Abstract
The human brain contains 100 billion neurons, and each neuron can have up to 200,000 connections to other neurons. Recent advancements in neuroscience—ranging from molecular studies in animal models to behavioral studies in humans—have given us deeper insights into the development of this extraordinarily intricate system. Studies show a complex interaction between biological predispositions and environment; while the gross neuroanatomy and low-level functions develop early prior to receiving environmental inputs, functional selectivity is shaped through experience, governed by the maturation of local excitatory and inhibitory circuits and synaptic plasticity during sensitive periods early in development. Plasticity does not end with the closing of the early sensitive period – the environment continues to play an important role in learning throughout the lifespan. Recent work delineating the cascade of events that initiates, controls and ends sensitive periods, offers new hope of eventually being able to remediate various clinical conditions by selectively reopening plasticity.
Keywords: Sensitive Period, Development, Plasticity, Sensory Deprivation, Cross-modal Plasticity
Introduction
Cortical functions are developed through a complex interaction between genetic predispositions and the environment. Recent findings, ranging from the study of molecules in animal models to behavior in humans, have given us a better understanding of the principles that govern the development and plasticity in the brain. Critical insights have been identified in four key areas (Figure 1): (1) the neuroanatomical and low-level functional organization that matures early in development, (2) the excitatory and inhibitory circuits that, in conjunction with synaptic sculpting, enable, control and eventually limit the fine-tuning of cortical functions based on experience, and (3) the effect of the environment, including (4) the manipulations such as sensory deprivation, on these processes.
Figure 1.
Recent findings have provided key insights into the developmental processes that co-ordinate molecular, structural and functional plasticity across the lifespan.
We begin by summarizing the evidence for how these mechanisms control plasticity throughout development, then review the attempts to induce plasticity outside of the sensitive window. Finally, we discuss the limits of developmental plasticity by examining the effects of early visual deprivation. While excellent recent reviews exist describing developmental plasticity from the perspective of cellular and molecular mechanisms (e.g., [1]), our goal in this review is to summarize the key points within that literature and how they can provide insight into what is known about plasticity in humans.
We center our discussion on the visual system—a region that has long been a focus of experimental studies of plasticity: due to being a particularly well characterized sensory system in which deprivation manipulations (e.g., eyelid suture in animal models) are relatively straightforward.
In this review, we refer to both critical and sensitive periods. Critical periods are well-defined limited time windows in development during which neural substrates or behaviors can be irreversibly disrupted by the absence of normal input. We use the term ‘sensitive period’ more loosely to refer to any one of three types of developmental event: (1) when normal development is most rapid, (2) periods when a system is susceptible to deprivation or damage (which includes critical periods, but also includes developmental periods where neural disruptions occur, but are reversible), and (3) periods during which recovery from deprivation or damage is still possible with the resumption of normal sensory experience. The sensitive periods for recovery are likely to depend not only on the brain area in question, but also on the environmental disruption that has occurred.
Neuroanatomical development precedes experience
The gross physiological architecture of the brain is determined early in development. For example, recent neuroimaging work in humans shows that both the major visual white matter tracts linking occipital cortex to other regions of the brain, and the connectivity patterns underlying the retinotopic topography of early visual areas (V1–V2–V3) begin to develop in the second trimester of gestation [2], even before the axons from the optic radiations innervate cortex. Labelling studies in infant monkeys also have demonstrated that adult-like V1–V2 connectivity exists by 2 weeks postnatally, with further refinement between 2–8 weeks of age [3]. Thus, the major pathways that connect visual cortical regions are established before the onset of visual experience. These early processes are largely guided by molecular signaling (as reviewed in [4,5]), although work in non-human primates has shown that spontaneous retinal activity during the third trimester clearly plays an important role in refining these connections [6].
Recent neuroimaging studies in humans demonstrate that the development of white matter structure preceding experience can also be observed outside the canonical visual areas. For instance, a recent study [7] found that the location of the visual word form area in older children who can read could be accurately predicted by its connectivity to low-level visual areas measured during their pre-reading years. Consistent with the notion of white matter structure developing prior to experience, functional magnetic resonance imaging (fMRI) found no evidence of functional selectivity for words or letters in the visual word form area at these earlier ages.
Neuroimaging findings in neonatal primates [8] have shown that resting state BOLD correlations are stronger within than across visual areas, and differences in resting state fluctuations between ventral and dorsal areas could be observed. These patterns indicate that the hierarchy of visual areas may be differentiated at birth. The study also observed the correlations in the resting state activity matched retinotopic organization measured later in life. However, it is worth noting that the correlations in resting state activity might also arise as a result of vascular organization [9].
Thus, on the whole, the evidence suggests that early white matter formation and initial functional specification precedes sensory experience. In the normal course of development, this large-scale scaffolding, together with a generic fine-scale canonical microarchitecture consisting of microcircuits of precisely interconnected excitatory neurons (rodents, [10]), constrains later experience-dependent plasticity. As discussed below, while the overall structure of this scaffold seems to persist, it can be successfully repurposed for novel functions in the case of early lesions or sensory deprivation.
The role of experience
Experience modulates and refines a pre-existing architecture. Since the classic work of Hubel and Wiesel, a large body of work in rodents, cats and primates has demonstrated a sculpting of the neuronal architecture in primary visual cortex that refines selectivity for low level properties, such as spatial frequency, orientation and eye of origin (i.e., ocular dominance; OD), with the onset of visual experience. This process takes place during a developmental sensitive period which can easily be disrupted by environmental manipulations.
Unsurprisingly, several recent studies in both humans and animals suggest that the period of sensitivity to visual experience differs across visual hierarchy. The effects of early bilateral cataracts suggest that dorsal tasks (e.g., global motion) develop more rapidly than comparable form tasks (e.g., global form) [11]. Similarly, recent findings in mice show qualitative differences in the development of higher visual areas across dorsal and ventral streams. In the dorsal stream, the overall magnitude of the visual response [12] and the functional segregation of areas [13] slowly develop over 10–14 days after the onset of visual experience. In the ventral stream, cells show strong responses shortly after eye opening, although orientation tuning becomes sharper and receptive field sizes gradually increase after the onset of visual experience [12].
Interestingly, in infant macaques, receptive field properties of individual neurons in extrastriate areas rapidly develop almost adult-like tuning for properties such as disparity, motion and object selectivity. On the other hand, the ability to perform the equivalent behavioral tasks develops over a much longer time scale [14]. It has been suggested that population-level analyses provide a more accurate basis for linking neural development to behavior [15]. Consistent with this, a recent fMRI study in macaques found a comparable time course between behavioral sensitivity to Glass patterns and the development of distinct multivoxel patterns of BOLD activity in V4 and MT [16]. Recent work has also demonstrated that more subtle population-level tuning properties, such as feature-selective spike synchrony, are also shaped by early visual experience (rodents, [17]).
The development during early sensitive periods also seem to have cascading effects throughout the visual hierarchy—visual experience immediately after birth is required to establish the neural architecture that mediates more complex aspects of vision that develop later in infancy. For example, in humans, it has been shown that the development of configural face processing is disrupted by early bilateral cataracts [18]. However, specialized processing areas also require specialized input: exposure to faces is necessary to develop face-selectivity in macaque inferotemporal cortex [19].
The sensitive period for higher visual areas that subserve more complex visual functions based on specialized visual input may extend later in development. Using fMRI, population receptive field properties (a marker for visual receptive field size) are shown to mature by age 5 in early visual processing areas, whereas the population receptive field structure of category-selective areas in the ventral visual stream continue to develop till at least the teenage years [20]. Similarly, the sensitive period for reading extends until well into adolescence, showing a progressive development of the visual word form area along with the development of reading skills [21].
The sensitive period involves a cascade of structural and neurochemical events
Studies in animal models have provided extraordinary insight into the molecular cascade that controls cortical plasticity during the sensitive period soon after eye opening (for recent reviews: [1,22–24]). As discussed later, the findings have important implications for understanding how plasticity might eventually be restored outside of the normal sensitive period in humans.
Figure 2 shows the time course of various markers of developmental plasticity, measured across a variety of species. To integrate developmental timelines across species, we normalized postnatal days (PN) for each species to a timeline based on rat postnatal days using the Workman translating time model [25]. Based on 18 mammalian species and 271 developmental events, this model has been shown to predict developmental time courses with high accuracy. Equivalent human postnatal years are shown in brackets. Figure 3 illustrates some of the structural changes discussed below.
Figure 2.
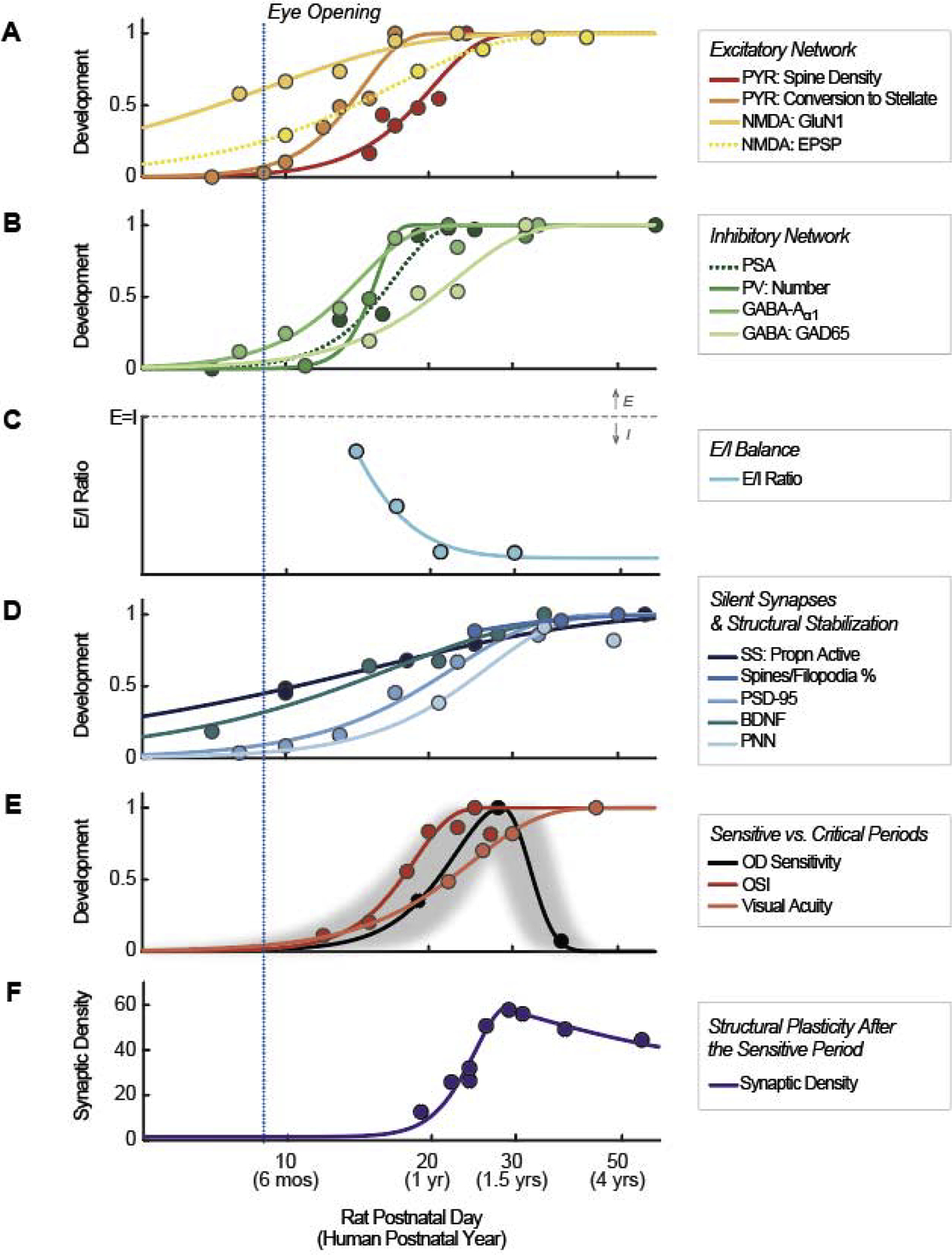
A summary of sensitive period events in typically developing primary visual cortex. Data were normalized in Panels A, B, D, and E. Solid lines in these panels represent values that increase in development, which were normalized to a maximum of 1, . Dotted lines in Panels A and B represent values that decline in development, which were linearly flipped and normalized, , such that min(yorig) → y = 1, and . Data in Panels C and F were not normalized. See Table 1 for detailed data and abbreviations.
Figure 3.
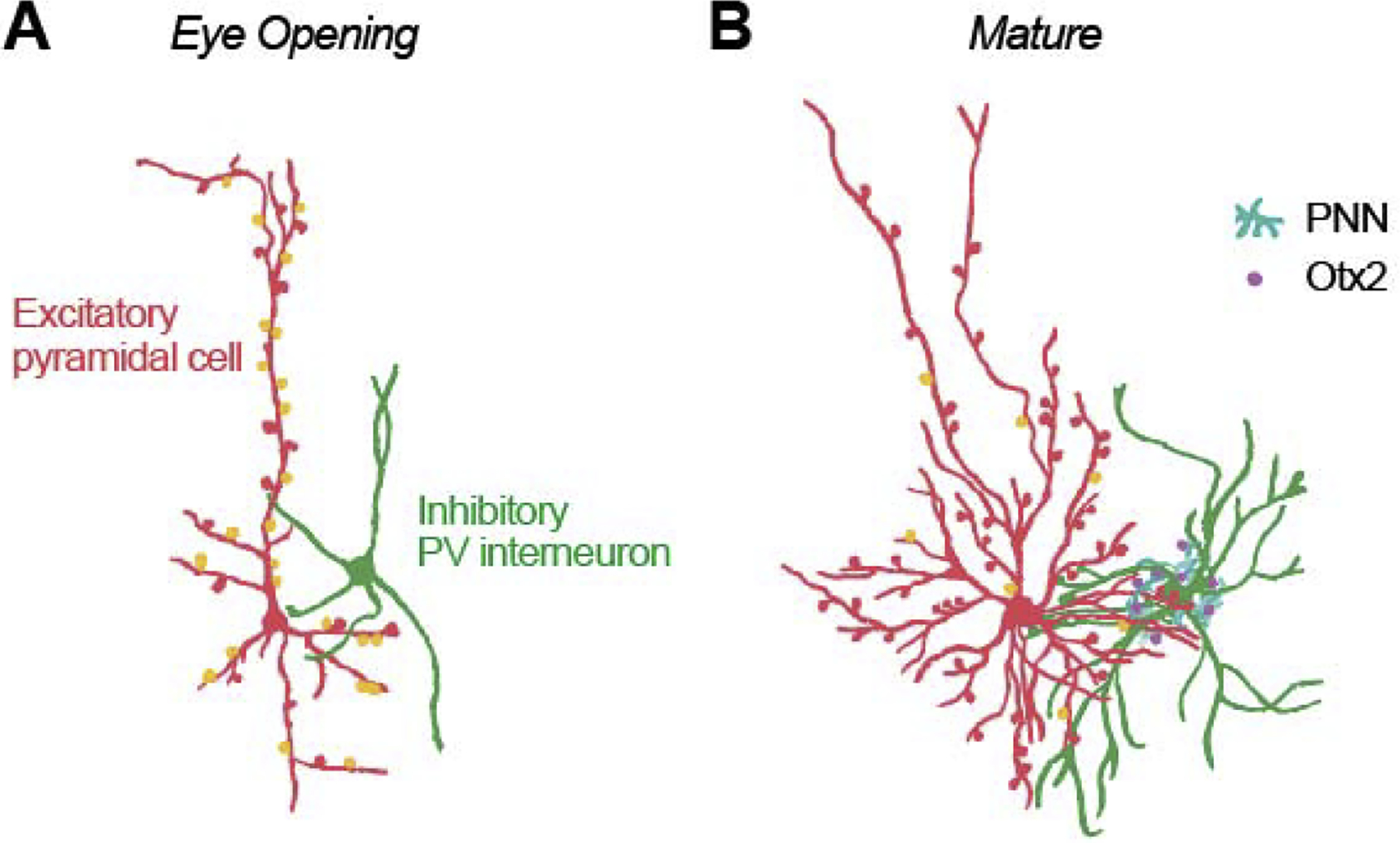
Illustration of structural changes in development within visual cortex just after eye opening (A) and at the end of the sensitive period (B). Initially, all excitatory neurons (red) appear pyramidal, with a prominent apical dendrite and few small basal dendrites. Later the majority of these neurons gradually sculpt to a stellate morphology [PYR: Conversion to Stellate, ferrets [27]]. Inhibitory PV cells (green) increase in complexity, and the number of inhibitory connections (mostly targeting the soma and proximal dendrites) increases [GABA: GAD65, mouse, [34]; GABA-Aα1, mice, [28]]. Over a slightly longer timeframe there is a decline in the proportion of excitatory synapses that lack AMPA (orange) and are functionally silent [SS: Propn Active; mice [28,39]]. While the number of synapses continues to increase for some time [Synaptic Density, human, [51]], eventually the growth of novel synapses is limited by perineuronal nets (cyan) [PNN, rodents, [44]], which permits the capture of Otx2 (purple).
Maturation of the excitatory network
Until relatively recently, work done in a variety of animal models suggested that both the structural (also see Figure 3) and the functional properties of excitatory pyramidal cells mature rapidly after eye opening. For example, pyramidal spine density [PYR: Spine Density, macaque [26]], the conversion of pyramidal cells to their mature stellate form [PYR: Conversion to Stellate, ferrets [27]], expression of NMDA (N-methyl-D-aspartate) receptor subunit GluN1 [NMDA: GluN1, mice [28]] and the shape of the excitatory postsynaptic potential [NMDA: EPSP, cats [29]] become close to adult-like approximately 20 days (rat) after eye opening (Figure 2A) corresponding to the first postnatal year in humans.
Maturation of the inhibitory network
Early synaptic activity within the excitatory system helps trigger the development of inhibitory pathways in a number of ways. First, excitatory activity triggers the downregulation of PSA-NCAM (polysialic acid on neural cell adhesion molecule) expression, thereby releasing a brake on precocious plasticity [PSA, mice, [30]] (Figure 2B). Excitatory activity also triggers the production of brain-derived neurotrophic factor (BDNF, mice, [31]; Figure 2D) which, in conjunction with neural activity, actively promotes the maturation of inhibitory GABAergic parvalbumin (PV)-containing interneurons. Similarly, the onset of visual experience triggers the transport of orthodenticle homeobox 2 (Otx2) from retinal to PV cells, where it promotes PV cell maturation (mice, [32]).
While visual experience acts as a trigger for maturation, innate timing mechanisms also influence the maturation of PV neurons: A recent study in mice has shown that transplanted PV neurons mature based on their intrinsic age, rather than that of the host animal [33].
Although PV cell numbers [PV: Number, mouse, [31]] and their GABA-Aα1 subunits [mice, [28]] mature rapidly, the full development of GABA-ergic transmission between PV and pyramidal cells takes longer to establish [GABA: GAD65, mice, [34]. GABAergic processes are regulated by molecular signaling, each governing different types of inhibitory interneurons (e.g., NRG1/Erb4 for PV neurons [35] and IGF1 gene for vasoactive intestinal peptide-expressing neurons [36] in mice).
E/I Balance
One major mechanism thought to be partially responsible for controlling the sensitive period is the changing balance between excitatory/inhibitory (E/I) responses in neural circuitry [24], [E/I Ratio, rodents, [37]] (Figure 2C). According to the E/I model of plasticity, early in development, excitation appears to dominate cortical circuits, which facilitates the heightened plasticity of the critical period. As inhibitory processes approach maturation, synaptic drive transitions from dominant excitation to dominant inhibition, helping to trigger the end of the sensitive period [38].
Maturation of silent synapses
Although many aspects of the excitatory network develop very quickly after eye-opening, recent work in rodent models has demonstrated that the maturation and pruning of pyramidal ‘silent synapses’ plays a key role in network refinement over a considerably longer timescale (see [1], for a more detailed review). In the mature animal, most excitatory synapses contain both AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA receptors. However, at eye opening, over 50% of primary visual cortex synapses lack AMPA receptors, and as a result, are functionally inactive [28,39].
In normal development, the proportion of these silent synapses declines over the first postnatal 30 days [SS: Propn Active, mice [28,39]] (Figure 2D). This process is experience dependent, since the proportion of silent synapses remains high in dark-reared animals (mice, [40]).
It is believed this decline in the proportion of silent synapses represents a combination of two processes. First, there is a developmental increase in both the amplitude of the unitary EPSC [39] as well as the amplitude of AMPA mediated EPSCs [40], suggesting that AMPA receptors are incorporated into silent synapses, making them functionally active. Second, it is suspected that experience dependent pruning also occurs, although the evidence for this is indirect. Within mice, it has been shown that, early in development, a large number of filopodia – transient dendritic protrusions – are generated. Some of these are converted to stable spines, but a significant proportion are eliminated [Spines/Filopodia %, mice, [41]] and eventually almost no filopodia remain.
The pace of silent synapse maturation appears to be controlled by a pair of DLG-MAGUK proteins – with PSD-95 [PSD-95, mice, [28]] promoting maturation and PSD-93 acting as a brake [39].
As described above, excitatory activity triggers the production of BDNF, which promotes the development of inhibitory cells. BDNF also plays a crucial role in maturing silent synapses [42] [BDNF, mice, [31]].
Structural stabilization
The maturation of inhibitory PV cells facilitates the gradual development of perineuronal nets (PNN)—net-like structures that typically sheathe PV cells in the primary visual cortex (mice [43]), resulting in synaptic stabilization [PNN, rodents, [44]] and structurally limiting the growth of novel synapses (Figure 2D). PNN, Otx2 and PV expression are mutually reinforcing. PNN permits the capture of Otx2, which serves to maintain both PV and PNN expression (mice, [45]). Thus, the maturation of inhibitory PV cells is intimately connected with the structural limits on plasticity provided by PNN and vice versa. Interestingly, the trajectory of PNN maturation closely matches that of visual acuity development (Figure 2E, [Visual Acuity, rats, [48]]), suggesting that structural stabilization only occurs once functional selectivity has been optimized.
Sensitive vs. critical periods
One thing worth noting in Figure 2 is that the critical period for monocular deprivation (i.e., the time in which the neural organization is most susceptible to change from environmental manipulations) occurs when neurochemical and structural plasticity is declining in the normally developing animal.
In normal development, excitatory networks are most plastic (slopes are steepest in our fitted curves) at day 14, and they show declining plasticity (reaching asymptote) between postnatal day 17–19, inhibitory networks are most plastic around day 17, and begin to asymptote around PN day 23, and the conversion of silent synapses is most rapid at day 18, and is close to complete by day 28.
Similarly, experience dependent changes in tuning properties, such as the refinement of orientation selectivity [OSI, ferrets [46]] (Figure 2E), and the binocular matching of orientation preference (mice, [47]) show peak plasticity around day 17–18, and are close to mature by day 30.
In contrast, susceptibility to a 10-day deprivation (Figure 2E, gray shaded area) is relatively low for a period between 14–24 days as compared to a later window of 23–33 PN (Figure 2E, [OD Sensitivity, rats, [48]), suggesting that the peak period of susceptibility to monocular deprivation is delayed compared to the period of maximum plasticity in the non-deprived animal.
Why this discrepancy? As described above, many of these processes, including the maturation of both inhibitory PV cells and silent synapses are triggered by visual experience. As a consequence, the lack of visual experience (i.e., visual deprivation) significantly delays or prolongs the period of maximum plasticity for many processes, including BDNF in the rat [49], silent synapse maturation in the mouse [39], neuronal tuning (rat, [48]; mice, [50]) and visual acuity (mice, [50]). Thus, (as also argued by [1]), visual deprivation can be thought of as an experimental “Schrödinger’s cat” where the experimental manipulation of deprivation delays the timeline of the processes being observed.
Structural plasticity after the sensitive period
Plasticity does not end with the closing of the sensitive period. In humans, synaptic density has been shown to increase up until ~1.5 years of age (equivalent to PN 29 in the rat) then systematically declines with age [Synaptic Density, humans, [51]] (Figure 2F). It is assumed that this pruning is one of the mediators of the plasticity that is observed through the childhood and teenage years [52], fine-tuning neural capacities to reflect complex and specialized environmental demands.
Over the last decade, a variety of human studies have measured apparent cortical thickness in young children using non-invasive magnetic resonance imaging measurements. Most studies (participants are generally over the age of 4) show a monotonic decrease in measured apparent cortical thickness [53], and have attributed this to pruning [54]. However, ‘apparent cortical thickness’ as a function of age and across clinical populations is likely to also reflect differences in myelination, with increased myelination reducing gray-white contrast and thereby reducing the apparent cortical thickness as measured in 1mm MR images [55].
Environmental learning after early development
Learning continues throughout the lifespan, especially in complex and changing environments. Animals reared in an enriched environment with larger social groups, a variety of stimulating objects, and continued exercise demonstrate markers for enhanced cortical development (for a review: [56]). Indeed, some of the effects of dark rearing may be attributable to a loss of enrichment rather than a loss of visual input; rats raised in standard cages have more occipital astrocytes than those reared in darkness, but with enriched environment as well as physical exercise, the animals reared in darkness have more astrocytes than those in the standard environment [57]. The role of exercise is consistent with the observation that physical exercise promotes plasticity in adult primary visual cortex by shifting the E/I balance towards a state of greater excitation (rodents, [58]; mice, [59]).
In humans, one powerful example of adult plasticity to meet specialized environmental demands comes from action video game playing, which seems to improve visual abilities in a variety of domains (for a review, [60]), including training to remediate amblyopia [61,62]. The heavy release of dopamine associated with these games may promote the generation of stimulus-reward associations [63], thereby facilitating the learning of task-related perceptual templates via re-weighting of connectivity across visual areas [64].
Advances in “reawakening” the sensitive period in humans
As scientists have begun to delineate the cascade of events that control the sensitive period, this has naturally led to an interest in reawakening plasticity after the sensitive period has ended. The goal has been to improve training-based clinical treatments for disorders such as amblyopia or visual field loss due to stroke (for reviews, amblyopia: [23]; stroke: [65]).
A variety of animal studies have hastened, extended or reawakened the critical period in regards to OD plasticity via direct pharmacological manipulation (such as activating inhibitory GABAA receptors with allosteric modulators such as benzodiazepines, increasing growth factor levels, removing cell adhesion or DNA binding proteins, see [24] for a review), or through transplantation of embryonic inhibitory neurons (mice, [66]). While many of these manipulations have been carried out with the motivation of understanding the critical period cascade, these findings have broader applications for ‘reawakening’ plasticity in humans.
Inspired by pharmacological successes in reawakening plasticity in animal models, some recent work has attempted to induce enhanced plasticity in adult humans via direct modulation of the E/I balance. One example is cholinergic enhancement via the use of pharmaceutical cholinesterase inhibitors, which some studies have suggested enhance visual perceptual learning [67]. Another potential method to transiently alter the E/I balance is via repetitive transcranial magnetic brain stimulation (rTMS). Indeed, daily rTMS has be shown to improve contrast sensitivity in amblyopia, with effects being surprisingly long lasting (on the order of weeks; [68]).
One of the simplest manipulations, brief sensory deprivation, is already being examined in the context of amblyopia. In humans, monocular patching for as short as three hours can alter the temporal dynamics of binocular rivalry in adults [69], with exercise during deprivation boosting the effect [70].
Longer term deprivation has an even more dramatic effect. Exposure to complete darkness for 10 days in previously eyelid sutured cats can reverse the visual acuity lost due to monocular deprivation [71]. However, although the sensitive period for deprivation-based recovery is later than the critical period for deprivation, the capacity for recovery nonetheless declines as animals age [72].
These adult-deprivation manipulations have been generally explained as being the likely consequence of deprivation shifting the E/I balance towards a state of greater excitation. However, it seems highly plausible that adult-onset deprivation may also result in synaptic remodeling via a ‘reawakening’ of remaining silent synapses.
Most non-invasive interventions in adulthood, such as temporary deprivation and rTMS are likely to operate by increasing adult plasticity, rather than reopening the critical period. Although they have been shown to be reasonably effective in reducing amblyopia, none are capable of entirely reversing it. Fully reawaking the sensitive period is likely to require significant pharmacological intervention. Thus, the effectiveness of any intervention may depend heavily on whether the goal is to facilitate learning within the context of a fully developed visual system, or to more drastically reconfigure neural circuits, as would be required for treatment of amblyopia or stroke.
Examining the limits of cortical plasticity: cross-modal changes after sensory deprivation
Early temporary visual deprivation delays visual development. In contrast, early and prolonged visual deprivation drastically reorganizes the neural pathways, such that the deprived visual areas in the brain respond to auditory or tactile stimuli (for a review, [73]). This dramatic cross-modal reorganization seems to require visual loss before early adolescence [74].
Studies on cross-modal plasticity after blindness suggest that many cortical areas which have a highly specialized role show “functional constancy” wherein they perform a similar functional role using a different modality [75]. Higher-level visual areas, such as hMT+ (a human analogue of primate middle temporal area, selective to motion), the fusiform gyrus (thought to be selective for faces) and the visual word form processing area, show cross-modal responses consistent with functional constancy, responding to auditory motion [76–79], voice recognition [80] and braille reading [81], respectively. Similar task-specificity is also observed in the auditory cortices of deaf individuals [82,83], suggesting that functional constancy may be one of the general principles of cortical plasticity following sensory deprivation early in life.
The role of cross-modal plasticity within non-specialized visual areas, such as V1 and V2, is far less clear [84], although these areas do show robust responses to auditory and tactile stimuli. The large range of tasks that recruit these areas has led to the suggestion that they may be recruited for general, higher-level cognitive processes, including mathematics, language, and verbal working memory [85–87].
How does the brain achieve functional constancy after sensory deprivation? Emerging evidence suggests that the development of functional specialization is at least partly driven by competition between cortical areas for a functional role. The recruitment of hMT+ for auditory motion processing in early blind individuals is accompanied by a loss of sensitivity to auditory motion in the right planum temporale, an area involved in auditory motion processing in sighted individuals [88,89].
A second example of plasticity occurring at the level of ‘role’ is cross-modal plasticity in hMT+ in early blind individuals. In normally sighted individuals, hMT+ neurons have complex receptive fields tuned for spatial frequency, retinotopic location, motion direction, and binocular disparity. In early blind individuals, hMT+ shows tuning to auditory frequency as well as auditory direction motion [90]. This finding indicates that the recruitment of hMT+ for auditory motion is not mediated at the level of closely analogous computations (e.g., direction of motion computations), since there is no close visual analogue to auditory frequency, but rather reflects hMT+ continuing to play the functional role of tracking moving objects in space—using audition rather than vision.
Historically, it has often been assumed that the enhanced auditory and tactile abilities of blind individuals are mediated by cross-modal plasticity. However, some recent studies suggest that plasticity as a result of sensory loss also includes compensatory enhancement of function in cortical areas that process the remaining senses (humans: [91]; opossums: [92]). It remains to be seen whether this enhanced functionality in non-deprived sensory areas is the result of deprivation per se, or simply the result of enhanced reliance on the remaining senses.
Conclusions
Over the last decade, much has been learned about the establishment of neuronal connections prior to visual experience, and how visual experience then refines and sculpts this pre-existing architecture at a local scale. What will the next decade bring? First, with the advent of large-scale recording techniques, we may see a shift in focus from plasticity within local mechanisms, describing how individual neurons organize to represent specific features of the sensory input, to a more global perspective, examining the mechanisms of plasticity across many millimeters of cortex. This broader perspective will be critical for gaining a deeper understanding of how cortical specialization occurs, during both normal and atypical development. Second, it is very likely that we will see significant advances in our understanding of environmental influences on the molecular and structural mechanisms that govern plasticity. There is now significant work suggesting that factors like environmental enrichment and exercise play a crucial role, and the field is positioned for rapid progress in elucidating the underlying mechanisms—work that is likely to be of clinical importance for both education and aging.
Table 1.
Summary of the data depicted in Figure 2. Included events should not be considered comprehensive of the literature: our goal was simply to include markers for major structural and biochemical changes. Studies were included if they contained at least time points between postnatal days 0–60 (translated to rat). Descriptive functions were generated using standard functions (Weibull, Gaussian, exponential) and were fitted based on standard function minimization (code available at https://github.com/VisCog/plasticity-molecules-to-behavior).
| Mechanisms | Data | Species | Reference |
|---|---|---|---|
| Maturation of the excitatory network, Figure 2A | |||
| PYR: Spine Density | Spine density (at a distance 50–60μm from the soma) | Macaque monkey | [26] Figure 4C |
| PYR: Conversion to Stellate | Ratio of stellate: pyramidal spiny neurons. At eye opening, no pyramidal stellate cells exist. By adulthood 78% of spiny cells are classified as stellate. | Ferret | [27] Figure 4D |
| NMDA: GluN1 | NMDA receptor subunit GluN1 | Mouse | [28] Figure 3C |
| NMDA: EPSP | Speed (half width) of NMDA-mediated EPSP under blockade of non-NMDA receptors | Cat | [29] Figure 7A |
| Maturation of the inhibitory network, Figure 2B | |||
| PSA | Polysialic acid (PSA) expression | Mouse | [30] Figure 1 |
| PV: Number | Number of PV cells | Mouse | [31] Figure 4B |
| GABA-Aα1 | GABA-A receptor subunit α1 | Mouse | [28] Figure 3A |
| GABA: GAD65 | GAD65 expression in presynaptic boutons of inhibitory interneurons | Mouse | Data from [31] replotted in [34] |
| Excitatory/Inhibitory balance, Figure 2C | |||
| E/I Ratio | Spontaneous EPSC / spontaneous IPSC charge. Data averaged across layers. | Rat | [37] Figure 3 |
| Maturation of silent synapses and structural stabilization, Figure 2D | |||
| SS: Propn Active | Proportion non-silent synapses. At postnatal day 3 only 20% of synapses are active, increasing to over 90% at PN 57. | Mouse | [28] Figure 2D [39] Figure S1 |
| Spines/Filipodia % | % Spines / Filopodia | Mouse | [41] Figure 1 |
| PSD-95 | Quantified protein level (normalized to adult) of PSD-95. One of a pair of DLG-MAGUK proteins. PSD-95 promotes and PSD-93 inhibits silent synapse development. | Mouse | [28] Figure 3A |
| BDNF | Expression of BDNF transgene | Mouse | [31] Figure 1 |
| PNN | Percentage of PV/WFA/PTPσ positive cells among PV cells | Rat | [44] Figure 6B |
| Sensitive vs. critical periods, Figure 2E | |||
| OSI | Orientation selectivity index | Ferret | [46] Figure 2 |
| Visual Acuity | Visual acuity (c/deg) | Rat | [48] Figure 3B |
| OD Sensitivity | Ipsilateral index. Data replotted to align to the middle of the 10-day monocular deprivation period. | Rat | [48] Figure 6B |
| Structural plasticity after the sensitive peiiod, Figure 2F | |||
| Synaptic density | Postmortem synaptic density | Human | [51] Figure 2 |
Highlights.
The development of gross neuroanatomy precedes experience-dependent plasticity.
A cascade of neurochemical and structural events, mediated by experience-driven neural activity, controls the sensitive period.
Environmental manipulations, such as sensory deprivation, have an impact on the timeline of the sensitive period.
Prolonged sensory deprivation early in development results in cross-modal plasticity.
Acknowledgements
National Eye Institute and Office of Director, Office of Behavioral and Social Sciences Research Grant R01EY014645 (to I.F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Reference
- 1.Huang X: Silent synapse: A new player in visual cortex critical period plasticity. Pharmacol Res 2019, 141:586–590. [DOI] [PubMed] [Google Scholar]
- 2.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L: The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 2014, 276:48–71. [DOI] [PubMed] [Google Scholar]
- 3**.Baldwin MKL, Kaskan PM, Zhang B, Chino YM, Kaas JH: Cortical and subcortical connections of V1 and V2 in early postnatal macaque monkeys. J Comp Neurol 2012, 520:544–569. [DOI] [PMC free article] [PubMed] [Google Scholar]; Subcortical and cortical connection patterns (V1–V4, MT) were largely adult-like as early as 2 weeks of age, though these connections seem to undergo refinement and pruning between 2–8 weeks of age.
- 4.O’Leary DDM, Yates PA, McLaughlin T: Molecular development of sensory maps: Representing sights and smells in the brain. Cell 1999, 96:255–269. [DOI] [PubMed] [Google Scholar]
- 5.Tessier-lavigne M, Goodman CS: The molecular biology of axon guidance. Science (80-) 1996, 274:1123–1133. [DOI] [PubMed] [Google Scholar]
- 6.Katz LC, Shatz CJ: Synaptic activity and the construction of cortical circuits. Science (80-) 1996, 274:1133–1138. [DOI] [PubMed] [Google Scholar]
- 7**.Saygin ZM, Osher DE, Norton ES, Youssoufian DA, Beach SD, Feather J, Gaab N, Gabrieli JDE, Kanwisher N: Connectivity precedes function in the development of the visual word form area. Nat Neurosci 2016, 19:1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]; The location of the visual word form area in children who can read can be predicted from their pre-reading white matter connectivity patterns (but not their pre-reading functional responses). This suggests that neuroanatomical development precedes functional development.
- 8.Arcaro MJ, Livingstone MS: A hierarchical, retinotopic proto-organization of the primate visual system at birth. Elife 2017, 6:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Tong Y, Hocke LM, Frederick BB: Low frequency systemic hemodynamic “noise” in resting state BOLD fMRI: Characteristics, causes, implications, mitigation strategies, and applications. Front Neurosci 2019, 13:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systemic low frequency oscillations of non-neural origin contribute significantly to resting state signals, even the most accepted resting state networks are likely to contain significant peripheral physiological contributions. Because vascular organization often reflects functional organization, these non-neural signals often closely resemble functional organization, making them difficult to differentiate.
- 10.Ishikawa AW, Yoshimura Y, Komatsu Y, Yoshimura Y, Yoshimura Y, Yoshimura Y: Experience-dependent emergence of fine-scale networks in visual cortex. J Neurosci 2014, 34:12576–12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis TL, Maurer D: Effects of early pattern deprivation on visual development. Optom Vis Sci 2009, 86:640–646. [DOI] [PubMed] [Google Scholar]
- 12.Smith IT, Townsend LB, Huh R, Zhu H, Smith SL: Stream-dependent development of higher visual cortical areas. Nat Neurosci 2017, 20:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami T, Matsui T, Ohki K: Functional segregation and development of mouse higher visual areas. J Neurosci 2017, 37:9424–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiorpes L, Movshon JA: Neural limitations on visual development in primates: Beyond striate cortex In The New Visual Neurosciences. Edited by W J, C L. Massachusetts Institute of Technology; 2014:1423–1431. [Google Scholar]
- 15.Kiorpes L: The puzzle of visual development: Behavior and neural limits. J Neurosci 2016, 36:11384–11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Grootel TJ, Meeson A, Munk MHJ, Kourtzi Z, Movshon JA, Logothetis NK, Kiorpes L: Development of visual cortical function in infant macaques: A BOLD fMRI study. PLoS One 2017, 12:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Ishikawa AW, Komatsu Y, Yoshimura Y: Experience-dependent development of feature-selective synchronization in the primary visual cortex. J Neurosci 2018, 38:7852–7869. [DOI] [PMC free article] [PubMed] [Google Scholar]; Feature-selective synchrony in neuronal firing is developed through experience over a few weeks after eye opening in rat visual cortex. The effect of experience was local; visual experience was necessary for the development of the synchronized firing for the upper (2 – 4) but not the lower (5 – 6) layers.
- 18.Mondloch CJ, Segalowitz SJ, Lewis TL, Dywan J, Le Grand R, Maurer D: The effect of early visual deprivation on the development of face detection. Dev Sci 2013, 16:728–742. [DOI] [PubMed] [Google Scholar]
- 19**.Arcaro MJ, Schade PF, Vincent JL, Ponce CR, Livingstone MS: Seeing faces is necessary for face-domain formation. Nat Neurosci 2017, 20:1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]; Monkeys raised without seeing faces do not develop face-selective clusters in the inferotemporal cortex, although retinotopic organization and normal responses to other categories are preserved. These results suggest the importance of experience for the development of face-selectivity early in development.
- 20.Gomez J, Natu V, Jeska B, Barnett M, Grill-Spector K: Development differentially sculpts receptive fields across early and high-level human visual cortex. Nat Commun 2018, 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, et al. : Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 2002, 52:101–110. [DOI] [PubMed] [Google Scholar]
- 22.Hooks BM, Chen C: Circuitry underlying experience-dependent plasticity in the mouse visual system. Neuron 2020, 106:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stryker MP, Löwel S: Amblyopia: New molecular/pharmacological and environmental approaches. Vis Neurosci 2018, 35:E018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hensch TK, Quinlan EM: Critical periods in amblyopia. Vis Neurosci 2018, 35:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL: Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 2013, 33:7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]; Neurodevelopment data is currently obtained from a variety of non-human species, making it difficult to relate developmental time periods across studies in different animals. The translating time model integrates over 1,000 empirically-derived neural events to a common neurodevelopment timescale across 18 mammalian species.
- 26.Boothe RG, Greenough WT, Lund JS, Wrege K: A quantitative investigation of spine and dendrite development of neurons in visual cortex (area 17) of Macaca nemestrina monkeys. J Comp Neurol 1979, 186:473–489. [DOI] [PubMed] [Google Scholar]
- 27.Callaway EM, Borrell V: Developmental sculpting of dendritic morphology of layer 4 neurons in visual cortex: Influence of retinal input. J Neurosci 2011, 31:7456–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Huang X, Stodieck SK, Goetze B, Cui L, Wong MH, Wenzel C, Hosang L, Dong Y, Löwel S, Schlüter OM: Progressive maturation of silent synapses governs the duration of a critical period. Proc Natl Acad Sci 2015, 112:E3131–E3140. [DOI] [PMC free article] [PubMed] [Google Scholar]; Early in development, many synapses do not contain AMPA and are functionally silent. Postsynaptic density protein-95 (PSD-95)–dependent maturation (in a later paper it is shown that PSD-93 is a brake) of these silent glutamatergic synapses onto principal neurons is sufficient to govern the duration of the critical period for ocular dominance plasticity. The consolidation of silent synapses marks the end of the critical period.
- 29.Iwakiri M, Komatsu Y: Postnatal development of NMDA receptor-mediated synaptic transmission in cat visual cortex. Dev Brain Res 1993, 74:89–97. [DOI] [PubMed] [Google Scholar]
- 30.Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, Bélanger MC, Wu CZ, Rutishauser U, Maffei L, Huang ZJ: Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci 2007, 10:1569–1577. [DOI] [PubMed] [Google Scholar]
- 31.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S: BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 1999, 98:739–755. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK: Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 2008, 134:508–520. [DOI] [PubMed] [Google Scholar]
- 33*.Figueroa Velez DX, Ellefsen KL, Hathaway ER, Carathedathu MC, Gandhi SP: Contribution of innate cortical mechanisms to the maturation of orientation selectivity in parvalbumin interneurons. J Neurosci 2017, 37:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]; By isolating the embryonic GABAergic interneurons and transplanting them into the host animal, this study demonstrates that innate cortical mechanisms contribute to the maturation of inhibitory interneurons. The result challenges previous proposals that this process is solely governed by the onset of visual experience.
- 34.Berardi N, Pizzorusso T, Ratto GM, Maffei L: Molecular basis of plasticity in the visual cortex. Trends Neurosci 2003, 26:369–378. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Ikrar T, Davis MF, Gong N, Zheng X, Luo ZD, Lai C, Mei L, Holmes TC, Gandhi SP, et al. : Neuregulin-1/ErbB4 signaling regulates visual cortical plasticity. Neuron 2016, 92:160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mardinly AR, Spiegel I, Patrizi A, Centofante E, Bazinet JE, Tzeng CP, Mandel-Brehm C, Harmin DA, Adesnik H, Fagiolini M, et al. : Sensory experience regulates cortical inhibition by inducing IGF1 in VIP neurons. Nature 2016, 531:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatti R, Swanson OK, Lee MSE, Maffei A: Layer-specific developmental changes in excitation and inhibition in rat primary visual cortex. eNeuro 2017, 4:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hensch TK: Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005, 6:877–888. [DOI] [PubMed] [Google Scholar]
- 39.Favaro PD, Huang X, Hosang L, Stodieck S, Cui L, Liu YZ, Engelhardt KA, Schmitz F, Dong Y, Löwel S, et al. : An opposing function of paralogs in balancing developmental synapse maturation. PLoS Biol 2018, 16:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funahashi R, Maruyama T, Yoshimura Y, Komatsu Y: Silent synapses persist into adulthood in layer 2/3 pyramidal neurons of visual cortex in dark-reared mice. J Neurophysiol 2013, 109:2064–2076. [DOI] [PubMed] [Google Scholar]
- 41.Grutzendler J, Kasthuri N, Gan WB: Long-term dendritic spine stability in the adult cortex. Nature 2002, 420:812–816. [DOI] [PubMed] [Google Scholar]
- 42.Nakata H, Nakamura S: Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett 2007, 581:2047–2054. [DOI] [PubMed] [Google Scholar]
- 43.Sigal YM, Bae H, Bogart LJ, Hensch TK, Zhuang X: Structural maturation of cortical perineuronal nets and their perforating synapses revealed by superresolution imaging. Proc Natl Acad Sci 2019, 116:7071–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Xu H, Yu T, Yao J, Zhao C, Yin ZQ: Expression of perineuronal nets, parvalbumin and protein tyrosine phosphatase σ in the rat visual cortex during development and after BFD. Curr Eye Res 2013, 38:1083–1094. [DOI] [PubMed] [Google Scholar]
- 45.Beurdeley M, Spatazza J, Lee HHC, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A: Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 2012, 32:9429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White LE, Coppola DM, Fitzpatrick D: The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature 2001, 411:1049–1052. [DOI] [PubMed] [Google Scholar]
- 47.Wang BS, Sarnaik R, Cang J: Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 2010, 65:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L: Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Vision Res 1994, 34:709–720. [DOI] [PubMed] [Google Scholar]
- 49.Castren E, Zafra F, Thoenen H, Lindholm D: Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci 1992, 89:9444–9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang E, Durand S, LeBlanc JJ, Hensch TK, Chen C, Fagiolini M: Visual acuity development and plasticity in the absence of sensory experience. J Neurosci 2013, 33:17789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huttenlocher PR, Dabholkar AS: Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997, 387:167–178. [DOI] [PubMed] [Google Scholar]
- 52.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK: Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 2010, 30:14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT: Through thick and thin: A need to reconcile contradictory results on trajectories in human cortical development. Cereb cortex 2017, 27:1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petanjek Z, Judaš M, Kostović I, Uylings HBM: Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer-specific pattern. Cereb Cortex 2008, 18:915–929. [DOI] [PubMed] [Google Scholar]
- 55**.Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, Zhen Z, Cox S, Weiner KS, Weiskopf N, et al. : Apparent thinning of human visual cortex during childhood is associated with myelination. Proc Natl Acad Sci 2019, 116:20750–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]; Many human neuroimaging measurements, including most diffusion measures and gray matter thickness, consist of a single measure that reflects a variety of underlying structural properties—a complication that tends to be overlooked. This paper presents evidence that MRI apparent cortical thickness measurements may be strongly influenced by concurrent developmental changes in myelination.
- 56.Sale A, Berardi N, Maffei L: Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol Rev 2014, 94:189–234. [DOI] [PubMed] [Google Scholar]
- 57.Argandoña EG, Bengoetxea H, Lafuente JV.: Physical exercise is required for environmental enrichment to offset the quantitative effects of dark-rearing on the S-100β astrocytic density in the rat visual cortex. J Anat 2009, 215:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baroncelli L, Bonaccorsi J, Milanese M, Bonifacino T, Giribaldi F, Manno I, Cenni MC, Berardi N, Bonanno G, Maffei L, et al. : Enriched experience and recovery from amblyopia in adult rats: Impact of motor, social and sensory components. Neuropharmacology 2012, 62:2388–2397. [DOI] [PubMed] [Google Scholar]
- 59.Kaneko M, Stryker MP: Sensory experience during locomotion promotes recovery of function in adult visual cortex. Elife 2014, 3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bavelier D, Green CS, Pouget A, Schrater P: Brain plasticity through the life span: learning to learn and action video games. Annu Rev Neurosci 2012, 35:391–416. [DOI] [PubMed] [Google Scholar]
- 61.Vedamurthy I, Nahum M, Huang SJ, Zheng F, Bayliss J, Bavelier D, Levi DM: A dichoptic custom-made action video game as a treatment for adult amblyopia. Vis Res 2015, 114:173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gambacorta C, Nahum M, Vedamurthy I, Bayliss J, Jordan J, Bavelier D, Levi DM: An action video game for the treatment of amblyopia in children: A feasibility study. Vis Res 2018, 148:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinstein A, Livny A, Weizman A: New developments in brain research of internet and gaming disorder. Neurosci Biobehav Rev 2017, 75:314–330. [DOI] [PubMed] [Google Scholar]
- 64.Bejjanki VR, Zhang R, Li R, Pouget A, Green CS, Lu Z-L, Bavelier D: Action video game play facilitates the development of better perceptual templates. Proc Natl Acad Sci 2014, 111:16961–16966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melnick MD, Tadin D, Huxlin KR: Relearning to see in cortical blindness. Neurosci 2016, 22:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis MF, Figueroa Velez DX, Guevarra RP, Yang MC, Habeeb M, Carathedathu MC, Gandhi SP: Inhibitory neuron transplantation into adult visual cortex creates a new critical period that rescues impaired vision. Neuron 2015, 86:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rokem A, Silver MA: Cholinergic enhancement augments magnitude and specificity of visual perceptual learning in healthy humans. Curr Biol 2010, 20:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clavagnier S, Thompson B, Hess RF: Long lasting effects of daily theta burst rTMS sessions in the human amblyopic cortex. Brain Stimul 2013, 6:860–867. [DOI] [PubMed] [Google Scholar]
- 69.Lunghi C, Emir UE, Morrone MC, Bridge H: Short-term monocular deprivation alters GABA in the adult human visual cortex. Curr Biol 2015, 25:1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lunghi C, Sale A: A cycling lane for brain rewiring. Curr Biol 2015, 25:R1122–R1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duffy KR, Mitchell DE: Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Curr Biol 2013, 23:382–386. [DOI] [PubMed] [Google Scholar]
- 72*.Mitchell DE, Crowder NA, Duffy KR: The critical period for darkness-induced recovery of the vision of the amblyopic eye following early monocular deprivation. J Vis 2019, 19:1–13. [DOI] [PubMed] [Google Scholar]; This work shows that a 10-day exposure to complete darkness can recover visual acuity in the amblyopic eye of monocularly deprived cats, with its effectiveness declining as the animal ages.
- 73.Fine I, Park J-M: Blindness and human brain plasticity. Annu Rev Vis Sci 2018, 4:337–356. [DOI] [PubMed] [Google Scholar]
- 74.Sadato N, Okada T, Honda M, Yonekura Y: Critical period for cross-modal plasticity in blind humans: a functional MRI study. Neuroimage 2002, 16:389–400. [DOI] [PubMed] [Google Scholar]
- 75*.Pascual-Leone A, Hamilton R: The metamodal organization of the brain. Prog Brain Res 2001, 134:427–445. [DOI] [PubMed] [Google Scholar]; This is one of the first papers to conceptualize the notion of metamodal plasticity—that regions of the brain perform specialized functions irrespective of the input modality. This framework has been influential in offering a theoretical framework for predictions of cross-modal plasticity after sensory loss.
- 76.Bedny M, Konkle T, Pelphrey K, Saxe R, Pascual-Leone A: Sensitive period for a multimodal response in human visual motion area MT/MST. Curr Biol 2010, 20:1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang F, Stecker GC, Fine I: Auditory motion processing after early blindness. J Vis 2014, 14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poirier C, Collignon O, Scheiber C, Renier L, Vanlierde A, Tranduy D, Veraart C, De Volder AG: Auditory motion perception activates visual motion areas in early blind subjects. Neuroimage 2006, 31:279–285. [DOI] [PubMed] [Google Scholar]
- 79.Saenz M, Lewis LB, Huth AG, Fine I, Koch C: Visual motion area MT+/V5 responds to auditory motion in human sight-recovery subjects. J Neurosci 2008, 28:5141–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hölig C, Föcker J, Best A, Röder B, Büchel C: Brain systems mediating voice identity processing in blind humans. Hum Brain Mapp 2014, 35:4607–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reich L, Szwed M, Cohen L, Amedi A: A ventral visual stream reading center independent of visual experience. Curr Biol 2011, 21:363–368. [DOI] [PubMed] [Google Scholar]
- 82.Bola Ł, Zimmermann M, Mostowski P, Jednoróg K, Marchewka A, Rutkowski P, Szwed M: Task-specific reorganization of the auditory cortex in deaf humans. Proc Natl Acad Sci 2017, 114:E600–E609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finney EM, Fine I, Dobkins KR: Visual stimuli activate auditory cortex in the deaf. Nat Neurosci 2001, 4:1171–1173. [DOI] [PubMed] [Google Scholar]
- 84.Bedny M: Evidence from blindness for a cognitively pluripotent cortex. Trends Cogn Sci 2017, 21:637–648. [DOI] [PubMed] [Google Scholar]
- 85.Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R: Language processing in the occipital cortex of congenitally blind adults. Proc Natl Acad Sci 2011, 108:4429–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanjlia S, Lane C, Feigenson L, Bedny M: Absence of visual experience modifies the neural basis of numerical thinking. Proc Natl Acad Sci 2016, 113:11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amedi A, Raz N, Pianka P, Malach R, Zohary E: Early “visual” cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci 2003, 6:758–766. [DOI] [PubMed] [Google Scholar]
- 88.Jiang F, Stecker GC, Boynton GM, Fine I: Early blindness results in developmental plasticity for auditory motion processing within auditory and occipital cortex. Front Hum Neurosci 2016, 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dormal G, Rezk M, Yakobov E, Lepore F, Collignon O: Auditory motion in the sighted and blind: Early visual deprivation triggers a large-scale imbalance between auditory and “visual” brain regions. Neuroimage 2016, 134:630–644. [DOI] [PubMed] [Google Scholar]
- 90.Huber E, Jiang F, Fine I: Responses in area hMT+ reflect tuning for both auditory frequency and motion after blindness early in life. Proc Natl Acad Sci 2019, 116:10081–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91**.Huber E, Chang K, Alvarez I, Hundle A, Bridge H, Fine I: Early blindness shapes cortical representations of auditory frequency within auditory cortex. J Neurosci 2019, 39:5143–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]; Early blind individuals have enhanced selectivity to auditory frequency in primary and secondary auditory cortices. This is one of the first human studies to provide evidence for compensatory plasticity as a result of early blindness within non-deprived sensory areas.
- 92**.Ramamurthy DL, Krubitzer LA: Neural coding of whisker-mediated touch in primary somatosensory cortex is altered following early blindness. J Neurosci 2018, 38:6172–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]; After blindness early in development, representations in the primary sensory cortex in short-tailed opossums show spatial sharpening of receptive fields, which was accompanied by an improvement in the animals’ ability to discriminate between stimulation of neighboring whiskers.


