google Scholar]
- Sinha M., Manna P., Sil P.C. Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride-induced hepatic and renal oxidative stress. J. Nat. Med. 2007;61:251–260. [Google Scholar]
- Šmejkal K., Grycová L., Marek R., Lemiere F., Jankovská D., Forejtníková H., Vanco J., Suchý V. C-geranyl compounds from Paulownia tomentosa fruits. J. Nat. Prod. 2007;70:1244–1248. doi: 10.1021/np070063w. [DOI] [PubMed] [Google Scholar]
- Somtimuang C., Olatunji O.J., Ovatlarnporn C. Evaluation of. Vitro α-Amylase and α-Glucosidase Inhibitory Potentials of 14 Medicinal Plants Constituted in Thai Folk Antidiabetic Formularies. Chem. Biodiversity. 2018;15:e1800025. doi: 10.1002/cbdv.201800025. [DOI] [PubMed] [Google Scholar]
- Song Y.H., Uddin Z., Jin Y.M., Li Z., Curtis-Long M.J., Kim K.D., Cho J.K., Park K.H. Inhibition of protein tyrosine phosphatase (PTP1B) and α-glucosidase by geranylated flavonoids from Paulownia tomentosa. J. Enzyme Inhib Med. Chem. 2017;32:1195–1202. doi: 10.1080/14756366.2017.1368502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridevi V., Chouhan H.S., Singh N.K., Singh S.K. Antioxidant and hepatoprotective effects of ethanol extract of Vitex glabrata on carbon tetrachloride-induced liver damage in rats. Nat. Prod. Res. 2012;26:1135–1140. doi: 10.1080/14786419.2011.560849. [DOI] [PubMed] [Google Scholar]
- Subramoniam A., Pushpangadan P., Rajasekharan S., Evans D., Latha P., Valsaraj R. Effects of Artemisia pallens Wall. on blood glucose levels in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 1996;50:13–17. doi: 10.1016/0378-8741(95)01329-6. [DOI] [PubMed] [Google Scholar]
- Tasnuva S., Qamar U., Ghafoor K., Sahena F., Jahurul M., Rukshana A., Juliana M., Al-Juhaimi F.Y., Jalifah L., Jalal K. α-glucosidase inhibitors isolated from Mimosa pudica L. Nat. Prod. Res. 2017:1–5. doi: 10.1080/14786419.2017.1419224. [DOI] [PubMed] [Google Scholar]
- Thissera B., Visvanathan R., Khanfar M.A., Qader M.M., Hassan M.H., Hassan H.M., Bawazeer M., Behery F.A., Yaseen M., Liyanage R., Abdelmohsen U.R. Sesbania grandiflora L. Poir leaves: a dietary supplement to alleviate type 2 diabetes through metabolic enzymes inhibition. S. Afr. J. Bot., Le. 2020;130:282–299. [Google Scholar]
- Torres-Piedra M., Ortiz-Andrade R., Villalobos-Molina R., Singh N., Medina-Franco J.L., Webster S.P., Binnie M., Navarrete-Vázquez G., Estrada-Soto S. A comparative study of flavonoid analogues on streptozotocin–nicotinamide induced diabetic rats: quercetin as a potential antidiabetic agent acting via 11β-hydroxysteroid dehydrogenase type 1 inhibition. Eur. J. Med. Chem. 2010;45:2606–2612. doi: 10.1016/j.ejmech.2010.02.049. [DOI] [PubMed] [Google Scholar]
- Vadivelan R., Krishnan R.G., Kannan R. Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of α-amylase and α-glucosidase. J. Tradit. Complement. Med. 2019;9:1–4. doi: 10.1016/j.jtcme.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanessa Fiorentino T., Prioletta A., Zuo P., Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharmaceut. Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- Vinayagam R., Xiao J., Xu B. An insight into anti-diabetic properties of dietary phytochemicals. Phytochemistry Rev. 2017;16:535–553. [Google Scholar]
- Wang K., Bao L., Ma K., Zhang J., Chen B., Han J., Ren J., Luo H., Liu H. A novel class of α-glucosidase and HMG-CoA reductase inhibitors from Ganoderma leucocontextum and the anti-diabetic properties of ganomycin I in KK-Ay mice. Eur. J. Med. Chem. 2017;127:1035–1046. doi: 10.1016/j.ejmech.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Wang K., Bao L., Zhou N., Zhang J., Liao M., Zheng Z., Wang Y., Liu C., Wang J., Wang L. Structural modification of natural product ganomycin I leading to discovery of a α-glucosidase and HMG-CoA reductase dual inhibitor improving obesity and metabolic dysfunction in vivo. J. Med. Chem. 2018;61:3609–3625. doi: 10.1021/acs.jmedchem.8b00107. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xiang L., Wang C., Tang C., He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PloS One. 2013;8:e71144. doi: 10.1371/journal.pone.0071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeier U., Piepersberg W. Biotechnology and molecular biology of the α-glucosidase inhibitor acarbose. Appl. Microbiol. Biotechnol. 2004;63:613–625. doi: 10.1007/s00253-003-1477-2. [DOI] [PubMed] [Google Scholar]
- Wen H., Tang B., Stewart A.J., Tao Y., Shao Y., Cui Y., Yue H., Pei J., Liu Z., Mei L. Erythritol attenuates postprandial blood glucose by inhibiting α-glucosidase. J. Agric. Food Chem. 2018;66:1401–1407. doi: 10.1021/acs.jafc.7b05033. [DOI] [PubMed] [Google Scholar]
- Wiczkowski W., Topolska J., Honke J. Anthocyanins profile and antioxidant capacity of red cabbages are influenced by genotype and vegetation period. J. Funct. Food. 2014;7:201–211. [Google Scholar]
- Williams S.J., Goddard-Borger E.D. α-glucosidase inhibitors as host-directed antiviral agents with potential for the treatment of COVID-19. Biochem. Soc. Trans. 2020;48:1287–1295. doi: 10.1042/BST20200505. [DOI] [PubMed] [Google Scholar]
- Wu X., Beecher G.R., Holden J.M., Haytowitz D.B., Gebhardt S.E., Prior R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- Xiong S.-L., Yue L.-M., Lim G.T., Yang J.-M., Lee J., Park Y.-D. Inhibitory effect of raspberry ketone on α-glucosidase: docking simulation integrating inhibition kinetics. Int. J. Biol. Macromol. 2018;113:212–218. doi: 10.1016/j.ijbiomac.2018.02.124. [DOI] [PubMed] [Google Scholar]
- Yee H.S., Fong N.T. A review of the safety and efficacy of acarbose in diabetes mellitus. Pharmacotherapy. 1996;16:792–805. [PubMed] [Google Scholar]
- Yin Z.-h., Wang J.-j., Gu X.-z., Gu H.-p., Kang W.-y. Antioxidant and a-glucosidase inhibitory activity of red raspberry (Harrywaters) fruits in vitro. Afr. J. Pharm. Pharmacol. 2012;6:3118–3123. [Google Scholar]
- Yokozawa T., Kim H.Y., Cho E.J. Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2002;50:5485–5489. doi: 10.1021/jf020168z. [DOI] [PubMed] [Google Scholar]
- Zaharudin N., Staerk D., Dragsted L.O. Inhibition of α-glucosidase activity by selected edible seaweeds and fucoxanthin. Food Chem. 2019;270:481–486. doi: 10.1016/j.foodchem.2018.07.142. [DOI] [PubMed] [Google Scholar]
- Zhang J., Sun L., Dong Y., Fang Z., Nisar T., Zhao T., Wang Z.C., Guo Y. Chemical compositions and α-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chem. 2019;299:125102. doi: 10.1016/j.foodchem.2019.125102. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J., Hogan S., Chung H., Welbaum G.E., Zhou K. Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chem. 2010;119:592–599. [Google Scholar]
- Zhang L., Xu L., Ye Y.H., Zhu M.F., Li J., Tu Z.C., Yang S.H., Liao H. Phytochemical profiles and screening of α-glucosidase inhibitors of four Acer species leaves with ultra-filtration combined with UPLC-QTOF-MS/MS. Ind. Crop. Prod. 2019;129:156–168. [Google Scholar]
- Zhang S., Li X.Z. Inhibition of α-glucosidase by polysaccharides from the fruit hull of Camellia oleifera Abel. Carbohydr. Polymer. 2015;115:38–43. doi: 10.1016/j.carbpol.2014.08.059. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Kong F., Ni H., Mo Z., Wan J.-B., Hua D., Yan C. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 2016;144:106–114. doi: 10.1016/j.carbpol.2016.02.030. [DOI] [PubMed] [Google Scholar]
- Zeng L., Ding H., Hu X., Zhang G., Gong D. Galangin inhibits α-glucosidase activity and formation of non-enzymatic glycation products. Food Chem. 2019;271:70–79. doi: 10.1016/j.foodchem.2018.07.148. [DOI] [PubMed] [Google Scholar]
- Zimmet P., Alberti K., Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.