Abstract
In 2002/2003 there was a pandemic denominate SARS (severe acute respiratory syndrome), caused by the SARS-CoV virus that belongs to the genera Betacoranavirus and the family Coronaviridae, generally responsible for influenza infections. In mid of 2019, a new disease by the coronavirus named by COVID-19 (SARS-CoV-2) emerged, both infections have flu symptoms, however they are infections that variable intensity, being medium to severe. In medium infections individuals have the virus and exhibit symptoms, however hospitalization is not necessary, in severe infections, individuals are hospitalized, have high pathology and in some cases progress to death. The virus is formed by simple positive RNA, enveloped, non-segmented, and presenting the largest genome of viruses constituting 32 Kb, consisting of envelope proteins, membrane, nucleocapsid and spike protein, which is essential in the interaction with the host cells. As for the origin of this virus, research has been intensified to determine this paradox and although the similarity with SARS-CoV, this virus did not has necessarily the same place of origin. As for the immune system, it is currently unknown how this new virus interacts. In this brief review, we demonstrate important considerations about the responses to this infection.
Highlights
-
•
SARS-CoV-2 versus SARS-CoV immune response.
-
•
Clinical features of COVID-19.
-
•
Immune response in SARS-CoV and SARS-CoV-2 infection.
-
•
Immune Memory in coronavirus infection.
1. Introduction
The acute respiratory syndrome is a disease caused by the SARS-CoV-2 virus (COVID-19), where symptoms include difficulty breathing, high fever, and cough [1]. Belonging to the genera Betacoranavirus and the family Coronaviridae [2] in Gorse et al., 2020 [3]; in Gorse et al., 2020 [4]; in Gorse et al., 2020; [5]. This pandemic has currently highlighted in the media due to its rapid propagation across the globe through migration processes, totaling 183 affected regions (countries, areas or territories) [6], (China, Japan, Republic of Korea, Italy, Spain, France, Germany, Brazil, among others). It has a mortality rate of around 3–4%, being more severe in the elderly and immunocompromised individuals [7]. Before approaching the current virus, it is necessary to report on its origin, starting from the discovery of this family.
1.1. General characteristics of the family coronaviridae
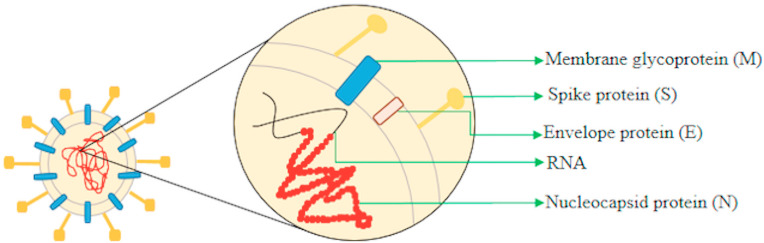
Discovered in the decade of the 1960s, this family subdivided into the genera Alphacoronavirus (HCoV-229E, HCoV-NL63) and Betacoronavirus (HCoV-OC43, HCoV-HKU1, SARS-CoV, MERS-CoV) responsible for infection in humans. However, there are other genera (Gammacoronavirus, Deltacoronavirus, Torovirus and Bafinivirus) that cause injuries to animals [8,9] in Phan et al., 2018 [10]; in Phan et al., 2018; [11]. Consist of viruses with the largest genomes (32 kb), with a simple positive sense RNA strand, not segmented and enveloped [12]. This structure is constituted of four proteins: the envelope (E) (9–12 kDa), membrane (M) (23–35 kDa), nucleocapsid (N) (50–60 kDa) and the spike (S) (180–220 kDa) (Fig. 1 ) [[12], [13], [14]].
Fig. 1.
Representative design of the essential structures of the coronavirus.
In 2002/2003 there was a SARS (Severe Acute Respiratory Syndrome) pandemic, caused by the SARS-CoV virus, affecting about 29 countries, with a mortality rate of 9.6% [15]. This virus has a definitive host insectivorous bat of the species Rhinolophus sinicus. The transmission to human probably occurred because of the manipulation or consumption of meat of the intermediate hosts, the species Nyctereutes procyonoides and Peribases larvata [8,16,17]. Later in mid of 2012, a new disease emerged to MERS (Middle East Respiratory Syndrome) [18] with confirmed cases in 27 countries [19]. It is a zoonotic disease, where the species Camelus dromedarius the dromedaries are definitive hosts, the transmission to humans occurs through contact with these animals [20,21], with a mortality rate of 34.4% [22]. In both infections mentioned, the dissemination of the viruses in other countries occurs through close contact between the infected person and non-infected person.
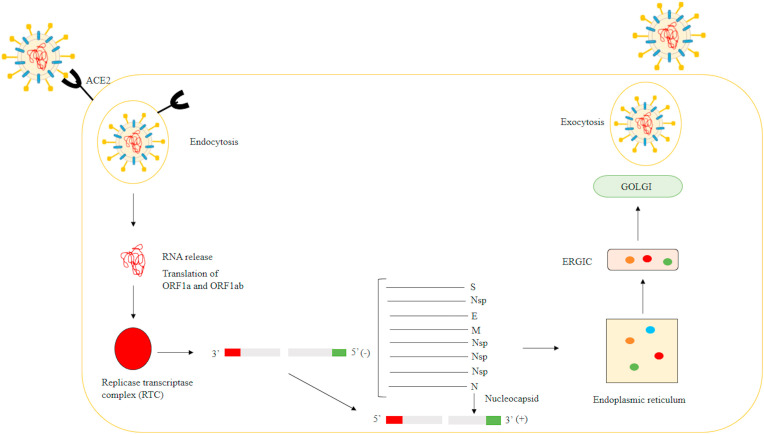
The infection starts when the virion enters in the host cell, provided by the connection of the viral S protein with the ACE2 receptor (angiotensin-converting enzyme 2) in the case of SARS-CoV however depending on the virus, the receptor of cell connection differs (Ex: MERS-CoV uses the DPP4 receptor), at this moment endocytosis of the virus occurs. Within the endosome, the S2 region of the S protein undergoes modifications, usually performed by proteases (cathepsin, TMPRRS2), in order to release its domains (RBD region, fusion domain) and to expose the fusion peptide. This peptide is inserted into the endosomatic membrane, where occurs your connection with the heptified hydrophobic repeating regions (HR1 and HR2) forming a nucleus with six helices. Through this transformation, the virus is now able to fuse with the host cell membrane and release the genomic RNA in the cytoplasm, at this moment this RNA will go through the translation process. The Open Reading Frames (ORF), ORF1a, and ORF1ab sequences are translated into pp1a and pp1ab (viral replicase polyproteins) that will be cleaved into smaller proteins, which join to form a replicase-transcriptase (RTC) complex. In this complex, the formation of a complete negative-strand RNA occurs and several copies are generate and used as a template for the synthesis of a complete positive-strand RNA. The subgenomic mRNA's are generated by discontinuous transcription and will be translated into structural proteins (S, E, M, N). The proteins will be transported to the lumen of the intermediate compartment of the endoplasmic reticulum-Golgi (ERGIC) and with the genomic RNA, virion formation occurs (the nucleocapsid is formed by the N protein in the cytoplasm and the genome). After complete formation, the virion is released from the cell by exocytosis and restarts the infection process in new cells (Fig. 2 ) [12,[23], [24], [25], [26], [27], [28]].
Fig. 2.
Representative design of the viral replication cycle (SARS-Cov).
Years after the infections caused by SARS-CoV and MERS-CoV, in 2019, a new disease established in China, caused by the virus later designate by SARS-CoV-2, disseminate to several countries [1,5]. Currently, the origin of SARS-CoV-2 is uncertain. Studies have demonstrated that it shares ~76% amino acid identity with SARS-CoV and both viruses uses ACE2 to interact with protein S and TMPRSS2, a protease that cleaves protein S to release its domains, essential processes in viral infection [29], so it would be a possibility that SARS-CoV-2 performs the same infection process in host cells. In addition, that due to this similarity to SARS-CoV and as previously mentioned the origin of this virus, SARS-CoV-2 probably arises from a natural selection in the animal host or a selection in humans [30].
This virus is preferred in cells of the respiratory tract, epithelial hair cells of the airways and type 2 alveolar pneumocytes in SARS-CoV infections [31] in Totura & Baric, 2012), in MERS-CoV we can cite pneumocytes and syncytial epithelial cells [32]. Because of this preference, the damages caused during this infection are of high predominance in the respiratory tract. In SARS infections we can mention infiltrations by monocytes, macrophages, and neutrophils and with a result of the presence of these cells, the increase in pro-inflammatory cytokines (IFN-γ, TNF-α, IL-1, IL-6, IL-12, TNF) and chemokine's (CCL2, CXCL9, CXL10). These are probably responsible for diffuse alveolar damage (edema, fibrosis, the formation of a hyaline membrane), in addition, the alveolar collapse, desquamation of epithelial and alveolar cells, and damage to other organs (spleen, liver, bone marrow, among others) [33,34]. In MERS infections, however, there is limited information about its pathogenesis, but it includes diffuse alveolar damage, epithelial denudation, hyaline membrane, edema, type II pneumocyte hyperplasia, severe acute hemorrhagic pneumonia, intra-alveolar macrophages, and lymphocyte infiltrates (CD3 +, CD4 +, CD8 +) in the pulmonary parenchyma, multinucleated syncytial cells [32,35]. As well in SARS infection, the inflammatory response is predominant with the production of TNF-α, IL-6, CXCL-10, CCL-2, CCL-3, CCL-5, and IL-8 contributing to tissue damage [36]. in Liu et al., 2020 [37]; in Liu et al., 2020).
1.2. Clinical features of coronavirus
In infections with these viruses, the clinical characteristics variate depending on the individual's immune response, presenting as asymptomatic (positive for the disease but without clinical manifestation), mild symptomatic (positive for the disease with clinical manifestations) or severe symptomatic (positive for the disease with manifestations in high degree). In MERS the classification in the population prevails as asymptomatic, mild symptomatic, for these, the symptoms generally include fever, cough, and shortness of breath, some have, pneumonia or diarrhea. The symptomatic presents respiratory failure, requiring mechanical ventilation in a specialized unit, in addition to leukopenia, lymphocytopenia, thrombocytopenia and high levels of creatinine, lactate dehydrogenase, liver enzymes, this stage usually develops in the elderly, immunodeficient, diabetics and individuals with chronic diseases or cancer [38,39]. In SARS-CoV infection, there are asymptomatic individuals, the mild symptomatic individuals present: fever, dry cough, shortness of breath, malaise, headache, diarrhea, and tremors. The severe symptom consists in fever, tachycardia, tachypnea, lymphocytopenia, thrombocytopenia, neutrophilia, and oxygen desaturation. These patients require oxygenation and in some cases treatment in the intensive care unit (ICU) (WHO; [40,41], usually this phase develops in individuals with advanced age, severe lymphopenia, chronic hepatitis B infection, high LDH peak, other infections, elevated neutrophils, among others [42]. In SARS-CoV-2 the same classification remains asymptomatic individuals, the mild symptomatic individual's presents dry cough, fever, and fatigue, difficulty breathing, some have diarrhea, sore throat, congestion, and runny nose. According to the National Health Comission of China severe symptomatic patients have difficulty breathing (greater than 30 times/minute), oxygen saturation at rest (less than 93%), the ratio between partial pressure of arterial oxygen and oxygen concentration in arterial blood (less than 300 mmHg) and greater than 50% of pulmonary image evolution in the short term. Critical individuals have a respiratory failure with the need for mechanical ventilation, organ failure or need for treatment in the ICU, individuals who develop severe and critical forms include the elderly, immunodeficient, individuals with high blood pressure, diabetes, heart disease, lung disease and cancer [1,[43], [44], [45]]. The symptomatology in infected people diverge and most of the severe symptomatic patients evolve to death.
1.3. Immune response in SARS-CoV and SARS-CoV-2 infection
Infections caused by a coronavirus, in general, will be mediated by T lymphocytes, which will become active the moment the pathogen presented by antigen-presenting cells (dendritic cells or macrophages) is recognized [46]. In the moment of activation, there will be the production of inflammatory mediators (IFN–I, TNF-β, IL-1, IL-6, CCL2) and probably the production of perforin and granzyme B, processes that usually occur in others airways infections [47,48]. What several studies indicate is not knows for sure how this response occurs in both SARS-CoV and SARS-Cov-2 infections. In the acute phase of responses, in both infections, occurs lymphopenia [46,47]. Is still believed that decrease of lymphocytes in SARS-CoV infections is must failures in their activation, through strategies developed by the virus as an escape from the immune response, for example the suppression of IFN-I, which impairs the activation of dendritic cells and the processes of activation, differentiation and expansion of T cells [49,50].
In more severe cases, high pathogenesis is observed caused by an intense inflammatory process that isn't controlled by the cytokine storm (release of inflammatory mediators: IFN-α, IL-1β, IL-6, TNF-α, CCL2, CCL5, among others), responsible for the development of lung injuries, which culminates in respiratory failure, organ failure and death [48,50]. In addition, the most severe SARS-CoV infection is observed in the lungs histology, a presence of the inflammatory infiltration, caused by macrophages and neutrophils which correlated with the increase in the number of cells in the peripheral blood [51] in Channappanavar & Pelrman, 2017 [52]; in Channappanavar & Pelrman, 2017 [53]; in Channappanavar & Pelrman, 2017 [54]; in Channappanavar & Pelrman, 2017).
Therefore, possibly in the later stage of the disease, the predominance of the immune response is directs by these cells. They are cells that participate in the innate immune response and are key components in activating an adaptive immune response, during this infection that response fails to activate T cells. With a constant stimulus caused by the virus infection, these cells continue to produce inflammatory mediators to reduce viral replication, however, this process causes tissue damage that evolves into an intensified pathogenesis. To avoid this process, it is necessary to have a balance in the immune response, with the production of anti-inflammatory mediators, such as IL-10 [55].
This fact probably is seen in individuals who have the disease in its non-severe form. In this case, the immune system is able to control the infection and minimize the damage caused by the inflammatory response. [56]; show an infected individual, with the mildest form of SARS-CoV-2 infection, which presented a frequency of activated CD4+ and CD8+ T cells, follicular T cells and increased antibody-secreting cells and minimal levels of inflammatory cytokines and chemokines. Enough to activate an efficient immune response to control viral replication and not intensify the exaggerated immune response.
In the most severe cases of the disease, this profile is not observed. However, this variation will depend on the individual's immune response and if it belongs to the risk group [57]. For example, in the article of Gong and collaborators 2020, severe patients present a high production of IL-10 and IL-6, patients with medium changes had low production of IL-6 (less than 100pg/mL) and for patients critical (who died) IL-6 levels were greater than 100pg/mL. The levels of IL-6 and IL-10 related to the severity of the disease, as well as TNF-α, IL-12R, ferroprotein, lymphocyte count, neutrophil, eosinophil, and procalcitonin. The reduction of peripheral CD4+ and CD8+ T cells in the blood is observed, but present in inflammatory infiltrate in the lung. While in these patients the inflammatory response is high [58], demonstrate that in asymptomatic individuals the inflammatory response is reduce in relation to healthy controls.
The article of [59]; shows an excessive activation of lymphocytes in infection by SARS-CoV-2 and in non-serious infections, there is a lymphocyte response. Therefore and what was been previously reported about infections with SARS-CoV-2, lymphocytes are essential cells for infection control and the immune responses performed by these cells vary from individual to individual. Thus, the immune system of a healthy individual has control of the infection, elderly and immunodeficient patients may develop the most severe form, in this case, the infection becomes intense, as well as the immune responses.
In children, the infection is generally mild or moderate, or even asymptomatic (which makes diagnosis difficult) and the amount of viral RNA is high in children under 5 years old in their nasopharynx [60]. In the work developed by Refs. [61]; the authors demonstrate a low activation of T cells and a high production of IL-12 and IL-1β in children in compare to adults infected with SARS-CoV-2. With this, we can raise a hypothesis that there is a control of the virus development by the immune system without an intense inflammatory process. In their article [62] raise hypotheses about possible mechanisms that may explain the less susceptibility of children to development COVID-19 disease and that this is probably based on the generation of antibodies by memory B cells and their rapid response and the rapid production of antibodies natural with wide reactivity and not selected. As a preliminary result, the authors suggest an early polyclonal B cell response with production of mainly IgM plamoblasts in children, and this profile is not observe in adults with severe disease. Some articles approach about trained immunity [[63], [64], [65]] or the angiotensin-converting enzyme (ACE-2) in children [64,66,67], however this hypotheses are not enough to obtain a conclusive response in SARS-CoV-2 infection in children.
After eliminating the pathogen performed by the adaptive immune response, the response resolves, in other words, the levels of inflammatory mediators become basal and the cells return to their place of origin. In contrast, in the case of lymphocytes, an extremely important characteristic is the generation of memory cells.
1.4. Immune Memory
Memory allows the immune system to develop a faster and more efficient response since the cells have already had first contact with the pathogen, the formation of a response will be a specific antigen, and therefore eliminating the pathogen is more effective than a primary response. In memory T lymphocytes, there is less demanding activation than a naive cell (lower antigen concentration and co-stimulatory signals) and gradual proliferation, while B cells proliferate/differentiate quickly and become plasma cells [[68], [69], [70], [71], [72]].
When memory T cells are activated, they produce inflammatory mediators, such as IFN-γ, CCL3, CCL4, CCL5, responsible by the activation and recruitment of other types of cells. Their proliferation and survival depends on cytokine stimulation, such as IL -15 and IL-7 and are cells with a half-life that vary from 8 to 15 years, that become the responsible for the great part of the eliminated pathogens throughout an individual's life [[69], [70], [71],73]. Memory B cells (usually IgG) differentiate into plasma cells (antibody producer) or return to the germinal center (usually IgM) they are also capable of producing cytokines and are long-lasting cells [74,75]. In the infection process is also generate neutralizing antibody and that remains after infection and they are responsible for connecting directly to the virus that prevents it from entering the host cell [75].
1.5. Memory in coronavirus infection
The potential of lymphocytes in SARS infection has been demonstrated, the generation of memory is essential in reinfection processes. Articles demonstrate the detection of IgG antibody titles in the 16th month after SARS infection [76]. In 2 years after infection together with neutralizing antibodies, in 6 years after infection and the presence of T memory lymphocytes of CD4+/CD8+ cytokine producers with a central memory phenotype, are more frequent in severe than mild infections [77]. In addition, the neutralizing antibodies generated in SARS infections would be specific to the RBD domain of protein S, demonstrating to be an immunogenic protein [78]. Hoffmann and collaborators 2020 raise the hypothesis that antibodies generated during this infection could have a certain influence on partial protection against SARS-CoV-2. There could be a blockage in the entry of the virus since the memory cell has already been exposed to the SARS-CoV protein S and because of similarity to SARS-CoV-2, the virus's performance would be partially reduced. It currently not proven the individuals already infected with SARS became infected again with SARS-CoV-2. However, a study by Ref. [79] demonstrated there is a cross reaction between the SARS-CoV-2 and SARS-CoV binding antibodies for the RBD and S1 regions in patients recovered from COVID-19, but there was no development of cross-neutralizing antibodies to the SARS-CoV-2 and SARS-CoV protein S. Still in the in vitro assays, the antibodies present in the plasma of individuals infected with SARS-CoV-2 were not able to neutralize the SARS-CoV infection. Demonstrating that S protein in both vírus, although has a similarity they will have a different response.
In relation to production of antibodies in SARS-CoV-2 infections, the detection of IgM antibodies occurs from the fourth day of infection, increasing with time until reaching the 20th day (approximate peak) and reducing, while the detection of IgG occurred from the seventh day to the peak on the twenty-fifth day and maintain high levels after 4 weeks of infection [80].
In the production of antibodies according to the severity of the disease, it is possible to observe that three days after the onset of symptoms, the IgM titers gradually increase in patients with mild and severe forms over time, in the positive rate is higher in groups with the mild form [81]. In the work developed by Ref. [82] it is possible to observe that the IgG and IgM titers are high in the severe group in relation to the non-severe group, with statistical difference in IgG two weeks after the onset of symptoms. Furthermore, the seronegative patients evaluated presented seroconversion of IgM or IgG twenty days after the onset of symptoms.
We can still report as demonstrated in the work of Liu & [83] that individuals with severe disease tend to have a more robust IgG response than mild individuals (except for some cases), in contrast, mild cases have a faster peak IgM response. In both cases, IgM levels disappear 4 weeks after the onset of symptoms. It is also important to note that the responses of IgG and IgM (detection during the peak) were high in patients in ICU compared to non-ICU patients [84].
In asymptomatic individuals, IgM and IgG are produced, however, IgG production was significantly higher in symptomatic individuals during the acute phase compared to asymptomatic individuals. In the initial phase of convalescence, IgG levels decreased in both groups (asymptomatic - 93.3% and symptomatic - 96.8%). It is extremely important to highlight that some individuals analyzed became seronegative for IgG (asymptomatic- 40.0% and symptomatic - 12.9%) [58].
In MERS infections, more than 20 antibodies isolated from humans or humanized have been described [48]. Antibody and neutralizing antibody titles generated and dosed 13 months after the MERS outbreak in Jordan are the same when subsequently dosed 34 months after infection [85]. However, in SARS-CoV-2 infection was demonstrate that is a reduction in the levels of neutralizing antibodies 2–3 months after infection [58].
In SARS CoV and MERS infection, the presence of memory cells is demonstrate and how they are essential over the infection and in reinfection, mainly the neutralizing antibodies. Currently, articles debate about the use of neutralizing antibody therapy, derived from the plasma of previously infected individuals, as a possible form of viral control. However the variability in sera is high [86], the response of this antibody after infection already installed would be reduced, so this method would be appropriate for prophylaxis and the immune system is unique in each individual, thus immunological reactions are predicted [87]. However, studies have demonstrated the use of convalescent serum in infections by SARS, MERS, and SARS-CoV-2, showing positive results [87].
In SARS-CoV-2 infections [88], demonstrated the process of reinfection in primates (rhesus monkeys) using the same strain, in this study observed that was no viral replication after reinfection and as well was no development of symptomatology, demonstrating an elimination of the virus by the immune system, probably performed by neutralizing antibodies [89]. e [90]; demonstrated that individuals previously infected with SARS-CoV-2, after curing, presented a positive test (presence of viral RNA), however without presenting clinical manifestations of the disease and without transmitting the disease to people close to them. Summarize these data, it is possible to observe with that post-infection immunity is essential for the elimination of the virus and the inhibition of its entry into the cell, probably performed by memory cells. It is still unclear if the infected individual, after curing, will be re-infected with a different strain of the same virus and if there will be the develop of clinical characteristics of the disease. In the current scenario, scientists have been engaged in the development of possible treatments for this disease.
Such as the use of drugs such as remdesivir (used in MERS and Ebola), nafamostat (anticoagulant), drugs that have been shown able to control infection in vitro [83,91,92] and other types of drugs like immunomodulators, antiparasitic, histamine receptor inhibitor (H2 type) [93]. The production of a suitable drug for treatment is complex for several reasons, such as, for example, the tested compound may have an effective dosage for controlling the virus, but it is toxic to the cell in vitro or induce viral resistance or in vivo the selected dose has several side effects [94]; Paintsil & Cheng, 2009). Depending on the compound we have an influence on immune responses, for example if you think about severe patients, a drug that has the ability to interrupt the intense inflammatory response (imunosuppression) would consequently reduce the pathology, however it could increase viral multiplication, since it would have an inhibition of the inflammatory response. Similarly, if there is a treatment that would stimulate the immune system in pacientes with non-severe disease, it could reduce the viral load, but it is not possible to say that it will not cause cellular damage. Therefore are several barriers prevent the development of an effective drug with the ability to control the virus and reduce the pathology.
There is an effort to the elaboration of vaccines, of which 123 are pré-clinical phase and 10 in clinical evaluation. Of these we can highlight the one that is in phase 2b/3, two are in phase 2, five in phase 1/2 and two in phase 1 [7]. Despite this, it is still unclear whether the vaccines will allow the development of protection over the years, as observed in other existing ones. It is noted that the neutralizing antibodies to SARS-CoV-2 are in circulation in up to 2–3 months reported previously, due to this it is possible to raise the hypothesis that probably the vaccine would not have a long protection, having to be applied other doses, but whether the antibodies generated by the vaccine are more durable cannot be confirmed.
1.6. Perspectives futures
It is remarkable that the participation of the immune response in the infection is essential for pathogenic elimination, cellular homeostasis, tissue repair, and generation of memory cells. Over the years, research of that complex system and how it interacts with infections is increasingly notable, this is of paramount importance, since this allows for a greater understanding of the pathogen, especially how it acts on this system and how we can acquire knowledge to develop effective methods to control and eradicate a disease.
In this article we focus on that current pandemic that is devastating in humanity, COVID-19, in addition to reporting on other pandemics belonging to this same family that has developed over the years, highlighting the principal points and immunological performance. It is notable that the participation of lymphocytes in these infections is essential for its control, this is demonstrated by the presence of asymptomatic individuals and with mild forms of the disease, here we hypothesize that the central targets of control are these cells.
In fact, an analysis as together is difficult, since each individual will have an answer to this virus, which can adapt it. What would be the reason behind the excellent activation of lymphocytes in asymptomatic individuals? Because they are central cells in a more effective response, they are able to mediate the activation of several components of the immune system, in addition to the generation of memory cells and the balance of the immune response. The big question is that in severe individuals the maintenance of the response probably occurs by other cell types (Ex: neutrophils, macrophages) or there is sequestration of lymphocytes. Because of the multiplication of the virus, this immune response becomes more and more intense, which it is responsible for the developed pathology, such as cell infiltration, fibrosis, among others, which prevents the proper functioning of the organ, causing the debilitation of the respiratory system. We can still report that in these individuals there is a production of anti-inflammatory mediators as a way to maintain the dynamic balance in anti and pro-inflammatory responses, the attenuation of the inflammatory response promotes viral persistence.
On the other hand, the immune system of individuals with the mild and asymptomatic form of the disease probably perform an efficient “control or elimination” of the virus, thus the course of the inflammatory response follows a continuous flow (inflammation to the resolution of the response) and there is no the development of severe pathologies associated with exacerbated inflammation. Therefore, the inflammatory response during the infection seems to be essential to define the course of the disease.
Thus, the major key point in the immune response is not only the activation factor, but also how the response is controlled, maintaining the balance of the anti- and pro-inflammatory components. The response will be efficient when this balance is established, generating less damage to the host.
Declaration of competing interest
All authors declare no commercial or financial conflict of interest.
Acknowledgments
The authors thank the funders Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [401494/2020–9 to JASG] and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG). The funders had no role in study, decision to publish, or preparation of the manuscript. JASG, NIM and DSO thank CNPq, Fundação Oswaldo Cruz (FIOCRUZ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarships.
References
- 1.WHO-World Health Organization Mers situation update. 2020. http://applications.emro.who.int/docs/Emrpub-Csr-241-2019-En.pdf?ua=1&ua=1&ua=1 Available in:
- 2.Chan K.H. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229e, Oc43, and Nl63. Clin. Diagn. Lab. Immunol. 2005;12(11):1317–1321. doi: 10.1128/CDLI.12.11.1317-1321.2005. In .Gorse, G. J.; Donovan, M.M.; Patel, G. B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. Journal of Medical Virology, feb.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim Y.X., Ng Y.L., Tam J.P., Liu D.X. Human coronaviruses: a review of virus–host interactions. Diseases. 2016;4(3):26. doi: 10.3390/diseases4030026. jul.2016.In Gorse, G. J.; Donovan, M.M.; Patel, G. B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. Journal of Medical Virology, feb.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J. Recovery from the Middle East respiratory syndrome is associated with antibody and T cell responses. Sci. Immunol. aug.2017;2(14) doi: 10.1126/sciimmunol.aan5393. In. Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In: Seminars in immunopathology. Springer Berlin Heidelberg, pp. 529–539, feb. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorbalenya A.E. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. mar.2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO-World Health Organization Q&A on coronaviruses (COVID-19) https://www.who.int/news-room/q-a-detail/q-a-coronaviruses#:~:text=symptoms Available in:
- 7.WHO-World Health Organization . 2020. Draft Landscape of COVID-19 Candidate Vaccines- 2 June 2020.https://www.who.int/who-documents-detail/draft-landscape-of-COVID-19-candidate-vaccines Available in: [Google Scholar]
- 8.Kahn J.S., Mcintosh K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. nov.2005;24(11):223–227. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- 9.De Groot R.J. 2012. Family Coronaviridae. Virus taxonomy; pp. 806–828. In .Phan, M. V.; Ngo Tri, T.; Hong Anh, P.; Baker, S.; Kellam, P.; Cotten, M. Identification and characterization of Coronaviridae genomes from Vietnamese bats and rats based on conserved protein domains. Virus evolution, v. 4, n. 2, p.0-35, dec.2018. [Google Scholar]
- 10.Drexler Jan Felix, Corman Victor Max, Drosten Christian. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral research. 2014;101:45–56. doi: 10.1016/j.antiviral.2013.10.013. Phan, M. V.; Ngo Tri, T.; Hong Anh, P.; Baker, S.; Kellam, P.; Cotten, M. Identification and characterization of Coronaviridae genomes from Vietnamese bats and rats based on conserved protein domains. Virus evolution, v. 4, n. 2, p.0-35, dec.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enjuanes S.L., Zuñiga S., Castaño-Rodriguez C., Gutierrez-Alvarez J., Canton J., Sola I. Chapter eight-Molecular basis of Coronavirus virulence and vaccine development. Adv. Virus Res. 2016;96:245–286. doi: 10.1016/bs.aivir.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehr A.R., Perlman S. vol. 1282. Humana Press; jan.2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. (Coronaviruses). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peiris J.S.M., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. nov.2004;10(12):88–97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavanagh D. Coronaviruses with Special Emphasis on First Insights Concerning SARS. Birkhäuser Basel; 2005. Coronaviridae: a review of coronaviruses and toroviruses; pp. 1–54. [Google Scholar]
- 15.CDC - Center for Disease Control and Prevention Update: severe acute respiratory syndrome---worldwide and United States. 2003. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5228a4.htm Available in:
- 16.Guan Y. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. oct.2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 17.Lau S.K.P., Woo P.C.Y., Li K.S.M., Huang Y., Tsoi H.W., Wong H.L., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. Unit. States Am. aug. 2005;102(39):14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaki A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 19.WHO-World Health Organization Coronavírus Disease 2019 (COVID-19). Situation Report 46. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-COVID-19.pdf?sfvrsn=96b04adf_2 Available in:
- 20.Mubarak A., Alturaiki W., Hemida M.G. Middle east respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J. Immunol. Res. apr.2019;2019 doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. mar.2019;9(1):35. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO-World Health Organization Coronavirus disease. Coronavírus disease (COVID-19) outbreak situation. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available in:
- 23.Van Der Meer Y., Van Tol H., Locker J.K., Snijder E.J. Orf1a-encoded replicase subunits are involved in the membrane association of the arterivirus replication complex. J. Virol. aug.1998;72(8):6689–6698. doi: 10.1128/jvi.72.8.6689-6698.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosch B.J., Van Der Zee R., De Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. aug, 2003;77(16):8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S., Lu L., Liu Q., Xu W., Du L. Receptor-binding domains of spike proteins of emerging or re-emerging viruses as targets for development of antiviral vaccines. Emerg. Microb. Infect. july.2012;1(1):1–8. doi: 10.1038/emi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X., Liu Q., Du L., Lu L., Jiang S. Receptor-binding domain as a target for developing SARS vaccines. J. Thorac. Dis. aug.2013;5(2):142. doi: 10.3978/j.issn.2072-1439.2013.06.06. In. Gorse, G. J.; Donovan, M.M.; Patel, G. B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. Journal of Medical Virology, feb.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos N.S.O., Romanos M.T.V., Wigg M.D. Virologia humana. Guanabara Koogan. jun.2017;3:336. [Google Scholar]
- 28.Song Z. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. jan.2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann M. Cell Press; mar.2020. SARS-CoV-2 Cell Entry Depends on Ace2 and Tmprss2 and Is Blocked by a Clinically Proven Protease Inhibitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. mar.2020:1–3. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow K.-C. Detection of severe acute respiratory syndrome–associated coronavirus in pneumocytes of the lung. Am. J. Clin. Pathol. jan.2004;121(4):574–580. doi: 10.1309/C0EDU0RAQBTXBHCE. In Totura, A.L.; Baric, R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Current opinion in virology, v. 2, n. 3, p. 264-275, june.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng D.L. april. 2014. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. apr.2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J. Med. Virol. may.2020;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alsaad K.O. Histopathology of Middle East respiratory syndrome coronavirus (MERS‐CoV) infection–clinic pathological and ultra-structural study. Histopathology. feb.2018;72(3):516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu H. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. apr.2014;1(454):197–205. doi: 10.1016/j.virol.2014.02.018. In Liu, J et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. Journal of Medical Virology, feb.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. may.2014;209(9):1331–1342. doi: 10.1093/infdis/jit504. In Liu, J et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. Journal of Medical Virology, feb.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alsolamy S., Arabi Y.M. Infection with Middle East respiratory syndrome coronavirus. Can. J. Respir. Ther.: Cjrt= Rev. Canadienne Ther. Respir. Rctr. 2015;51(4):102. [PMC free article] [PubMed] [Google Scholar]
- 39.WHO-World Health Organization Middle East respiratory syndrome coronavirus (MERS-CoV) https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(Mers-CoV Available in:
- 40.Lau A.C.-W., Yam L.Y.C., So L.K.-Y. Management of critically ill patients with severe acute respiratory syndrome (SARS) Int. J. Med. Sci. 2004;1(1):1. doi: 10.7150/ijms.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Che X.-Y. A patient with asymptomatic severe acute respiratory syndrome (SARS) and antigenemia from the 2003–2004 community outbreak of SARS in Guangzhou, China. Clin. Infect. Dis. july.2006;43(1):1–5. doi: 10.1086/504943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vijayanand P., Wilkins E., Woodhead M. Severe acute respiratory syndrome (SARS): a review. Clin. Med. mar/apr.2004;4(2):152. doi: 10.7861/clinmedicine.4-2-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coronavirus disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-nCoV/index.html Available in: Access in: 9 apr/
- 44.Gong J. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. medrxiv. 2020:1–17. doi: 10.1186/s12879-020-05681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimball A. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility. Morbity Mortal. Weekly Rep. (Mmwr) mar. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G. Coronavirus infections and immune responses. J. Med. Virol. jan.2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. may 2014;59(1–3):118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharmaceut. Anal. apr. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teijaro J.R. Type I interferons in viral control and immune regulation. Curr. Opin. Virol. feb.2016;16:31–40. doi: 10.1016/j.coviro.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. Mar.2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 51.Cui W., Fan Y., Wu W., Zhang F., Wang J.-Y., Ni A.-P. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin. Infect. Dis. sept.2003;37(6):857–859. doi: 10.1086/378587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholls J.M. Lung pathology of fatal severe acute respiratory syndrome. Lancet. may.2003;361(9371):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. In. Channappanavar, R.; Paintsil, E.; Cheng, Y.-C. Antiviral Agents. Encyclopedia of Microbiology, p. 176, feb.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y.-H., Lin A.-S., Chao T.-Y., Lu S.-N., Liu J.-W., Chen S.-S., Lin M.-C. A cluster of patients with severe acute respiratory syndrome in a chest ward in southern Taiwan. Intensive Care Med. apr.2004;30(6):1228–1231. doi: 10.1007/s00134-004-2311-8. In. Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In: Seminars in immunopathology. Springer Berlin Heidelberg, pp. 529–539, feb. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu J. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. jul.2005;202(3):415–424. doi: 10.1084/jem.20050828. Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In: Seminars in immunopathology. Springer Berlin Heidelberg, pp. 529–539, feb. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010;10(7):514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thevarajan I. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. mar.2020:1–3. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. mar.2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long Q.-X., Tang X.-J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 59.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet respir. Med. feb.2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heald-Sargent T. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19) Jama Pediatr. jul.2020;179(9):902–903. doi: 10.1001/jamapediatrics.2020.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moratto D. Immune response in children with COVID-19 is characterized by lower levels of T cell activation than infected adults. Eur. J. Imunnol. jun. 2020;50:1412–1414. doi: 10.1002/eji.202048724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carsetti R. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolescent Health. may.2020;4(6):414–416. doi: 10.1016/S2352-4642(20)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Q., Chen Y.-C., Chen C.-L., Chiu C.-H. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J. Formos. Med. Assoc. mar.2020;119(3):670. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dhochak N. Pathophysiology of COVID-19: why children fare better than adults? Indian J. Pediatr. may.2020:1. doi: 10.1007/s12098-020-03322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer A. Resistance of children to COVID-19. How? Mucosal Immunol. 2020:1–3. doi: 10.1038/s41385-020-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee P.-I., Hu Y.-L., Chen P.-Y., Huang Y.-C., Hsueh P.-R. Are children less susceptible to COVID-19? J. Microbiol. Immunol. Infect. feb.2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muus C. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. Biorxiv. apr.2020:1–87. [Google Scholar]
- 68.Janeway C.A., Capra J.D., Travers P., Walport M. fifth ed. Garland Science; 2001. Immunological Memory. Immunobiology: the Immune System in Health and Disease. [Google Scholar]
- 69.Boyman O., Létourneau S., Carsten K., Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur. J. Immunol. aug. 2009;39(8):2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 70.Macleod M.K.L., Kappler J.W., Marrack P. Memory Cd4 T cells: generation, reactivation and re‐assignment. Immunology. may.2010;130(1):10–15. doi: 10.1111/j.1365-2567.2010.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pennock N.D., White J.T., Cross E.W., Cheney E.E., Tamburini B.A., Kedi R.M. T cell responses: naive to memory and everything in between. Adv. Physiol. Educ. dec.2013;37(4):273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palm A.-K.E., Henry C. Remembrance of things past: long-term B cell memory after infection and vaccination. Front. Immunol. july.2019;10:1787. doi: 10.3389/fimmu.2019.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauvau G., Soudja S.M.H. Mechanisms of memory T cell activation and effective immunity. Crossroads Innate Adapt. Immun. V. apr. 2015;850:73–80. doi: 10.1007/978-3-319-15774-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurosaki T., Kometani K., Ise W. Memory B cells. Nat. Rev. Immunol. feb.2015;15(3):149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 75.Weisel F., Shlomchik M. Memory B cells of mice and humans. Annu. Rev. Immunol. jan.2017;35:255–284. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]
- 76.Liu W. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J. Infect. Dis. mar.2006;193(6):792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: comparison with SARS and MERS. Rev. Med. Virol. apr.2020;30(3):2107. doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao Z., Liu L., Du L., Zhang C., Jiang S., Li T., He Y. Potent and persistent antibody responses against the receptor-binding domain of SARS-CoV spike protein in recovered patients. Virol. J. nov.2010;7(1):299. doi: 10.1186/1743-422X-7-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu F. apr.2020. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. [Google Scholar]
- 80.Liu X., Wang J., Xu X., Liao G., Chen Y., Hu C.-H. Patterns of Igg and Igm antibody response in COVID-19 patients. Emerg. Microb. Infect. 2020;9(1):1269–1274. doi: 10.1080/22221751.2020.1773324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen L. Delayed specific Igm antibody responses observed among COVID-19 patients with severe progression. Emerg. Microb. Infect. jun.2020;9(1):1096–1101. doi: 10.1080/22221751.2020.1766382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long Q.-X., Liu B.-Z. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020:1–4. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 83.Wang M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. feb.2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lynch K.L. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. medrxiv. june.2020:1–18. doi: 10.1093/cid/ciaa979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Payne D.C. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. oct.2016;22(10):1824. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. mar.2020;16(10):1718. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casadevall A., Pirofski L.-A. The convalescent sera option for containing COVID-19. J. Clin. Invest. mar.2020;130(4) doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bao L. biorxiv; may.2020. Lack of Reinfection in Rhesus Macaques Infected with SARS-CoV-2. [Google Scholar]
- 89.Lan L. Positive Rt-Pcr test results in patients recovered from COVID-19. J. Am. Med. Assoc. apr.2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.An J. Medrxiv; mar.2020. Clinical Characteristics of the Recovered COVID-19 Patients with Re-detectable Positive Rna Test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamamoto M. biorxiv; apr. 2020. The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 Infection in Vitro: an Existing Drug with Multiple Possible Therapeutic Effects. [Google Scholar]
- 92.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. Antiviral Research; apr.2020. The Fda-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro; p. 104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaffer L. 15 drugs being tested to treat COVID-19 and how they would work. Nat. Med. may.2020 doi: 10.1038/d41591-020-00019-9. https://www.nature.com/articles/d41591-020-00019-9 Available in. [DOI] [PubMed] [Google Scholar]
- 94.Garré B. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, Pmedap and foscarnet. Vet. Microbiol. may.2007;122(1–2):43–51. doi: 10.1016/j.vetmic.2007.01.004. [DOI] [PubMed] [Google Scholar]