Abstract
Objective: To determine if cannabis may be used as an alternative or adjunct treatment for intermittent and chronic prescription opioid users.
Design: Retrospective cohort study.
Setting: A single-center cannabis medical practice site in California.
Patients: A total of 180 patients who had a chief complaint of low back pain were identified (International Classification of Diseases, 10th Revision, code M54.5). Sixty-one patients who used prescription opioids were analyzed.
Interventions: Cannabis recommendations were provided to patients as a way to mitigate their low back pain.
Outcome Measures: Number of patients who stopped opioids and change in morphine equivalents.
Results: There were no between-group differences based on demographic, experiential, or attitudinal variables. We found that 50.8% were able to stop all opioid usage, which took a median of 6.4 years (IQR=1.75–11 years) after excluding two patients who transitioned off opioids by utilizing opioid agonists. For those 29 patients (47.5%) who did not stop opioids, 9 (31%) were able to reduce opioid use, 3 (10%) held the same baseline, and 17 (59%) increased their usage. Forty-eight percent of patients subjectively felt like cannabis helped them mitigate their opioid intake but this sentiment did not predict who actually stopped opioid usage. There were no variables that predicted who stopped opioids, except that those who used higher doses of cannabis were more likely to stop, which suggests that some patients might be able to stop opioids by using cannabis, particularly those who are dosed at higher levels.
Conclusions: In this long-term observational study, cannabis use worked as an alternative to prescription opioids in just over half of patients with low back pain and as an adjunct to diminish use in some chronic opioid users.
Keywords: medical cannabis, marijuana, prescription opioid use, opiate abuse, low back pain, chronic pain
Introduction
In 2016, there were an estimated 11.8 million opioid misusers aged 12 years and older, representing 4.4% of the U.S. population.1 In the same year the Centers for Disease Control reported 42,249 deaths related to opioids, a fivefold increase compared with 1999.2 With rising numbers of deaths each year, there seems to be no easy solution to combat this national health crisis.
Since the publication of an article reporting an association between states with medical cannabis laws and a 24.8% decrease in annual opioid deaths,3 there has been increased interest in medical cannabis as a potential treatment alternative or adjunct. Another study that found an association between states with medical cannabis dispensaries and decreased opioid overdoses concluded that cannabis may be acting as a substitute for opioids.4 Neither of these studies was able to show a direct cause and effect relationship.
Cannabis has been used for centuries as an analgesic5 and has been shown to reduce chronic pain.6–9 Although cannabis works by acting on the CB1 and CB2 receptors of the endocannabinoid system and does not bind opioid receptors of the noradrenergic system directly, there is thought that the two systems may overlap synergistically or indirectly function in concert.10,11 It has therefore been postulated that pain relief may be achieved with lower doses of opioids in the setting of concomitant cannabis use.10,12 A number of studies have supported this idea of a combined approach8,13–21 or found cannabis may be used in place of prescription opioids.22,23
To find patients who might be taking opioids, we chose to study patients with back pain. Low back pain was reportedly experienced by 71 million people surveyed 18 years and older in the previous 3 months during 2016, representing 28.4% of the U.S. population.24 Low back and back symptoms were the reported principal reason for 20.4 million doctors' office visits in 2014, the most common pain complaint in the survey.25 Because opioids are commonly prescribed for back pain26 (although not shown to be more efficacious to nonopioid medication therapy27), we chose to study patients who had a primary complaint of lower back pain and were given the provisional diagnosis corresponding to the International Classification of Diseases, 10th Revision (ICD-10) code (M54.5). The primary purpose of this study was to determine whether the use of cannabis may be used as an alternative to opioids. A secondary purpose was to see if cannabis would lower opioid usage in intermittent and chronic opioid users.
Materials and Methods
Study design and participants
This was a retrospective cohort study of a single practitioner (J.Y.H.) who has practiced cannabis medicine in California since 1997 and has seen >3000 patients. We selected patients from the active database of patient records who had a chief complaint of lower back pain and carried the corresponding ICD-10 code (M54.5). The cannabis practitioner verified the diagnosis through medical records from the patient's primary care physician's office the majority of the time when patients had them sent. However, because care was not predicated on receiving these records, the low back pain diagnosis was not confirmed by the primary care doctor in all cases. A detailed chart review was conducted on the study patients in October 2018 over the course of 2 weeks. Additional data collection was performed for 2 days in February 2019.
Setting and variables
Medical cannabis use was first legalized in the state of California in 1996 following the passage of a voter-approved initiative called the “Compassionate Use Act.”28 Until 2018, the medical cannabis laws in California required patients to receive a recommendation from a physician certifying the need for medical cannabis. If the physician agreed, the patient would be granted a 1-year approval for medical cannabis and the conditional document afforded the patient the right to grow a limited amount of cannabis and/or purchase medical cannabis at a California dispensary.
Before their first scheduled appointment, patients were asked to fill out a detailed health history questionnaire that mirrors a standard medical intake interview with additional questions about cannabis. It included demographic information, employment history, insurance status, medical history, medications, social habits, cannabis use patterns, and how cannabis affected him or her. One additional question asked, “Does the use of cannabis modify your use of other drugs?” The “yes/no” closed-ended response was followed by a blank space for patients to elect to elucidate the answer.
On the day of the initial intake, details of the health history questionnaire were verified and clarified when necessary. Reasons for wanting to use medical cannabis were discussed. The history was followed by a standard physical examination. Once the intake evaluation was completed, the patient was given a medical cannabis recommendation if the physician thought it would benefit his or her medical condition. Since the Compassionate Use Act did not address expiration for the medical cannabis approval, California physicians would write the approval for 1 year in deference to the guidelines of the Medical Board of California. Patient follow-up was recommended based on the complaint and timely need for re-evaluation.
All patients saw the cannabis physician to medically justify and thus legalize their use of cannabis to mitigate their back pain. Although decreasing or stopping opioid usage was not an explicit goal from the physician, many patients were motivated to discontinue or diminish their opioid use while augmenting their pain management regimen with cannabis. Typically, the patient was encouraged to use opioids and cannabis at the same time with the assumption that better pain control could be achieved.
Data sources
For patients taking opioids, we divided them into two groups. One group comprised patients who we called “intermittent users”: defined as irregular or pro re nata use of opioids taken as needed. The intermittent group was further subdivided into two groups. “Short-term intermittent” users took opioids of any amount for <6 months. “Infrequent intermittent” users took opioids less than three times a day for any length of time. The second group comprised patients who we termed “chronic users”: defined as regular use of opioids taken for at least 6 months with an opioid dosing frequency of three or more doses per day.
All prescription opioid use was converted into “morphine equivalents” (MEs) using standardized guidelines.29 The amount of time it took for patients to stop opioids (or who continued usage) was calculated as the difference between when a patient was initially seen and treated by the cannabis physician and the date of visit when the patient was no longer taking opioids (or their last visit if they were still on opioids).
Because cannabis usage between patients was highly variable in the methods of consumption, types of products used, frequency of usage and percentages of cannabinoid concentrations, we report the grams of cannabis used per day, recognizing that the bioavailability of various methods of consumption such as inhalation (e.g., smoking and vaporization) are highly variable within and between groups such as ingestion (e.g., tinctures, pills, and edibles). For smoking, we assumed that one joint was 0.32 g of cannabis.30 For ingestion, we used the reported amount of cannabis per patient-reported product.
To determine differences between patients who stopped using opioids and those who continued with regard to various demographics, cannabis use, and opioid use, Fisher's exact test was used for categorical variables, t-test for normally distributed continuous variables, and Wilcoxon rank sum for non-normal distributions. To ascertain whether patients reduced their morphine equivalents after starting cannabis, regardless of whether patients stopped using opioids, the sign test and the Wilcoxon sign rank test was used. To determine which patient factors might predict who would stop opioids, logistic regression models were developed. All analyses were performed using SAS statistical software (version 9.4; SAS Institute, Cary, NC). The Western Institutional Review Board approved this study as exempt.
Results
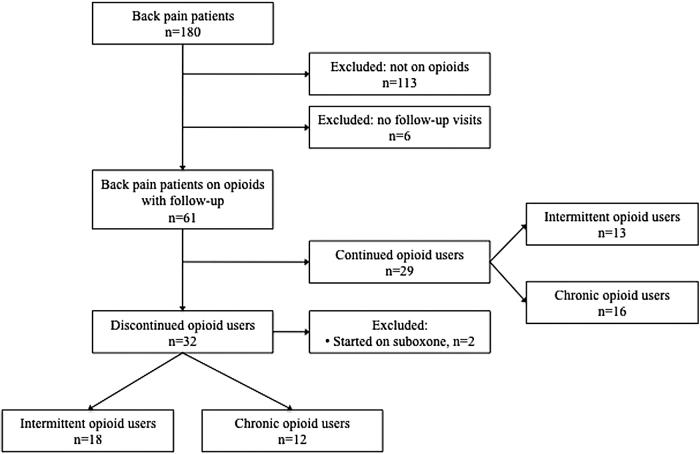
The active database of patient records showed that 180 patients had a chief complaint of lower back pain and carried the corresponding ICD-10 code (M54.5). Of 180 patients, 113 patients were excluded because they were not taking any prescribed opioid medications when they were initially evaluated. Six patients were excluded because they did not have any follow-up visits after their initial evaluation for a final sample size of 61 patients (Fig. 1).
FIG. 1.
Flow sheet of patients.
Patients were 50.1±11.4 years of age (range=49–86), 62% men with the majority (85%) being white. The mean level of education was 13.7±2.5 years. Fifteen percent had military service. For religious affiliation, 52.3% were Christian, 31.8% were none, and 15.9% were other. For employment status, 77.8% were employed and 22.2% were unemployed, retired, or had unknown employment status. For insurance type, 75.4% had private, 21.1% public, and 3.5% had none. Forty-five percent smoked, 34.4% drank any alcohol, and 19.6% had ever used any illegal drugs excluding cannabis.
The patients reported median years of chronic pain was 11 (range=1–50). The median time patients were on opioids was 3 years (range=0.1–20). The median morphine equivalents used was 21 mg/day (range=1.1–500). The median amount of cannabis used was 1.45 g/day (range=0.01–18.7 g) with the majority smoking (44/61=72%). Lesser used modalities included vaping (16/61=26%), tinctures (11/61=18%), edibles (9/61=15%), and capsules (2/61=3%; totals do not equal 100% because patients used multiple modalities of consumption). When asked if cannabis modified other drug use, 48% of those who answered the question felt it diminished their pain/opioid medications, 28% felt like it had no effect on their pain medications, and 24% were unsure.
Thirty patients (49%) were classified as chronic users and 31 (51%) were classified as intermittent users. Of the intermittent users, 20 (64.5%) were short-term intermittent users and 11 (35.5%) were infrequent intermittent users.
Of the 61 patients who were taking opioids on their initial visit, 32 (52.5%) were able to completely stop opioids. Of the 32 patients who stopped opioids, 2 were able to stop by transitioning to the opioid partial agonist suboxone in combination with cannabis use. We excluded these two patients from the remaining analyses because suboxone is a partial opioid agonist and we wanted to study the effect of cannabis alone in reducing opioids. Of these 30 remaining patients who stopped opioids, the median time it took patients to stop opioids was 6.4 years (range=0.4–15.7).
There were no significant differences in age, gender, race, education, military service status, religion, employment status, insurance type, smoking status, alcohol use, history of substance abuse, amount of time of chronic pain, amount of time on opioids, frequency of cannabis usage, amount of cannabis usage, attitude if cannabis use affected opioid use and morphine equivalents, between intermittent and chronic users and between the groups of patients who were able to stop opioids compared with those who did not with one exception. The 32 patients who stopped opioids used a median of 1.4 g (IQR=0.64–1.89) of cannabis per day compared with 0.64 g (IQR=0.25–0.98) for the 29 patients who did not stop opioids. Using the Wilcoxon rank sign test, these differences were significant (p=0.019) (Table 1).
Table 1.
Characteristics of Those Off Opioids and Those Who Continued Opioids
| Off opioids (n=32) |
Continued opioid users (n=29) |
p | |||
|---|---|---|---|---|---|
| n | %a | n | %a | ||
| Age, mean±SD | 48.2±12.3 | 52.3±10.1 | 0.15 | ||
| Gender, female | 9 | 27.3 | 14 | 50.0 | 0.11 |
| Race, white | 27 | 84.4 | 24 | 85.7 | >0.99 |
| Education, years, mean±SD | 13.9±2.9 | 13.5±2.1 | 0.54 | ||
| Military service | 5 | 15.6 | 4 | 14.8 | >0.99 |
| Religion, Christian | 11 | 52.4 | 12 | 52.2 | >0.99 |
| Employed | 23 | 79.3 | 19 | 76.0 | >0.99 |
| Insurance, private | 23 | 76.7 | 19 | 74.1 | >0.99 |
| Smokers | 15 | 46.9 | 12 | 42.9 | 0.80 |
| Alcohol usage | 13 | 27.5 | 8 | 40.6 | 0.42 |
| History of substance use | 5 | 17.9 | 4 | 22.2 | 0.72 |
| Type user chronic | 12 | 37.5 | 16 | 55.2 | 0.44 |
| Years of chronic pain, median (IQR) | 12 (7–20) | 10 (4–19) | 0.38 | ||
| Years on opioids, median (IQR) | 6.0 (1–10) | 3 (1.3–5) | 0.46 | ||
| Cannabis amount used, g/day, median (IQR) | 1.4 (0.64–1.89) | 0.64 (0.25–0.98) | 0.019 | ||
| Attitude if cannabis lowers opioid use | 14 | 40.0 | 15 | 51.7 | 0.65 |
| Morphine equivalents, mg/day median (IQR) | 18 (12–36) | 27 (15–95) | 0.45 | ||
Percentages may not total 100% because of missing data.
IQR, interquartile range; SD, standard deviation.
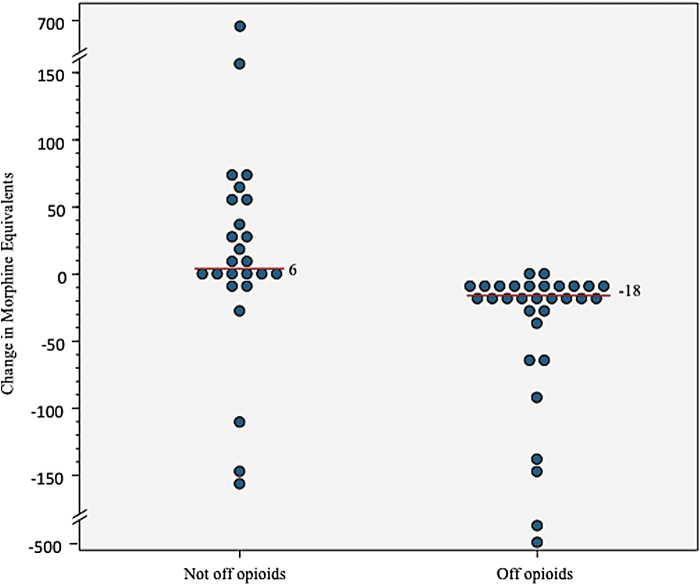
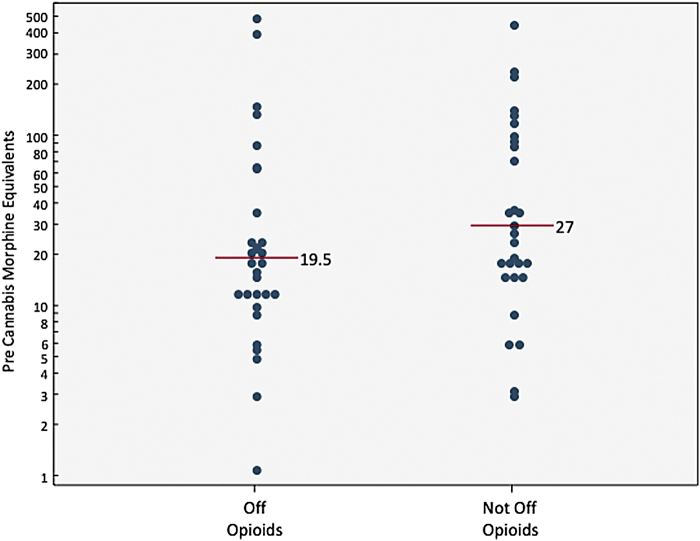
Overall, the median reduction of morphine equivalents was −9.0 (IQR=−24 to 6, sign test: p=0.004, Wilcoxon sign rank test: p=0.09). Of the 30 patients off opioids, median change was −18 MEs (IQR=−36 to −12). Of those not off opioids, 9 (31%) were able to reduce their MEs (median=−24, IQR=−108.5 to −9), 3 (10%) remained the same, and 17 (59%) increased their amount (median=54, IQR=24–72; Fig. 2). Discontinuing opioids was not related to MEs before cannabis use (median=19.5 vs. 27; Fig. 3).
FIG. 2.
Change in morphine equivalents pre/postcannabis use.
FIG. 3.
Morphine equivalents before cannabis use.
When subgroup analysis using a Wilcoxon sign rank test was performed, both chronic (median = − 21, IQR = − 90 to 5) and intermittent opioid users (median = − 5.8, IQR = − 12 to 6) diminished their use of opioids after starting cannabis and chronic users were significantly more likely to reduce by larger amounts (p=0.048). Logistic regression models, including age, gender, pre-MEs, and type of opioid user, did not reveal any individual predictors of the ability to stop taking opioids using cannabis.
Discussion
To our knowledge, this is the longest study period of patients on prescription opioids who reported on average over a decade of chronic pain. For those able to stop using opioids with medical cannabis, it took an average of 6 years. We postulate that the combined effects of opioids plus cannabis on reducing pain—and possibly reducing muscle spasms, anxiety, depression, and improved sleep that are often associated with chronic lower back pain—may have provided the relief necessary to allow for a reduction in the prescribed doses of opioids. We therefore believe this supports a synergistic relationship between opioids and cannabis.
Our findings that there was only one variable that predicted who would stop opioids was surprising to us. We had expected that at least intermittent users would have an easier time stopping opioids and that chronic users would have more difficulty transitioning off opioids, especially given the long duration of chronic pain and years of habituating to opioids. What our findings suggest is that cannabis may help transition some patients off opioids without regard to any of the demographic variables we studied including age, gender, amount of opioids being taken, and how often opioids are taken. Perhaps the patients who were not able to transition off opioids, who used about half the amount of cannabis as those who did stop opioids, were not titrated to levels sufficient to achieve the possible synergistic effects of cannabis necessary to stop opioids.
Clearly, the addition of cannabis did not help all patients. Seventeen of 61 patients (28%) increased their opioid use and 3 (4.9%) had no change in opioids. Given the addictive nature of opioids, this is not entirely surprising. We were encouraged that 9 of 30 (30%) who remained on opioids reduced their usage, which suggests cannabis works as an adjunct to reduce opioid usage in some people.
The results of this study generally support the findings of other observational studies already mentioned: that cannabis can be used as an alternative or adjunct to diminish opioid usage.8,13–23 But of those studies, only four8,13,17,20 appear to follow patients for any length of time in a practice and the follow-up was considerably shorter than is reported in this article.
Unlike other articles that describe patient claims of cannabis reducing opioid usage, our article did not show a correlation between self-reports and actual opioid cessation. We do suspect the two measures to be linked but we would not be completely surprised at the possibility that the two may not be correlated. Perhaps the addition of cannabis makes patients feel improved and provides the feeling as if they need less opioids but patients may not actually reduce them. Alternatively, perhaps enough time had not passed for patients to diminish or stop using opioids.
Our results are substantially different from one large Australian observational study of chronic noncancer pain patients taking opioids.31 They reported patients who used cannabis had more pain and less self-efficacy to manage their pain, concluding that cannabis neither reduced pain nor helped to reduce opioid usage. We interpreted their data differently, which led us to different conclusions. Their tables show that cannabis users at baseline had significantly more anxiety, more pain (on Brief Pain Inventory), and less self-efficacy, which likely drove them to seek alternatives to treatment.
In addition, this was an observational cohort study that only asked if patients used cannabis. The authors acknowledged in their article that cannabis use was likely not overseen by a medical practitioner to guide them on how to use cannabis properly for their pain, which we believe is essential to teach patients' successful methods that have been used to diminish opioid use. Furthermore, because it was obtained illegally and presumably with minimal if any choice of chemovars, we further have doubts that cannabis was used methodically to address let alone optimize pain control. We believe this article represents one of questionable associations and clearly no causation.
A limitation to our study is being an observational study in a single-center practice site without a large number of patients. We recognize that the results reflect correlation and not necessarily causation. That is, cannabis may not have been the only reason patients stopped using opioids. For example, the patients' primary care physicians may have been working with their patients to taper their opioids. However, we suspect that with a greater number of patients, significant results may have been more probable.
It is possible that some of the patients who stopped opioids would have weaned themselves off without cannabis. It is also possible that the introduction of the supportive care of the cannabis physician may have had a contributing effect to cessation or diminution of opioids. This study was also not primarily directed toward those who were known or recognized to be opioid dependent.
The patients in this study represent motivated patients who were seeking out cannabis as an alternative approach to treat their pain. Although there was never an explicit goal to wean them from opioids, the practitioner did use cannabis to try to control and then mitigate pain. It is possible that the pain relief they received from cannabis was because of poor response from other pain regimens or from failed stress adaptation.32 We understand that the results may be skewed, reflecting motivated patients. We further acknowledge that the opioids used were self-reported by patients to their cannabis physician. It is possible that their data might not have been accurate.
Finally, we recognize that the group classifications for intermittent compared with chronic opioid users are arbitrary. Simply because someone uses opioids three times a day for a minimum of 6 months does not mean they are opioid dependent or addicted. However, given the status of cannabis as a Schedule I drug, it is unlikely that a randomized controlled trial study of opioid-dependent patients could be performed given our current laws.
Conclusion
In this long-term observational study of a single-center cannabis medical practice site, the addition of cannabis use worked as an alternative to prescription opioids in 50% of patients with chronic back pain. It worked as an adjunct to diminish use in some chronic opioid users. There was only one variable that predicted those who were able to stop opioids suggesting that some patients might be able to stop opioids by using cannabis and that those who do not stop opioids may not be titrated at doses of cannabis high enough to achieve the desired effect necessary to diminish or stop their opioid usage.
Abbreviations Used
- ICD-10
International Classification of Diseases, 10th Revision
- IQR
interquartile range
- ME
morphine equivalents
- SD
standard deviation
Author Disclosure Statement
K.M.T. has a financial interest in the medical cannabis company MGC LLC that did not exist when this study was performed.
Funding Information
The Society of Cannabis Clinicians paid for the IRB review of this project and statistical analysis.
Cite this article as: Takakuwa KM, Hergenrather JY, Shofer FS, Schears RM (2019) The impact of medical cannabis on intermittent and chronic opioid users with back pain: how cannabis diminished prescription opioid usage, Cannabis and Cannabinoid Research 5:3, 263–270, DOI: 10.1089/can.2019.0039.
References
- 1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, 2017. https://www.samhsa.gov/data Accessed February25, 2018
- 2. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachhuber MA, Saloner B, Cunningham CO, et al. . Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174:1668–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell D, Pacula RL, Jacobson M. Do medical marijuana laws reduce addictions and deaths related to pain killers? RAND Corporation. J Health Econ. 2018;58:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russo EB. History of cannabis and its preparations in saga, science and sobriquet. Chem Biodivers. 2007;4:2624–2648 [DOI] [PubMed] [Google Scholar]
- 6. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Press: Washington, DC, 2017 [PubMed] [Google Scholar]
- 7. Aggarwal SK. Cannabinergic pain medicine: a concise clinical primer and survey of randomized-controlled trial results. Clin J Pain. 2013;29:162–171 [DOI] [PubMed] [Google Scholar]
- 8. Haroutounian S, Ratz Y, Ginosar Y, et al. . The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016;32:1036–1043 [DOI] [PubMed] [Google Scholar]
- 9. Busse JW, Wang L, Kamaleldin M, et al. . Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320:2448–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russo EB, Hohmann AG. Role of cannabinoids in pain management. In Deer TR, et al. (eds.), Comprehensive Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. American Academy of Pain Medicine. Springer: New York, 2013, p. 181 [Google Scholar]
- 11. Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530:54–58 [DOI] [PubMed] [Google Scholar]
- 13. Lynch ME, Clark AJ. Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. J Pain Symptom Manage. 2003;25:496–498 [DOI] [PubMed] [Google Scholar]
- 14. Abrams DI, Couey P, Shade SB, et al. . Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844–851 [DOI] [PubMed] [Google Scholar]
- 15. Kral AH, Wenger L, Novak SP, et al. . Is cannabis use associated with less opioid use among people who inject drugs? Drug Alcohol Depend. 2015;153:236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17:739–744 [DOI] [PubMed] [Google Scholar]
- 17. Gruber SA, Sagar KA, Dahlgren MK, et al. . Splendor in the grass? A pilot study assessing the impact of medical marijuana on executive function. Front Pharmacol. 2016;7:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res. 2017;2:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen S, Sabioni P, Trigo JM, et al. . Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology. 2017;42:1752–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellnier T, Brown GW, Ortega TR. Preliminary evaluation of the efficacy, safety, and costs associated with the treatment of chronic pain with medical cannabis. Ment Health Clin. 2018;8:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooper ZD, Bedi G, Ramesh D, et al. . Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology. 2018;43:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corroon JM Jr, Mischley LK, Sexton M. Cannabis as a substitute for prescription drugs—a cross-sectional study. J Pain Res. 2017;10:989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int J Drug Policy 2017;42:30–35 [DOI] [PubMed] [Google Scholar]
- 24. NHIS. Summary Health Statistics: National Health Interview Survey, 2016. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_A-5.pdf Accessed February25, 2018
- 25. National Ambulatory Medical Care Survey. 2014 State and National Summary Tables. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf Accessed February25, 2018
- 26. Ivanova JI, Birnbaum HG, Schiller M, et al. . Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 2011;11:622–632 [DOI] [PubMed] [Google Scholar]
- 27. Krebs EE, Gravely A, Nugent S, et al. . Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. State of California Health and Safety Code, Section 11362.5. https://leginfo.legislature.ca.gov/faces/codes_displaySection.xhtml?sectionNum=11362.5.&lawCode=HSC Accessed January26, 2019
- 29. Nielsen S, Degenhardt L, Hoban B, et al. . A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25:733–737 [DOI] [PubMed] [Google Scholar]
- 30. Ridgeway G, Kilmer B. Bayesian inference for the distribution of grams of marijuana in a joint. Drug Alcohol Depend. 2016;165:175–180 [DOI] [PubMed] [Google Scholar]
- 31. Campbell G, Hall WD, Peacock A, et al. . Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health. 2018;3:e341–e350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70:2409–2438 [DOI] [PubMed] [Google Scholar]