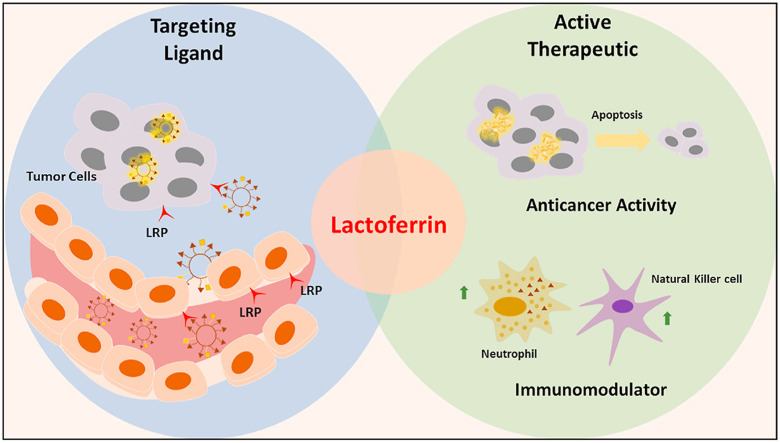
Abstract
Recent progress in protein-based nanomedicine, inspired by the success of Abraxane® albumin-paclitaxel nanoparticles, have resulted in novel therapeutics used for treatment of challenging diseases like cancer and viral infections. However, absence of specific drug targeting, poor pharmacokinetics, premature drug release, and off-target toxicity are still formidable challenges in the clinic. Therefore, alternative protein-based nanomedicines were developed to overcome those challenges. In this regard, lactoferrin (Lf), a glycoprotein of transferrin family, offers a promising biodegradable well tolerated material that could be exploited both as an active therapeutic and drug nanocarrier. This review highlights the major pharmacological actions of Lf including anti-cancer, antiviral, and immunomodulatory actions. Delivery technologies of Lf to improve its pries and enhance its efficacy were also reviewed. Moreover, different nano-engineering strategies used for fabrication of drug-loaded Lf nanocarriers were discussed. In addition, the use of Lf for functionalization of drug nanocarriers with emphasis on tumor-targeted drug delivery was illustrated. Besides its wide application in oncology nano-therapeutics, we discussed the recent advances of Lf-based nanocarriers as efficient platforms for delivery of anti-parkinsonian, anti-Alzheimer, anti-viral drugs, immunomodulatory and bone engineering applications.
Keywords: Lactoferrin, Nanoparticles, Drug delivery, Cancer therapy, Tumor targeting, Anti-viral drugs, Immunomodulatory functions, Bone engineering
Graphical abstract
1. Introduction
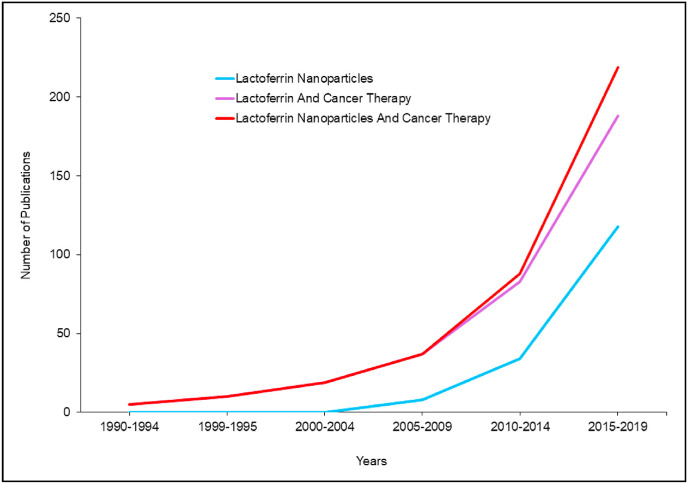
Though discovered in 1939 in bovine milk, lactoferrin (Lf) was reported to be an iron-containing protein [1]. Then after, its structure and chemical properties were detailed in a study published in 1960 [2]. Subsequently, Lf was found to be related to the superfamily of iron-binding glycoproteins, namely transferrins [3]. Beyond this point, Lf emerged as a focus in many research disciplines, aiming to discover its multitude functions. In this regard, we carried out a search on PubMed library to assess the progress in Lf research, in particular, Lf-based nanoparticles in cancer therapy. Our search results showed an increasing pattern of research on Lf role in cancer therapy, since emerged as early as 1992 (Fig. 1 ). Furthermore, the rising progress to the field of nanomedicine by 2005 dragged the researchers’ attention towards Lf and its potential application as a drug nanocarrier, and more specifically, the implications of Lf nanoparticles in cancer therapy [4]. Notably, Lf research patterns reached its maximum interest in 2018, with about 21 published articles discussing various roles of Lf in cancer therapy, and 32 research papers about Lf nanoparticles of which 11 articles focusing on cancer therapeutics.
Fig. 1.
The research progress timeline of Lf nanoparticles in cancer therapy field.
Since Lf is a natural protein present in the milk, the chances of eliciting any adverse immune reaction would be minimal [5]. Primarily, the particles fabricated based on Lf protein prepared by mild methods that do not involve any chemical reactions. Studies of our laboratory and other research groups on Lf NPs have shown excellent safety properties even after systemically administered at high doses concentrations with the liver and hematological biochemical parameters are retained [6].
2. Structural and biological properties
Lf is a red to salmon-pink whey protein with a large molecular size of ~80 KDa. It is found in milk and in a smaller percentage in bile and tears [7]. It has an isoelectric point (pI) of 8.0–8.5, hence it is positively charged at physiological pH 7.4 [8].
2.1. Molecular structure
In 1984, the molecular structure and amino acid sequence of human Lf were discovered. Lf is made of two globular lobes of ~700 amino acids stabilized by disulfide bonds. The two globular lobes are linked by a flexible alpha helix, and they are called amino and carboxy terminal regions or simply N-lobe and C-lobe [9]. Lf undergoes denaturation at two different temperatures for the two lobes; ~60 °C and ~90 °C [7]. Regarding its amino acid composition, cysteine provides thiol groups for the 16 and 17 intramolecular disulfide bonds stabilizing the lobes in human & bovine Lf, respectively with no free sulfhydryl groups. Asparagine provides potential glycosylation sites on N and C-lobes. Histidine, two tyrosine molecules and aspartic acid are essential for iron binding. Arginine is essential for carbonate binding. In addition, the carbohydrate content showed a residue of terminal sialic acid [9,10].
2.2. Iron binding
Lf binds to a wide variety of ions and substances including cations (e.g. Iron, & copper), anions (e.g. carbonate & bicarbonate) and substances (e.g. lipopolysacharides, heparin, glycosaminoglycans and DNA). Structurally, Lf is two times more capable to bind to and transfer iron than transferrin [7,9]. Lf has three isoforms, Lf-α, Lf-β and Lf-γ, which have different iron binding ability and ribonuclease activity. Lf-α can bind to iron but has no ribonuclease efficacy unlike Lf-β and Lf-γ which have ribonuclease action and cannot bind to iron [9]. When Lf binds to a ferric ion, it binds to a carbonate ion synergistically. Lf binds to the ferric ions by a strong bond that can resist pH as low as 4 while binds to other metals with much less affinity [8,9].
2.3. Tertiary structure & glycosylation
Lf can bind to iron in a reversible manner resulting in different 3D conformations; apo-Lf (iron-free), monoferric Lf and holo-Lf which is saturated with two ferric ions. Apo-Lf has an open conformation and is susceptible to proteolysis while holo-Lf has a closed conformation and is more resistant to proteolysis [9,11]. Human milk Lf is linked to poly-N-acetyl-lactosaminic glycans in each lobe while bovine Lf contains α-1,3-linked galactose moieties in the terminal non-reducing location. These glycans increase the stability of Lf against proteases and acidic pH [9,12].
2.4. Receptors
Initially, Lf interacts with sulfated proteoglycans at the cell surface and then binds specifically with membrane receptors to stimulate ERK1/2 and PI3K/Akt pathways in the cells. Lf membrane internalization receptors include low density lipoprotein related proteins (LRP1/CD91, LRP2/Megalin), transferrin binding receptors (TFR1, TFR2), as well as ferritin and ferroportin required for iron transfer. The expression of receptors is commonly higher at the surface of cancer cells due to their high metabolic rate [13,14]. In addition, Lf can enter the cells via charge-based interaction where its positive charge enables its interaction with negatively charged cell surface glycosaminoglycans [15]. Meng et al. developed Lf-conjugated N-trimethylated chitosan NPs that have displayed significantly higher cellular uptake into 16HBE and SH-SY5Y cells compared with the negatively charged PLGA NPs [16]. The cationic NPs were easily attracted to cells because of the electrostatic interactions between the negatively charged cell membranes and positively charged NPs. An additional Lf receptor is intelectin 1 (Omentin-1), which is a lectin expressed in intestinal epithelia responsible for Lf uptake [17]. Moreover, the intestinal cells also express iron absorption receptors such as divalent metal ion receptor (DMT1) receptors that enhance intestinal uptake of Fe-Lf [18]. Furthermore, Lf binds to Toll-like receptor 4 (TLR4) that is responsible for Lf-mediated induction of NF-kB pathway and C-X-C-motif cytokine receptor 4 (CXCR4) that facilitates Lf-mediated stimulation of Akt signaling [19,20].
2.5. Absorption & oral bioavailability
After oral dose of bLf, the absolute oral bioavailability was nearly 1% because of its protein nature [21,22]. In addition to its hydrolysis by pepsin, ionization of the amino acids responsible for holding Lf structure is changed by the stomach low pH leading to changes in its secondary and tertiary structure [21,23]. Next, Lf is further completely broken down by the small intestine proteases (trypsin, chymotrypsin, amino and carboxyl peptidase). Other studies found that Lf was partially degraded by trypsin into fragments of different sizes, where the larger fragments (>~30 kDa) showed resistance to proteolysis [21,23]. In contrast, Lf was found to survive enzymes in the small intestine of adult rats [21]. Moreover, holo-Lf can survive pepsin digestion in the stomach if the acidity is not enough [24]. Intact Lf molecule reached peripheral blood from the intestine 10–20 min after intragastric intubation and localized in different tissues [22]. After bLf resists the intestinal enzymatic degradation, it binds to LPR receptors on M cells in payer's patches and absorbed into blood and tissues in an immunoreactive form. Takeuchi et al. showed that intraduodenally administered bLf enters the body through the intestinal lymphatic pathway and reaches the whole body through the thoracic lymph fluid [21]. Overall, gastric-mediated degradation of Lf is controversial. A clinical study conducted by Troost et al. found that Lf is systematically absorbed through the gastrointestinal tract (more than 60%), in its intact form, after ~ 30 min. The pH range (4.0–7.0) that allowed the absorption of Lf was higher than the pH needed for digestion with pepsin (1.5–2.0) [25]. However, another clinical trial demonstrated contradictory results [26]. The mechanism of Lf degradation, as well as its degradation products, are still poorly understood.
2.6. Half-life and metabolism
Lf has a short half-life in the body estimated to be about 10 min after i.v. administration [21]. According to Peen et al., recombinant human Lf has a half-life of about 12.6 min and completely removed after 7 h of injection [27]. Once it is transferred by the lymphatic pathway across the intestinal wall, it is rapidly internalized by the liver and excreted into the gall bladder [21]. In the liver, Lf is rapidly cleared from circulation by the action of LRP-mediated internalization by hepatocytes [28]. Intact and fragments of Lf of maternal origin were found in breast-fed infants' urine, so kidneys must be responsible for part of the excretion of Lf [9,27]. Another way to clear Lf from the circulation and interstitial space is by the phagocytes where Lf is engulfed by receptor-mediated endocytosis. The iron carried by the Lf is then carried by ferritin [9,27]. With this very short half-life, there is a thought that the Lf carried inside lymphocytes survive for longer time and carry out its function in the body [21].
3. Pharmacological activities of lactoferrin
Lf possesses a wide array of different functions including anticancer activity, anti-inflammatory, a potential role in bone health preservation and cognitive function improvement in patients with Alzheimer's disease (Fig. 2 ).
Fig. 2.
A schematic diagram illustrating different pharmacological actions of Lf.
3.1. Iron homeostasis
Being an iron-binding glycoprotein, Lf was hypothesized to possess a key role in intestinal absorption of iron across the brush border membrane of human enterocytes owing to the presence of specific receptors for Lf [29]. A recent meta-analysis study concluded that daily oral administration of bLf showed similar efficacy to ferrous sulfate with lower gastrointestinal complications in pregnant women with iron deficiency [30]. On the contrary, using a genetic mouse model of Lf deficiency (LFKO), the ablation of Lf resulted in a mild iron overload [31]. These studies anticipate that the role of Lf in iron absorption is controversial, and that Lf might function by scavenging free iron in the gut, controlling microbial pathogenesis and prevent free-radical induced cellular damage [32,33].
3.2. Antimicrobial activity
3.2.1. Anti-bacterial activity
Lf exerts its antimicrobial action towards a wide array of microbes such as bacteria, fungi, viruses and parasites by two different mechanisms [34,35]. (a) A bacteriostatic activity mediated by sequestering free iron essential for bacterial growth and proliferation; (b) A bactericidal function, Lf may interact directly, through its highly cationic N-terminus, with the negatively charged lipopolysaccharide of gram-negative bacteria causing cell membrane damage [36]. In addition, supplementation with Lf may decrease the risk of necrotizing enterocolitis by altering the intestinal microbiota in premature infants [37]. A randomized study on H. Pylori positive patients reported that bLf in combination with a 7-day triple therapy was effective with higher eradication rates than other regimens [38].
A specific bactericidal activity was reported for Lf and Lf chimera (synthetic Lf peptides). The Lf bacterial killing mechanism was attributed to membrane disruption followed by intracellular internalization [39,40]. Then, Lf or Lf chimera would result in alteration of gene expression. For instance, PspA, an antigen which protects pneumococci against host complement system, was down-regulated by treatment with Lf chimera. Whereas, treatment with Lf resulted in decreased expression of luxS gene, and therefore, inhibiting biofilm synthesis [39]. The bactericidal activity of Lf-chimera, could be extended towards their ability to cause perturbation for bacterial cells, leading to programmed cell death type II. Moreover, membrane perturbation of V. Cholera was due to binding of Lf and Lf-peptides to specific sites on V. Cholera membrane [40].
3.2.2. Antiviral activity
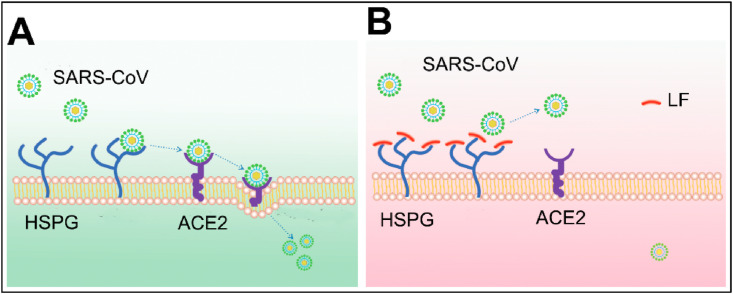
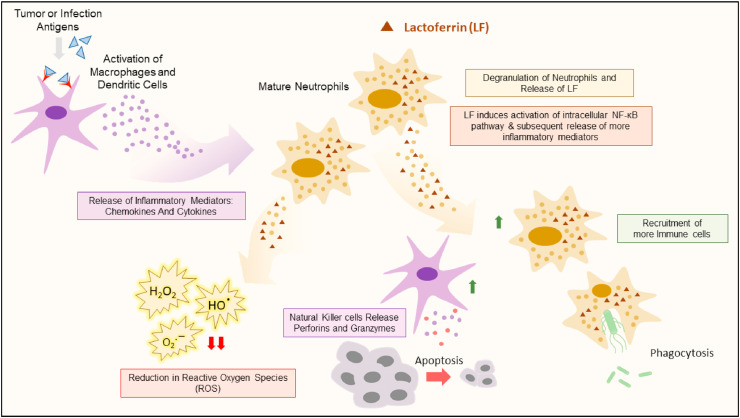
Lf showed antiviral efficacy against HIV, HCV, HBV, Influenza viruses, HPV, and poliovirus, by direct binding to the surface of the virus [41], iron scavenging or by competition for binding to host cells [42,43]. This is mediated by its ability to activate NK cells and enhance aggregation and adhesion of neutrophils. According to Lang et al., Lf blocks the binding of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) to the host cell [44]. Thus, Lf could prevent the infection of SARS-CoV by inhibiting the early attachment of viral spike protein to the heparan sulfate proteoglycans (HSPGs) at the host cell surface (Fig. 3 ). The over-expression of the anti-apoptotic protein Bcl-2 has showed an activity against the viral nucleoprotein, preventing the translocation of the viral components from the nucleus to the cytoplasm [45]. Similarly, it was demonstrated that Lf can inhibit caspase-3 activity, preventing influenza virus-induced apoptosis and hence, it results in sequestration of viral nucleoproteins into the nucleus, preventing virus assembly and propagation in MDCK cells [46]. Additionally, Lf binds to viral III and IIIa polypeptides of Adenovirus causing infection neutralization [47]. An anti-HCV activity was demonstrated by a decrease in the RNA titer with Lf monotherapy; suggesting the effective use of Lf as an adjuvant therapy for Ribavirin and Interferon in HCV treatment [48].
Fig. 3.
Antiviral activity of Lf towards SARS-CoV. (A) SARS-Cov utilizes Heparan Sulfate Proteoglycans (HSPG) to roll on host cells and subsequently identify potential specific entry receptors. (B) Upregulation of Lf and allocations to HSPGs to prevent initial contact between virus particles and subsequent infection [44].
3.2.3. Antiparasitic and antifungal activity
Lf may exert its antiparasitic action through iron scavenging and depletion [49,50]. Additionally, an amoebicidal activity was explained by binding of apo-Lf to the lipid membrane of the parasite causing its disruption and damage [51]. The spectrum of Lf activity was further extended to plasmodium spp. Lf inhibits the sporozoite invasion by competition on heparan sulfate proteoglycans (HSPG) found on hepatocytes [52] and also inhibits the growth of P. falciparum by generation of oxygen free radicals and damaging the membrane of the parasite [53]. In addition, an antifungal activity of Lf was demonstrated against Candida spp. by competing with the pathogen for the iron [54]. Recently, Lf was shown to act synergistically with lactoperoxide; for the prevention of oral candidiasis [55].
3.3. Anticarcinogenic activity
Lf was reported to exhibit anti-cancer efficacy via different mechanisms [[56], [57], [58]]. Table 1 summarizes some of the most promising anti-carcinogenic mechanisms of Lf.
Table 1.
Anti-carcinogenic activity of Lf against various tumor types.
| Cancer Type | Protein | Outcome | Ref. |
|---|---|---|---|
| Breast | hLf | Arrest cancer cells in the G0/G1 phase, induction apoptosis and modulation of Bcl-2 and Bax expressions. | [59] |
| bLf | Suppression of V–H + ATPase and reducing the acidity of tumor microenvironment. | [60] | |
| Cervix | hLf | Increased expression of Fas & reduced ratio of Bcl-2/Bax. | [61] |
| hLF | Upregulation of NK cells, increased infiltration of CD4+ & CD8+ peripheral T lymphocytes, elevated serum IFN-γ, IL-2 and TNF-α in tumor-bearing mice. | [62] | |
| Prostate | bLf | Inhibits the plasma membrane V-ATPase, reducing the tumoral acidity and suppressing tumor progression and metastasis in PC-3 cells. | [63] |
| Colon | hLf, bLf and CbLf | Increased expression of TGF-β1, stimulated IL-18 secretion in Caco-2 cells. | [64] |
| bLf | Enhanced infiltration of CD4+ & CD8+ cells, increased production of IL-18. | [65] | |
| NPC | hLf | Downregulation of PDPK1 via the MAPK/c-Jun pathway and suppression of K18-facilitated AKT stimulation. | [66] |
| Leukemia | hLf | LF-induced apoptosis of Jurkat T cells by interactions with the E2F1 or Bcl-2 target genes. | [67] |
| Lung | hLf | Antiproliferative effects attributed to the elevated levels of hypophosphorylated Rb in H1299 cells. | [68] |
| bLf | Decreased levels of TNF-α, IL-4, IL-6, and IL-10 cytokines, limiting inflammation and restricting tumor proliferation. | [69] | |
| OSCC | bLf | Selective suppression of growth through mTOR/S6K & JAK/STAT3 signaling pathways and triggering of apoptosis in OSCC. | [70] |
| Head & Neck | Recombinant hLf | LF induced growth inhibitory effects via p27/cyclin E-dependent signaling pathway by regulating Akt phosphorylation. | [71] |
| GBM | hLf | Suppression of the proliferation of NMD and FN primary cells as well as U87MG cells by reducing the expression of cyclin D1 and D4. | [72] |
hLf: Human Lactoferrin; bLf: Bovine Lactoferrin; NPC: Nasopharyngeal Carcinoma; OSCC: Oral Squamous Cell Carcinoma; GBM: Glioblastoma; TMZ: Temozolomide.
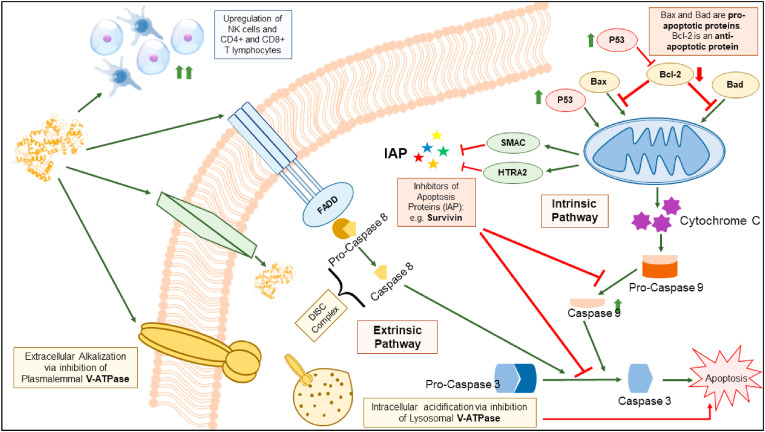
Some studies showed that bLf functions as a specific inhibitor of the activity of the plasma membrane V-ATPase, and reducing the acidity of tumor microenvironment, thus suppressing tumor growth and metastasis. This was supported by preferential cytotoxicity bLf against the highly metastatic cancer cells PC-3, Mg-63, Hs 578T and MDA-MB-231, characterized by higher levels of V-ATPase compared to the non-cancer cells [73,74]. The selectivity of bLf compared to other conventional V-ATPase inhibitors is also mediated by suppression of the lysosomal V-ATPase [75]. Moreover, the anticancer apoptotic activity of Lf was shown to be indirectly related to the inhibition of inhibitors of apoptosis (IAP), for instance, Survivin. Survivin is expressed in various cancer cells and inhibits the effect of caspase leading to cancer evade apoptosis. Lf causes down-regulation of Survivin gene expression. The anti-survivin activity was reported by nano-formulated bLf against colon CSCs and tumors (Fig. 4 ). More importantly, Lf up-regulates inhibitors of IAP, HTRA2 and SMAC, which activates the cleavage of caspases, mainly caspases 8, 9, and 3. Therefore, induction of apoptosis in cancer cells via Lf was attributed, mainly, to inhibition of IAPs [76].
Fig. 4.
Schematic representation of the major anti-cancer mechanisms of Lf.
Lf also rapidly internalizes into cancer cells and increases the sensitivity of resistant tumors to the action of chemotherapeutics such as doxorubicin (DOX) thus overcoming chemo-resistance [77]. In addition, Lf nanocarriers could increase the expression of cytokines TNF-α, and IFN-γ with anticancer activity. Interestingly, the iron saturation of Lf could be linked to its anticancer efficacy so that the iron saturated holo-Lf exhibited higher anti-tumor efficacy than apo-Lf or native Lf while the mechanism is still unclear [78].
3.4. Wound healing
The ability of Lf to promote wound healing is well reported through different mechanisms [79]. Talactoferrin (TLf), a recombinant human Lf, helps production of pro-inflammatory cytokines IL-6, IL-8, TNF-α and MIP-1α. It also increases the expression of hyaluronan required for forming granulation tissue, upregulates platelet derived growth factor and promotes keratinocyte proliferation and migration essential for wound re-epithelization, in addition to cellular protection from apoptosis [80]. TLf was found to be safe and effective in Phase II clinical trials in patients with diabetic ulcers [81].
4. Delivery of lactoferrin as active therapeutic
4.1. Microparticles
Chitosan microparticles (mean size 4.9 μm) were developed via emulsification-solvent evaporation for controlled release of Lf (Table 2 ) [82]. The microparticles demonstrated high Lf loading (16.7%, w/w) together with gradual controlled release of Lf over a period of 24 h. However, the microparticles may be disintegrated in the gastric acidic conditions due to solubility of chitosan in acidic pH so it was not suitable for oral delivery. Therefore, Lf was encapsulated into calcium alginate microparticles prepared by electrostatic complexation then coated with chitosan. The microparticles maintained their integrity in the GIT at both pH 1.2 and 6.8 which would be useful for the intestinal delivery of Lf [83,84].
Table 2.
Representative examples of drug delivery systems developed as Lf carriers.
| Drug delivery system | Preparation technique | Indication | Outcome | Ref. |
|---|---|---|---|---|
| Chitosan/alginate/calcium complex microparticles | Emulsification–evaporation | Anti-inflammatory effect | The MPs exhibited more powerful inhibition of edema and faster recovery than Lf solution. | [84] |
| Bioadhesive tablets | Direct compression | Anti-inflammatory | Tablet has reduced Swelling of the oral ulcer, due to the immune regulatory function of b-Lf. | [94] |
| AEC-CCo-CP-bLf-NCs | Nanoprecipitn./ionic gelation | Antiparasitic Toxoplasma gondii | Two-fold increased bioavailability of bLf, significantly decreased parasitic load and increased survival of mice up to 25 days. | [95] |
| AEC-CCo-CP-bLf-NCs | Nanoprecipitn./ionic gelation | Treatment of Osteoarthritis | Significantly reduced joint inflammation and decreased expression of catabolic genes. | [86] |
| AEC-CCo-CP-bLf-NCs | Nanoprecipitn./ionic gelation | Colon cancer therapy | None of the treated mice developed tumors or exhibited any toxic effects. | [85] |
| 20k-PEG-bLf | Carbodiimide coupling | – | 5.4-fold prolonged serum half-life and 2-fold increased proteolytic half-life over unmodified Lf. | [91] |
| 40 k-PEG-bLf | Carbodiimide coupling | – | 8.7-fold longer plasma half-life and 6-fold increased proteolytic half-life than unmodified Lf. | [92] |
| Multi-lamellar liposomes | Thin-film hydration | Anti-inflammatory effects | Liposomal bLf pretreatment significantly suppressed any GPT or GOT increase in serum induced by CCl4. | [87] |
| Liposomes | Thin-film hydration | Anti-tumoral effects | Entrapment of Apo bLf into liposomes increased its anticancer effect on melanoma cells. | [88] |
| Multi-lamellar liposomes | Thin-film hydration | Hepatoprotective effects | Liposo. Lf exhibited powerful anti-inflammatory action against CCl4-induced liver injury. | [89] |
Alginate chitosan calcium phosphate bLf nanocapsules: AEC-CCo-CP-bLf-NCs.
4.2. Nanoparticles
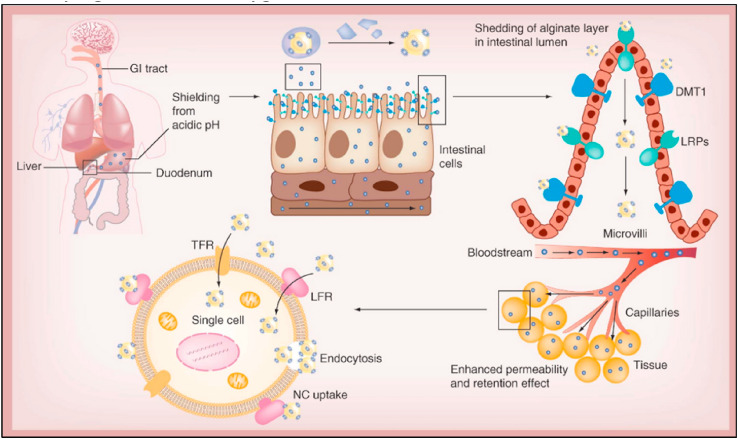
To increase its oral absorption, cationic Fe-bLf was electrostatically adsorbed onto calcium phosphate nanocrystals (NCs) and then coated with a thin layer of chitosan. The chitosan-coated NCs were further encapsulated in alginate gel to provide protection against GIT degradation [18]. In the acidic stomach environment, alginate exists in unionized state forming a matrix around the inner core to protect the entrapped Fe-bLf. Reaching the alkaline intestinal pH, the ionized sodium alginate is formed releasing the Lf content. The mucoadhesive nature of FebLf NCs, mediated by the interaction between the positively charged of both iron and chitosan with the negative charged of the mucus, enabled increased internalization in the intestinal tissue layer of the nanocarriers. Moreover, LPR and DMT1 receptors on intestinal cells help the absorption process of the nanocarriers (Fig. 5 ) [14]. The alginate/chitosan-coated Lf NCs displayed promising activity against different types of solid tumors [14,85] and osteoarthritis [86].
Fig. 5.
Proposed mechanisms of absorption of Fe-bLf loaded polymetric NPs in cancer therapy. Maximal Uptake was achieved through iron receptors; DMT1 in addition to LRP receptors. Through EPR mediated effect, the NPs reached cancer tissue and subsequently internalized through pinocytosis utilizing Tf and Lf receptors [14].
4.3. Liposomes
Incorporation of bLf into liposomes was found to improve its anti-inflammatory action following its oral or intra-articular administration by improving its resistance to gastric digestion and hence increasing its intestinal absorption [87]. Moreover, liposomal Lf showed higher cytotoxicity against cancer cells by increasing its intracellular accumulation and protecting from lysosomal or proteasomal degradation [88,89].
4.4. PEGylation
PEGylated Lf can be synthesized by direct conjugation between and the free amino moieties of bLf and the branched PEG N-hydroxysuccinimide (NHS) esters. Alternatively, bLf could be coupled to two p-nitrophenyl esters of linear 5 and 30 kDa PEG [90]. Compared to PEG-NHS, slower reaction rates but remarkably higher degradation stabilities could be achieved using PEG-p-nitrophenyl active esters. PEGylation of Lf improved its oral bioavailability so that the proteolytic half-life of 20k and 40 kDa-PEG-bLf has been increased by 2-fold and 6-fold, respectively compared to unmodified Lf [91,92]. Intragastric injection of 20k-PEG-bLf at a dose of 30 mg/kg into rat stomach increased its intestinal absorbed amount by 10-fold and prolonged plasma half-life by 5.4-fold relative to unmodified Lf. PEGylated bLf enhanced its hepatoprotective effect against CCl4-induced liver injury by increasing the SOD activities and ROS scavenging in mitochondria, leading to the reduced activity of AST and ALT [93]. The 40k-PEG-bLf displayed enhanced hepatoprotection and improved pharmacokinetics in comparison with both unmodified bLf and 20 k-PEG-bLf conjugates.
5. Preparation techniques of lactoferrin nanocarriers
5.1. Sol-in-oil emulsion
In this technique, the drug is simply mixed with the protein aqueous solution in the presence of oil phase. When the oil phase was added to the soluble mixtures of drug and protein, the drug was adsorbed to protein molecules forming emulsified aggregates. The complex aggregates were disaggregated by sonication and then cooled resulting in their precipitation with formation of solidified drug-protein particles. Finally, the formed particles are cleaned off oil. Different drugs e.g. temozolamide [96], fluorouracil (5-FU) [97], Oxaliplatin [98] and siRNA [6] have been successfully loaded into Lf by this method (Table 3 ). The Lf NPs prepared using this method offered several advantages including small particle size 60–90 nm and high drug loading (50% loading). moreover, the NPs displayed pH-responsive drug release with higher release at endosomal pH (pH 5 and 6) which can be advantageous for tumor targeted drug delivery. This behavior can be attributed to the conformational change or shrinkage of Lf in that acidic milieu. Since this technique does not need any harsh chemical treatments such as using stabilizing or cross-linking agents, we anticipate that Lf molecules may revert back to their native conformation and regain their receptor binding epitopes [6]. However, some limitations of this technique can arise from the difficulty of oil removal and the effect of sonication on the structural integrity of protein molecules.
Table 3.
Representative examples of Lf-based nanocarriers for drug delivery applications.
| Method of preparation | Drug | Indication | Outcomes | Ref. |
|---|---|---|---|---|
| Lf NPs Sol-oil chemistry |
DOX | HCC | Minimized the cardiotoxicity of DOX and enhanced its efficacy and bioavailability. | [122] |
| Lf NPs Sol-oil chemistry |
5-FU | Melanoma | Higher intracellular uptake, prolonged retention and 2.7-fold improved cytotoxicity against B16F10 melanoma cells. | [97] |
| Lf NPs Sol-oil chemistry |
EFV | HIV Therapy | > two-fold increased anti-HIV-1 activity in comparison with free drug. | [123] |
| Lf NPs Sol-oil chemistry |
TMZ | Glioma treatment | Significant reduction of tumor volume and improved median survival time. | [96] |
| Lf NPs Sol-oil chemistry |
Carboplatin | Retinoblastoma | Higher anti-proliferative activity against retinoblastoma cells than soluble carboplatin. | [124] |
| Lf NPs Desolvation |
CUR | In vitro optimization | The NPs exhibited a particle size of 214.7 nm with encapsulation efficiency of 53.7%. | [99] |
| Mann-Lf NPs Thermal treatment |
Shikonin JQ1 |
Colorectal cancer | The NPs elicited immunogenic cell death and repolarizing TAMs to M1 macrophages. | [102] |
| TPGS-TAT Lf NPs Thermal treatment |
Simvastatin Fenretinide |
Glioma | The NPs repolarized TAMs to M1 phenotype & increased ROS-induced mitochondrial apoptosis. | [101] |
| Lf NPs Nab technology |
Gambogic acid | HCC | Enhanced oral bioavailability & anti-cancer effect of GA, thus decreasing its toxicity. | [104] |
| Lf NPs Nab technology |
Oleanolic acid | Improved bioavailability | Increased dissolution and enhanced oral absorption of OA. | [105] |
| Lf-Drug conjugate Carbodiimide coupling | CUR | Colorectal cancer | Lower IC50 of NPs (0.5 μg/ml) against HCT116 cells than free curcumin (3.3 μg/ml) after 48 h. | [112] |
| Lf-Drug conjugate Carbodiimide coupling | DOX | Prostate cancer | Orally Fe-bLf-DOX inhibited tumor growth, prolonged survival, reduced DOX toxicity. | [77] |
| Lf-Drug conjugate Free-radical graft copolymerization | EGCG CA Gallic acid |
Antioxidant | Higher antioxidant activity of Lf-polyphenol conjugates than control Lf in both ABTS scavenging and reducing power assays. | [116] |
| Lf-Drug conjugate coated MSNPs | PMT & Ellagic acid | Breast cancer | The Lf-MSNPs showed higher cytotoxic effect against MCF-7 breast cancer cells. | [114] |
| Lf-shell oily core nanocapsules | Sorafenib & Quercetin | HCC | The LA/Lf-NCs exhibited better uptake into HepG2 cells with 2-fold decrease of IC50 compared to free drugs. | [121] |
| Lf/ChS electrostatic nanocomplex | DOX & Ellagic acid | NSCLC | Higher cytotoxic effect and uptake into A549 cancer cells triggered by Tf & CD44 receptors. | [110] |
| Lf-zein amphiphilic micelles | Rapamycin & Wogonin Dasatinib & Fe3O4 NPs |
Breast cancer | Enhanced synergistic cytotoxicity & suppression of MCF-7 cells & EAT tumor growth. 1.35-fold higher cytotoxicity against MDA-MB-231 via external magnetic field. |
[118] [119] |
Doxorubicin: DOX; 5-Fluorouracil: 5-FU; Efavirenz: EFV; Temozolomide: TMZ; Curcumin: CUR; Epigallocatechin gallate: EGCG; Pemetrexed: PMT; Chlorogenic acid: CA; Ehrlich Ascites Tumor: EAT.
5.2. Desolvation
Lf nanoparticles could be prepared by desolvation technique by addition of a miscible organic solvent of the drug to the aqueous protein solution at adjusted pH. When the solution turns turbid, revealing particle formation, a crosslinking agent e.g. glutaraldehyde solution was added to harden the particles. Curcumin-loaded Lf NPs prepared by desolvation exhibited a particle size of 214.7 nm with encapsulation efficiency of 53.7% [99]. The nanoparticles were optimized by varying different factors e.g. protein concentration, solvent ratio, temperature, sonication strength, flow rate, cross-linking agent and pH. Smaller particles were formed by heating the protein at 35–60 °C. At high temperature, protein unfolding increased the exposure of the SH groups resulting in increased intra-crosslinking of protein macromolecules thus producing smaller nanoparticles [100]. Generally, desolvation is advantageous to other preparation techniques of protein NPs by its simplicity with no need for harsh conditions including high shearing or heating, that might influence the structural stability of proteins.
5.3. Thermal denaturation
Lf NPs can be prepared by controlled thermal denaturation at 61 and 93 °C corresponding to its two lobes. The hydrophobic domains usually become exposed upon protein unfolding resulting in its aggregation via hydrophobic interaction. The protein nanoparticles prepared by thermal denaturation exhibited high pH stability that can be attributed to the strong hydrophobic bonding formed between protein macromolecules following their unfolding. Moreover, the outer glycan components of Lf extending into the external aqueous medium can enhance the stability of the protein nanoparticles by increasing the steric repulsion.
Simvastatin and fenretinide were co-encapsulated into TPGS-TAT-modified Lf NPs (177 nm) prepared by heat denaturation-driven protein self-assembly process [101]. An aqueous Lf solution was stirred on the oil bath at 80 °C for 10 min followed by dropwise addition of organic solution of both drug and cell-penetrating peptide TPGS-TAT (developed by TAT was conjugated to d-α-tocopheryl polyethylene glycol succinate). In a consequent study, the same thermal denaturation procedure was used for preparation of mannosylated Lf NPs (150 nm) for codelivery of shikonin and JQ1 [102]. Loading of the antioxidant polyphenol cichoric acid to Lf by heating to 95 °C at pH 7 resulted in formation of NPs with a diameter of about 67 nm with higher antioxidant capacity than free cichoric acid [103]. This technique can be considered as a green one that does not require toxic cross-linkers or organic solvents.
5.4. Albumin-bound nanoparticle (Nab) technology
Being glycoprotein rich in cysteine with 14 disulfide bonds, Lf has a great tendency to form nanoparticles (NPs) via albumin-bound technology (Nab). Homogenization of protein solution induces the formation of a protein layer coating the drug particles where the S–S bonds were broken and new bonds were formed due to the local heat generated because of the high shearing. Gambogic acid and oleanolic acid (OA) were loaded into Lf nanoparticles with size about 100–200 nm by Nab technology [104,105]. The solution of OA, in mixture of methylene dichloride and ethanol (7:3), was cooled to 25 °C and then slowly added to the aqueous Lf solution. The two solutions were mixed and homogenized at 50 MPa for three cycles followed by evaporated at 45 °C under reduced pressure then freeze-dried without adding any cryoprotectant. In addition, the hydrophilic structure of LF make the particles readily redispersed in aqueous solution.
5.5. Electrostatic nanocomplexes
Polyelectrolyte complexes (PEC) were formed based on electrostatic interactions between positively charged Lf, with higher isoelectric point (pI ≈ 8.5) than most of other proteins (pI ≈ 5) and negatively charged polysaccharides in aqueous solutions. Aqueous solutions of Lf and polysaccharide can be mixed at ambient temperature to form electrostatic nanocomplexes followed by heating up to 92 °C above the thermal denaturation temperature of Lf to promote protein aggregation and particulate formation [106]. For example, Lf/N-succinyl chitosan/galactomannan nanocomplexes were formed by their simple mixing at 1:2:3 and 1:3:2 ratios prior to thermal treatment [107]. Alternatively, Lf NPs with a diameter of 200–400 nm were first formed by thermal denaturation followed by electrostatic complexation with negatively charged polysaccharides e.g. alginate, carrageenan, or pectin [108]. Reversal of the particle surface charge from positive to a negative value indicated successful coating by a layer of the polysaccharide. Yan et al. has used heat-denatured Lf/pectin PEC NPs as a carrier for curcumin [109]. Curcumin was released in a pH-dependent manner showing higher release at acidic pH. This pattern can be explained by the weakened Lf/pectin electrostatic attraction at acidic pH due to protonation of pectin carboxylic groups. The encapsulated curcumin showed higher antioxidant capacities through DPPH radical scavenging ability.
Lf can also form electrostatic nanocomplexes with anionic polysaccharides without need for further heating. In our laboratory, novel nanocomplexes were developed based on the electrostatic complexation between positively charged Lf and negatively charged chondroitin sulfate [110]. The hydrophobic phytomedicine ellagic acid, converted into water soluble nanosuspension, was co-encapsulated together with water soluble cytotoxic drug DOX into the hydrophilic Lf-ChS nanoparticles. As the pH of Lf solution decreases far below its isoelectric point, Lf macromolecules become strongly positively charged resulting in formation of a strong and stable nanocomplex at pH 3.5 with smaller size 138.2 nm. Similarly, Lf-hyaluronic acid-EGCG ternary nanocomplexes were formed via hydrophobic and electrostatic interactions as well as hydrogen bonding [111].
5.6. Lactoferrin-drug nanoconjugates
The structure of Lf makes it capable to chemically conjugate with various hydrophobic moieties or drugs. Utilizing the hydrophilic property of Lf in enhancing the water solubility of the hydrophobic drugs where the conjugated structures drive the self-assembly forming nano-micelles in aqueous media. Chaharband et al. has prepared Lf-curcumin conjugate where the carboxylic moiety of Lf was activated via carbodiimide reagent and then coupled to the hydroxyl group of curcumin forming an ester bond [112]. The conjugate of curcumin to Lf macromolecule was self-assembled into nanostructures with a size of 165 nm. By increasing the Cur-Lf molar ratio from 10 to 40, the conjugation ratio was also increased from 0.85 to 4.52, respectively. Similarly, Lf-deferasirox conjugate was prepared via ester bond carbodiimide coupling and self-assembled into nanoparticles with a size of 100–500 nm [113]. In another investigation, DOX-Lf conjugate were prepared also via carbodiimide coupling reaction forming amide bond through activating the carboxylic moiety of Lf and attaching it to the amino group of DOX [77].
In our laboratory, the chemotherapeutic drug pemetrexed was chemically coupled to Lf via carbodiimide coupling. The Lf-drug conjugate was anchored onto the surface of aminated mesoporous silica nanoparticles entrapping the herbal drug ellagic acid [114]. The synergistic combination showed enhanced internalization and cytotoxicity against MCF-7 breast cancer cells as revealed by lower combination index value (CI = 0.885) in comparison with soluble drugs.
In addition to carbodiimide coupling, Lf conjugates with the polyphenolic drugs epigallocatechin gallate (EGCG), chlorogenic acid (CA) and gallic acid (GA) were successfully elaborated by free-radical graft copolymerization in aqueous media using ascorbic acid/H2O2 redox pair system [115,116]. Moreover, Maillard reaction was used for covalent bond formation between carbohydrate (glucose or polydextrose) and Lf-CA conjugate resulting in higher thermal stability [117].
5.7. Amphiphilic micelles
Nanosized micelles can be developed by the aqueous self-assembly of amphiphilic co-polymers. In our laboratory, an amphiphilic co-polymer was synthesized by chemical carbodiimide coupling of the hydrophobic corn protein zein to the hydrophilic Lf protein [118,119]. Upon dispersion in aqueous solution, the chemical conjugate was self-assembled into nano-micelles (276.6 nm) with a low CMC value. This property protected the micelles from dis-assembly after dilution as a result of injection into systemic circulation. Two lipophilic drugs; rapamycin and wogonin were efficiently entrapped into the hydrophobic core of zein-Lf micelles whereas the hydrophilic Lf shell enabled prolonged circulation. The protein amino groups of Lf allowed further crosslinking of the micelles using glutaraldehyde leading to lower particle diameter and higher colloidal stability. In comparison to the commonly used synthetic amphiphilic co-polymers (e.g. PLA-PEG), zein-Lf co-polymer is considered GRAS (Generally recognized as safe) material composed of natural protein components with much less cost and toxicity [120].
5.8. Lactoferrin shell-oily core nanocapsules
Polymeric oily-core nanocapsules are commonly used for solubilization and controlled delivery of hydrophobic drugs. The NCs are composed of a polymeric shell enveloping an oily core for drug encapsulation. The hydrophobic synthetic polymers (e.g. PLGA or PCL) are among the most commonly used shell forming materials for that type of NCs. However, besides their high cost, numerous toxicity issues have been raised related to their acidic degradation products or immunological reactions. In our laboratory, an oily core composed of Capryol PGMC was used for solubilization of the hydrophobic drugs sorafenib and quercetin [121]. SFB was pre-complexed with phospholipid to maximize its incorporation in the oily phase. A surrounding shell of cationic Lf was electrostatically attached onto the negatively charged oily core to form liver tumor-targeted NCs exploiting the Lf interaction with its receptors upregulated by liver cancer cells. To further enhance tumor targeting, lactobionic or glycyrrhetinic acid was chemically anchored to the Lf shell where they bind with ASGP or GA receptors on HCC cells, respectively [121].
5.9. Lactoferrin-inorganic nanocomposites
Inspired by the emerging role of inorganic materials in therapy and diagnosis, hybrid nanocomposites were fabricated by chemical coupling of Lf to various types of inorganic nanoparticles (Table 4 ). As an extraordinary theranostic agent, tiny gadolinium oxide (Gd2O3) NPs (13.4 nm) were synthesized and stabilized with coating layers of poly(acrylic acid) and reduced albumin. Carbodiimide reaction was then used to develop nanocomposites by chemical coupling of Lf and RGD dimer to the surface of the NPs to enhance BBB permeation and tumor internalization, respectively [125]. Similarly, cisplatin was loaded into hybrid Fe3O4/Gd2O3 NPs modified with Lf and RGD dimer to enhance ferroptosis of brain tumor [126]. Cisplatin induced generation of H2O2 while Fe2+ and Fe3+ released from the NPs resulted in ROS generation. As a result, synergistic inhibition of cancer growth was achieved.
Table 4.
Lf-inorganic nanocomposites for drug delivery & tissue imaging applications.
| Inorganic NPs | Drug | Major outcomes | Ref. |
|---|---|---|---|
| Gd2O3 NPs | – | Ultra-BBB permeable MRI theranostics which enhanced the effect of radiotherapy in orthotopic glioblastoma models. | [125] |
| Fe3O4/Gd2O3 NPs | CDDP | Enhanced ferroptosis of cancer cells. | [126] |
| Mesoporous Fe3O4@SiO2 NPs Mesoporous maghemite NPs |
DOX | Combined DOX/magnetic/PTT therapy significantly inhibited growth and metastasis of breast cancer to lung while reduced cardiotoxicity. | [127,128] |
| GO@Fe3O4 Nanocomposites |
DOX | Increased cytotoxic efficacy to glioma cells. | [129] |
| Graphene nanosponge-supported lipid bilayer | DTX PFH |
LF conjugation increased the tumor accumulation and penetration of NPs by increasing its bilayer fluidity. | [130] |
| Silver nanoparticles (AgNPs) | – | Enhanced antibacterial and antibiofilm properties and reduced toxicity. | [132,133] |
| CdTe QDs | CXB HNK | The theranostic LF-QD nanocapsules enabled fluorescence imaging of breast cancer cells after their uptake with enhanced cytotoxicity. | [134] |
| Mesoporous Silica NPs | PMT ELA |
The NPs exhibited high internalization and synergistic killing of breast cancer cells. | [135,136] |
| Gold quantum clusters | - | LF hybridization successfully stabilized the gold nanoclusters in wide pH range. | [137,138] |
Cisplatin: CDDP; Doxorubicin: DOX; Docetaxel: DTX; Perfluorohexane: PFH; Celecoxib: CXB; Honokiol: HNK; Pemetrexed: PMT; Ellagic acid: ELA.
For combined cancer therapy, Lf-inorganic NPs can be combined with chemotherapy to elicit synergistic anticancer effect. Porous magnetic NPs showed excellent capacity of absorbing magnetic and optical wavelengths resulting in heat generation to kill cancer cells. Therefore, mesoporous Fe3O4@SiO2 NPs (PMNSs) and maghemite NPs (MMNPs) were synthesized by hydrothermal method then chemically modified with Lf for tumor targeting and then physically loaded with DOX [127,128]. The combination of chemo- and photothermal/magnetic therapy showed enhanced antitumor efficacy. Lf was also used to chemically modify the surface of graphene oxide/Fe3O4 NPs (GO@Fe3O4 NPs) to increase their accumulation into glioma cells [129]. The resultant nanocomposites showed acid-responsive release of DOX with enhanced killing of glioma C6 cells. In another investigation, ultrasmall graphene nanosponge-supported lipid bilayers (40 nm) were co-loaded with docetaxel (DTX) and gasified perfluorohexane (PFH). This nanoplatform acted as a photothermal agent for combined gasification/chemo-thermotherapy of cancer by releasing both drug and heat under exposure to near-infrared laser irradiation [130]. The surface of nanosponge was further modified with Lf via chemical conjugation to the lipid bilayer. Lf increased the lateral bilayer fluidity and enhanced its penetration into tumor spheroids mediated by transcytosis.
Lf-silver nanocomplexes have been reported for their efficient antibacterial activity [131]. For example, Lf was used to modify the surface of silver nanoparticles (AgNPs) resulting in a synergistic anti-biofilm efficacy against S. aureus and P. aeruginosa [132]. Moreover, the adsorbed Lf layer was found to decrease the cytotoxicity of silver NPs [133]. Quantum dots, as potent fluorescent imaging agents, were chemically coupled to Lf via a tumor cleavable bond to prevent systemic toxicity of its Cd component [134]. The cationic Lf-QD conjugate was used to electrostatically decorate drug-loaded nanocapsules to enhance their tumor accumulation. The QD-induced fluorescence enabled tracing the biodistribution of nanocarriers and their internalization into breast cancer cells. Silica nanoparticles were also coupled to Lf to enhance their brain delivery. The Lf-modified silica NPs (25 nm) exhibited the highest transport across the cerebral endothelial cells via Lf receptor-mediated transcytosis [135]. In addition, Lf was successfully used to modify the surface of ellagic acid-loaded mesoporous silica NPs (MSNPs) to enhance their accumulation into breast tumor tissue. The carbodiimide-activated carboxylic group of Lf was coupled to the amino groups of APTES-modified MSNPs [136]. On the other hand, few studies reported the use of Lf protein to stabilize the highly luminescent gold nanoclusters [137,138]. Those Lf-gold nanocomposites might have future applications in tissue imaging and cancer theranostics.
6. Design of Lf-targeted nanocarriers
By virtue of its receptors overexpressed by cancer cells and some other tissues such as brain, Lf was increasingly exploited to modify the surface of drug nanocarriers. Two main strategies have been investigated for the design of Lf surface-modified nanocarriers; covalent conjugation and electrostatic complexation. Chemical conjugation was utilized using various coupling reactions such as carbodiimide and maleimide thiol coupling methods. High yield and conjugation efficiency was obtained when carbodiimide and maleimide thiol coupling were used for conjugating Lf to SLNs [139] and PEGylated liposomes [140] with conjugation efficiency of 71.02% and 74%, respectively. In addition, Lf was coupled to the surface of poly(propylene imine) dendrimer using N-c-maleimidobutyryl-oxysuccinimide ester (GMBS) as a linker. This heterobifunctional cross-linking agents inhibit the polymerization that can be obtained using homo-bifunctional cross-linking reagents e.g. dimethyl suberimidate [141].
7. Pharmaceutical applications
7.1. Brain delivery
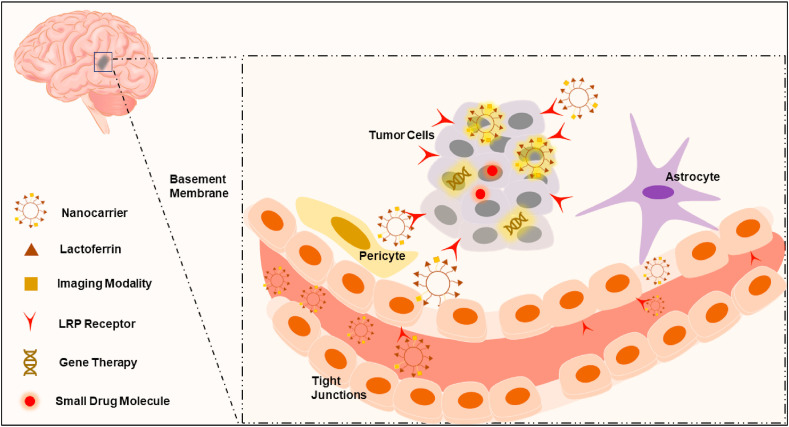
Many challenges including the tight junctions between brain endothelial cells, their low pinocytic activity, presence of efflux pump systems and inactivating enzymes hamper efficient delivery of drugs and genes to the brain across the blood brain barrier (BBB) [142]. Lf receptors including LRP1 and LRP2 (megalin) are well expressed in glioma cells, brain microvessels and neurons (Fig. 6 ) [143]. In addition, Lf bears positive charge in physiologic pH, which facilitates its uptake into negatively charged brain capillary endothelial cells (BCECs). Therefore, Lf-modified nanocarriers are internalized by receptor- and adsorptive-mediated endocytosis into BCECs and glioma cells. In comparison with Tf, Lf-conjugated dendrimers for gene delivery showed 2.2-fold increased uptake and 2.3-fold increased brain gene expression in BCECs with lesser accumulation in liver, spleen and kidney [144]. In contrast to Tf, the lower plasma concentration of endogenous Lf, its positive charge as well as unidirectional transport of Lf across the BCEC monolayer might result in higher brain accumulation of Lf-conjugated vector [145,146]. Chan and coworkers have recently investigated the vasculature of U87-MG glioblastoma xenograft model [147]. The authors found that 97% of the NPs could enter the tumors through an active trans-endothelial process rather than the commonly known passive extravasation transport mechanism through the inter-endothelial gaps. This suggested mechanism may account for the entry of Lf-modified nanocarriers to brain through the brain capillary endothelial cells. The NPs may bind to endothelial cells followed by their transport into the tumor through vesicles and transcellular channels. Therefore, this mechanism can further strengthen the concept of Lf-mediated transcytosis, and its role in enhancing penetration into deep tumor tissues. Lf-targeted huperzine A-loaded nanoemulsion has shown higher accumulation in hCMEC/D3 brain cells via transcytosis compared with untargeted nanoemulsion [16]. Furthermore, kumari et al. has confirmed that Lf NPs accumulated in brain tissue and delivered high temozolamide concentrations to tumor through transcytosis across BBB reaching the brain tumor site [96].
Fig. 6.
Schematic illustration of the brain targeting mechanism of Lf.
7.1.1. Brain tumors
Lf-conjugated PEG-PCL polymersomes (POS), with around 40 Lf molecules per POS, were co-loaded with DOX and Tetrandrine (Tet) (Table 5 ). Although particles had 220 nm diameter which increases the mononuclear phagocytic system (MPS) clearance, the full PEGylation protected it and increased their circulation time [148]. Notably, Lf modification increased the concentration of POS more in right hemisphere compared to the un-modified ones, while Tet, as MDR inhibitor, enhanced their cellular uptake by decreasing cellular resistance thus resulting in the smallest tumor volume and longest survival time. In another investigation, Lf-modified procationic liposomes (PCLs) loaded with DOX, showed longest survival time on Wistar male rats inoculated with C6 glioma cells compared with control group. Lf-conjugation increases cellular uptake into glioma C6 cells; while PCLs uptake occur via adsorption-mediated endocytosis solely, Lf-PCLs uptake occur via receptor- and adsorption-mediated endocytosis [149]. To further enhance the penetration of Lf-modified nanocarriers, tLyP-1, a tumor-homing peptide, was co-administered with Lf-modified paclitaxel (PTX)-loaded polyethylene glycol-polylactic acid (PEG-PLA) NPs. tLyP-1 enhanced the tissue penetration of NPs via the neuropilin-1-dependent (NRP-1) internalization pathway, especially because NRP-1 receptors are specifically expressed on endothelial cells of tumor blood vessels [150]. While Lf modification improved cellular uptake of the NPs relative to unmodified NPs.
Table 5.
Representative examples of Lf-modified nanocarriers for brain targeted drug delivery.
| Delivery system | Cargo | Lf surface modification | Model of study | Disease | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Lf-PEG-PAMAM dendrimer | hGDNF | Maleimide thiol coupling | Rotenone induced PD rats | Parkinson's disease | 4-fold higher enhanced expression of reporter genes in the brain. | [161] |
| Lf-PDNCs SPIO NPs | CUR | Carbodiimide coupling | RG2 cells bearing rats | Orthotopic brain tumor | 30% prolonged animal survival. Self-responsive fluorescence & MRI. | [170] |
| Lf/FA-PLGA NPs | ETP | Carbodiimide coupling | HBMECs & U87MG cells | Glioblastoma | two-fold increased permeability coefficient across the BBB. | [171] |
| Lf-SLNs | DTX | Carbodiimide coupling | U-87 MG cells & mice | Glioblastoma | enhanced cytotoxicity of DTX with lower IC50 values. | [139] |
| Lf-TMX -SLNs | CRM | Carbodiimide coupling | U87MG cells and | Glioblastoma multiforme | 10 times increase in BBB permeability coefficient. | [172] |
| Lf-PEG-BSA NPs |
DOX | Electrostatic complexation | BCECs, C6 glioma cells & SD rats | Glioma | Strongest cytotoxicity and highest uptake both in BCECs and C6 cells. | [173] |
| Lf-MPNA nanogels | Fe3O4 NPs | Carbodiimide coupling | C6 cells, NIH/3T3 cells & rats | Glioma diagnosis | Novel specific MRI contrast agent for glioma diagnosis in vivo. | [174] |
| Lf-modified liposomes | Senktide | Maleimide thiol coupling | Male SD rats & HBMECs | Schizophrenia | Significant increase of dialysate dopamine in nucleus accumbens shell. | [175] |
| LF-PAMAM dendrimer | hGDNF | Maleimide thiol coupling | BCECs, Male 6-OHDA rat model | Parkinson's disease | Enhanced locomotor activity, lowered dopaminergic neuronal loss and increased monoamine levels. | [176] |
| Lf-PEG-PLGA & PEG-PLGA NPs | Urocortin | Maleimide thiol coupling | bEnd.3 cells, 6-OHDA rat | Parkinson's disease | The uptake was 2.5-fold higher and caused a transient inflammation in liver, kidney, and spleen. | [177] |
| Lf-PEG-PCL & PEG-PCL NPs | NAP peptide | Maleimide thiol coupling | 16HBE14o-cells, Male ICR mice | Alzheimer disease | The intranasal administration lead to around 1.5-fold increase of uptake to the brain. | [178] |
| Lf-PEG-PLA & PEG-PLA NPs | PTX | Maleimide thiol coupling | BCECs & C6 cells | Glioblastoma | Enhanced cancer targeting, vascular extravasation & deeper penetration. | [150] |
| Lf-PEG-PCL & PEG-PCL Polymersomes |
DOX & TET | carbodiimide coupling | C6 Cells, & SD rats | Glioma | 2.3-fold stronger cell inhibition with the lowest IC50. Addition of LF reduced spleen accumulation. | [148] |
| Lf-PEG-PLGA & PEG-PLGA Polymersomes |
S14G-humanin | Maleimide thiol coupling | Male Kunming mice | Brain diseases | Better brain uptake Vs Tf or BSA & lower leakage during circulation due to lower proton permeability. | [168,169] |
| Lf-CHETA/PC procationic liposomes | Coumarin-6 | Electrostatic complexation | Co-culture of BCECs & astrocytes | Brain diseases | Lf lip. showed receptor & absorption mediated endocytosis, with enhanced activity & lower cytotoxicity. | [179] |
Polyamidoamine: PAMAM; Sprague Dawley: SD; Polydiacetylene: PDNCs; Curcumin: CUR; Poly (lactide-co-glycolide): PLGA; Etoposide: ETP; Human brain-microvascular endothelial cells: HBMECs; Solid lipid NPs: SLNs; Docetaxel: DTX; Tamoxifen: TMX; Carmustine: CRM; Doxorubicin: DOX; 6-hydroxydopamine: 6-OHDA; Polycaprolactone: PCL; Human bronchial epithelial cells: 16HBE14o-cells; Brain capillary endothelial cells: BCECs; Phosphatidylcholine: PC; Tetrandrine: TET; Paclitaxel: PTX.
In addition to drug delivery, the cationic nature of Lf enabled both high binding capacity of nucleic acids as well as endosomolytic activity mediated by proton sponge effect that contributed in escape of Lf NPs from endolysosomal compartment into the cytoplasm where siRNA can bind with RNA-induced silencing (RISC) complex. As an efficient combination therapy for glioblastoma multiforme (GBM) was enabled by development of Aurora Kinase B (AKB) siRNA–loaded Lf NPs, to enhance BBB crossing, co-administered with TMZ treatment [6]. AKB was found to by overexpressd in malignant glioma where it induces degradation of the tumor suppressor gene p53 thus triggering tumor growth. As a result, the combined TMZ treatment and AKB silencing has significantly improved the survival of treated mice from 14 to 33 days.
Lf could be also used to enhance brain accumulation of nanoparticles through intranasal route based on the high expression of LfR on the nasal epithelial cells and olfactory linings. Both Lf and hyaluronate were coupled to the surface of acid-responsive lenalidomide-conjugated FePt alloy nanoparticles. While hyaluronic acid enhanced brain tumor targeting by its CD44 binding ability, Lf facilitated the olfactory uptake through enhanced mucosal and nasal penetration, which led to brain accumulation of the nanoconjugates. Also, LfR are overexpressed on the surface of glioma cells, thus increasing their specific uptake by cancer cells. In addition to this direct intranasal transport, Lf-modified nanoconjugates absorbed into blood circulation will most likely target brain delivery since LfR are overexpressed on the surface of brain endothelium [151]. The acidic microenvironment of lysozome resulted in cleavage of the pH sensitive hydrazone bond between drug and NPs to trigger the drug release. The NPs efficiently generated reactive oxygen species (ROS) due to release of Fe and Pt which increased its cytotoxicity against U87MG cells.
7.1.2. Parkinson disease (PD)
The Lf receptors are found to be upregulated by neurons involved in the pathophysiology of Parkinson and Alzheimer's diseases [152,153]. Both diseases are characterized by iron overload in specific areas in the brain. Since Lf plays an important role in iron metabolism, its therapeutic effect in Parkinson Disease (PD) and Alzheimer has been studied as metal chelator [154]. In case of PD, Lf is overexpressed in activated microglia where it plays an important neuroprotective role. This was evident for both apo- (iron-free) and holo- (iron-saturated) Lf. The suggested Lf neuroprotective mechanisms could be mediated through the increase of the mitochondrial transmembrane potential, indicating better mitochondrial activity [155]. Another hypothesis for the neuroprotective activity of Lf is iron-dependent, where Lf suppressed transferrin receptors and divalent metal transporter, leading to enhanced iron internalization [156]. Further, Lf is associated with upregulation of brain-derived neurotrophic factor (BDNF), in addition to activation of the pathway of regulated protein kinases-mitogen activated protein kinase (ERK/MAPK). Interestingly, Lf protects dopaminergic neurons in the nigrostriatal area by decreasing levels of α-synuclein through upregulation of hypoxia-inducible factor 1α [157].
In addition to its intrinsic activity, Lf and borneol were used together to modify the surface of dopamine-loaded nanoparticles (Lf-BNPs) for enhanced permeability and striatum-specificity for treatment of PD [158]. The NPs could alleviate the 6-hydroxydopamine-induced striatum lesion in rats by effective delivery of dopamine after intranasal administration. Similarly, dopaminergic SK-N-SH cells were protected against rotenone-induced neurotoxicity in dopaminergic neurons using Lf nanoparticles encapsulating curcumin prepared by sol/oil chemistry [159].
The use of genes such as glial cell line-derived neurotrophic factor (GDNF) encoding gene showed a great promise in PD therapy due to the stimulatory effect of GDNF on dopaminergic neurons [160]. In order to achieve successful brain delivery, hGDNF was encapsulated in Lf-functionalized PAMAM nanoparticles. Lf-modified NPs showed the highest GDNF expression compared to saline and Tf-modified nanoparticles. Compared to viral vectors, higher expression of hGDNF was achieved by continuous administration of Lf-modified NPs in chronic rotenone-induced model. This extended administration enhanced locomotor activity, decreased loss of dopaminergic neurons in addition to improved levels of monoamine neurotransmitters in PD rats [161].
7.1.3. Alzheimer
Increased iron in Alzheimer disease is associated with oxidative stress, reactive oxygen species and nitric oxide synthase production. Lf has an anti-oxidative effect as it acts as iron chelator in the brain to restore iron hemostasis. It also has anti-inflammatory activity, leading to decreased levels of TNF-α and IL-6 [162]. These results were further confirmed in a three-month clinical trial, with decreased serum amyloid β, p-tau, and MAPK1 [163]. Wang et al. found that the intranasal administration of Lf led to a reduction in β-amyloid deposition through activation of α-secretase which increased the metabolism of amyloid precursor protein. This led to amelioration in cognitive function and learning ability in treated animals [164]. Recently, Bartolome et al. found that salivary Lf could be a promising biomarker for non-invasive early detection of Alzheimer's disease, as it was shown to have specifically lower salivary levels [165].
Lf-polyamidoamine dendrimeric nanoparticles (PAMAM-Lf) were designed for the efficient delivery of rivastigmine (RIV) to Alzheimer's model. Administration of Lf-PAMAM NPs in Alzheimer's animal model enhanced the brain bio-availability of RIV and increased AChE activity resulting in higher Ach level and improved memory deficit than unmodified dendrimers [166]. Application of the hydrophilic peptide, neuron growth factor (NGF), for management of Alzheimer's disease is a feasible strategy. However, delivering therapeutic peptides to the brain faces many challenges such as degradation in serum and failure to bypass BBB. To deliver NGF across BBB, Lf-decorated liposomes were developed. The NGF Permeability across HBMECs/HAs monolayer showed higher penetration for Lf/NGF liposomes compared to non-targeted liposomes and free NGF [167]. In another hand, the viability of Aβ-insulted SK-N-MC cells was increased upon incubation with Lf/NGF-liposomes. Similarly, encapsulation of another neuroprotective peptide, S14G-humanin, into Lf-modified polymersomes inhibited the overexpression of caspase-3 and Bax and enhanced cholinesterase activity. As a result, they efficiently protected the hippocampuses of rats against learning and memory deficits triggered by using amyloid-β25-35 [168,169].
7.2. Colon cancer targeting
Lf was proposed as a safe alternative with powerful antitumor activity specifically against colon cancer. First, the anti-inflammatory effect of Lf on colon cancer was widely reported in literature. In Lf knockout mice, NF-ĸB was upregulated resulting in an aggravated inflammatory response as evidenced by the increased levels of the nuclear p65 protein expression, p65+ cell numbers, and IKKα/β. Furthermore, the pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) were found to be upregulated, which can cause AKT and mTOR deregulation and subsequent tumor progression [180]. Lf may also induce apoptosis in colon cancer cells by several pathways including induction of caspase 3/7, activation of p53 tumor suppressor gene, and enhancing expression of the pro-apoptotic genes; TP5313, PMAIP-1, and SFN thereby promoting subsequent cell death in HT-29 cells [57].
Ingestion of Lf was correlated with an elevation in the activity of NK cells, and increases in CD16+/CD56+ subset of NK cells in the blood, which was consistent with regression of colorectal polyps. bLf was concluded not to induce activation/inhibition of immune system but rather increase the responsiveness of the immune system [181,182]. In addition to its anti-inflammatory and immunomodulatory effects, bLf was shown to have antioxidant effect by increasing the transcription of antioxidation related genes: GCLC and GCLM, involved in glutathione synthesis and scavenging of reactive oxygen species (ROS) [57]. Selenium-saturated bovine Lf (Se-bLf) showed a promising anti-oxidative property in HT-29 cells treated with 250 μM of H2O2, by increasing the activity of the enzymes glutathione peroxidase, glutathione s-transferase and glutathione reductase showing a protective effect against cancer development [183].
Nano-formulations of bLf were found to confer a promising efficacy against colon cancer; owing to its both colon targeting and antitumor effects. Lf can target cells colon cancer cells and may internalize itself utilizing these receptors regulated by intestinal epithelial cells [57]. Liposomal bovine Lf was able to inhibit the expression of TNF-α mRNA expression in colorectal RKO and RCN-9 cells resulting in a lower inflammation, as demonstrated by reduced numbers of neutrophils associated with suppression of colorectal polyps [184]. In a study by Kanwar et al., alginate coated, chitosan conjugated, calcium phosphate, Fe saturated bLf nanocapsules (AEC-CCo-CP-Fe-bLf NCs) with a size of 396.1 nm were effective against triple positive cancer stem like Caco-2 cells [14]. These nanocarriers were able to localize at the tumor site by EPR effect and then internalize utilizing LRP1, transferrin and Lf receptors in addition to iron receptors: DMT1, ferroprotein, and ferritin receptors, which were believed to play a major role in the absorption of Fe-bLf NCs [185]. These NCs showed enhanced efficacy compared to Fe-bLf NCs; due to better efficient delivery of Lf by the coating polymers, chitosan and alginate. Additionally, treatment of Caco-2 bearing mice with the NPs has downregulated anti-apoptotic factors including Survivin and Bcl-2, while upregulated pro-apoptotic Bax, Fas, caspase-3 and caspase-9 [14]. Consequently, modification of the surface of AEC-CCo-CP-Fe-bLf nanocapsules with LNA (EpCAM and nucleolin)-aptamer enhanced tumor targeting via binding to the cell surface receptors; EpCAM and nucleolin and hence translocate into the nucleus [186]. The uptake of the targeted NCs into Caco-2 cells was enhanced by 2.84 folds through receptor mediated internalization. The mice treated with both non-targeted and targeted NCs showed a significant decrease in the tumor volume by 6.8 and 2.91 folds, respectively.
The nanocapsules were then modified by addition of zinc ferrite to a solution of apo-Lf in the presence of sodium bicarbonate to produce zinc ferrite saturated bovine Lf (Zn–Fe-bLf) which was then encapsulated in a similar way into alginate-coated chitosan nanogel. The antitumor activity of the Zn–Fe-bLf NCs 24 nm was enhanced as demonstrated by dramatic reduction in tumor volume in human xenograft colonic adenocarcinoma to 0.10 mm3 compared to the vehicle NCs (52.28 mm3) and control group (93.51 mm3). Application of magnetic field and photothermal laser after treatment with Zn–Fe-bLf NCs resulted in further tumor reduction. Moreover, the presence of Zn may improve CT scanning signal intensity of the NCs compared to Fe-bLf revealing efficient multimodule nanotheranostic properties [185].
Lf NPs could also be used as a carrier for chemotherapeutic drugs such as oxaliplatin and 5-Flourouracil to enhance their targetability to colon cancer. The NPs exhibited enhanced cytotoxicity against COLO-205 cells as shown by lower IC50 (1.08 and 0.98 μg/ml for Lf-5FU and Lf-oxalo NPs, respectively) compared to free drug (IC50 of = 2.27 and 2.22 μg/ml for free 5FU and free oxaliplatin, respectively). The nano-formulations showed a 7-fold decrease in AFC (structural deformity which turns into colon adenocarcinoma) compared to a 3-fold decrease by the free drugs in azoxymethane induced AFC in Wistar rats [98].
7.3. Liver cancer targeting
Lf binds specifically to LRP and asialoglycoprotein receptors (ASGP-R) that facilitate its internalization into hepatocellular carcinoma (HCC). Therefore, modification of PEGylated liposomes with Lf improved their uptake into ASGPR-positive cells including HepG2, BEL7402, and SMMC7721 but not in ASGPR-negative cells [187]. DOX-loaded Lf-PEG-liposomes exhibited higher anticancer efficacy in HepG2 bearing mice relative to free drug.
For combined HCC therapy, we have prepared Lf-coated oily core nanocapsules for synergistic delivery of sorafenib and quercetin [121]. The dual targeted lactobionic acid/Lf-NCs exhibited enhanced uptake into HepG2 cells and higher anti-tumor effect in HCC mice model compared to free drugs. For oral targeted HCC therapy, Lf-coated gliadin NPs co-encapsulating celecoxib and diosmin showed a synergistic anticancer action due to their common inhibitory effects on COX-2, NF-κB and TNF-α [188,189]. The high stability of Lf-GLNPs is probably due to surface coating of GL-NPs by Lf providing both hydrophilic surface and charge-based stabilization. In addition, the glycan chains of Lf may add steric stabilization mechanism. The Lf-coated NPs exhibited enhanced internalization and improved anti-tumor efficacy against HepG2 cells at 48 h and HCC-bearing mice. Lf-targeted gliadin NPs demonstrated a remarkable inhibition of NF-κB and TNF-α expression compared with the positive control.
7.4. Breast cancer targeting
After oral administration, the small size FebLf NCs (80 nm) induced apoptosis of claudin-low, triple-negative MDA-MB–231 breast cancer cells through mitochondrial depolarization, downregulation of the apoptosis inhibitory proteins; survivin, and livin as well as PI3K followed by activation of caspase-3 [18]. By virtue of their superparamagnetic properties, FebLf NCs can be considered as suitable cancer nanotheranostic agent for MRI contrast imaging and magnetic guided cancer therapy. In another investigation, Lf-coated DOX-loaded porous magnetite nanospheres (PMNSs) were developed for synergistic chemo-magnetic field photothermal therapy of breast cancer [190]. The injected Lf-DOX-PMNSs significantly into 4T1 bearing mice suppressed the proliferation of 4T1 cells and tumor weight by activating both the extrinsic pathway of apoptosis via upregulation of the TNF-α mRNA and enhancing the intrinsic Bax mRNA expression. The Lf coating layer not only enhanced the drug targeting to tumor site but also prolonged its circulation by forming protein corona that can evade clearance by RES and showed acid responsive drug release at tumor microenvironment.
In addition to its intrinsic anticancer effect, Lf was exploited as a hydrophilic shell of amphiphilic micelles co-encapsulating two water insoluble drugs rapamycin and wogonin within its hydrophobic core composed of the plant protein zein [118]. In addition to its active tumor targeting properties, the brush-like hydrophilic Lf corona increased the micellar stability, inhibited their opsonization and prolonged their systemic circulation leading to maximized accumulation at tumor site by the EPR effect. In a consequent study, oleic acid-coated Fe3O4 NPs were encapsulated together with dasatinib in the micellar hydrophobic zein core [119]. Attributed to their superparamagnetic characteristics, the micelles showed enhanced cytotoxicity against MDA-MB-231 cells upon application of external magnetic field. In another approach, Lf was electrostatically anchored onto the surface of zein nanospheres co-loaded were the hydrophobic aromatase inhibitor exemestane and the herbal flavonoid luteolin [191]. Lf-modified NPs enhanced their targeting to breast cancer cells resulting in improved cytotoxic effect against MCF-7 and 4T1 breast cancer cells with 2.5-fold higher selectivity index SI to cancer cells rather than normal cells compared to the free drugs.
Another interesting application of holo-Lf is its ability to relief of hypoxia in TME of solid tumors. Hypoxia is known to reduce the efficacy of radiotherapy by weakening the radiation-induced cell DNA damage. The presence of Fe in the structure of holo-Lf can induce decomposition of H2O2 to oxygen thus alleviating the hypoxic TME. Through its oxygen generation capacity, Lf-PEGylated DOX-loaded liposomes showed synergistic anticancer effect and increased survival rate of mice when combined with 4 Gy X-ray radiation in breast cancer bearing mice which could be confirmed by photoacoustic imaging [78]. Lf NPs were also used to enhance the efficacy of breast cancer photodynamic therapy by encapsulation of photosensitizer (PS) Chlorine e6 (Ce6). The NPs inhibited the aggregation of the hydrophobic Ce6 in aqueous media thus improving its efficacy to generate ROS [192]. Under light irradiation, the NPs triggered a remarkable cell death in SK-OV-3 and MDA-MD 231 cells 4 folds higher than that of free Ce6.
7.5. Lung cancer targeting
Lf receptors were found to be upregulated on the bronchial epithelial cells therefore, Lf-modified lipid nanocarriers were used for the targeted delivery of PTX to lung cancer therapy [193]. Lf-targeted lipid NPs showed enhanced cytotoxicity against BEAS-2B human bronchial epithelial cells, relative to non-targeted NPs and free PTX. After intravenous administration into rats, the Lf-NPs showed higher amounts of drug accumulated in lung tissue relative to non-targeted NPs.
Beside the use of Lf-targeted nanocarriers for systemic therapy of lung cancer, the inhalable Lf-targeted nanocomposites have emerged as a promising alternative to avoid the systemic toxicity and enhance deep lung deposition [194]. For this purpose, different Lf-targeted NPs were prepared for synergistic lung cancer treatment and then transformed into inhalable nanocomposites via spray-drying with suitable pulmonary inert carriers. Examples of those nanocarriers include; (i) HA/Lf layer-by-layer coated lipid nanoparticles co-encapsulating rapamycin-phospholipid complex and berberine-sodium lauryl sulfate hydrophobic ion pair [195], (ii) Lf-chondroitin electrostatic nanocomplex co-loaded with DOX hydrochloride and ellagic acid nanocrystal soluble form [110], and (iii) Lf/chondroitin layer-by-layer functionalized monoolein liquid crystalline NPs co-entrapping pemetrexed and resveratrol (Fig. 7 ) [196]. All those nanocarriers exhibited favorable physicochemical properties such as small size, improved drug release pattern, improved uptake and enhanced cytotoxic effect against A549 lung cancer cells. for deep lung deposition, those nanocarriers were mixed with drying aids such as mannitol, maltodextrin, leucine or their combinations prior to spray-drying to form inhalable nanocomposites. The inhalable powder demonstrated favorable aerosolization efficiency (MMAD of 2.68–3.28 μm and FPF of 55.5–89.58% %) revealing efficient particle deposition into deep lung tissues. After reaching lung tissues, the carriers dissolved releasing the nanoparticles followed by internalization into lung cancer cells by virtue of their Lf, HA, or chondroitin-based receptor mediated endocytosis. In lung cancer bearing mice, all the prepared inhalable nanocomposites displayed powerful anti-cancer efficacy by reducing the lung weight, decreasing the number and diameters of lung adenomatous foci, downregulating the level of proliferative and angiogenic markers and increasing the apoptotic markers compared to intravenously administered nanocarriers.
Fig. 7.
Schematic diagram illustrating the formation of inhalable PEM/RES-loaded liquid crystalline monoolein NPs for lung cancer targeting [196].
7.6. Prostate cancer targeting
Lf demonstrated direct cytotoxic action against highly metastatic PC-3 prostate cancer cells via inhibition of V-ATPase and subsequent reduction of the ability of those cells to acidify the TME. Moreover, Lf induced phenotypic changes of DU-145 and LNCaP reducing their invasive properties. Lf also downregulated the expression of steroid hormone receptors (e.g. estrogen receptor ERα and, progesterone receptor PR) and increased the expression of oncosuppressive miRNAs (e.g. miR-133a and miR-200b) in DU-145 cells [197].
As a targeting ligand for prostate cancer cells, Lf modification of diaminobutyric polypropylenimine dendriplexes enhanced the delivery and transfection of the encapsulated plasmid DNA encoding TNFa, TRAIL or IL-12 to prostate cancer cells after intravenous administration [198]. The Lf-targeted dendriplexes resulted in much stronger suppression of PC-3 and DU145 tumors in comparison with Tf-modified dendriplexes. Conjugates of DOX to iron saturated Lf (Fe-bLf-DOX) showed higher cytotoxicity against DU145 prostate cancer cells and lower toxicity against non-cancerous RWPE-1 cells compared to iron free Apo-bLf-DOX and free DOX [77]. Besides it increases uptake into cancer cells via interaction with additional receptors, the iron acts as an essential effector of DOX induced cytotoxicity. As a result, Fe-bLf-DOX exhibited superior efficacy in decreasing Bcl-2 expression levels associated with elevated PTEN expression. After internalization into the endo-lysosome, DOX was released upon cleavage of the covalent amide bond between drug and the bLf protein by the hydrolytic enzymes and the acidic environment thus resulting in increased nuclear accumulation of DOX. Therefore, the Lf-DOX conjugate could be considered as DOX prodrug while including a functional carrier bLf with cytotoxic nature thus causing decrease in the LC50 of DOX by more than 4 folds. Another interesting property of Lf conjugate is inhibiting the efficacy of membrane efflux pumps in efflux DOX out of cells resulting in higher retention of drug in the prostate cancer cells [77]. Thus, bLf-DOX conjugate induced both down-regulation of P-gp as well as the bypass of P-gp [77].
7.7. Cancer theranostics
Modification of the surface of curcumin-loaded magnetic polydiacetylene nanocarriers (PDNCs) with Lf significantly improved their brain accumulation by enhancing BBB penetration resulting in powerful suppression of orthotopic brain tumor and increasing the animal survival by about 30%. At the same time, the NPs ability to exhibit both self-responsive fluorescence and MRI contrast imaging upon cellular internalization enabled cell trafficking and imaging-assisted cancer therapy. Thus, the Lf-targeted magnetic PDNCs can be promising theranostic application for targeting gliomas [170].
Another promising imaging probe for cancer, highly fluorescent CdTe quantum dots (QDs) were conjugated to Lf-targeted nanocapsules. The oily core of nanocapsules co-solubilizing celecoxib and honokiol was electrostatically coated with anionic chondroitin sulfate layer followed by a cationic Lf-QDs conjugate corona [199]. The covalent bond formed between Lf and QDs prohibited the systemic release of Cd into circulation and guarantee the site-specific release of QDs only at tumor sites. The QDs luminescence was quenched in vitro upon conjugation with Lf (OFF state) due to electron/energy transfer mechanism. Upon intracellular uptake into MCF-7 cells, luminescence was restored (ON state) due to detachment of surface-bound ligands from QDs in cytosolic environment confirm and monitor intracellular uptake of our nanoparticles that was confirmed using confocal microscopy. In vivo, tumor tissues of Lf–QDs–CS–NCs treated mice group showed the highest fluorescence intensity compared with liver and kidney that proved their effective localization in the tumor tissue.
Recently, Lf and RGD dimer dual-modified extremely small (13.4 nm) gadolinium oxide nanoparticles were prepared as highly efficient theranostics of GBM. The NPs successfully crossed the BBB due to its small diameter and Lf binding to its receptors then enter brain tumor cells by RGD2-integrin αvβ3 interaction, and thus can be used for MRI-guided radiosensitization of brain tumors [125]. The NPs exhibited high contrast MRI as indicated by extraordinary relaxivities thus showing much better signal enhancement for T1-weighted MRI of tumors than commercial Gd-chelates.
7.8. Nuclear targeting
One of the most interesting properties of Lf is its ability to reach the nucleus. On this regard, Lf has great potential for macromolecular delivery to cytoplasm and nucleus such as nucleic acids (siRNA, mRNA, shRNA and DNA) and gene editing machinery (Cas9 and sgRNA). Furthermore, by virtue of its cationic charge, Lf can bind to RNA and DNA and activates transcription. It was reported early in 1992 that Lf can easily traffic to the nucleus of K562 cells in temperature-dependent manner [200]. In 5637 bladder carcinoma, U87MG, MCF7, NIH3T3 and HeLa cells, a pentapeptide enriched in basic amino acids (Gly-Arg-Arg-Arg-Arg), derived from the N-terminal portion of human Lf was internalized within 10 min into the nucleus and mainly nucleoli [201]. Nuclear localization of Lf was also observed in human THP-1 monocytic and bovine rectal epithelial cells [202,203].
In addition, a cell penetrating peptide derived from lactoferricin termed as L5a CPP (RRWQW) was shown to efficiently deliver DNA into the nucleus of human A549 lung cancer cells without induction of cytotoxic effect [204]. Compared to Tf, Lf was shown to be apically internalized into Caco-2 intestinal epithelial cells and then localized to the nuclei while Tf was internalized from the basolateral side and localized in the cytoplasm [205]. Nuclear trafficking of Lf was shown to be mediated by non-endocytic mechanism in some studies, which offers an easy way to escape from endosomes and late lysosomes for functional delivery of nucleic acids to cytoplasm and nucleus. More sophisticated investigations are required to fully understand the nuclear targeting mechanism of Lf and its nanoparticulate form in different cell lines to make the best use of Lf for wide therapeutic applications.
7.9. Immunomodulatory applications
Being a key component in the mammalian innate immune system, Lf plays an essential role in the host defense against pathogens and inflammation where its deficiency may precipitate infection recurrence [206]. The cationic nature of Lf contributes to its ability to bind to surface molecules found on immune cells, triggering signaling cascades leading to immune cells activation and maturation [207]. Bovine Lf exerts its immunostimulatory effect through its immunogenic forms which are formed by enzymatic digestion in the GIT [208]. Oral Lf is thought to send signals through receptors on the epithelium of the intestine and immune cells as macrophages and lymphocytes [22]. Moreover, the absorbed Lf can prime the immune system where an enhanced cytokine release was demonstrated including cytokines related to early (TNF-α, IL-6, IL-10) and adaptive (IL-12p40, IFN-γ, IL-4) immunity. Overall, two possible explanations may account for the immunomodulatory effects of Lf: a) Immune cells express Lf receptors. Therefore, a receptor-mediated interaction between Lf and immune cells or indirect uptake of Lf by immune cells, results in the priming of immune system, b) Secondly, Lf may help educate the immune cells present in the GIT microenvironment. The immune cells will be transported via the lymphatic system to lymph nodes or spleen [209].
A study by Mulder et al. showed that oral supplementation of bLf in humans provided immunomodulatory effect through T-cell activation and anti-oxidant effect [210]. Moreover, Lf was shown to contribute to inflammatory response by granulocyte accumulation and activation, inducing phagocytosis and augmentation of natural killer cells activity (Fig. 8 ) [211,212]. Being an innate immunity component, the immuno-modulatory activity of Fe-bLf dramatically augmented the anti-tumor efficacy of Fe-bLf-DOX in prostate cancer bearing mice [77]. This was demonstrated as reduced serum levels of the tumor promoting cytokines IL-5, IL-6 and IL-17 and elevated levels of IFNγ and TNFα as well as GM-CSF, CCL17 and CCL4 responsible for triggering intratumoral infiltration of cytotoxic T cells and macrophages.
Fig. 8.
Immuno-modulatory mechanisms of Lf. Lf triggers differentiation, maturation and activation of immune cells through NF-ĸB pathway, leading to enhanced activity of immune cell; NK and PMN cells.
Lf nanocarriers were also used for remodeling of the immunosuppressive tumor microenvironment. The surface of Lf nanoparticles was modified with TPGS-coupled TAT cell penetrating peptide to enhance the BBB penetration of simvastatin and fenretinide drug combination. Interaction of Lf with LRP-1 receptors expressed by brain capillary endothelial cells (BCEC) and U87 glioma cells enhanced BBB permeation while TAT peptide increased the intracranial tumor penetration by 1.5 folds higher than that of un-modified Lf nanoparticles [101]. Simvastatin was reported to remodel TME and functional plasticity of TAMs whereas fenretinide induces ROS production and also can reverse the pro-tumoral M2-macrophages to the anti-tumoral M1 TAMs and reduce angiogenesis. The nanoparticles showed powerful synergistic cytotoxic effect against glioma cells in vitro and inhibited the tumor growth in vivo. Mechanistically, the dual drug loaded NPs succeeded to remodel since they effectively repolarized M2-TAM to M1-TAM as demonstrated by downregulating CD206 (TAM2 biomarker) and increasing the expression of iNOs and STAT1 (TAM1 biomarkers). The nanoparticles also improved the infiltration of cytotoxic NK cells into the tumor as revealed by overexpression of the NK cell marker NKp44. Moreover, it increased ROS production which in turn induced mitochondrial apoptosis.
Another example of application of Lf NPs in TME immune-reprogramming includes the use of mannosylated Lf nanoparticles for combined delivery of shikonin and JQ1 for both metabolic and of TME [102]. The nanoparticles targeted mannose and LRP-1 receptors overexpressed by TAMs and cancer cells, respectively. The dual drug loaded nanoparticles could remodel the TME via different pathways. First, the NPs elicited antitumor immunity mediated by SHK ability to induce immunogenic cell death. As a result, the nanoparticles enhanced maturation of dendritic cells and hence increased the amount of tumor infiltrating CD8+ T cells [102]. Moreover, the SHK-loaded NPs suppressed cancer glucose metabolism through inhibition of pyruvate kinase M2 resulting in reduced lactate production and hence repolarizing M2-TAMs. On the other hand, JQ1 can decrease PD-L1 expression on cancer cells and downregulate Foxp3 and CTLA-4 in the tumor-infiltrating regulatory T cells (Tregs) so the NPs dramatically reduced the percentage of the intratumor infiltrating Tregs.
7.10. Antiviral drug delivery
The anti-HIV drug Indinavir (IDV) suffers from poor entry into brain due to efflux by the P-glycoprotein expressed in BBB. Chemical coupling of Lf to the surface of indinavir-loaded nanoemulsion (Lf-IDV-NEs) has enhanced its BBB penetration and increased its residence time in brain [213]. As a result, after administration into rat model, Lf-coupled nanoemulasion remarkably increased the brain concentration of IDV compared to free drug. Encapsulation of the antiretroviral drug Zidovudine (AZT) into Lf NPs for oral delivery resulted in improved pharmacokinetics (4 fold increase in AUC and 2-fold increase in Tmax and t1/2), higher efficacy and 2-fold lower organs related toxicities compared to free drug while keeping the antiviral activity unaltered [214].
Lf nanoparticles co-encapsulating the anti-HIV-1 drug Efavirenz and the anti-microbial drug curcumin (ECNPs) were used as a vaginal microbicide for multipurpose prevention technologies (MPT) [215]. After topical application in rats, the NPs could deliver higher drug amounts in vaginal lavage while showed significantly lower drug absorption in vaginal tissue and plasma relative to its free form. An anti-retroviral drug combination (zidovudine, efavirenz and lamivudine) was encapsulated into Lf NPs to improve their absorption and hence increase their anti-HIV efficacy [216]. The NPs showed pH-dependent drug release behavior with very low initial burst effect while most of the entrapped drugs were released at pH 5 with minimal toxicity to the erythrocytes and less tissue-related inflammation.
7.11. Bone engineering
Lf exhibits favorable modulatory effects on bone cells and enhances bone growth thus it can be used as a bone growth factor for bone regeneration and bone engineering. Addition of Recombinant human Lf (rhLf) to the culture media of NHOst human osteoblast cells resulted in increased level of alkaline phosphatase (ALP), a marker for osteogenic activity, and enhanced ERK1/2 activation, which is a part of proliferative signaling. Treatment of piglet with rhLf has increased the serum calcium and bone mineral density compared to control piglets [217].
Treatment of MC3T3 osteoblast precursor cells with rhLf for 24 h resulted in increase in the anti-apoptotic Wnt5a together with an increase in Akt phosphorylation, while down-regulated caspase 3, which is a marker of cell apoptosis. Therefore, tyramine-coupled rhLf was enzymatically cross-linked using horseradish peroxidase and hydrogen peroxide into injectable hydrogel for encapsulation of MC3T3 cells. A remarkable increased expression of ERK (a proliferation marker) and RunX2 (a differentiation marker) was observed in the encapsulated MC3T3 cells. The cells maintained their differentiation ability as revealed by expression of collagen I protein, and osteocalcin after 21 days post culture in mineralization media [218,219]. A number of studies suggested that Lf-induced osteogenic activity and proliferation of osteoblasts may be mediated by binding with TGF-β receptors and stimulating TGF-β canonical pathways, or by activation of MAPK pathways [220].
Due to its antiapoptotic effect on osteoblasts and suppression of osteoclastogenesis, Lf can be considered as anabolic factor for anti-osteoporotic therapy [221]. Different studies found that hydroxyapatite nanocrystals (HA) and Lf had a synergistic effect on bone homeostasis. To increase its bioavailability and prolong its action, Lf was electrostatically adsorbed onto HA nanocrystals forming a single protein layer well exposed to cells. Lf/HA combination resulted in several beneficial effects [222]. This includes enhancing the expression of RUNX2 mRNA, Osterix and IBSP, common markers of osteoblast differentiation confirming a synergistic effect on osteogenic differentiation and formation of bone matrix. Moreover, Lf reduced the apoptotic effect of HA on osteoblasts, suppressed the stimulatory effect of HA on osteoclasts and stimulated osteoblasts to produce osteoprotegerin (OPG) that reduce the activity of osteoclasts [86].
8. Challenges and limitations
Despite the promising role of Lf as a drug nanocarrier and tumor-targeting ligand with anti-cancer and immunomodulatory functions, some challenges have to be overcome prior to clinical translation. First, the cationic nature of Lf can cause toxicity and adverse inflammatory response [223]. Positively charged NPs are known to suffer from higher non-specific uptake and rapid clearance from blood by macrophages of liver, spleen and lung compared to anionic and neutral particles [224]. Mitochondrial damage and cell necrosis were observed after injection of cationic liposomes or chitosan NPs mostly mediated by inhibition of the cation-binding site of Na+/K+-ATPase [225]. Moreover, high expression levels of the inflammatory cytokines e.g. IL6, CXCL2 and CCL2 were detected in lung, spleen and liver of rats after administration of cationic micelles and liposomes [223]. We anticipate that the effect of positive charge of Lf macromolecules can be alleviated via its electrostatic complexation with polyanionic polysaccharides to form nanocomplexes with negative or low positive charge according to the complexation ratio. Another approach might involve the formation of multi-stage nanocarriers via encapsulation of the cationic Lf NPs within negatively charged or PEGylated phospholipid bilayers. This approach avoids the toxicity of the released cationic Lf NPs into circulation while makes use of their positive charge in enhancing their uptake into cancer cells via charge-mediated transcytosis. The positive charge of Lf can also be beneficial for endosomal escape particularly for gene delivery.
Second, the short half-life of Lf after systemic administration due to the action of proteolytic enzymes and its oral susceptibility after oral intake may lead to inadequate delivery. Many effective strategies have been successfully implemented to prolong its half-life and increase its oral stability including PEGylation, encapsulation or chemical crosslinking [90,226]. Finally, although the reported safety and biocompatibility of Lf, the use of bovine Lf might induce possible immunogenicity with undesired reactions. Antibodies against bovine Lf have been detected in the serum of mice treated with Lf-coated nanocapsules [134]. Therefore, the use of recombinant Lf can overcome this limitation (discussed below).
9. Clinical trials & future perspectives
Bovine Lf (bLf), is commercially available and well tolerated form of Lf with similar biological functions to human Lf but with lower cost. Based on the results of several toxicological studies on bovine milk derived Lf (cMDLf), including an acute, a 4 week, a 13-week, and two chronic oral toxicity studies in rats, as well as hypersensitivity, allergenicity and mutagenicity studies, it can be concluded that cMDLf does not produce adverse effects, not mutagenic and unlikely to be a clinically relevant allergen or the primary causative agent of any immunologically driven hypersensitivity consumption. bLf is recognized by the US Food and Drug Admission and the European Food Safety Authority as GRAS (generally recognized as safe) component for numerous indications [[227], [228], [229]]. Table S1 lists some of the Lf-based products available in the pharmaceutical market. Many studies and clinical trials have demonstrated the safety and tolerability of orally administered rhLf in treatment of non-small cell lung cancer and renal cell carcinoma [5,75]. We have summarized the most important outcomes of the clinical trials based on Lf products for various applications (Table S2).
Talactoferrin (TLf) is a recombinant human Lf which is intended for oral administration. Production of recombinant human Lf may proceed using different expression systems, including fungi, animals, plants and viruses. Nevertheless, recombinant human Lf, produced by Aspergillus awamori, has been directed toward therapeutic uses [230,231]. More prominently, TLf has shown to modulate the immunity in various pathological conditions. For instance, TLf was demonstrated to induce INF-γ dependent activation of NK cells and CD8+ cells in the gut associated lymphoid tissue (GALT), Peyer's patches as well as systematically. In addition, the subsequent effect was further extended towards inhibition of neu+ tumors [232]. Moreover, TLf showed a comparable antitumor effect to human Lf and bovine Lf in colon and liver cancer cells, through induction of apoptosis, chemokine C–C motif ligand 20 (CCL20) secretion and TGF-β1 expression [233]. Furthermore, it was shown that topically, TLf enhanced the release of key inflammatory mediators, such as IL-8, IL-6, TNF-α and macrophage inflammatory protein-1 alpha, which are responsible for tissue repair, and subsequently wound healing [81].
In addition to recombinant human Lf, a lot of research is directed to Lf-derived peptides. The peptides Lf (1-11), Lactoferricin (17–41) and Lactoferrampin (268–284), are the three major peptides derived from Lf showing pharmacological actions: antibacterial, antiviral, antiparasitic, antifungal and anticarcinogenic activities. It worth noting that Lactoferricin B, corresponding to residues 17–41 of bLf, was reported to exert more potent antimicrobial activity than Lf [234]. Table S3 elucidates biological functions of Lf peptides. Being chemically modified, LTX-315 is a 9-mer cationic peptide derived from bovine Lf and possess an anti-tumor activity, especially against solid tumors [235,236]. The mechanism of oncolytic property resides in the activation of local anti-tumor immunity, alone or combined with chemotherapeutic agents. In this regard, LTX-315 was shown to promote the infiltration of CD4+ and CD8+ cells to the tumor sites, in addition to a reduction in myeloid derived-suppressor cells (MDSC) and CD4+ regulatory T cells. The modulation of the tumor micro-environment exhibited by LTX-315 paved the way to a more pronounced anti-tumor activity when combined with DOX [236]. Furthermore, it was demonstrated that mitochondrial permeabilization through Bcl-2 family protein dependent pathway, was an additional anti-tumor mechanism of LTX-315 [237].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2020.120355.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sorensen M.S. The Proteins in Whey, Whey. Compte rendu des Travaux du Laboratoire de Carlsberg Ser. Chim. 1939;23:55–99. [Google Scholar]
- 2.Groves M.L. The isolation of a red protein from Milk2. J. Am. Chem. Soc. 1960;82(13):3345–3350. [Google Scholar]
- 3.Baker E.N., Lindley P.F. New perspectives on the structure and function of transferrins. J. Inorg. Biochem. 1992;47(1):147–160. doi: 10.1016/0162-0134(92)84061-q. [DOI] [PubMed] [Google Scholar]
- 4.Astruc D. Introduction to Nanomedicine. Molecules. 2015;21(1) doi: 10.3390/molecules21010004. E4-E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aboda A., Taha W., Attia I., Gad A., Mahmoud Mostafa M., Abdelfattah Abdelwadod M., Mohsen M., Kaur Kanwar R., Kanwar J.R. In: Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents. Singh M.R., Singh D., Kanwar J.R., Chauhan N.S., editors. Academic Press; 2020. Chapter 8 - iron bond bovine lactoferrin for the treatment of cancers and anemia associated with cancer cachexia; pp. 243–254. [Google Scholar]
- 6.Kumari S., Bhattacharya D., Rangaraj N., Chakarvarty S., Kondapi A.K., Rao N.M. Aurora kinase B siRNA-loaded lactoferrin nanoparticles potentiate the efficacy of temozolomide in treating glioblastoma. Nanomedicine. 2018;13(20):2579–2596. doi: 10.2217/nnm-2018-0110. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy N.A., Kelly A.L., O'Mahony J.A., Fenelon M.A. Sensitivity of emulsions stabilised by bovine β-casein and lactoferrin to heat and CaCl2. Food Hydrocolloids. 2014;35:420–428. [Google Scholar]
- 8.González-Chávez S.A., Arévalo-Gallegos S., Rascón-Cruz Q. Lactoferrin: structure, function and applications. Int. J. Antimicrob. Agents. 2009;33(4):301. e1–301. e8. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Adlerova L., Bartoskova A., Faldyna M. Lactoferrin: a review. Vet. Med. 2008;53(9):457–468. [Google Scholar]
- 10.Castellino F.J., Fish W.W., Mann K.G. Structural studies on bovine lactoferrin. J. Biol. Chem. 1970;245(17):4269–4275. [PubMed] [Google Scholar]
- 11.Anderson B.F., Baker H.M., Norris G.E., Rice D.W., Baker E.N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2· 8 Å resolution. J. Mol. Biol. 1989;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- 12.Lönnerdal B., Iyer S. Lactoferrin: molecular structure and biological function. Annu. Rev. Nutr. 1995;15(1):93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 13.Gupta I., Sehgal R., Kanwar R.K., Punj V., Kanwar J.R. Nanocapsules loaded with iron-saturated bovine lactoferrin have antimicrobial therapeutic potential and maintain calcium, zinc and iron metabolism. Nanomedicine. 2015;10(8):1289–1314. doi: 10.2217/nnm.14.209. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar J.R., Mahidhara G., Roy K., Sasidharan S., Krishnakumar S., Prasad N., Sehgal R., Kanwar R.K. Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine. 2015;10(1):35–55. doi: 10.2217/nnm.14.132. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar J., Roy K., Patel Y., Zhou S., Rawat Singh M., Singh D., Nasir M., Sehgal R., Sehgal A., Singh R., Garg S., Kanwar R. Multifunctional iron bound lactoferrin and nanomedicinal a pproaches to enhance its bioactive functions. Molecules. 2015;20:9703–9731. doi: 10.3390/molecules20069703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng Q., Wang A., Hua H., Jiang Y., Wang Y., Mu H., Wu Z., Sun K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer's disease. Int. J. Nanomed. 2018;13:705–718. doi: 10.2147/IJN.S151474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama Y., Oshima K., Kuhara T., Shin K., Abe F., Iwatsuki K., Nadano D., Matsuda T. A lactoferrin-receptor, intelectin 1, affects uptake, sub-cellular localization and release of immunochemically detectable lactoferrin by intestinal epithelial Caco-2 cells. J. Biochem. 2013;154(5):437–448. doi: 10.1093/jb/mvt073. [DOI] [PubMed] [Google Scholar]
- 18.Kanwar J.R., Kamalapuram S.K., Krishnakumar S., Kanwar R.K. Multimodal iron oxide (Fe3O4)-saturated lactoferrin nanocapsules as nanotheranostics for real-time imaging and breast cancer therapy of claudin-low, triple-negative (ER(-)/PR(-)/HER2(-)) Nanomedicine. 2016;11(3):249–268. doi: 10.2217/nnm.15.199. [DOI] [PubMed] [Google Scholar]
- 19.Takayama Y., Aoki R., Uchida R., Tajima A., Aoki-Yoshida A. Role of CXC chemokine receptor type 4 as a lactoferrin receptor. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2017;95(1):57–63. doi: 10.1139/bcb-2016-0039. [DOI] [PubMed] [Google Scholar]
- 20.Ando K., Hasegawa K., Shindo K., Furusawa T., Fujino T., Kikugawa K., Nakano H., Takeuchi O., Akira S., Akiyama T., Gohda J., Inoue J., Hayakawa M. Human lactoferrin activates NF-kappaB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 2010;277(9):2051–2066. doi: 10.1111/j.1742-4658.2010.07620.x. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi T., Jyonotsuka T., Kamemori N., Kawano G., Shimizu H., Ando K., Harada E. Enteric‐formulated lactoferrin was more effectively transported into blood circulation from gastrointestinal tract in adult rats. Exp. Physiol. 2006;91(6):1033–1040. doi: 10.1113/expphysiol.2006.035543. [DOI] [PubMed] [Google Scholar]
- 22.Fischer R., Debbabi H., Blais A., Dubarry M., Rautureau M., Boyaka P.N., Tome D. Uptake of ingested bovine lactoferrin and its accumulation in adult mouse tissues. Int. Immunopharm. 2007;7(10):1387–1393. doi: 10.1016/j.intimp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Yao X. University of Auckland; 2015. Development of a Novel Drug Delivery System to Enhance the Oral Bioavailability of Lactoferrin. [Google Scholar]
- 24.Lönnerdal B. Springer; 1994. Lactoferrin Receptors in Intestinal Brush Border Membranes, Lactoferrin; pp. 171–175. [DOI] [PubMed] [Google Scholar]
- 25.Troost F.J., Steijns J., Saris W.H., Brummer R.J. Gastric digestion of bovine lactoferrin in vivo in adults. J. Nutr. 2001;131(8):2101–2104. doi: 10.1093/jn/131.8.2101. [DOI] [PubMed] [Google Scholar]
- 26.Troost F.J., Saris W.H., Brummer R.J. Orally ingested human lactoferrin is digested and secreted in the upper gastrointestinal tract in vivo in women with ileostomies. J. Nutr. 2002;132(9):2597–2600. doi: 10.1093/jn/132.9.2597. [DOI] [PubMed] [Google Scholar]
- 27.Levay P.F., Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80(3):252–267. [PubMed] [Google Scholar]
- 28.Kanwar J.R., Samarasinghe R.M., Sehgal R., Kanwar R.K. Nano-lactoferrin in diagnostic, imaging and targeted delivery for cancer and infectious diseases. J. Canc. Sci. Ther. 2012;4(3):31–42. [Google Scholar]
- 29.Levay P.F., Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80(3):252–267. [PubMed] [Google Scholar]
- 30.Abu Hashim H., Foda O., Ghayaty E. Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: a meta-analysis of randomized trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;219:45–52. doi: 10.1016/j.ejogrb.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Ward P.P., Mendoza-Meneses M., Cunningham G.A., Conneely O.M. Iron status in mice carrying a targeted disruption of lactoferrin. Mol. Cell Biol. 2003;23(1):178–185. doi: 10.1128/MCB.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez L., Calvo M., Brock J.H. Biological role of lactoferrin. Arch. Dis. Child. 1992;67(5):657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldwin D.A., Jenny E.R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J. Biol. Chem. 1984;259(21):13391–13394. [PubMed] [Google Scholar]
- 34.Valenti P., Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cell. Mol. Life Sci. : CMLS. 2005;62(22):2576–2587. doi: 10.1007/s00018-005-5372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker E.N., Baker H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie. 2009;91(1):3–10. doi: 10.1016/j.biochi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Ellison R.T., 3rd, Giehl T.J., LaForce F.M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988;56(11):2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vongbhavit K., Underwood M.A. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin. Therapeut. 2016;38(4):716–732. doi: 10.1016/j.clinthera.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Mario F., Aragona G., Dal Bo N., Cavestro G.M., Cavallaro L., Iori V., Comparato G., Leandro G., Pilotto A., Franze A. Use of bovine lactoferrin for Helicobacter pylori eradication, Digestive and liver disease. official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2003;35(10):706–710. doi: 10.1016/s1590-8658(03)00409-2. [DOI] [PubMed] [Google Scholar]
- 39.León-Sicairos N., Angulo-Zamudio U.A., Vidal J.E., López-Torres C.A., Bolscher J.G., Nazmi K., Reyes-Cortes R., Reyes-López M., de la Garza M., Canizalez-Román A. Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in luxS gene expression by lactoferrin. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2014;27(5):969–980. doi: 10.1007/s10534-014-9775-y. [DOI] [PubMed] [Google Scholar]
- 40.Acosta-Smith E., Viveros-Jiménez K., Canizalez-Román A., Reyes-Lopez M., Bolscher J.G.M., Nazmi K., Flores-Villaseñor H., Alapizco-Castro G., de la Garza M., Martínez-Garcia J.J., Velazquez-Roman J., Leon-Sicairos N. Bovine lactoferrin and lactoferrin-derived peptides inhibit the growth of Vibrio cholerae and other Vibrio species. Front. Microbiol. 2017;8:2633. doi: 10.3389/fmicb.2017.02633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward P.P., Paz E., Conneely O.M. Multifunctional roles of lactoferrin: a critical overview. Cell. Mol. Life Sci. : CMLS. 2005;62(22):2540–2548. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanwar J.R., Samarasinghe R.M., Sehgal R. Nano-lactoferrin in diagnostic, imaging and targeted delivery for cancer and infectious diseases. J. Canc. Sci. Ther. 2012;4(3):031–042. 04(03) [Google Scholar]
- 43.Kanwar R.K., Singh N., Gurudevan S., Kanwar J.R. Targeting hepatitis B virus and human papillomavirus induced carcinogenesis: novel patented therapeutics. Recent Pat. Anti-Infect. Drug Discov. 2011;6(2):158–174. doi: 10.2174/157489111796064560. [DOI] [PubMed] [Google Scholar]
- 44.Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PloS One. 2011;6(8) doi: 10.1371/journal.pone.0023710. e23710-e23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wurzer W.J., Planz O., Ehrhardt C., Giner M., Silberzahn T., Pleschka S., Ludwig S. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22(11):2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietrantoni A., Dofrelli E., Tinari A., Ammendolia M.G., Puzelli S., Fabiani C., Donatelli I., Superti F. Bovine lactoferrin inhibits influenza A virus induced programmed cell death in vitro. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2010;23(3):465–475. doi: 10.1007/s10534-010-9323-3. [DOI] [PubMed] [Google Scholar]
- 47.Pietrantoni A., Di Biase A.M., Tinari A., Marchetti M., Valenti P., Seganti L., Superti F. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob. Agents Chemother. 2003;47(8):2688–2691. doi: 10.1128/AAC.47.8.2688-2691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaito M., Iwasa M., Fujita N., Kobayashi Y., Kojima Y., Ikoma J., Imoto I., Adachi Y., Hamano H., Yamauchi K. Effect of lactoferrin in patients with chronic hepatitis C: combination therapy with interferon and ribavirin. J. Gastroenterol. Hepatol. 2007;22(11):1894–1897. doi: 10.1111/j.1440-1746.2007.04858.x. [DOI] [PubMed] [Google Scholar]
- 49.Cirioni O., Giacometti A., Barchiesi F., Scalise G. Inhibition of growth of Pneumocystis carinii by lactoferrins alone and in combination with pyrimethamine, clarithromycin and minocycline. J. Antimicrob. Chemother. 2000;46(4):577–582. doi: 10.1093/jac/46.4.577. [DOI] [PubMed] [Google Scholar]
- 50.Leon-Sicairos N., Reyes-Lopez M., Canizalez-Roman A., Bermudez-Cruz R.M., Serrano-Luna J., Arroyo R., de la Garza M. Human hololactoferrin: endocytosis and use as an iron source by the parasite Entamoeba histolytica. Microbiology. 2005;151(Pt 12):3859–3871. doi: 10.1099/mic.0.28121-0. [DOI] [PubMed] [Google Scholar]
- 51.Leon-Sicairos N., Lopez-Soto F., Reyes-Lopez M., Godinez-Vargas D., Ordaz-Pichardo C., de la Garza M. Amoebicidal activity of milk, apo-lactoferrin, sIgA and lysozyme. Clin. Med. Res. 2006;4(2):106–113. doi: 10.3121/cmr.4.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinnis P., Willnow T.E., Briones M.R., Herz J., Nussenzweig V. Remnant lipoproteins inhibit malaria sporozoite invasion of hepatocytes. J. Exp. Med. 1996;184(3):945–954. doi: 10.1084/jem.184.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fritsch G., Sawatzki G., Treumer J., Jung A., Spira D.T. Plasmodium falciparum: inhibition in vitro with lactoferrin, desferriferrithiocin, and desferricrocin. Exp. Parasitol. 1987;63(1):1–9. doi: 10.1016/0014-4894(87)90072-5. [DOI] [PubMed] [Google Scholar]
- 54.Kirkpatrick C.H., Green I., Rich R.R., Schade A.L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 1971;124(6):539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- 55.Nakano M., Suzuki M., Wakabayashi H., Hayama K., Yamauchi K., Abe F., Abe S. 2019. Synergistic Anti-candida Activities of Lactoferrin and the Lactoperoxidase System. [DOI] [PubMed] [Google Scholar]
- 56.Luzi C., Brisdelli F., Iorio R., Bozzi A., Carnicelli V., Di Giulio A., Lizzi A.R. Apoptotic effects of bovine apo-lactoferrin on HeLa tumor cells. Cell Biochem. Funct. 2017;35(1):33–41. doi: 10.1002/cbf.3242. [DOI] [PubMed] [Google Scholar]
- 57.Jiang R., Lonnerdal B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell. Biol. 2017;95(1):99–109. doi: 10.1139/bcb-2016-0094. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Nicolau A., Lima C.F., Rodrigues L.R. Bovine lactoferrin induces cell cycle arrest and inhibits mTOR signaling in breast cancer cells. Nutr. Canc. 2014;66(8):1371–1385. doi: 10.1080/01635581.2014.956260. [DOI] [PubMed] [Google Scholar]
- 59.Wang J., Li Q., Li K., Ou Y., Han Z., Gao D., Li J. Effects of adenovirus vectors mediated human lactoferrin cDNA on mice bearing EMT6 breast carcinoma. Pharmazie. 2011;66(9):704–709. [PubMed] [Google Scholar]
- 60.Pereira C.S., Guedes J.P., Gonçalves M., Loureiro L., Castro L., Gerós H., Rodrigues L.R., Côrte-Real M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget. 2016;7(38):62144–62158. doi: 10.18632/oncotarget.11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W.Y., Li Q.W., Han Z.S., Jiang Z.L., Yang H., Li J., Zhang X.B. Growth suppression effects of recombinant adenovirus expressing human lactoferrin on cervical cancer in vitro and in vivo. Cancer Biother. Radiopharm. 2011;26(4):477–483. doi: 10.1089/cbr.2010.0937. [DOI] [PubMed] [Google Scholar]
- 62.Shi H., Li W. Inhibitory effects of human lactoferrin on U14 cervical carcinoma through upregulation of the immune response. Oncology letters. 2014;7(3):820–826. doi: 10.3892/ol.2013.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guedes J.P., Pereira C.S., Rodrigues L.R., Côrte-Real M. Bovine milk lactoferrin selectively kills highly metastatic prostate cancer PC-3 and osteosarcoma MG-63 cells in vitro. Frontiers in oncology. 2018;8 doi: 10.3389/fonc.2018.00200. 200-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lonnerdal B., Jiang R., Du X. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J. Pediatr. Gastroenterol. Nutr. 2011;53(6):606–614. doi: 10.1097/MPG.0b013e318230a419. [DOI] [PubMed] [Google Scholar]
- 65.Kuhara T., Iigo M., Itoh T., Ushida Y., Sekine K., Terada N., Okamura H., Tsuda H. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr. Canc. 2000;38(2):192–199. doi: 10.1207/S15327914NC382_8. [DOI] [PubMed] [Google Scholar]
- 66.Deng M., Zhang W., Tang H., Ye Q., Liao Q., Zhou Y., Wu M., Xiong W., Zheng Y., Guo X., Qin Z., He W., Zhou M., Xiang J., Li X., Ma J., Li G. Lactotransferrin acts as a tumor suppressor in nasopharyngeal carcinoma by repressing AKT through multiple mechanisms. Oncogene. 2013;32(36):4273–4283. doi: 10.1038/onc.2012.434. [DOI] [PubMed] [Google Scholar]
- 67.Lee S.H., Hwang H.M., Pyo C.W., Hahm D.H., Choi S.Y. E2F1-directed activation of Bcl-2 is correlated with lactoferrin-induced apoptosis in Jurkat leukemia T lymphocytes. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2010;23(3):507–514. doi: 10.1007/s10534-010-9341-1. [DOI] [PubMed] [Google Scholar]
- 68.Son H.J., Lee S.H., Choi S.Y. Human lactoferrin controls the level of retinoblastoma protein and its activity. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2006;84(3):345–350. doi: 10.1139/o06-048. [DOI] [PubMed] [Google Scholar]
- 69.Tung Y.T., Chen H.L., Yen C.C., Lee P.Y., Tsai H.C., Lin M.F., Chen C.M. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Dairy Sci. 2013;96(4):2095–2106. doi: 10.3168/jds.2012-6153. [DOI] [PubMed] [Google Scholar]
- 70.Chea C., Miyauchi M., Inubushi T., Febriyanti Ayuningtyas N., Subarnbhesaj A., Nguyen P.T., Shrestha M., Haing S., Ohta K., Takata T. Molecular mechanism of inhibitory effects of bovine lactoferrin on the growth of oral squamous cell carcinoma. PloS One. 2018;13(1) doi: 10.1371/journal.pone.0191683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao Y., Monitto C.L., Minhas K.M., Sidransky D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin. Canc. Res. : an official journal of the American Association for Cancer Research. 2004;10(24):8683–8686. doi: 10.1158/1078-0432.CCR-04-0988. [DOI] [PubMed] [Google Scholar]
- 72.Arcella A., Oliva M.A., Staffieri S., Aalberti S., Grillea G., Madonna M., Bartolo M., Pavone L., Giangaspero F., Cantore G., Frati A. In vitro and in vivo effect of human lactoferrin on glioblastoma growth. J. Neurosurg. 2015;123(4):1026–1035. doi: 10.3171/2014.12.JNS14512. [DOI] [PubMed] [Google Scholar]
- 73.Pereira C.S., Guedes J.P., Goncalves M., Loureiro L., Castro L., Geros H., Rodrigues L.R., Corte-Real M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget. 2016;7(38):62144–62158. doi: 10.18632/oncotarget.11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stransky L., Cotter K., Forgac M. The function of V-ATPases in cancer. Physiol. Rev. 2016;96(3):1071–1091. doi: 10.1152/physrev.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guedes J.P., Pereira C.S., Rodrigues L.R., Côrte-Real M. Bovine milk lactoferrin selectively kills highly metastatic prostate cancer PC-3 and osteosarcoma MG-63 cells in vitro. Frontiers in oncology. 2018;8:200. doi: 10.3389/fonc.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibbons J.A., Kanwar J.R., Kanwar R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Canc. 2015;15:425. doi: 10.1186/s12885-015-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shankaranarayanan J.S., Kanwar J.R., Al-Juhaishi A.J., Kanwar R.K. Doxorubicin conjugated to immunomodulatory anticancer lactoferrin displays improved cytotoxicity overcoming prostate cancer chemo resistance and inhibits tumour development in TRAMP mice. Sci. Rep. 2016;6:32062. doi: 10.1038/srep32062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Z., Yang J., Min Q., Ling C., Maiti D., Xu J., Qin L., Yang K. Holo-lactoferrin modified liposome for relieving tumor hypoxia and enhancing radiochemotherapy of cancer. Small. 2019;15(6) doi: 10.1002/smll.201803703. [DOI] [PubMed] [Google Scholar]
- 79.Takayama Y., Aoki R. Roles of lactoferrin on skin wound healing. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2012;90(3):497–503. doi: 10.1139/o11-054. [DOI] [PubMed] [Google Scholar]
- 80.Tang L., Wu J.J., Ma Q., Cui T., Andreopoulos F.M., Gil J., Valdes J., Davis S.C., Li J. Human lactoferrin stimulates skin keratinocyte function and wound re-epithelialization. Br. J. Dermatol. 2010;163(1):38–47. doi: 10.1111/j.1365-2133.2010.09748.x. [DOI] [PubMed] [Google Scholar]
- 81.Engelmayer J., Blezinger P., Varadhachary A. Talactoferrin stimulates wound healing with modulation of inflammation. J. Surg. Res. 2008;149(2):278–286. doi: 10.1016/j.jss.2007.12.754. [DOI] [PubMed] [Google Scholar]
- 82.Onishi H., Machida Y., Koyama K. Preparation and in vitro characteristics of lactoferrin-loaded chitosan microparticles. Drug Dev. Ind. Pharm. 2007;33(6):641–647. doi: 10.1080/03639040601085334. [DOI] [PubMed] [Google Scholar]
- 83.Koyama K., Onishi H., Sakata O., Machida Y. Preparation and in vitro evaluation of chitosan-coated alginate/calcium complex microparticles loaded with fluorescein-labeled lactoferrin. Yakugaku Zasshi. 2009;129(12):1507–1514. doi: 10.1248/yakushi.129.1507. [DOI] [PubMed] [Google Scholar]
- 84.Onishi H., Koyama K., Sakata O., Machida Y. Preparation of chitosan/alginate/calcium complex microparticles loaded with lactoferrin and their efficacy on carrageenan-induced edema in rats. Drug Dev. Ind. Pharm. 2010;36(8):879–884. doi: 10.3109/03639040903567109. [DOI] [PubMed] [Google Scholar]
- 85.Kanwar J.R., Mahidhara G., Kanwar R.K. Novel alginate-enclosed chitosan-calcium phosphate-loaded iron-saturated bovine lactoferrin nanocarriers for oral delivery in colon cancer therapy. Nanomedicine. 2012;7(10):1521–1550. doi: 10.2217/nnm.12.29. [DOI] [PubMed] [Google Scholar]
- 86.Samarasinghe R.M., Kanwar R.K., Kanwar J.R. The effect of oral administration of iron saturated-bovine lactoferrin encapsulated chitosan-nanocarriers on osteoarthritis. Biomaterials. 2014;35(26):7522–7534. doi: 10.1016/j.biomaterials.2014.04.109. [DOI] [PubMed] [Google Scholar]
- 87.Ishikado A., Imanaka H., Takeuchi T., Harada E., Makino T. Liposomalization of lactoferrin enhanced it's anti-inflammatory effects via oral administration. Biol. Pharm. Bull. 2005;28(9):1717–1721. doi: 10.1248/bpb.28.1717. [DOI] [PubMed] [Google Scholar]
- 88.Roseanu A., Florian P.E., Moisei M., Sima L.E., Evans R.W., Trif M. Liposomalization of lactoferrin enhanced its anti-tumoral effects on melanoma cells. Biometals. 2010;23(3):485–492. doi: 10.1007/s10534-010-9312-6. [DOI] [PubMed] [Google Scholar]
- 89.Ma J., Guan R., Shen H., Lu F., Xiao C., Liu M., Kang T. Comparison of anticancer activity between lactoferrin nanoliposome and lactoferrin in Caco-2 cells in vitro. Food Chem. Toxicol. 2013;59:72–77. doi: 10.1016/j.fct.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 90.Kato K., Tamaki N., Saito Y., Fujimoto T., Sato A. Amino group PEGylation of bovine lactoferrin by linear polyethylene glycol-p-nitrophenyl active esters. Biol. Pharmaceut. Bull. 2010;33(7):1253–1255. doi: 10.1248/bpb.33.1253. [DOI] [PubMed] [Google Scholar]
- 91.Nojima Y., Suzuki Y., Iguchi K., Shiga T., Iwata A., Fujimoto T., Yoshida K., Shimizu H., Takeuchi T., Sato A. Development of poly(ethylene glycol) conjugated lactoferrin for oral administration. Bioconjugate Chem. 2008;19(11):2253–2259. doi: 10.1021/bc800258v. [DOI] [PubMed] [Google Scholar]
- 92.Nojima Y., Suzuki Y., Yoshida K., Abe F., Shiga T., Takeuchi T., Sugiyama A., Shimizu H., Sato A. Lactoferrin conjugated with 40-kDa branched poly(ethylene glycol) has an improved circulating half-life. Pharm. Res. (N. Y.) 2009;26(9):2125–2132. doi: 10.1007/s11095-009-9925-z. [DOI] [PubMed] [Google Scholar]
- 93.Sugiyama A., Sato A., Takeuchi T. PEGylated lactoferrin enhanced its hepatoprotective effects on acute liver injury induced by carbon tetrachloride in rats. Food Chem. Toxicol. 2009;47(7):1453–1458. doi: 10.1016/j.fct.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi Y., Takeda C., Seto I., Kawano G., Machida Y. Formulation and evaluation of lactoferrin bioadhesive tablets. Int. J. Pharm. 2007;343(1–2):220–227. doi: 10.1016/j.ijpharm.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 95.Anand N., Sehgal R., Kanwar R.K., Dubey M.L., Vasishta R.K., Kanwar J.R. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite Toxoplasma gondii. Int. J. Nanomed. 2015;10:6355–6369. doi: 10.2147/IJN.S85286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumari S., Ahsan S.M., Kumar J.M., Kondapi A.K., Rao N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433) Sci. Rep. 2017;7(1):6602. doi: 10.1038/s41598-017-06888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumari S., Kondapi A.K. Lactoferrin nanoparticle mediated targeted delivery of 5-fluorouracil for enhanced therapeutic efficacy. Int. J. Biol. Macromol. 2017;95:232–237. doi: 10.1016/j.ijbiomac.2016.10.110. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed F., Kumari S., Kondapi A.K. Evaluation of antiproliferative activity, safety and biodistribution of oxaliplatin and 5-fluorouracil loaded lactoferrin nanoparticles for the management of colon adenocarcinoma: an in vitro and an in vivo study. Pharm. Res. (N. Y.) 2018;35(9):178. doi: 10.1007/s11095-018-2457-7. [DOI] [PubMed] [Google Scholar]
- 99.Pandey A.P., More M.P., Karande K.P., Chitalkar R.V., Patil P.O., Deshmukh P.K. Optimization of desolvation process for fabrication of lactoferrin nanoparticles using quality by design approach. Artificial cells, nanomedicine, and biotechnology. 2017;45(6):1–14. doi: 10.1080/21691401.2016.1202259. [DOI] [PubMed] [Google Scholar]
- 100.Alting A.C., Hamer R.J., de Kruif C.G., Visschers R.W. Cold-set globular protein gels: interactions, structure and rheology as a function of protein concentration. J. Agric. Food Chem. 2003;51(10):3150–3156. doi: 10.1021/jf0209342. [DOI] [PubMed] [Google Scholar]
- 101.Mo X., Zheng Z., He Y., Zhong H., Kang X., Shi M., Liu T., Jiao Z., Huang Y. Antiglioma via regulating oxidative stress and remodeling tumor-associated macrophage using lactoferrin-mediated biomimetic codelivery of simvastatin/fenretinide. J. Contr. Release. 2018;287:12–23. doi: 10.1016/j.jconrel.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Wang H., Tang Y., Fang Y., Zhang M., Wang H., He Z., Wang B., Xu Q., Huang Y. Reprogramming tumor immune microenvironment (TIME) and metabolism via biomimetic targeting codelivery of shikonin/JQ1. Nano Lett. 2019;19(5):2935–2944. doi: 10.1021/acs.nanolett.9b00021. [DOI] [PubMed] [Google Scholar]
- 103.Li J., Zhao C., Wei L., Li X., Liu F., Zhang M., Liu X., Wang Y. Preservation of cichoric acid antioxidant properties loaded in heat treated lactoferrin nanoparticles. Molecules. 2018;23(10) doi: 10.3390/molecules23102678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Z.H., Wang X.P., Ayman W.Y., Munyendo W.L., Lv H.X., Zhou J.P. Studies on lactoferrin nanoparticles of gambogic acid for oral delivery. Drug Deliv. 2013;20(2):86–93. doi: 10.3109/10717544.2013.766781. [DOI] [PubMed] [Google Scholar]
- 105.Xia X., Liu H., Lv H., Zhang J., Zhou J., Zhao Z. Preparation, characterization, and in vitro/vivo studies of oleanolic acid-loaded lactoferrin nanoparticles. Drug Des. Dev. Ther. 2017;11:1417–1427. doi: 10.2147/DDDT.S133997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bengoechea C., Jones O.G., Guerrero A., McClements D.J. Formation and characterization of lactoferrin/pectin electrostatic complexes: impact of composition, pH and thermal treatment. Food Hydrocolloids. 2011;25(5):1227–1232. [Google Scholar]
- 107.Il'ina A.V., Kurek D.V., Zubareva A.A., M M., Mestechkina N.M., Varlamov V.P. vol. 102. 2016. pp. 33–38. (Preparation and Characterization of Biopolymer Nanoparticles Based on Lactoferrin-Polysaccharide Complexes, Reactive and Functional Polymers). [Google Scholar]
- 108.Peinado I., Lesmes U., Andres A., McClements J.D. Fabrication and morphological characterization of biopolymer particles formed by electrostatic complexation of heat treated lactoferrin and anionic polysaccharides. Langmuir. 2010;26(12):9827–9834. doi: 10.1021/la1001013. [DOI] [PubMed] [Google Scholar]
- 109.Yan J.K., Qiu W.Y., Wang Y.Y., Wu J.Y. Biocompatible polyelectrolyte complex nanoparticles from lactoferrin and pectin as potential vehicles for antioxidative curcumin. J. Agric. Food Chem. 2017;65(28):5720–5730. doi: 10.1021/acs.jafc.7b01848. [DOI] [PubMed] [Google Scholar]
- 110.Abd Elwakil M.M., Mabrouk M.T., Helmy M.W., Abdelfattah E.A., Khiste S.K., Elkhodairy K.A., Elzoghby A.O. Inhalable lactoferrin-chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine. 2018;13(16):2015–2035. doi: 10.2217/nnm-2018-0039. [DOI] [PubMed] [Google Scholar]
- 111.Liu R., Yan X., Liu Z., McClements D.J., Liu F., Liu X. Fabrication and characterization of functional protein-polysaccharide-polyphenol complexes assembled from lactoferrin, hyaluronic acid and (-)-epigallocatechin gallate. Food Funct. 2019;10(2):1098–1108. doi: 10.1039/c8fo02146e. [DOI] [PubMed] [Google Scholar]
- 112.Chaharband F., Kamalinia G., Atyabi F., Mortazavi S.A., Mirzaie Z.H., Dinarvand R. Formulation and in vitro evaluation of curcumin-lactoferrin conjugated nanostructures for cancerous cells, Artificial cells. nanomedicine, and biotechnology. 2018;46(3):626–636. doi: 10.1080/21691401.2017.1337020. [DOI] [PubMed] [Google Scholar]
- 113.Kamalinia G., Khodagholi F., Atyabi F., Amini M., Shaerzadeh F., Sharifzadeh M., Dinarvand R. Enhanced brain delivery of deferasirox-lactoferrin conjugates for iron chelation therapy in neurodegenerative disorders: in vitro and in vivo studies. Mol. Pharm. 2013;10(12):4418–4431. doi: 10.1021/mp4002014. [DOI] [PubMed] [Google Scholar]
- 114.Ali O.M., Bekhit A.A., Khattab S.N., Helmy M.W., Abdel-Ghany Y.S., Teleb M., Elzoghby A.O. Synthesis of lactoferrin mesoporous silica nanoparticles for pemetrexed/ellagic acid synergistic breast cancer therapy. Colloids Surf. B Biointerfaces. 2020;188:110824. doi: 10.1016/j.colsurfb.2020.110824. [DOI] [PubMed] [Google Scholar]
- 115.Liu F., Wang D., Sun C., McClements D.J., Gao Y. Utilization of interfacial engineering to improve physicochemical stability of beta-carotene emulsions: multilayer coatings formed using protein and protein-polyphenol conjugates. Food Chem. 2016;205:129–139. doi: 10.1016/j.foodchem.2016.02.155. [DOI] [PubMed] [Google Scholar]
- 116.Liu F., Sun C., Yang W., Yuan F., Gao Y. Structural characterization and functional evaluation of lactoferrin–polyphenol conjugates formed by free-radical graft copolymerization. RSC Adv. 2015;5(20):15641–15651. [Google Scholar]
- 117.Liu F., Sun C., Wang D., Yuan F., Gao Y. Glycosylation improves the functional characteristics of chlorogenic acid–lactoferrin conjugate. RSC Adv. 2015;5(95):78215–78228. [Google Scholar]
- 118.Sabra S.A., Elzoghby A.O., Sheweita S.A., Haroun M., Helmy M.W., Eldemellawy M.A., Xia Y., Goodale D., Allan A.L., Rohani S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur. J. Pharm. Biopharm. : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2018;128:156–169. doi: 10.1016/j.ejpb.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 119.Sabra S.A., Sheweita S.A., Haroun M., Ragab D., Eldemellawy M.A., Xia Y., Goodale D., Allan A.L., Elzoghby A.O., Rohani S. Magnetically guided self-assembled protein micelles for enhanced delivery of dasatinib to human triple-negative breast cancer cells. J. Pharmaceut. Sci. 2019;108(5):1713–1725. doi: 10.1016/j.xphs.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 120.Alqahtani M.S., Islam M.S., Podaralla S., Kaushik R.S., Reineke J., Woyengo T., Perumal O. Food protein based core-shell nanocarriers for oral drug delivery: effect of shell composition on in vitro and in vivo functional performance of zein nanocarriers. Mol. Pharm. 2017;14(3):757–769. doi: 10.1021/acs.molpharmaceut.6b01017. [DOI] [PubMed] [Google Scholar]
- 121.Abdelmoneem M.A., Elnaggar M.A., Hammady R.S., Kamel S.M., Helmy M.W., Abdulkader M.A., Zaky A., Fang J.Y., Elkhodairy K.A., Elzoghby A.O. Dual-targeted lactoferrin shell-oily core nanocapsules for synergistic targeted/herbal therapy of hepatocellular carcinoma. ACS Appl. Mater. Interfaces. 2019;11(30):26731–26744. doi: 10.1021/acsami.9b10164. [DOI] [PubMed] [Google Scholar]
- 122.Golla K., Cherukuvada B., Ahmed F., Kondapi A.K. Efficacy, safety and anticancer activity of protein nanoparticle-based delivery of doxorubicin through intravenous administration in rats. PloS One. 2012;7(12) doi: 10.1371/journal.pone.0051960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar P., Lakshmi Y.S., Kondapi A.K. An oral formulation of efavirenz-loaded lactoferrin nanoparticles with improved biodistribution and pharmacokinetic profile. HIV Med. 2017;18(7):452–462. doi: 10.1111/hiv.12475. [DOI] [PubMed] [Google Scholar]
- 124.Ahmed F., Ali M.J., Kondapi A.K. Carboplatin loaded protein nanoparticles exhibit improve anti-proliferative activity in retinoblastoma cells. Int. J. Biol. Macromol. 2014;70:572–582. doi: 10.1016/j.ijbiomac.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 125.Shen Z., Liu T., Yang Z., Zhou Z., Tang W., Fan W., Liu Y., Mu J., Li L., Bregadze V.I., Mandal S.K., Druzina A.A., Wei Z., Qiu X., Wu A., Chen X. Small-sized gadolinium oxide based nanoparticles for high-efficiency theranostics of orthotopic glioblastoma. Biomaterials. 2020;235:119783. doi: 10.1016/j.biomaterials.2020.119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shen Z., Liu T., Li Y., Lau J., Yang Z., Fan W., Zhou Z., Shi C., Ke C., Bregadze V.I., Mandal S.K., Liu Y., Li Z., Xue T., Zhu G., Munasinghe J., Niu G., Wu A., Chen X. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano. 2018;12(11):11355–11365. doi: 10.1021/acsnano.8b06201. [DOI] [PubMed] [Google Scholar]
- 127.Sharifi M., Hasan A., Nanakali N.M.Q., Salihi A., Qadir F.A., Muhammad H.A., Shekha M.S., Aziz F.M., Amen K.M., Najafi F., Yousefi-Manesh H., Falahati M. Combined chemo-magnetic field-photothermal breast cancer therapy based on porous magnetite nanospheres. Sci. Rep. 2020;10(1):5925. doi: 10.1038/s41598-020-62429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharifi M., Jafari S., Hasan A., Paray B.A., Gong G., Zheng Y., Falahati M.J.A.B.S. 2020. Engineering, Antimetastatic Activity of Lactoferrin-Coated Mesoporous Maghemite Nanoparticles in Breast Cancer Enabled by Combination Therapy. [DOI] [PubMed] [Google Scholar]
- 129.Song M.M., Xu H.L., Liang J.X., Xiang H.H., Liu R., Shen Y.X. Lactoferrin modified graphene oxide iron oxide nanocomposite for glioma-targeted drug delivery, Materials science & engineering. C, Materials for biological applications. 2017;77:904–911. doi: 10.1016/j.msec.2017.03.309. [DOI] [PubMed] [Google Scholar]
- 130.Su Y.L., Chen K.T., Sheu Y.C., Sung S.Y., Hsu R.S., Chiang C.S., Hu S.H. The penetrated delivery of drug and energy to tumors by lipo-graphene nanosponges for photolytic therapy. ACS Nano. 2016;10(10):9420–9433. doi: 10.1021/acsnano.6b04414. [DOI] [PubMed] [Google Scholar]
- 131.Pomastowski P., Sprynskyy M., Žuvela P., Rafińska K., Milanowski M., Liu J.J., Yi M., Buszewski B. Silver-lactoferrin nanocomplexes as a potent antimicrobial agent. J. Am. Chem. Soc. 2016;138(25):7899–7909. doi: 10.1021/jacs.6b02699. [DOI] [PubMed] [Google Scholar]
- 132.Abdalla S.S.I., Katas H., Chan J.Y., Ganasan P., Azmi F., Busra M.F.M. Antimicrobial activity of multifaceted lactoferrin or graphene oxide functionalized silver nanocomposites biosynthesized using mushroom waste and chitosan. 2020;10(9):4969–4983. doi: 10.1039/c9ra08680c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nayak P.S., Borah S.M., Gogoi H., Asthana S., Bhatnagar R., Jha A.N., Jha S. Lactoferrin adsorption onto silver nanoparticle interface: implications of corona on protein conformation, nanoparticle cytotoxicity and the formulation adjuvanticity. Chem. Eng. J. 2019;361:470–484. [Google Scholar]
- 134.AbdElhamid A.S., Zayed D.G., Helmy M.W., Ebrahim S.M., Bahey-El-Din M., Zein-El-Dein E.A., El-Gizawy S.A., Elzoghby A.O.J.N. Lactoferrin-tagged quantum dots-based theranostic nanocapsules for combined COX-2 inhibitor/herbal therapy of breast cancer. 2018;13(20):2637–2656. doi: 10.2217/nnm-2018-0196. [DOI] [PubMed] [Google Scholar]
- 135.Song Y., Du D., Li L., Xu J., Dutta P., Lin Y. Vitro study of receptor-mediated silica nanoparticles delivery across blood–brain barrier. ACS Appl. Mater. Interfaces. 2017;9(24):20410–20416. doi: 10.1021/acsami.7b03504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ali O.M., Bekhit A.A., Khattab S.N., Helmy M.W., Abdel-Ghany Y.S., Teleb M., Elzoghby A.O.J.C., Biointerfaces S.B. vol. 188. 2020. p. 110824. (Synthesis of Lactoferrin Mesoporous Silica Nanoparticles for Pemetrexed/ellagic Acid Synergistic Breast Cancer Therapy). [DOI] [PubMed] [Google Scholar]
- 137.Xavier P.L., Chaudhari K., Verma P.K., Pal S.K., Pradeep T. Luminescent quantum clusters of gold in transferrin family protein, lactoferrin exhibiting FRET. Nanoscale. 2010;2(12):2769–2776. doi: 10.1039/c0nr00377h. [DOI] [PubMed] [Google Scholar]
- 138.Chaudhari K., Xavier P.L., Pradeep T. Understanding the evolution of luminescent gold quantum clusters in protein templates. ACS Nano. 2011;5(11):8816–8827. doi: 10.1021/nn202901a. [DOI] [PubMed] [Google Scholar]
- 139.Singh I., Swami R., Pooja D., Jeengar M.K., Khan W., Sistla R. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. J. Drug Target. 2016;24(3):212–223. doi: 10.3109/1061186X.2015.1068320. [DOI] [PubMed] [Google Scholar]
- 140.Huang F.Y., Chen W.J., Lee W.Y., Lo S.T., Lee T.W., Lo J.M. In vitro and in vivo evaluation of lactoferrin-conjugated liposomes as a novel carrier to improve the brain delivery. Int. J. Mol. Sci. 2013;14(2):2862–2874. doi: 10.3390/ijms14022862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lim L.Y., Koh P.Y., Somani S., Al Robaian M., Karim R., Yean Y.L., Mitchell J., Tate R.J., Edrada-Ebel R., Blatchford D.R., Mullin M., Dufes C. Tumor regression following intravenous administration of lactoferrin- and lactoferricin-bearing dendriplexes. Nanomedicine. 2015;11(6):1445–1454. doi: 10.1016/j.nano.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kabanov A.V., Batrakova E.V. New technologies for drug delivery across the blood brain barrier. Curr. Pharmaceut. Des. 2004;10(12):1355–1363. doi: 10.2174/1381612043384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suzuki Y.A., Lopez V., Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell. Mol. Life Sci. 2005;62(22):2560–2575. doi: 10.1007/s00018-005-5371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang R., Ke W., Liu Y., Jiang C., Pei Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials. 2008;29(2):238–246. doi: 10.1016/j.biomaterials.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 145.Fillebeen C., Descamps L., Dehouck M.P., Fenart L., Benaissa M., Spik G., Cecchelli R., Pierce A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999;274(11):7011–7017. doi: 10.1074/jbc.274.11.7011. [DOI] [PubMed] [Google Scholar]
- 146.Elzoghby A.O., Abd-Elwakil M.M., Abd-Elsalam K., Elsayed M.T., Hashem Y., Mohamed O. Natural polymeric nanoparticles for brain-targeting: implications on drug and gene delivery. Curr. Pharmaceut. Des. 2016;22(22):3305–3323. doi: 10.2174/1381612822666160204120829. [DOI] [PubMed] [Google Scholar]
- 147.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J., MacMillan P., Zhang Y., Rajesh N.U., Hoang T., Wu J.L.Y., Wilhelm S., Zilman A., Gadde S., Sulaiman A., Ouyang B., Lin Z., Wang L., Egeblad M., Chan W.C.W. The entry of nanoparticles into solid tumours. Nat. Mater. 2020;19(5):566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 148.Pang Z., Feng L., Hua R., Chen J., Gao H., Pan S., Jiang X., Zhang P. Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol. Pharm. 2010;7(6):1995–2005. doi: 10.1021/mp100277h. [DOI] [PubMed] [Google Scholar]
- 149.Chen H., Qin Y., Zhang Q., Jiang W., Tang L., Liu J., He Q. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur. J. Pharmaceut. Sci. 2011;44(1–2):164–173. doi: 10.1016/j.ejps.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 150.Miao D., Jiang M., Liu Z., Gu G., Hu Q., Kang T., Song Q., Yao L., Li W., Gao X., Sun M., Chen J. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol. Pharm. 2014;11(1):90–101. doi: 10.1021/mp400189j. [DOI] [PubMed] [Google Scholar]
- 151.Pandey A., Singh K., Patel S., Singh R., Patel K., Sawant K. Hyaluronic acid tethered pH-responsive alloy-drug nanoconjugates for multimodal therapy of glioblastoma: an intranasal route approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;98:419–436. doi: 10.1016/j.msec.2018.12.139. [DOI] [PubMed] [Google Scholar]
- 152.Bi C., Wang A., Chu Y., Liu S., Mu H., Liu W., Wu Z., Sun K., Li Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson's disease treatment. Int. J. Nanomed. 2016;11:6547–6559. doi: 10.2147/IJN.S120939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yan X., Xu L., Bi C., Duan D., Chu L., Yu X., Wu Z., Wang A., Sun K. Lactoferrin-modified rotigotine nanoparticles for enhanced nose-to-brain delivery: LESA-MS/MS-based drug biodistribution, pharmacodynamics, and neuroprotective effects. Int. J. Nanomed. 2018;13:273–281. doi: 10.2147/IJN.S151475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martorell P., Llopis S., Gonzalez N., Ramón D., Serrano G., Torrens A., Serrano J.M., Navarro M., Genovés S. A nutritional supplement containing lactoferrin stimulates the immune system, extends lifespan, and reduces amyloid β peptide toxicity in Caenorhabditis elegans. Food science & nutrition. 2017;5(2):255–265. doi: 10.1002/fsn3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang J., Bi M., Liu H., Song N., Xie J. The protective effect of lactoferrin on ventral mesencephalon neurons against MPP + is not connected with its iron binding ability. Sci. Rep. 2015;5:10729. doi: 10.1038/srep10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu H., Wu H., Zhu N., Xu Z., Wang Y., Qu Y., Wang J. Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson's disease in mice. J. Neurochem. 2020;152(3):397–415. doi: 10.1111/jnc.14857. [DOI] [PubMed] [Google Scholar]
- 157.Xu S.F., Zhang Y.H., Wang S., Pang Z.Q., Fan Y.G., Li J.Y., Wang Z.Y., Guo C. Lactoferrin ameliorates dopaminergic neurodegeneration and motor deficits in MPTP-treated mice. Redox biology. 2019;21:101090. doi: 10.1016/j.redox.2018.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang S., Wang A., Yan X., Chu L., Yang X., Song Y., Sun K., Yu X., Liu R., Wu Z., Xue P. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson's disease. Drug Deliv. 2019;26(1):700–707. doi: 10.1080/10717544.2019.1636420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bollimpelli V.S., Kumar P., Kumari S., Kondapi A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016;95:37–45. doi: 10.1016/j.neuint.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 160.Kordower J.H., Emborg M.E., Bloch J., Ma S.Y., Chu Y., Leventhal L., McBride J., Chen E.-Y., Palfi S., Roitberg B.Z. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290(5492):767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 161.Huang R., Ke W., Liu Y., Wu D., Feng L., Jiang C., Pei Y. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci. 2010;290(1–2):123–130. doi: 10.1016/j.jns.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 162.Lepanto M.S., Rosa L., Paesano R., Valenti P., Cutone A. Lactoferrin in aseptic and septic inflammation. Molecules. 2019;24(7) doi: 10.3390/molecules24071323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mohamed W.A., Salama R.M., Schaalan M.F. A pilot study on the effect of lactoferrin on Alzheimer's disease pathological sequelae: impact of the p-Akt/PTEN pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;111:714–723. doi: 10.1016/j.biopha.2018.12.118. [DOI] [PubMed] [Google Scholar]
- 164.Guo C., Yang Z.H., Zhang S., Chai R., Xue H., Zhang Y.H., Li J.Y., Wang Z.Y. Intranasal lactoferrin enhances α-secretase-dependent amyloid precursor protein processing via the ERK1/2-CREB and HIF-1α pathways in an Alzheimer's disease mouse model. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(13):2504–2515. doi: 10.1038/npp.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.González-Sánchez M., Bartolome F., Antequera D., Puertas-Martín V., González P., Gómez-Grande A., Llamas-Velasco S., Herrero-San Martín A., Pérez-Martínez D., Villarejo-Galende A., Atienza M., Palomar-Bonet M., Cantero J.L., Perry G., Orive G., Ibañez B., Bueno H., Fuster V., Carro E. Decreased salivary lactoferrin levels are specific to Alzheimer's disease. EBioMedicine. 2020;57:102834. doi: 10.1016/j.ebiom.2020.102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gothwal A., Singh H., Jain S.K., Dutta A., Borah A., Gupta U. Behavioral and biochemical implications of dendrimeric rivastigmine in memory-deficit and Alzheimer's induced rodents. ACS Chem. Neurosci. 2019;10(8):3789–3795. doi: 10.1021/acschemneuro.9b00286. [DOI] [PubMed] [Google Scholar]
- 167.Kuo Y.C., Wang C.T. Protection of SK-N-MC cells against beta-amyloid peptide-induced degeneration using neuron growth factor-loaded liposomes with surface lactoferrin. Biomaterials. 2014;35(22):5954–5964. doi: 10.1016/j.biomaterials.2014.03.082. [DOI] [PubMed] [Google Scholar]
- 168.Yu Y., Pang Z., Lu W., Yin Q., Gao H., Jiang X. Self-assembled polymersomes conjugated with lactoferrin as novel drug carrier for brain delivery. Pharm. Res. (N. Y.) 2012;29(1):83–96. doi: 10.1007/s11095-011-0513-7. [DOI] [PubMed] [Google Scholar]
- 169.Yu Y., Jiang X., Gong S., Feng L., Zhong Y., Pang Z. The proton permeability of self-assembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood-brain barrier after modification with lactoferrin. Nanoscale. 2014;6(6):3250–3258. doi: 10.1039/c3nr05196j. [DOI] [PubMed] [Google Scholar]
- 170.Fang J.H., Chiu T.L., Huang W.C., Lai Y.H., Hu S.H., Chen Y.Y., Chen S.Y. Dual-targeting lactoferrin-conjugated polymerized magnetic polydiacetylene-assembled nanocarriers with self-responsive fluorescence/magnetic resonance imaging for in vivo brain tumor therapy. Adv Healthc Mater. 2016;5(6):688–695. doi: 10.1002/adhm.201500750. [DOI] [PubMed] [Google Scholar]
- 171.Kuo Y.C., Chen Y.C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015;479(1):138–149. doi: 10.1016/j.ijpharm.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 172.Kuo Y.C., Cheng S.J. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int. J. Pharm. 2016;499(1–2):10–19. doi: 10.1016/j.ijpharm.2015.12.054. [DOI] [PubMed] [Google Scholar]
- 173.Su Z., Xing L., Chen Y., Xu Y., Yang F., Zhang C., Ping Q., Xiao Y. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol. Pharm. 2014;11(6):1823–1834. doi: 10.1021/mp500238m. [DOI] [PubMed] [Google Scholar]
- 174.Jiang L., Zhou Q., Mu K., Xie H., Zhu Y., Zhu W., Zhao Y., Xu H., Yang X. pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials. 2013;34(30):7418–7428. doi: 10.1016/j.biomaterials.2013.05.078. [DOI] [PubMed] [Google Scholar]
- 175.De Luca M.A., Lai F., Corrias F., Caboni P., Bimpisidis Z., Maccioni E., Fadda A.M., Di Chiara G. Lactoferrin- and antitransferrin-modified liposomes for brain targeting of the NK3 receptor agonist senktide: preparation and in vivo evaluation. Int. J. Pharm. 2015;479(1):129–137. doi: 10.1016/j.ijpharm.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 176.Huang R., Han L., Li J., Ren F., Ke W., Jiang C., Pei Y. Neuroprotection in a 6-hydroxydopamine-lesioned Parkinson model using lactoferrin-modified nanoparticles. J. Gene Med. 2009;11(9):754–763. doi: 10.1002/jgm.1361. [DOI] [PubMed] [Google Scholar]
- 177.Hu K., Shi Y., Jiang W., Han J., Huang S., Jiang X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: preparation, characterization and efficacy in Parkinson's disease. Int. J. Pharm. 2011;415(1–2):273–283. doi: 10.1016/j.ijpharm.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 178.Liu Z., Jiang M., Kang T., Miao D., Gu G., Song Q., Yao L., Hu Q., Tu Y., Pang Z., Chen H., Jiang X., Gao X., Chen J. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials. 2013;34(15):3870–3881. doi: 10.1016/j.biomaterials.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 179.Chen H., Tang L., Qin Y., Yin Y., Tang J., Tang W., Sun X., Zhang Z., Liu J., He Q. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur. J. Pharmaceut. Sci. 2010;40(2):94–102. doi: 10.1016/j.ejps.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 180.Ye Q., Zheng Y., Fan S., Qin Z., Li N., Tang A., Ai F., Zhang X., Bian Y., Dang W., Huang J., Zhou M., Zhou Y., Xiong W., Yan Q., Ma J., Li G. Lactoferrin deficiency promotes colitis-associated colorectal dysplasia in mice. PloS One. 2014;9(7) doi: 10.1371/journal.pone.0103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iigo M., Alexander D.B., Xu J., Futakuchi M., Suzui M., Kozu T., Akasu T., Saito D., Kakizoe T., Yamauchi K., Abe F., Takase M., Sekine K., Tsuda H. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2014;27(5):1017–1029. doi: 10.1007/s10534-014-9747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kozu T., Iinuma G., Ohashi Y., Saito Y., Akasu T., Saito D., Alexander D.B., Iigo M., Kakizoe T., Tsuda H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Canc. Prev. Res. 2009;2(11):975–983. doi: 10.1158/1940-6207.CAPR-08-0208. Philadelphia, Pa. [DOI] [PubMed] [Google Scholar]
- 183.Burrow H., Kanwar R.K., Mahidhara G., Kanwar J.R. Effect of selenium-saturated bovine lactoferrin (Se-bLF) on antioxidant enzyme activities in human gut epithelial cells under oxidative stress. Anti Canc. Agents Med. Chem. 2011;11(8):762–771. doi: 10.2174/187152011797378616. [DOI] [PubMed] [Google Scholar]
- 184.Sugihara Y., Zuo X., Takata T., Jin S., Miyauti M., Isikado A., Imanaka H., Tatsuka M., Qi G., Shimamoto F. Inhibition of DMH-DSS-induced colorectal cancer by liposomal bovine lactoferrin in rats. Oncology letters. 2017;14(5):5688–5694. doi: 10.3892/ol.2017.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kamalapuram S.K., Kanwar R.K., Roy K., Chaudhary R., Sehgal R., Kanwar J.R. Theranostic multimodular potential of zinc-doped ferrite-saturated metal-binding protein-loaded novel nanocapsules in cancers. Int. J. Nanomed. 2016;11:1349–1366. doi: 10.2147/IJN.S95253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roy K., Kanwar R.K., Kanwar J.R. LNA aptamer based multi-modal, Fe3O4-saturated lactoferrin (Fe3O4-bLf) nanocarriers for triple positive (EpCAM, CD133, CD44) colon tumor targeting and NIR, MRI and CT imaging. Biomaterials. 2015;71:84–99. doi: 10.1016/j.biomaterials.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 187.Wei M., Guo X., Tu L., Zou Q., Li Q., Tang C., Chen B., Xu Y., Wu C. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 2015;10:5123–5137. doi: 10.2147/IJN.S87011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abdelmoneem M.A., Mahmoud M., Zaky A., Helmy M.W., Sallam M., Fang J.Y., Elkhodairy K.A., Elzoghby A.O. Decorating protein nanospheres with lactoferrin enhances oral COX-2 inhibitor/herbal therapy of hepatocellular carcinoma. Nanomedicine. 2018;13(19):2377–2395. doi: 10.2217/nnm-2018-0134. [DOI] [PubMed] [Google Scholar]
- 189.Abdelmoneem M.A., Mahmoud M., Zaky A., Helmy M.W., Sallam M., Fang J.Y., Elkhodairy K.A., Elzoghby A.O. Dual-targeted casein micelles as green nanomedicine for synergistic phytotherapy of hepatocellular carcinoma. J. Contr. Release. 2018;287:78–93. doi: 10.1016/j.jconrel.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 190.Sharifi M., Hasan A., Nanakali N.M.Q., Salihi A., Qadir F.A., Muhammad H.A., Shekha M.S., Aziz F.M., Amen K.M., Najafi F., Yousefi-Manesh H., Falahati M. Combined chemo-magnetic field-photothermal breast cancer therapy based on porous magnetite nanospheres. Sci. Rep. 2020;10(1):5925. doi: 10.1038/s41598-020-62429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.El-Lakany S.A., Elgindy N.A., Helmy M.W., Abu-Serie M.M., Elzoghby A.O. Lactoferrin-decorated vs PEGylated zein nanospheres for combined aromatase inhibitor and herbal therapy of breast cancer. Expet Opin. Drug Deliv. 2018;15(9):835–850. doi: 10.1080/17425247.2018.1505858. [DOI] [PubMed] [Google Scholar]
- 192.Adimoolam M.G., A V., Nalam M.R., Sunkara M.V. Chlorin e6 loaded lactoferrin nanoparticles for enhanced photodynamic therapy. J. Mater. Chem. B. 2017;5(46):9189–9196. doi: 10.1039/c7tb02599h. [DOI] [PubMed] [Google Scholar]
- 193.Pandey V., Gajbhiye K.R., Soni V. Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv. 2015;22(2):199–205. doi: 10.3109/10717544.2013.877100. [DOI] [PubMed] [Google Scholar]
- 194.Abdelaziz H.M., Gaber M., Abd-Elwakil M.M., Mabrouk M.T., Elgohary M.M., Kamel N.M., Kabary D.M., Freag M.S., Samaha M.W., Mortada S.M., Elkhodairy K.A., Fang J.Y., Elzoghby A.O. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Contr. Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 195.Kabary D.M., Helmy M.W., Abdelfattah E.A., Fang J.Y., Elkhodairy K.A., Elzoghby A.O. Inhalable multi-compartmental phospholipid enveloped lipid core nanocomposites for localized mTOR inhibitor/herbal combined therapy of lung carcinoma. Eur. J. Pharm. Biopharm. 2018;130:152–164. doi: 10.1016/j.ejpb.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 196.Abdelaziz H.M., Elzoghby A.O., Helmy M.W., Abdelfattah E.-Z.A., Fang J.-Y., Samaha M.W., Freag M.S. Inhalable lactoferrin/chondroitin-functionalized monoolein nanocomposites for localized lung cancer targeting. ACS Biomater. Sci. Eng. 2020;6(2):1030–1042. doi: 10.1021/acsbiomaterials.9b01639. [DOI] [PubMed] [Google Scholar]
- 197.Zadvornyi T.V., Lukianova N.Y., Borikun T.V., Chekhun V.F. Effects of exogenous lactoferrin on phenotypic profile and invasiveness of human prostate cancer cells (DU145 and LNCaP) in vitro. Exp. Oncol. 2018;40(3):184–189. [PubMed] [Google Scholar]
- 198.Altwaijry N., Somani S., Parkinson J.A., Tate R.J., Keating P., Warzecha M., Mackenzie G.R., Leung H.Y., Dufes C. Regression of prostate tumors after intravenous administration of lactoferrin-bearing polypropylenimine dendriplexes encoding TNF-alpha, TRAIL, and interleukin-12. Drug Deliv. 2018;25(1):679–689. doi: 10.1080/10717544.2018.1440666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.AbdElhamid A.S., Zayed D.G., Helmy M.W., Ebrahim S.M., Bahey-El-Din M., Zein-El-Dein E.A., El-Gizawy S.A., Elzoghby A.O. Lactoferrin-tagged quantum dots-based theranostic nanocapsules for combined COX-2 inhibitor/herbal therapy of breast cancer. Nanomedicine. 2018;13(20):2637–2656. doi: 10.2217/nnm-2018-0196. [DOI] [PubMed] [Google Scholar]
- 200.Garré C., Bianchi-Scarrá G., Sirito M., Musso M., Ravazzolo R. Lactoferrin binding sites and nuclear localization in K562(S) cells. J. Cell. Physiol. 1992;153(3):477–482. doi: 10.1002/jcp.1041530306. [DOI] [PubMed] [Google Scholar]
- 201.Penco S., Scarfi S., Giovine M., Damonte G., Millo E., Villaggio B., Passalacqua M., Pozzolini M., Garrè C., Benatti U. Identification of an import signal for, and the nuclear localization of, human lactoferrin. Biotechnol. Appl. Biochem. 2001;34(3):151–159. doi: 10.1042/ba20010038. [DOI] [PubMed] [Google Scholar]
- 202.Håversen L., Ohlsson B.G., Hahn-Zoric M., Hanson L.A., Mattsby-Baltzer I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell. Immunol. 2002;220(2):83–95. doi: 10.1016/s0008-8749(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 203.Rybarczyk J., Khalenkow D., Kieckens E., Skirtach A.G., Cox E., Vanrompay D. Lactoferrin translocates to the nucleus of bovine rectal epithelial cells in the presence of Escherichia coli O157:H7. Vet. Res. 2019;50(1):75. doi: 10.1186/s13567-019-0694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu B.R., Huang Y.W., Aronstam R.S., Lee H.J. Identification of a short cell-penetrating peptide from bovine lactoferricin for intracellular delivery of DNA in human A549 cells. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0150439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ashida K., Sasaki H., Suzuki Y.A., Lönnerdal B. Cellular internalization of lactoferrin in intestinal epithelial cells. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2004;17(3):311–315. doi: 10.1023/b:biom.0000027710.13543.3f. [DOI] [PubMed] [Google Scholar]
- 206.Puddu P., Valenti P., Gessani S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie. 2009;91(1):11–18. doi: 10.1016/j.biochi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 207.Legrand D., Elass E., Carpentier M., Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell. Mol. Life Sci. : CMLS. 2005;62(22):2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Debbabi H., Dubarry M., Rautureau M., Tomé D. Bovine lactoferrin induces both mucosal and systemic immune response in mice. J. Dairy Res. 1998;65(2):283–293. doi: 10.1017/s0022029997002732. [DOI] [PubMed] [Google Scholar]
- 209.Comstock S.S., Reznikov E.A., Contractor N., Donovan S.M. Dietary bovine lactoferrin alters mucosal and systemic immune cell responses in neonatal piglets. J. Nutr. 2014;144(4):525–532. doi: 10.3945/jn.113.190264. [DOI] [PubMed] [Google Scholar]
- 210.Mulder A.M., Connellan P.A., Oliver C.J., Morris C.A., Stevenson L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. (N.Y.) 2008;28(9):583–589. doi: 10.1016/j.nutres.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 211.Kurose I., Yamada T., Wolf R., Granger D.N. P-selectin-dependent leukocyte recruitment and intestinal mucosal injury induced by lactoferrin. J. Leukoc. Biol. 1994;55(6):771–777. doi: 10.1002/jlb.55.6.771. [DOI] [PubMed] [Google Scholar]
- 212.Szuster-Ciesielska A., Kaminska T., Kandefer-Szerszen M. Phagocytosis-enhancing effect of lactoferrin on bovine peripheral blood monocytes in vitro and in vivo. Arch. Vet. Pol. 1995;35(1–2):63–71. [PubMed] [Google Scholar]
- 213.Karami Z., Saghatchi Zanjani M.R., Rezaee S., Rostamizadeh K., Hamidi M. Neuropharmacokinetic evaluation of lactoferrin-treated indinavir-loaded nanoemulsions: remarkable brain delivery enhancement. Drug Dev. Ind. Pharm. 2019;45(5):736–744. doi: 10.1080/03639045.2019.1569039. [DOI] [PubMed] [Google Scholar]
- 214.Kumar P., Lakshmi Y.S., C B., Golla K., Kondapi A.K. Improved safety, bioavailability and pharmacokinetics of zidovudine through lactoferrin nanoparticles during oral administration in rats. PloS One. 2015;10(10) doi: 10.1371/journal.pone.0140399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lakshmi Y.S., Kumar P., Kishore G., Bhaskar C., Kondapi A.K. Triple combination MPT vaginal microbicide using curcumin and efavirenz loaded lactoferrin nanoparticles. Sci. Rep. 2016;6:25479. doi: 10.1038/srep25479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kumar P., Lakshmi Y.S., Kondapi A.K. Triple drug combination of zidovudine, efavirenz and lamivudine loaded lactoferrin nanoparticles: an effective nano first-line regimen for HIV therapy. Pharm. Res. (N. Y.) 2017;34(2):257–268. doi: 10.1007/s11095-016-2048-4. [DOI] [PubMed] [Google Scholar]
- 217.Li Q., Zhao J., Hu W., Wang J., Yu T., Dai Y., Li N. Effects of recombinant human lactoferrin on osteoblast growth and bone status in piglets. Anim. Biotechnol. 2018;29(2):90–99. doi: 10.1080/10495398.2017.1313269. [DOI] [PubMed] [Google Scholar]
- 218.Amini A.A., Nair L.S. Recombinant human lactoferrin as a biomaterial for bone tissue engineering: mechanism of antiapoptotic and osteogenic activity. Advanced healthcare materials. 2014;3(6):897–905. doi: 10.1002/adhm.201300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Amini A.A., Kan H.M., Cui Z., Maye P., Nair L.S. Enzymatically cross-linked bovine lactoferrin as injectable hydrogel for cell delivery. Tissue Eng. 2014;20(21–22):2830–2839. doi: 10.1089/ten.tea.2013.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li Y., Zhang W., Ren F., Guo H. Activation of TGF-beta canonical and noncanonical signaling in bovine lactoferrin-induced osteogenic activity of C3H10T1/2 mesenchymal stem cells. Int. J. Mol. Sci. 2019;20(12) doi: 10.3390/ijms20122880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Montesi M., Panseri S., Iafisco M., Adamiano A., Tampieri A. Effect of hydroxyapatite nanocrystals functionalized with lactoferrin in osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. 2015;103(1):224–234. doi: 10.1002/jbm.a.35170. [DOI] [PubMed] [Google Scholar]
- 222.Montesi M., Panseri S., Iafisco M., Adamiano A., Tampieri A. Coupling hydroxyapatite nanocrystals with lactoferrin as a promising strategy to fine regulate bone homeostasis. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0132633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Knudsen K.B., Northeved H., Kumar P.E., Permin A., Gjetting T., Andresen T.L., Larsen S., Wegener K.M., Lykkesfeldt J., Jantzen K., Loft S., Møller P., Roursgaard M. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Biol. Med. 2015;11(2):467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 224.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wei X., Shao B., He Z., Ye T., Luo M., Sang Y., Liang X., Wang W., Luo S., Yang S., Zhang S., Gong C., Gou M., Deng H., Zhao Y., Yang H., Deng S., Zhao C., Yang L., Qian Z., Li J., Sun X., Han J., Jiang C., Wu M., Zhang Z. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25(2):237–253. doi: 10.1038/cr.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Takeuchi T., Jyonotsuka T., Kamemori N., Kawano G., Shimizu H., Ando K., Harada E. Enteric-formulated lactoferrin was more effectively transported into blood circulation from gastrointestinal tract in adult rats. Exp. Physiol. 2006;91(6):1033–1040. doi: 10.1113/expphysiol.2006.035543. [DOI] [PubMed] [Google Scholar]
- 227.Manzoni P., Rinaldi M., Cattani S., Pugni L., Romeo M.G., Messner H., Stolfi I., Decembrino L., Laforgia N., Vagnarelli F., Memo L., Bordignon L., Saia O.S., Maule M., Gallo E., Mostert M., Magnani C., Quercia M., Bollani L., Pedicino R., Renzullo L., Betta P., Mosca F., Ferrari F., Magaldi R., Stronati M., Farina D. S. For the Italian task force for the, t.I.S.o.N. Prevention of neonatal fungal infections, bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. J. Am. Med. Assoc. 2009;302(13):1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 228.Manzoni P., Stolfi I., Messner H., Cattani S., Laforgia N., Romeo M.G., Bollani L., Rinaldi M., Gallo E., Quercia M., Maule M., Mostert M., Decembrino L., Magaldi R., Mosca F., Vagnarelli F., Memo L., Betta P.M., Stronati M., Farina D. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. 2012;129(1):116. doi: 10.1542/peds.2011-0279. [DOI] [PubMed] [Google Scholar]
- 229.M. A, Agency Response Letter GRAS Notice No. GRN 000611 | FDA. 2016. https://www.fda.gov/food/gras-notice-inventory/agency-response-letter-gras-notice-no-grn-000611 WWW Document.
- 230.Solomons H.D. Talactoferrin, Germs. 2012;2(3):121. doi: 10.11599/germs.2012.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Conesa C., Calvo M., Sánchez L. Recombinant human lactoferrin: a valuable protein for pharmaceutical products and functional foods. Biotechnol. Adv. 2010;28(6):831–838. doi: 10.1016/j.biotechadv.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 232.Spadaro M., Curcio C., Varadhachary A., Cavallo F., Engelmayer J., Blezinger P., Pericle F., Forni G. Requirement for IFN-gamma, CD8+ T lymphocytes, and NKT cells in talactoferrin-induced inhibition of neu+ tumors. Canc. Res. 2007;67(13):6425–6432. doi: 10.1158/0008-5472.CAN-06-4080. [DOI] [PubMed] [Google Scholar]
- 233.Jiang R., Du X., Lonnerdal B. Comparison of bioactivities of talactoferrin and lactoferrins from human and bovine milk. J. Pediatr. Gastroenterol. Nutr. 2014;59(5):642–652. doi: 10.1097/MPG.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 234.Bellamy W., Takase M., Wakabayashi H., Kawase K., Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992;73(6):472–479. doi: 10.1111/j.1365-2672.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- 235.Liao H.W., Garris C., Pfirschke C., Rickelt S., Arlauckas S., Siwicki M., Kohler R.H., Weissleder R., Sundvold-Gjerstad V., Sveinbjornsson B., Rekdal O., Pittet M.J. LTX-315 sequentially promotes lymphocyte-independent and lymphocyte-dependent antitumor effects. Cell Stress. 2019;3(11):348–360. doi: 10.15698/cst2019.11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Camilio K.A., Wang M.Y., Mauseth B., Waagene S., Kvalheim G., Rekdal O., Sveinbjornsson B., Maelandsmo G.M. Combining the oncolytic peptide LTX-315 with doxorubicin demonstrates therapeutic potential in a triple-negative breast cancer model. Breast Cancer Res. 2019;21(1):9. doi: 10.1186/s13058-018-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhou H., Forveille S., Sauvat A., Sica V., Izzo V., Durand S., Müller K., Liu P., Zitvogel L., Rekdal Ø., Kepp O., Kroemer G. The oncolytic peptide LTX-315 kills cancer cells through Bax/Bak-regulated mitochondrial membrane permeabilization. Oncotarget. 2015;6(29):26599–26614. doi: 10.18632/oncotarget.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.