Abstract
SARS-CoV-2 mainly invades respiratory epithelial cells by adhesion to angiotensin-converting enzyme 2 (ACE-2) and thus, infected patients may develop mild to severe inflammatory responses and acute lung injury. Afferent impulses that result from the stimulation of pulmonary mechano-chemoreceptors, peripheral and central chemoreceptors by inflammatory cytokines are conducted to the brainstem. Integration and processing of these input signals occur within the central nervous system, especially in the limbic system and sensorimotor cortex, and importantly feedback regulation exists between O2, CO2, and blood pH. Despite the intensity of hypoxemia in COVID-19, the intensity of dyspnea sensation is inappropriate to the degree of hypoxemia in some patients (silent hypoxemia). We hypothesize that SARS-CoV-2 may cause neuronal damage in the corticolimbic network and subsequently alter the perception of dyspnea and the control of respiration. SARS-CoV-2 neuronal infection may change the secretion of numerous endogenous neuropeptides or neurotransmitters that distribute through large areas of the nervous system to produce cellular and perceptual effects. SARS-CoV-2 mainly enter to CNS via direct (neuronal and hematologic route) and indirect route. We theorize that SARS-CoV-2 infection-induced neuronal cell damage and may change the balance of endogenous neuropeptides or neurotransmitters that distribute through large areas of the nervous system to produce cellular and perceptual effects. Thus, SARS-CoV-2-associated neuronal damage may influence the control of respiration by interacting in neuromodulation. This would open up possible lines of study for the progress in the central mechanism of COVID-19-induced hypoxia. Future research is desirable to confirm or disprove such a hypothesis.
Keywords: COVID-19, SARS-CoV-2, Hypoxemia, Neural Invasion, Central Nervous System
1. Introduction
The Coronavirus disease 2019 (COVID-19), initially identified in Wuhan in December 2019, was recently introduced as a pandemic by the World Health Organization. The virus that causes this disease, defined as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can infect human respiratory epithelial cells through an interaction with the angiotensin-converting enzyme (ACE)- 2 [1,2].
The ACE2 receptor has a key role in the adhesion of SARS-CoV-2 and its entrance into cells [3,4]. This virus mainly invades alveolar epithelial cells and then disseminates throughout the body via ACE2 expressing endothelial, CNS, myocardial, lymphocytes, and intestinal cells [[5], [6], [7]].
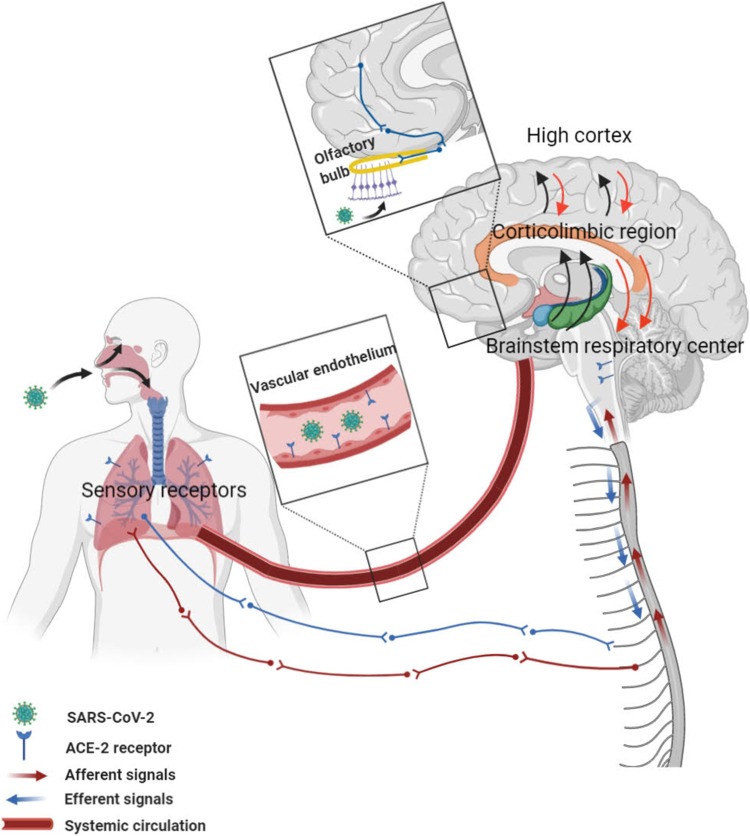
It is well-described that pulmonary, cardiac, renal, vascular endothelial, brain, and small intestine cells express ACE2 receptors and are potential targets for SARS-CoV-2 [8,9]. Another possible mechanism of SARS-CoV-2 brain involvement could be direct spreading across the cribriform plate of the ethmoidal sinus [10,11] (Fig. 1 ).
Fig. 1.
A neurobiological model framework shows the route of entry of SARS-CoV-2 via the systemic circulation and olfactory bulb to the brain. Afferent impulses from chemoreceptors and mechanoreceptors are transmitted to the brainstem, limbic system, and cerebral cortex for integration and processing. The central nervous system directs efferent motor commands via the phrenic and thoracic spinal nerves to regulate ventilation and modify dyspnea.
Recently, many studies have revealed that a significant number of patients with SARS-CoV-2 infection had anosmia as an initial symptom of the infection [[12], [13], [14], [15]]. Olfactory alterations are frequent in patients with SARS-CoV-2 infection. The absence of catarrhal symptoms was a prominent finding in half of the patients with COVID-19 [16]. The presence of virus in neural and capillary endothelial cells in frontal lobe tissue was reported [17]. Moreover, a direct invasion of the virus that enters through the olfactory mucosa was reported. Based on these autopsy findings, virions of SARS-CoV-2 were exist in olfactory pathways and medulla oblongata [18].
Although neurological presentations are not common in the COVID-19 epidemic, a considerable number of COVID-9 infected patients may complain from the early or delayed onset of neurological manifestations which correlated with the presence of the SARS-CoV-2 in the CNS [19]. According to a recent report of a COVID-19 patient with anosmia, the brain MRI revealed bilateral inflammatory obstruction of the olfactory clefts, with no anomalies of the olfactory bulbs and tracts [20]. SARS-CoV-2 can also damage glial cells and neurons by interacting with host ACE2 without developing substantial inflammation or clinical evidence of encephalitis [21,22,11]. The distribution of ACE2 in the brain is still obscure but some important brain areas were relatively expressed highly ACE2 receptors both in excitatory and inhibitory neurons in the substantia nigra and brain ventricles [23,24]. The high expression of ACE2 receptor in central glial substance and brain ventricles suggests two potential novel routes the entering of SARS-CoV-2to CSF and/or spreading around the brain [23,24].
In this review, we try to argue that clinical profiles and respiratory prognosis are related to the entry of the virus into the CNS and involvement of some specific areas and therefore we argue the dyspneic and silent hypoxia mechanism of CNS in COVID-19.
2. Mechanism of hypoxemia in COVID-19
The SARS-CoV-2 frequently leads to mild to severe inflammatory responses and diffuse alveolar damage. The autopsy reports of patients with SARS-CoV-2 have indicated mild to moderate mononuclear response consisting of notable aggregates of CD4+ cells around the thrombosed small vessels and foci of hemorrhage. Pulmonary microvascular thrombosis that is restricted to the lungs can be an additional mechanism that occurs in the severe type of COVID-19 and can undermine the pulmonary ventilation [25,26].
Hypoxemia in COVID-19 patients with diffuse lung injury could be related to three different types. The first main type of hypoxemia in COVID-19 patients is hypoxic and hypocapnic respiratory failure mainly due to the increased pulmonary shunt fraction and ventilation-perfusion mismatch (V/Q defects) in the injured lung [27,28]. Hypoxic and hypercapnic respiratory failure is the second type of hypoxemia that occurs less commonly among COVID-19 patients. Although hypercapnia contributes to breathlessness in normal individuals, some COVID-19 patients do not complain of dyspnea even with the development of severe hypoxemia [29]. The third type of hypoxemia is pure hypoventilation and is characterized by hypoxemia in the presence of a normal arteriovenous oxygen gradient, which is extremely rare among these patients [30,29].
Invasion of the alveolar epithelium and pulmonary parenchyma, and subsequent inflammatory response induces the stimulation of pulmonary mechano-chemoreceptors and other chemoreceptors. Input signals from these receptors are then transmitted to the brainstem by sensory afferents, and input impulses are further processed mainly in the limbic system and sensorimotor cortex [31,32]. This issue has been considered to be closely associated with dyspnea perception [33].
Feedback regulation of O2 and CO2 is operated by carotid bodies (CB) and central respiratory chemoreceptors (CRC). CRCs control this process by measuring the PCO2 and power of hydrogen ion (PH) in the brain. In hypoxic conditions, carotid bodies are activated selectively and CRCs are inhibited, contributing to hyperventilation [31,32,34].
Diminution in dyspnea perception in COVID-19 may be explained by two mechanisms; the direct invasion of SARS-CoV-2 to ACE2 expressing brain cells in the limbic system (especially the insular area), or by the indirect toxic effect of cytokine storm in the corticolimbic network that has the main role in expressing the perception of dyspnea [35,5].
3. Neurobiology of dyspnea
The experience of dyspnea occurs when there is an imbalance between the central neural drive and the respiratory muscle function. Sensory receptors (activated due to hypercapnia, hypoxemia, or even parenchymal disease) provide afferent signals and transmit the signal to the breathing centers in the brainstem, cerebral regions in the limbic system, and cortex. Input signals from the cardiopulmonary system continuously and precisely regulate oxygen and carbon dioxide and hydroxide ion concentration.
Neuroimaging studies have shown that respiratory input signals are processed within the anterior insular cortex and associated operculum, anterior cingulate cortex, amygdala, and dorsolateral prefrontal cortex [36,37]. Input signals are processed in the cortex by two main pathways: discriminative processing that is related to awareness of intensity components and the structures involved in this pathway include brainstem medulla, and ventroposterior area of the thalamus, and affective processing, which is associated with qualitative components and feelings and begins with the relay of afferent information via the vagal pathway to several structures of the limbic system, such as amygdala [38,39].
Afferent signals are transmitted via the vagal nerve to the central nervous system (CNS) and are then sent to the amygdala and medial dorsal regions of the thalamus and finally ascend specific parts of the limbic system, including the insular and cingulate cortices [40,33]. These structures are thought to process the unpleasant aspect of dyspnea and it seems that COVID-19 infection modifies the function of these areas of the brain [41,42].
The thalamus and hippocampus are critical neural areas for receiving respiratory sensory input [33]. After effective integration and processing of afferent inputs in the CNS, these structures subsequently produce efferent outputs and send impulses via the phrenic and thoracic spinal nerves to the diaphragm and intercostal muscles, respectively (Fig. 1).
4. Hypoxemia and effects on the brain
The effective function of synaptic transmission needs at least 30% cerebral oxygen delivery and hypoxemia can decrease the amount of brain adenosine triphosphate (ATP) down to 10 percent in 5 minutes [43].
COVID-19 patients experience moderate to severe hypoxemia that can contribute to multiple organ dysfunction, particularly the CNS. Hypoxemia induces a functional change in Na+ and K+ pump oxygen-sensitive ion channels and impairs the excitation and inhibition of neuronal and glial cells; also, this can activate glutamate transporters which split glutamate inside the synaptic region and cause excitotoxicity [44]. Under hypoxic conditions, the Krebs cycle is inhibited, leading to a reduction in 2-oxoglutarate [45].
During hypoxia, hypoxia-inducible factor (HIF)-1 becomes stabilized and leads to the expression of genes related to this condition (e.g. erythropoietin, vascular endothelial growth factor, and insulin-like growth factor 1) via hypoxemic response element (HRE) [46]. One of the neuroprotective agents is adenosine (also one of the neurotransmitters). Activation of this route further activates phospholipase C, which inhibits the release of glutamate and other neurotransmitters. HIF-1 also increases adenosine by breaking down the ATP of the extracellular fluid [47].
In hypoxemic conditions, HIF binds to HRE and allows the upregulation of cytokines, such as IL-β, IL-6, IL-8, and tumor necrosis factor (TNF)-α. TNF-α is one of the most important inflammatory cytokines associated with neural damage [44]. HIF acts as a cornerstone of the hypoxia-sensing machinery and regulatory transcriptional program, but the role of certain types of microRNAs has been demonstrated in hypoxia response, especially miR-210 [44,48].
In the condition of hypoxia, a shift from oxidative phosphorylation, which takes place in the mitochondria, to glycolysis is observed as a result of HIF activation. HIF-1 alpha induces miR-210 and causes this shift by repressing several steps of the mitochondrial metabolism and mitochondrial enzymes [44]. Also, miR-210 affects capillary wall formation and has a role in the regulation of apoptosis and DNA repair [49,48].
5. Neurotropism of SARS-CoV-2
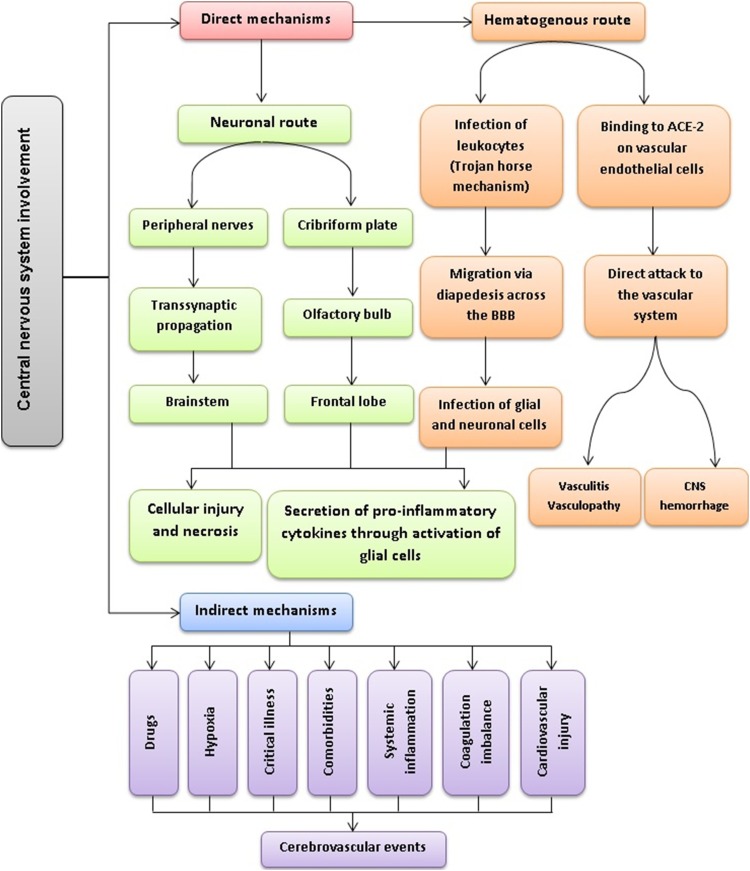
Currently, different mechanisms have been proposed for CNS invasion in patients with COVID-19, which could be categorized as direct or indirect mechanisms (Fig. 2 ). The direct entry of SARS-CoV-2 to the CNS could be achieved either through the hematogenous or the neuronal route. In the hematogenous route, SARS-CoV-2 can invade the vascular endothelium by binding to ACE-2, leading to increased permeability of the blood-brain barrier (BBB) and then infecting glial cells in the CNS or directly attacking the vascular system. Another potential mechanism, also known as the Trojan horse mechanism, is that SARS-CoV-2 can infect leukocytes within the bloodstream and then cross the BBB via diapedesis. In the neuronal route, SARS-CoV-2 could transmit from the peripheral nerves to the brainstem via retrograde transsynaptic propagation or it could directly invade the frontal lobe of the cortex via the cribriform plate and olfactory bulb; importantly, both of these mechanisms have been previously shown in other beta coronaviruses [50]. With the spread of the virus to the brainstem, the involvement of the nucleus of the solitary tract and the nucleus ambiguous, which receive afferent signals from pulmonary mechano-chemoreceptors, may eventually lead to respiratory failure and even death [22]. In addition, histopathologic examination of brain specimens of patients with COVID-19 revealed the loss of neurons in the cerebral cortex, hippocampus, and cerebellar Purkinje cell layer; also, SARS-CoV-2 was detected in several brain sections by RT-PCR [51]. These findings further support the notion that COVID-19 could induce direct neuronal damage. Regarding the indirect mechanisms, many factors including drugs, hypoxia, systemic inflammation, coagulation imbalance, comorbidities, cardiovascular injury, and critical illness could potentially cause cerebrovascular events. For example, the macrophage activation syndrome or cytokine storm, which has been reported in COVID-19, could result in neuroinflammation and brain tissue injury. Dixon et al. have argued that the negative RT-PCR results obtained from CSF samples in patients with neurological manifestations are in favor of a hyperinflammatory response, rather than a direct neurotropic effect [52].
Fig. 2.
Potential mechanisms of central nervous system involvement by SARS-CoV-2.
6. Respiratory failure in COVID-19-related neurological diseases
Emerging data suggest that SARS-CoV-2 can trigger autoimmune neurologic diseases such as Guillain-Barre syndrome (GBS). Such autoimmune reactions are likely due to molecular mimicry between SARS-CoV-2 spike proteins and glycolipids found on the surface of peripheral nerves [53]. Recent studies showed that patients with COVID-19 that presented with GBS developed severe and progressive respiratory failure [[54], [55], [56]]. Hence, neuromuscular dysfunction could be considered as an alternative cause of respiratory insufficiency in patients with COVID-19 and GBS, especially in those who have minimal chest imaging findings. As mentioned earlier, other coronaviruses such as SARS-CoV-1 and MERS-CoV could result in neurological complications via initially invading peripheral nerves and then entering the CNS through transsynaptic propagations [50]. Whether SARS-CoV-2 can also act similary, is still not fully understood; however, manifestations such as anosmia, ageusia, and headache that have been increasingly recognized in patients with COVID-19 imply the neuroinvasive potential of SARS-CoV-2. Other than GBS, several case reports have described patients with other neurologic disorders associated with COVID-19, including meningitis, encephalitis, acute disseminated encephalomyelitis, and myelitis [[57], [58], [59], [60]]. However, the exact role of SARS-CoV-2 in the development of these neurologic diseases is not clear yet.
7. Conclusions
COVID-19 mainly invades the alveolar epithelial cells and then disseminates throughout the body via the systemic circulation, neural transmission, and adherence to ACE2 expressing cells. SARS-CoV-2 could directly spread to the central nervous system by the olfactory epithelium. The indirect effects of cytokine storm and direct neuroinvasion of SARS-CoV-2 to ACE2 expressing brain cells in the limbic and insular area in the corticolimbic network could be one of the possible mechanism involved in the development of respiratory failure and silent hypoxemia. Also, SARS-CoV-2 can trigger autoimmune neurologic diseases such as GBS. Moreover, neuromuscular dysfunction could be considered as an alternative cause if respiratory insufficiency in patients with COVID-19. This would open up possible lines of study for the progress in the central mechanism of COVID-19-induced hypoxia. Future research is desirable to confirm or disprove such a hypothesis.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Funding
None.
References
- 1.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell discovery. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Military Medical Research. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nouri-Vaskeh M., Alizadeh L. Fecal transmission in COVID-19: A potential shedding route. J Med Virol. 2020;92(10):1731–1732. doi: 10.1002/jmv.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imanpour H., Rezaee H., Nouri-Vaskeh M. Angiotensin 1-7: A novel strategy in COVID-19 Treatment. Adv pharm bull. 2020;10(4):488–489. doi: 10.34172/apb.2020.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory medicine. 2020 doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nature reviews Cardiology. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS chemical neuroscience. 2020 doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 10.Kanninen K.M., Lampinen R., Rantanen L.M., Odendaal L., Jalava P., Chew S., White A.R. Olfactory cell cultures to investigate health effects of air pollution exposure: implications for neurodegeneration. Neurochemistry international. 2020;104729 doi: 10.1016/j.neuint.2020.104729. [DOI] [PubMed] [Google Scholar]
- 11.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of virology. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C., Herman P., Manley G.T., Lyon D.M., Hopkins C. Smell and taste dysfunction in patients with COVID-19. The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. The Laryngoscope. 2020 doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. European Archives of Oto-Rhino-Laryngology. 2020;1 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gane S., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020 doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Iglesias P., Porta-Etessam J., Montalvo T., Valls-Carbó A., Gajate V., Matías-Guiu J.A., Parejo-Carbonell B., González-García N., Ezpeleta D., Láinez J.M., Matías-Guiu J.A. An Online Observational Study of Patients With Olfactory and Gustory Alterations Secondary to SARS-CoV-2 Infection. Frontiers in public health. 2020;8 doi: 10.3389/fpubh.2020.00243. 243-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulfamante G., Chiumello D., Canevini M.P., Priori A., Mazzanti M., Centanni S., Felisati G. First ultrastructural autoptic findings of SARS -Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86(6):678–679. doi: 10.23736/s0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- 19.Matías-Guiu J., Gomez-Pinedo U., Montero-Escribano P., Gomez-Iglesias P., Porta-Etessam J., Matias-Guiu J.A. Should we expect neurological symptoms in the SARS-CoV-2 epidemic? Neurologia. 2020;35(3):170–175. doi: 10.1016/j.nrl.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliezer M., Hautefort C., Hamel A.L., Verillaud B., Herman P., Houdart E., Eloit C. Sudden and Complete Olfactory Loss Function as a Possible Symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 21.Lu W., Zhang S., Chen B., Chen J., Xian J., Lin Y., Shan H., Su Z.Z. A Clinical Study of Noninvasive Assessment of Lung Lesions in Patients with Coronavirus Disease-19 (COVID-19) by Bedside Ultrasound (Nicht-invasive Beurteilung von pulmonalen Läsionen bei Patienten mit Coronavirus-Erkrankung (COVID-19) durch Ultraschall direkt am Krankenbett) Ultraschall in Med (EFirst). 2020 doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- 22.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. Journal of medical virology. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Pinedo U., Matias-Guiu J., Sanclemente-Alaman I., Moreno-Jimenez L., Montero-Escribano P., Matias-Guiu J.A. Is the brain a reservoir organ for SARS-CoV2? Journal of medical virology. 2020 doi: 10.1002/jmv.26046. doi:10.1002/jmv.26046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R., Yu J., Wang K., Howard D., French L., Chen Z., Wen C., Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. BioRxiv. 2020 doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu SC Mou HM, Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Li Q.R., Huang X., Cui Y., Li X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. [A pathological report of three COVID-19 cases by minimally invasive autopsies] Zhonghua Bing Li X.Z.ue Za Zhi. 2020;49(0):E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 26.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. medRxiv. 2020;2020 doi: 10.1101/2020.04.06.20050575. 2004.2006.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. American journal of respiratory and critical care medicine (ja) 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Möhlenkamp S., Thiele H. Ventilation of COVID-19 patients in intensive care units (Beatmung von COVID-19-Patienten auf Intensivstationen) Herz:1-3. 2020 doi: 10.1007/s00059-020-04923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henig N.R., Pierson D.J. Mechanisms of hypoxemia. Respir Care Clin N Am. 2000;6(4):501–521. doi: 10.1016/s1078-5337(05)70087-3. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar M., Niranjan N., Banyal P.K. Mechanisms of hypoxemia. Lung India : official organ of Indian Chest Society. 2017;34(1):47–60. doi: 10.4103/0970-2113.197116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burki N.K., Lee L.Y. Mechanisms of dyspnea. Chest. 2010;138(5):1196–1201. doi: 10.1378/chest.10-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning H.L., Mahler D.A. Pathophysiology of dyspnea. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace. 2001;56(4):325–330. [PubMed] [Google Scholar]
- 33.Stoeckel M.C., Esser R.W., Gamer M., Büchel C., von Leupoldt A. Brain mechanisms of short-term habituation and sensitization toward dyspnea. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00748. 748-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gigliotti F. Mechanisms of dyspnea in healthy subjects. Multidisciplinary respiratory medicine. 2010;5(3):195–201. doi: 10.1186/2049-6958-5-3-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burki T. Outbreak of coronavirus disease 2019. The Lancet Infectious diseases. 2020;20(3):292–293. doi: 10.1016/s1473-3099(20)30076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prys-Picard Co, Kellett F., Niven Rm. Disproportionate breathlessness associated with deep sighing breathing in a patient presenting with difficult-to-treat asthma. Chest. 2006;130(6):1723–1725. doi: 10.1378/chest.130.6.1723. [DOI] [PubMed] [Google Scholar]
- 37.Wright G.W., Branscomb B.V. The origin of the sensations of dyspnea. Trans Am Clin Climatol Assoc. 1954;66:116–125. [PMC free article] [PubMed] [Google Scholar]
- 38.von Leupoldt A., Dahme B. Cortical substrates for the perception of dyspnea. Chest. 2005;128(1):345–354. doi: 10.1378/chest.128.1.345. [DOI] [PubMed] [Google Scholar]
- 39.Davenport P.W., Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respiratory physiology & neurobiology. 2009;167(1):72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peiffer C., Costes N., Herve P., Garcia-Larrea L. Relief of dyspnea involves a characteristic brain activation and a specific quality of sensation. Am J Respir Crit Care Med. 2008;177(4):440–449. doi: 10.1164/rccm.200612-1774OC. [DOI] [PubMed] [Google Scholar]
- 42.von Leupoldt A., Sommer T., Kegat S., Baumann H.J., Klose H., Dahme B., Buchel C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med. 2008;177(9):1026–1032. doi: 10.1164/rccm.200712-1821OC. [DOI] [PubMed] [Google Scholar]
- 43.Lipton P., Whittingham T.S. The effect of hypoxia on evoked potentials in the in vitro hippocampus. The Journal of physiology. 1979;287:427–438. doi: 10.1113/jphysiol.1979.sp012668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukandala G., Tynan R., Lanigan S., O’Connor J.J. The Effects of Hypoxia and Inflammation on Synaptic Signaling in the CNS. Brain Sci. 2016;6(1):6. doi: 10.3390/brainsci6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corcoran A., O’Connor J.J. Hypoxia-inducible factor signalling mechanisms in the central nervous system. Acta physiologica (Oxford, England) 2013;208(4):298–310. doi: 10.1111/apha.12117. [DOI] [PubMed] [Google Scholar]
- 46.Forsythe J.A., Jiang B.H., Iyer N.V., Agani F., Leung S.W., Koos R.D., Semenza G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and cellular biology. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feoktistov I., Ryzhov S., Zhong H., Goldstein A.E., Matafonov A., Zeng D., Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension (Dallas, Tex : 1979) 2004;44(5):649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- 48.Devlin C., Greco S., Martelli F., Ivan M. miR-210: More than a silent player in hypoxia. IUBMB life. 2011;63(2):94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivan M., Harris A.L., Martelli F., Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. Journal of cellular and molecular medicine. 2008;12(5a):1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurology. 2020 doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Sabeti P. Neuropathological Features of Covid-19. New England Journal of Medicine. 2020 doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D., Luqmani A., Jenkins Ih, Nicholas R., Jones B., Everitt A. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurology - Neuroimmunology Neuroinflammation. 2020;7(5):e789. doi: 10.1212/nxi.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalakas M.C. Guillain-Barré syndrome: The first documented COVID-19–triggered autoimmune neurologic disease. More to come with myositis in the offing. 2020;7(5):e781. doi: 10.1212/nxi.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virani A., Rabold E., Hanson T., Haag A., Elrufay R., Cheema T., Balaan M., Bhanot N. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00771. e00771-e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P., Viganò M., Giovannelli G., Pirro F., Montisano D.A., Appollonio I., Ferrarese C. Guillain-Barré syndrome related to COVID-19 infection. Neurology - Neuroimmunology Neuroinflammation. 2020;7(4):e741. doi: 10.1212/nxi.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., Abu-Rumeileh S., Piscaglia M.G., Querzani P., Callegarini C., Foschi M. Guillain-Barré syndrome following COVID-19: new infection, old complication? Journal of neurology. 2020;267(7):1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav Immun. 2020;87 doi: 10.1016/j.bbi.2020.05.012. 149-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. International Journal of Infectious Diseases. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., Fontanella M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. MedRxiv. 2020 [Google Scholar]