Abstract
Introduction: In the absence of effective antivirals and vaccination, the pandemic of COVID-19 remains the most significant challenge to our health care system in decades. There is an urgent need for definitive therapeutic intervention. Clinical reports indicate that the cytokine storm associated with acute respiratory distress syndrome (ARDS) is the leading cause of mortality in severe cases of some respiratory viral infections, including COVID-19. In recent years, cannabinoids have been investigated extensively due to their potential effects on the human body. Among all cannabinoids, cannabidiol (CBD) has demonstrated potent anti-inflammatory effects in a variety of pathological conditions. Therefore, it is logical to explore whether CBD can reduce the cytokine storm and treat ARDS.
Materials and Methods: In this study, we show that intranasal application of Poly(I:C), a synthetic analogue of viral double-stranded RNA, simulated symptoms of severe viral infections inducing signs of ARDS and cytokine storm.
Discussion: The administration of CBD downregulated the level of proinflammatory cytokines and ameliorated the clinical symptoms of Poly I:C-induced ARDS.
Conclusion: Our results suggest a potential protective role for CBD during ARDS that may extend CBD as part of the treatment of COVID-19 by reducing the cytokine storm, protecting pulmonary tissues, and re-establishing inflammatory homeostasis.
Keywords: ARDS, cannabidiol, CBD, COVID-19, cytokine storm, inflammation
Introduction
Acute respiratory distress syndrome (ARDS) is a serious inflammatory lung condition responsible for the highest rate of medical complications and mortality among critically ill patients.1 In the case of viral respiratory infections, symptoms are usually mild, self-limiting, and confined to the upper airways. However, in more severe respiratory viral infections, such as the current COVID-19 pandemic, the infection can affect the bronchoalveolar units of lower respiratory tracks, causing ARDS. There is increased pulmonary vascular permeability, alveolar infiltrates, hypoxemia, abnormal coagulation and fibrinolysis, and endothelial and epithelial damages.2,3
In patients with severe COVID-19, the transition to ARDS is mainly due to the occurrence of cytokine storm and excessive inflammatory responses, including massive production of proinflammatory cytokines such as interleukin (IL)-6 and IL-1β, IL-17, as well as infiltration of neutrophils and monocytes into the lung tissue.3–5 Currently, other than supportive measures, there is no definitive cure for ARDS,6,7 illustrating the urgent need for creative and effective therapeutic modalities to treat this complex condition.
Numerous studies report that cannabinoids may function as immune modulators, limiting the adverse effects of inflammatory diseases.8 Endocannabinoids are produced in the respiratory system and cannabinoids-induced bronchial dilation suggests a significant therapeutic potential for cannabinoids in the treatment of respiratory diseases, including ARDS in case of patients with severe form of COVID-19.9 Importantly, several reports demonstrated that cannabidiol (CBD), a phytocannabinoid produced by the cannabis plant, can block IL-6 in several models of inflammatory diseases.9 Therefore, it is very reasonable to investigate whether cannabinoids can be as therapeutic agents to treat severe viral respiratory infections including current COVID-19 and ARDS symptoms.
In this study, we have two objectives: to develop a murine model to simulate viral infection-induced ARDS and to test the potential of cannabinoids in mitigating ARDS symptoms. We used polyriboinosinic:polyribocytidylic acid [poly(I:C)] in a murine model to simulate the pathophysiological viral disease state and clinical symptoms of ARDS. Poly(I:C) is stable synthetic double-stranded RNA segments with average size between 1.5 and 8 kb that can bind to toll-like receptor 3 (TLR3) with high affinity.10
Our first hypothesis is: intranasal application of Poly(I:C) can induce a significant inflammatory response and affect the functional capacity of the lung tissue and airways in a similar manner as seen in COVID-19 and other viral ARDS. Our second hypothesis is: CBD can ameliorate the ARDS by reducing the inflammatory cytokines, limiting damages in the lung, and improving the functional capacity of airways.
Materials and Methods
Animal model and application of Poly(I:C) and CBD
Wild-type C57BL/6 mice (male, 12 weeks old) were divided into three experimental groups of sham, control, and treatment (n=5). All animals were housed in pathogen-free conditions at the animal facility of the Augusta University, and all experiments were performed in accordance with the rules and regulations of the Augusta University Institutional Animal Care and Use Committee (IACUC). All mice were anesthetized with isoflurane. Sham group received phosphate-buffered saline (PBS) while control and treatment groups were administered Poly(I:C) (Sigma Aldrich, St. Louis, MO, USA) (100 μg in 50 μL sterile PBS) intranasally (I/N) three once-daily doses.
CBD (isolate, tetrahydrocannabinol-free Canabidiol Ltd., Dublin, Ireland) was delivered intraperitoneally (5 mg/kg), first dose 2 h after the second Poly(I:C) treatment and every other day for a total of three doses to the treatment group. Sham and control groups received PBS only. All mice were sacrificed at 8 days after the first Poly(I:C) application. Blood and lung tissues were harvested and subjected to further analysis.
Measurement of vital signs
Vital signs including temperature and blood oxygen saturation were measured before and after any treatment. Central body temperature was measured rectally and blood oxygen saturation was determined using portable pulse oximeter (Contec Medic Sys Ltd., Qinhuangdao, China) through carotid arteries.
Histology and immunohistochemistry
Tissue from left lobes of the lung was fixed in 10% neutral buffered formalin. Samples were processed by routine methods, oriented to provide coronal sections. For histological examination, multiple 5 μm midcoronal sections were cut and stained with hematoxylin and eosin, or trichrome. As for inflammatory indices, immunohistochemistry was performed by incubating the samples with specific antibodies against murine IL-6 (Cat. No. 554402; BD BioSciences Pharmingen) and neutrophils (Cat. No. G102; Leinco Technologies). Preparations were counterstained with hematoxylin (Cat. No. 7221; Richard-Allan Scientific, Kalamazoo, MI, USA) and mounted in Faramount (Cat. No. S3025; DAKO), analyzed, and imaged by brightfield microscopy.
Analytical flow cytometry
Single-cell suspension was prepared from lung tissues. In brief, tissue samples were sieved through a 100 μM cell strainer (BD Biosciences, San Diego, CA, USA), followed by centrifugation (252 g, 10 min) to prepare single-cell suspensions. All cells were then stained with fluorescent antibodies to quantify neutrophils, macrophages, lymphocytes, and cytokine expression.
In brief, all cells were stained with anti-Gr1 (neutrophils), anti-F4/80 (macrophages), and anti-CD3/CD4/CD8 (lymphocytes, all from Biolegend USA). Then cells were fixed and permeabilized and stained intracellularly for cytokines including IL-6, tumor necrosis factor alpha (TNFα), IL-2, and interferon gamma (IFNγ) (proinflammatory cytokines). All samples were run through a 4-Laser LSR II flow cytometer. Cells were gated based on forward and side scatter properties and on marker combinations to select cells of interest. All acquired flow cytometry data were analyzed using FlowJo V10.
Statistical analysis
Graphs and summary statistics were also used to assess the results. All statistical tests were two sided. Except for where noted, all p-values presented are unadjusted for multiple comparisons.
Results
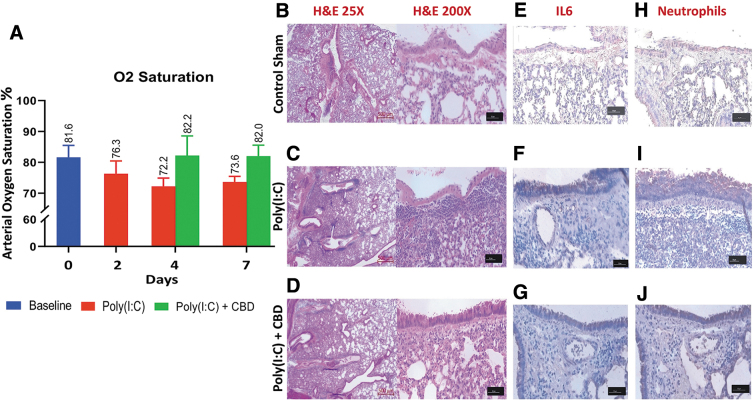
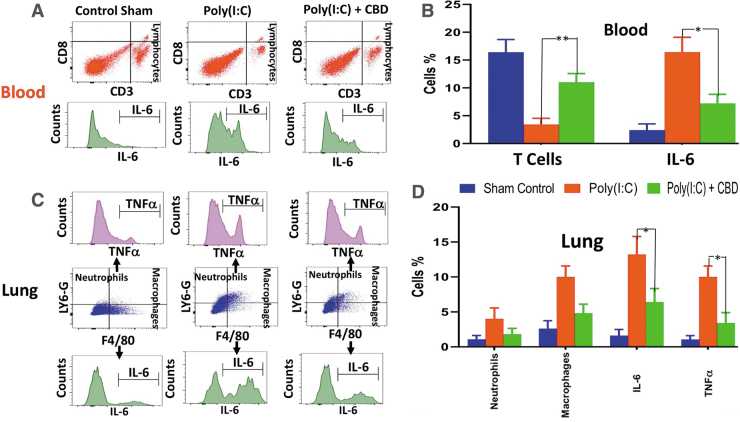
Intranasal administration of Poly(I:C) induced ARDS-like symptoms very similar to those of severe COVID-19. Poly(I:C) reduced the blood oxygen saturation by 10% from ±81.6% to ±72.2% (Fig. 1A), and the histological examination of lung tissues demonstrated that Poly(I:C) caused a significant perivascular and peribronchiolar interstitial inflammatory infiltrate compared with the normal tissue (Fig. 1B, C). Poly(I:C) produced structural damages to the lung, including, not limited to, fibrosis, hypertrophy, and pulmonary edema evidenced by the widened interstitial space surrounding the airways and vasculature (Fig. 1C). These symptoms were totally or partially reversed and returned to the level and condition of the normal after treatment with CBD (Fig. 1D). Furthermore, immunohistochemistry analysis of lung tissues revealed that Poly(I:C) treatment resulted in a marked increase in IL-6 and infiltrating neutrophils compared with the normal tissue (Fig. 1E, F). CBD treatment reduced the expression of IL-6 and lowered the frequencies of neutrophils in the lung (Fig. 1G). Flow cytometry analysis of blood showed significant reduction in number of lymphocytes (severe lymphopenia) (p<0.01), mild reduction in neutrophils, marked increase in monocytes, and significant increases in the level of IL-6 (p<0.03), IFNγ, and TNFα after Poly(I:C) treatment compared with the normal tissues (Fig. 2A, B).
FIG. 1.
CBD improves lung structure and function after intranasal Poly(I:C) treatment. (A) Blood oxygen saturation was reduced (≥10%) by intranasal administration of Poly(I:C). CBD was able to reverse the effect and return the rate of blood oxygen saturation toward the normal level. (B–D) Histological analysis (H, E) of normal lung tissue (B) demonstrated the destruction of normal morphology and structure of lung, hypertrophy, fibrosis, and pulmonary edema, as compared with Poly(I:C)-treated mice (C). CBD treatment (D) improved the structure toward the normal architecture. (E, G) Immunohistochemical analysis of lung tissue showed an increase in the expression level of IL-6 in Poly(I:C)-treated lung compared with normal tissue (E, F). CBD treatment (G) reduced the IL-6 expression in Poly(I:C)-treated lung, indicating the potential protective effect of CBD. (H–J) Immunohistochemical staining for neutrophils (Gr1+LY6G+) showed Poly(I:C) treatment of lung increased neutrophil mobilization compared with the untreated normal lung tissue. CBD treatment curtailed the neutrophil infiltration, suggesting a counter inflammatory role for CBD to limit the ARDS progression. ARDS, acute respiratory distress syndrome; CBD, cannabidiol.
FIG. 2.
Anti-inflammatory effect of CBD after intranasal Poly(I:C) treatment. (A, B) Flow cytometry analysis showed that intranasal administration of Poly(I:C) effectuated lymphopenia and increased IL-6 production. These effects were reversed with intraperitoneal administration of CBD, resulting in marked higher number of T cells (**p<0.01) and lower IL-6 level (*p<0.05) in the blood compared with Poly(I:C) with no CBD treatment group. (C, D) CBD treatment attenuated neutrophil and macrophage infiltration into the lung tissue after treatment with Poly(I:C), demonstrated by flow cytometry analysis. In addition, CBD reduced the level of inflammatory cytokines (e.g., interleukin-6, tumor necrosis factor alpha, and interferon gamma) (*p<0.05), suggesting a potential for controlling the cytokine storm in COVID-19 patients.
Flow cytometry analysis of lung demonstrated an increase in the frequencies of infiltrating neutrophils (p<0.01), macrophages, and significant elevation (p<0.01) in the expression of proinflammatory cytokines (e.g., IL-6, TNFα, and IFNγ) (Fig. 2C, D). CBD treatment reversed all these inflammatory indices and partially re-established homeostasis. In the blood, CBD treatment enhanced the lymphocyte frequencies markedly (p<0.01) while reducing the number of neutrophils and monocytes as well as the level of proinflammatory cytokines significantly (e.g., IL-6, IFNγ, and TNFα) (Fig. 2E, F). In the lung, CBD treatment downregulated the number of infiltrating neutrophils and macrophages markedly, and reduced the level of cytokines significantly (p<0.05) (Fig. 2G, H).
Discussion
Current studies strongly suggest that intranasal administration of high-dose Poly(I:C) in a murine system may be a reliable working and practical model to investigate and help better understand mechanisms responsible for SARS-CoV-2 and other respiratory virus-induced ARDS-like symptoms. Decrease in blood oxygen saturation, lymphopenia, and cytokine storm (marked production of proinflammatory cytokines), and destruction of lung tissue architecture are all cardinal clinical signs and symptoms of severe COVID-19 and other virus-induced ARDS.
In addition, being a synthetic analogue of double-stranded RNA, not only can Poly(I:C) provide a very robust foundation to study the immunopathology and physiology of COVID-19, but also, when compared with actual viral agents, it is a considerably safer and cheaper alternative for research on SARS-Cov-2 and other virus-induced infections.
Present findings propose a potential immunotherapeutic role for CBD in the treatment of severe respiratory viral infections and ARDS. The current data support the notion that the anti-inflammatory function of CBD may reduce cytokine storm and mitigate the effects of exaggerated inflammation. Recent reports suggest that the interaction between immune system and COVID-19 is a two-phased process of immune activation and immune dysregulation. Increasingly, clinical and preclinical studies show regulatory functions of CBD: inhibiting leukocyte migration and limiting inflammatory cytokine production.11–14
Considering all potential regulatory effects of CBD as well as the vast distribution of endocannabinoid system in the body, it is plausible that CBD may be used as a therapeutic candidate in the treatment of various inflammatory conditions including COVID-19 and other virus-induced ARDS.
In conclusion, we introduce a new animal model of pathogen-associated molecular patterns (PAMP)- Toll-like receptor 3 (TLR3)-induced ARDS using poly (I:C), as the PAMP ligand for TLR3. Applying this model, we investigated, and demonstrated, the potential of cannabinoids in the treatment of complex simulated viral respiratory diseases such as those seen in COVID-19. Obviously, more studies are required to expand and validate this therapeutic strategy.
Abbreviations Used
- ARDS
acute respiratory distress syndrome
- CBD
cannabidiol
- PBS
phosphate-buffered saline
- poly(I:C)
polyriboinosinic:polyribocytidylic acid
- IL
interleukin
- TLR3
toll-like receptor 3
- TNFα
tumor necrosis factor alpha
- IFNγ
interferon gamma
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was sponsored by institutional seed money of Dental College of Georgia (DCG) and Medical College of Georgia (MCG) jointly, as well as by National Institute of Health award (R01NS110378). The funding organizations had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Cite this article as: Khodadadi H, Salles ÉL, Jarrahi A, Chibane F, Costigliola V, Yu JC, Vaibhav K, Hess DC, Dhandapani KM, Baban B (2020) Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic RNA, Cannabis and Cannabinoid Research 5:3, 197–201, DOI: 10.1089/can.2020.0043.
References
- 1. Gan T, Yang Y, Hu F, et al. TLR3 regulated Poly I:C-induced neutrophil extracellular traps and acute lung injury partly through p38 MAP kinase. Front Microbiol. 2018;9:3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–82 [DOI] [PubMed] [Google Scholar]
- 3. Spadaro S, Park M, Turrini C, et al. Biomarkers for acute respiratory distress syndrome and prospects for personalised medicine. J Inflamm (Lond). 2019;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghadimi-Moghadam A, Haghani M, Bevelacqua JJ, et al. Tragic pandemic: concerns over unintentional “Directed Accelerated Evolution” of Novel Coronavirus (SARS-CoV-2) and introducing a modified treatment method for ARDS. J Biomed Phys Eng. 2020;10:241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020. pii: [DOI] [PMC free article] [PubMed]
- 6. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaari L, Golubnitschaja O. Covid-19 pandemic by the “real-time” monitoring: the Tunisian case and lessons for global epidemics in the context of 3PM strategies. EPMA J. 2020:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pini A, Mannaioni G, Pellegrini-Giampietro D, et al. The role of cannabinoids in inflammatory modulation of allergic respiratory disorders, inflammatory pain and ischemic stroke. Curr Drug Targets. 2012;13:984–993 [DOI] [PubMed] [Google Scholar]
- 9. Bozkurt TE. Endocannabinoid system in the airways. Molecules. 201917;24. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stowell NC, Seideman J, Raymond HA, et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir Res. 2009;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols JM, Kaplan BLF. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020;512–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McHugh D, Tanner C, Mechoulam R, et al. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–450 [DOI] [PubMed] [Google Scholar]
- 13. Baban B, Hoda N, Malik A, et al. Impact of cannabidiol treatment on regulatory T-17 cells and neutrophil polarization in acute kidney injury. Am J Physiol Renal Physiol. 2018;315:F1149–F1158 [DOI] [PubMed] [Google Scholar]
- 14. Mabou Tagne A, Marino F, Legnaro M, et al. Extract and its constituent cannabidiol inhibit human polymorphonuclear leukocyte functions. Int J Mol Sci. 2019;20:1833. [DOI] [PMC free article] [PubMed] [Google Scholar]