Abstract
Purpose:
A common pattern of recurrence in lung cancer after receiving full dose external beam radiation therapy (EBRT) to targeted sites is isolated mediastinal and hilar recurrence (IMHR). Treatment options for these patients are limited to palliative radiation, chemotherapy, and/or best supportive care. We describe our experience with treating IMHR with bronchoscopic endobronchial ultrasound (EBUS) guided intratumoral injection of cisplatin (ITC).
Methods:
Patients treated between Jan 2009–September 2014 with ITC for IMHR were included. Patient demographics, tumor histology, size, concurrent therapy, location, number of sites treated, treatment sessions, and encounters were abstracted. Responses were analyzed on follow-up scans 8–12 weeks after the last treatment session using RECIST 1.1 criteria. Locoregional recurrence, progression-free survival (PFS), and overall survival were measured.
Results:
50 sites were treated in 36 patients (19 males, 17 females) with mean age 61.9 ± 8.5 years. Eight sites treated on subsequent encounters were excluded and one patient had an unevaluable response, leaving 35 patients and 41 sites for final analysis. 24/35 (69%) had complete or partial response (responders), whereas 11/35 (31%) had stable or progressive disease (non-responders). There were no significant differences in response based on histology, size, and concurrent therapy. Median survival for the group was 8 months (95% CI of 6–11 mo). Responders had significantly higher survival and PFS than non-responders. Two patients treated with concurrent EBRT, developed broncho-mediastinal fistula.
Conclusion:
EBUS guided intratumoral cisplatin for IMHR appears to be safe and effective, and may represent a new treatment paradigm for this patient population.
Keywords: Lung cancer recurrence, Sisplatin, endobronchial ultrasound
1. Introduction
Lung cancer remains one of the most prevalent and deadly malignancies worldwide with 1.8 million new cases in 2012. It is responsible for nearly one in five cancer-related deaths (19.4%), or 1.59 million deaths annually worldwide, with a case fatality rate of 0.87 [1,2]. The majority of deaths due to lung cancer are secondary to disease recurrence after initial treatment, irrespective of histology, stage or initial treatment. Recurrent lung cancer has been viewed almost universally fatal secondary to a lack of curative treatment modalities. More importantly, recurrence is often associated with significant patient discomfort requiring substantial supportive treatment and decreased quality of life, thus limiting the feasibility and benefit of further interventions [3].
In 1982, the Radiation Therapy Oncology Group reported an incidence of 34% for locoregional recurrence and 16% for locoregional plus distant failure for NSCLC after irradiation [4]. Although tumor control has improved since then with improved techniques and increases in the dose delivered, there remains a high incidence of local failure after radiotherapy [5,6]. Treatment options for locoregionally recurrent lung carcinomas are limited. The objective response rate of second-line systemic chemotherapy is reported at 10% [7,8]. A recent review concluded that while repeat chest irradiation with EBRT is feasible, most studies in the review focused on symptom relief and palliation rather than disease control [3].
At the University of Florida (UF), we have treated patients with isolated mediastinal and hilar recurrence (IMHR) accessible through bronchoscopy with endobronchial ultrasound (EBUS) guided transbronchial intratumoral injection of cisplatin, with or without concurrent treatment with systemic chemotherapy and/or EBRT. Studies have shown that intratumoral chemotherapy (ITC) is safe, feasible, and effective at debulking of airway tumors [9,10]. Hohenforst-Schmidt et al. demonstrated that ITC in mediastinal nodes can be delivered safely [11], however there is limited to no data regarding its use for disease control for IMHR. The aim of the present work was therefore to study the effectiveness, safety, and feasibility of ITC with cisplatin for isolated mediastinal and hilar recurrences We also analyzed our data to determine whether ITC has any effect on overall survival and/or progression-free survival (PFS). This report describes our institutional experience and management standard for regional failures.
2. Methods and materials
2.1. Patient eligibility and data acquisition
Between January 2009 and September 2014, all patients treated with ITC for IMHR were reviewed. The institutional review board at the University of Florida approved this study (#IRB201400823). All data was prospectively collected and retrospectively analyzed. Patients were enrolled if they satisfied all the following criteria: age 18–80 years, pathologically confirmed NSCLC or small cell lung carcinoma (SCLC), and lung cancer recurrence after definitive EBRT to the site considered for treatment. All patients included in our study had received full dose EBRT to hilar and mediastinal structures at least 6 months before intratumoral therapy was administered. In addition, all recurrences were confirmed by histologic or cytologic examination. Only patients with recurrences limited to the hilar, mediastinal, and peribronchial structures (lymph nodes, nodules, and masses), without evidence of distant metastases, were considered for treatment with ITC. Each patient was restaged with diagnostic computed tomography (CT) of the chest and positron emission tomography (PET)/CT imaging. Each patient was presented at multi-disciplinary thoracic oncology tumor board for their IMHR and considered to have very limited therapeutic options. In all patients, the treatment goal was disease control, with or without palliation of symptoms. All patients were followed with a chest CT or a PET/CT scan 8–12 weeks after the last treatment session to evaluate response. Subsequent follow-up and imaging was completed at the discretion of the patient’s treating oncologist. Detailed chart review was performed for all patients to evaluate for toxicity, locoregional recurrence, distant recurrence, PFS, and mortality. For the purposes of this study, local recurrence was defined as the recurrence at the site of treatment and regional recurrence was defined as recurrence in the mediastinum, hilum or supraclavicular fossa. Other sites of recurrence, including contralateral lung and metastatic lymph nodes in the neck or axilla, were defined as distant recurrence.
The primary outcome was response to treatment as measured on repeat CT Chest or PET scan done 8–12 weeks after the final session of intratumoral therapy. The response was classified as complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD), or unable to assess response, based on RECIST 1.1 criteria as described in Table 1 [12]. For purposes of statistical analysis, we classified patients as “responders” (those with CR and PR) and “non-responders” (those with SD and or PD). We evaluated overall survival and PFS in all patients after the final session of ITC, and whether responders had better overall survival as compared to non-responders. We strictly evaluated each patient’s first encounter with ITC. Survival (both overall and PFS) were measured from the completion of their first encounter with ITC. Some patients were treated later, but these represent a biased subset (as we believe that patients who had good response to first encounter were the ones more likely to undergo subsequent encounters) and those sites were not included in any analysis for the purposes of this study. Secondary outcomes included response based on tumor histology, size of recurrence, and concurrent systemic therapy. We also measured safety outcomes and feasibility and report them here as secondary outcomes.
Table 1.
RECIST 1.1. Definitions of Response in Target Lesions and Target Lymph Nodes.
| Target lesion | Target lymph nodes | |
|---|---|---|
| Complete response (CR) | Disappearance ofall target lesions. | Reduction in short axis to <10mm. |
| Partial response (PR) | At least 30% decrease in sum of diameters of target lesions from baseline. | Same as PR for target lesion, but should include short axis diameter of the nodes. |
| Progressive disease (PD) | At least 20% increase in sum of diameters of target lesions and an absolute increase of at least 5 mm from baseline. | Same as PD for target lesion, but should include short axis diameter of the nodes. |
| Stable disease (SD) | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. | Same as SD for target lesion, but should include short axis diameter of the nodes. |
2.2. Procedural considerations and technical aspects
Convex-probe EBUS, which has a built-in ultrasound probe on a flexible bronchoscope and enables real-time visualization of hilar, mediastinal, and peribronchial structures, was used for ITC. The procedure was performed under moderate sedation. Monitoring, local anesthesia, and oxygenation were performed as with standard bronchoscopy. Once the target lesion was located, a 22 gauge EBUS needle, which is housed in a sheath, was advanced through the working channel of the scope and locked in position. Under real time ultrasound guidance, the tracheobronchial wall was punctured and the needle placed in the target lesion. The stylet within the needle was removed and cisplatin was then injected into the lesion. Each site was accessed one to four times at different locations to facilitate injection of the medication throughout. The needle was then retracted back into the sheath. One to two sites were treated per session. Patients recovered per hospital protocol and were discharged home on the same day.
In this study, cisplatin (aqueous solution at a concentration of 1 mg/ml) was used for ITC with a maximal dose of 40 mg per session based on previous published literature [13,14]. The lyophilized cisplatin powder was dissolved in 0.9% NaCl solution just before use. Each site was treated with one to four punctures per session, depending on the size and location of the lesion. If more than one site was treated per session, each site was injected with 20 mg of cisplatin with total dose per session not to exceed 40 mg. A single encounter of ITC consisted of 4 weekly sessions during a 3-week period (on days 1, 8, 15, and 22). Patients were given 10 mg of dexamethasone intravenously as well as 8 mg of ondansetron intravenously at the beginning of the procedure to prevent nausea.
Some patients treated with ITC experienced an isolated recurrence several months later in a different mediastinal or hilar site (separate from the previously treated IMHR) and subsequently underwent another 4 sessions of therapy with ITC. Those patients were classified as having second or subsequent ITC encounters. Once treated, a particular site was never considered for retreatment with ITC on subsequent encounters.
3. Statistical analysis
Kaplan–Meier curves were used to estimate survival probabilities over time, overall, and stratified by response (complete/partial responders vs. stable/progressive disease). We compared survival and PFS for responders vs. non-responders by the log-rank test, but no causality can be inferred. In patients with multiple sites treated during their first encounter, we included the patient’s worst response for statistical analysis. Associations between response rates and other factors were conducted by the Fisher’s exact test. For patients who were treated at a separate site on a subsequent encounter, the overall survival (OS) and PFS analysis was performed based upon time of first encounter. Including multiple encounters for the same patient confounds the independence of the observations, creating selection bias, as the reason for a subsequent encounter is likely correlated with what happened on the first encounter. Sites treated on subsequent encounters were therefore excluded from final analysis.
4. Results
A total of 50 sites were treated in 36 patients (19 males and 17 females) with mean age 61.9 ± 8.5 years. Eighteen patients had adenocarcinoma, 13 had squamous cell, 4 had small cell, and 1 had large cell. The anatomical distribution of the treated sites included the following locations: 11 in the right paratracheal region, 11 in the right hilar area, 7 in the subcarina, 4 in the left paratracheal area, 5 in the left hilum, 2 in the right upper lobe, 1 in the right middle lobe, 2 in the right lower lobe, 4 in the left upper lobe, 2 in the left lower lobe, and 1 in the posterior tracheoesophageal area. Eight of these 50 sites were treated on subsequent encounters and were excluded from the final analysis. One patient had a response on imaging which was not evaluable based on RECIST criteria and was excluded from the analysis. Our final analysis included 35 patients and 41 sites, as shown in Fig. 1. Eight patients received concurrent systemic chemotherapy and 2 patients received concurrent EBRT. The remaining 26 patients were deemed ineligible for additional systemic chemotherapy or declined systemic chemotherapy due to prior side effects.
Fig. 1.
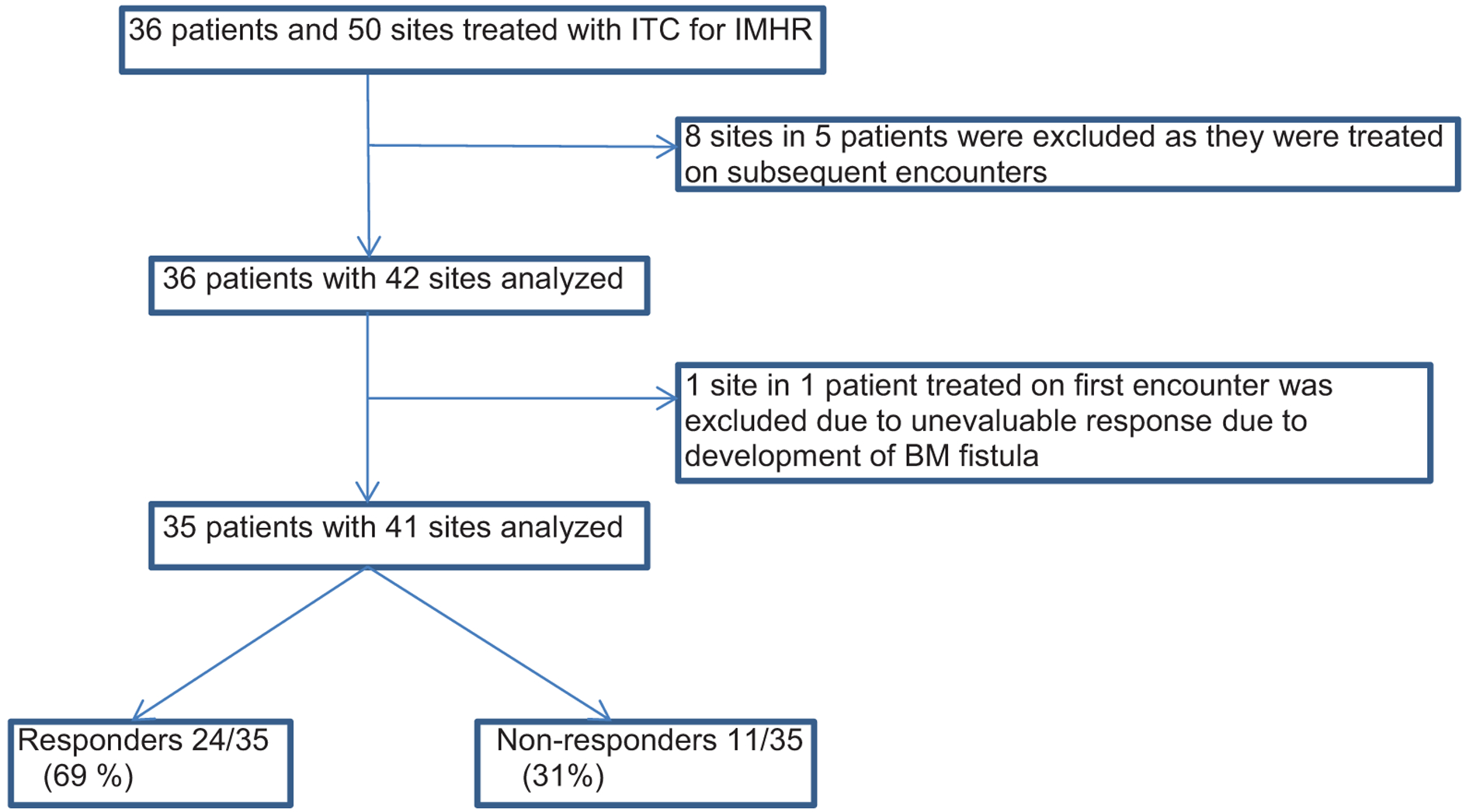
Total number of patients screened and included in the analysis and their final outcome.
Twenty-four of 35 patients (69%) were classified as responders having either a complete (n = 11) or partial response (n = 13), whereas the remaining 11 patients (31%) were classified as non-responders with either stable disease (n = 8) or progressive disease (n = 3). Variables including histologic subtypes of cancer, concurrent systemic therapy, and size of the treated lesion, stratified by response, are shown in Table 2. There were no statistically significant differences in response based on type of lung cancer, size of recurrence, or concurrent systemic therapy, although there was a trend toward more responders in patients with lesion size <2 cm. Of the 36 distinct patients (including one inevaluable for response); 8 were on concurrent chemotherapy (3 survived), 3 were on concurrent XRT (none survived), and 25 were on neither (3 survived). By exact chi-square analysis, there was no significant difference in survival (P = 0.17) based on concurrent systemic therapy. None of the sites treated in the patients with CR, PR, or SD had local recurrence, suggesting excellent local control. Eight patients subsequently had regional recurrence, 15 had distant recurrence and 2 had both regional and distant recurrence during the period of the study.
Table 2.
Response to ITC with cisplatin based on histology, concurrent systemic therapy and size. There is no statistical difference in response rates based on above variables.
| Responders | Non responders | P value | |
|---|---|---|---|
| 1. Histology | 0.84 | ||
| Adenoca | 13(72%) | 5(28%) | |
| Squamous | 7(58%) | 5(42%) | |
| Small cell | 3(75%) | 1(25%) | |
| Large cell | 1(100%) | 0 | |
| 2. Concurrent systemic Rx | 1.00 | ||
| Chemo | 5(63%) | 3(37%) | |
| Radiation | 2(100%) | 0 | |
| None | 17(68%) | 8(32%) | |
| 3. Size oflesion | .066 | ||
| < = 1 cm | 8(89%) | 1(11%) | |
| 1.1–2cm | 10(83%) | 2(17%) | |
| 2.1–3cm | 3(43%) | 4(57%) | |
| >3cm | 3(43%) | 4(57%) |
Overall median survival for the cohort was 8 months (95% CI of 6–11 months). Six patients were alive at the time of this analysis. Median survival was 10 months for responders (95% CI of 8–13 months) and 6 months for non-responders (95% CI of 3–10 months), with a log-rank p value of 0.029, suggesting that patients who responded to ITC had statistically better overall survival. Fig. 2 shows Kaplan–Meier (KM) curves for overall survival in responders and non-responders. We calculated PFS in all of our patients, which for the purposes of this study was defined as the time to progression by growth of the tumor, development of new tumors, death from cancer, or death from other causes. Median PFS for the cohort was 6 months (95% CI of 4–8 months). Median PFS was 7 months for the responders (95% CI of 5–11 months) and 3 months months the non-responders (95% CI of 2–4 months). PFS for the responders was statistically different as compared to non-responders p = 0.0030). Fig. 3 shows the KM curves for PFS in responders and non-responders.
Fig. 2.
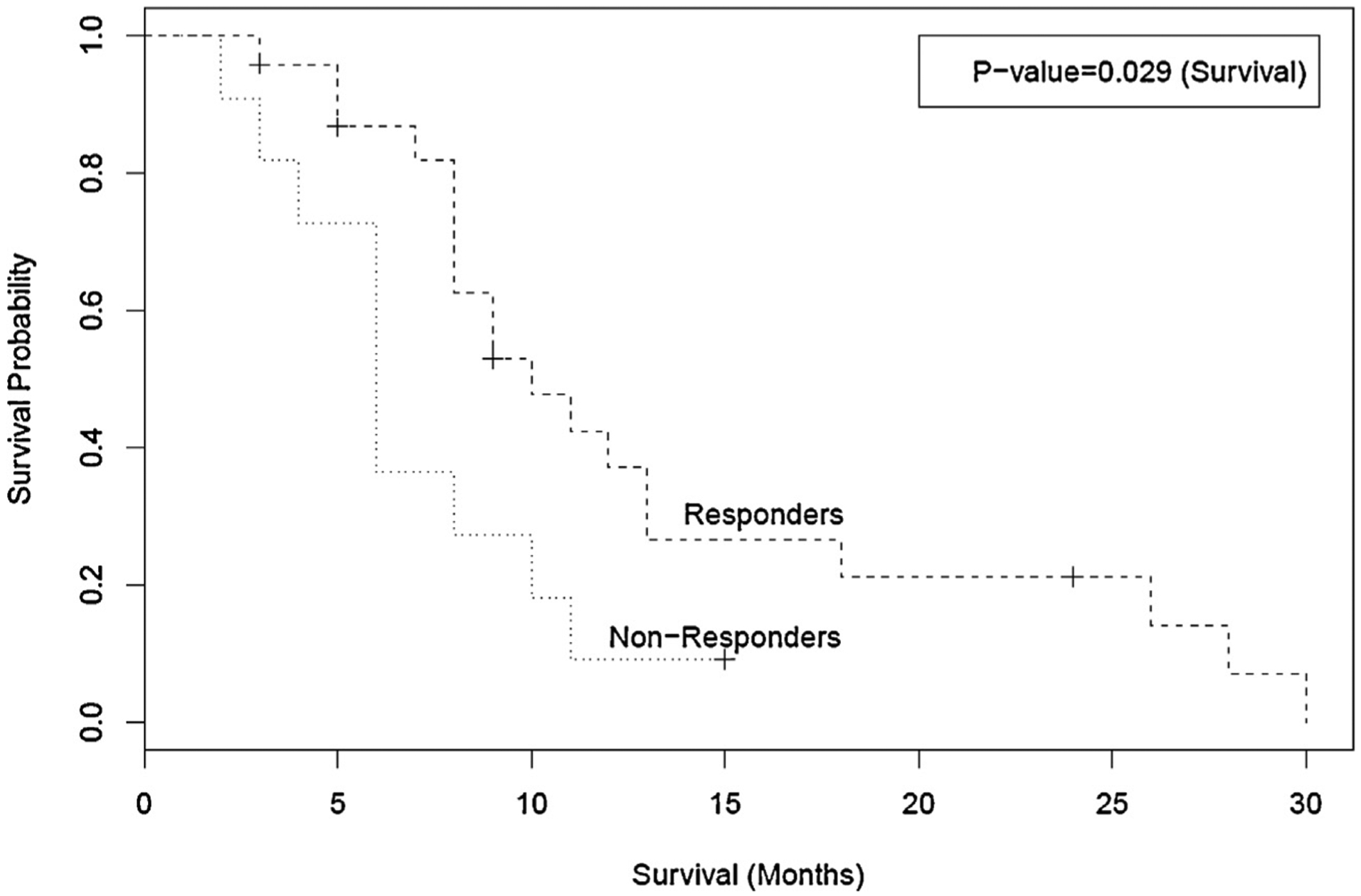
Kaplan–Meier curves for overall survival in months amongst responders and non-responders. Responders had statistically significant overall survival as compared to non-responders.
Fig. 3.
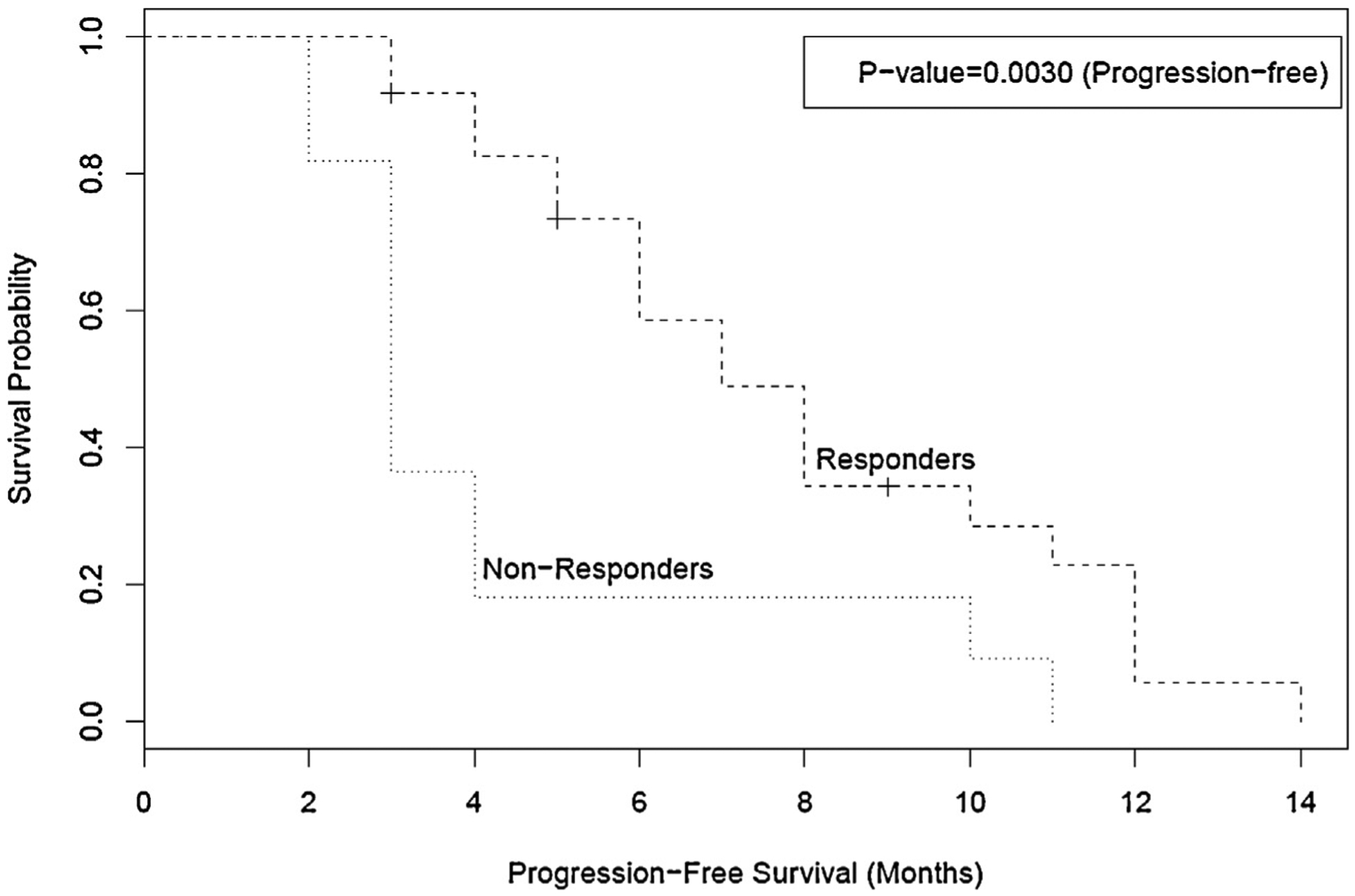
Kaplan–Meier curves for progression free survival in months amongst responders and non-responders. Responders had statistically significant progression free survival as compared to non-responders.
The majority of patients tolerated the procedure very well. A few patients had transient nausea post-procedure, which improved significantly after a course of oral ondansetron. Two patients developed broncho-mediastinal fistula toward the end of treatment, one on first encounter and the other on a subsequent encounter. Both of these patients were treated for recurrence in the subcarinal location with concurrent repeat palliative EBRT and intratumoral cisplatin. Both were managed conservatively with antibiotics and close bronchoscopic surveillance. Although contributing to increased morbidity for these patients, fistula development did not lead to additional hospitalizations and was not directly responsible for mortality. No additional adverse events such as interstitial pneumonia, bone marrow suppression, worsening of inflammatory findings, nephrotoxicity, or neurotoxicity were reported in any of the patients treated with intratumoral injection with cisplatin.
5. Discussion
Our study focuses on the management of isolated mediastinal and hilar recurrences in patients with previously treated lung cancer who received full-dose EBRT to the hilum and mediastinum, with or without surgery and/or systemic chemotherapy. The incidence of IMHR in patients with previously treated lung cancer is approximately 9% [15]. Unfortunately, systemic chemotherapy is unable to provide durable tumor control in these patients, and has modest response rates at the cost of substantial toxicity [16]. Chest reirradiation has been shown to be feasible, safe, and effective at relieving symptoms associated with recurrence, however has not been shown to achieve disease control or remission. It has been studied only in small retrospective cohorts, with the primary goal of symptom palliation, not tumor control [3].
The last decade has brought a proliferation of intratumoral chemotherapy and immunotherapy research [17]. Studies suggest that when chemotherapeutic agents are injected locally into a tumor mass, therapeutic doses within the tumor may be 6–10 times higher, without systemic toxicity. High local drug concentrations with minimal toxicity are therefore a key potential benefit of intratumoral therapy [17]. Use of several different chemotherapeutic agents for ITC, including cisplatin, methotrexate, bleomycin, mitox-antrone, mitomycin, and 5-fluorouracil, has been described in the literature [9,10]. We chose to use cisplatin for ITC as it has been demonstrated to be one of the most active single agents against lung cancer, and is frequently used in systemic combination chemotherapy [18,19]. Moreover, intratumoral chemotherapy with cisplatin has been successfully used in the treatment of a variety of localized malignant tumors. It has been administered by direct injection in lung cancers [13], head and neck cancers [20], and malignant liver tumors (under CT guidance) [21]. It has additionally been utilized endoscopically for palliation of obstructive esophageal cancers [22], and injected into gastric tumors [23]. Based on available evidence and experience, we advocate for the use of cisplatin as the chemotherapeutic agent of choice for bronchoscopic ITC.
Bronchoscopic ITC has been studied in a variety of settings in patients with lung cancer, including palliation of life-threatening central airways obstruction developing or persisting after conventional cancer treatment [9,10]. It has also been studied in patients with newly diagnosed obstructive inoperable lung cancer as part of a combination therapy regimen (intratumoral chemotherapy with cisplatin followed by irradiation) [13], and direct treatment of involved lymph nodes by EBUS transbronchial needle [11]. All prior studies have demonstrated that bronchoscopic ITC is safe, feasible, well tolerated, and largely effective. ITC however has never been studied for isolated mediastinal and hilar recurrence following full-dose EBRT with the purpose of achieving long-term control and/or disease remission.
In our experience, the overall safety profile of ITC with cisplatin was favorable. Two patients developed broncho-mediastinal fistula within 2–4 weeks after therapy. Both were managed conservatively and although this contributed to increased morbidity, fistula development was not linked to mortality in these patients. These cases occurred during the initial phase of our experience with ITC. We hypothesize that development of airway fistulae in these patients was secondary to concurrent administration of ITC with cisplatin and palliative EBRT to the same site. Based on this experience, we recommend against treating recurrence sites with concurrent ITC with cisplatin and EBRT. After modifying our practice to exclude combination therapy with palliative EBRT, we did not observe any further incidence of bronchial fistulae.
Our study suggests that ITC with cisplatin is safe, effective and well tolerated. It demonstrates that significant rates of disease remission can be achieved in this group of patients with very limited treatment options. Excluding the 3 patients with progressive disease at the site of treatment, no local recurrence was observed in any of the other treated sites. There was no statistically significant difference in response based on histology, concurrent systemic therapy, or size of recurrence. A trend toward having a greater number of responders with smaller lesions was noted.
Survival among patients with isolated mediastinal or hilar recurrence following EBRT, with or without chemotherapy, is not specifically known. In a prospective, randomized study of docetaxel versus best support care in patients with stage III or stage IV NSCLC previously treated with chemotherapy, median survival was 7.0 and 4.6 months, respectively [24]. In a randomized study comparing pemetrexed to docetaxel in previously treated patients with NSCLC, survival among the subgroup of patients with stage III disease was 9.3 and 10.3 months, respectively, with an overall PFS of 2.9 months (stage III and stage IV) [25]. Although external comparisons are not statistically valid, our overall median survival of 8 months seems to be comparable to these studies and would suggest a survival benefit compared to best supportive care only. Survival analysis was performed on patients who had multiple sites treated on first encounter. We had 5 such patients with 4 of them having 2 sites treated and one having 3 sites treated on first encounter. Median PFS in that cohort was 8 months and median overall survival of 11 months, with 1 of 5 patients alive at the time of analysis suggesting that multiple sites treated on first encounter is feasible and may yield similar survival. Additionally, localized cisplatin injection appears to be simple, less toxic and may be superior to standard intravenous regimens with respect to quality of life and side effects. One significant finding of our study was that patients who responded to ITC had an improved overall survival and PFS compared to non-responders.
Among the limitations of this study, we should highlight that this is a descriptive, retrospective series. Study assessed outcomes based on RECIST criteria which rely solely on radiographic measurements and since one of our patients had associated atelectasis with the tumor the response was not evaluable for the study. Serum and tumor levels of cisplatin were not measured and some patients received systemic therapies, which can be a potential con-founder. However, since much higher concentrations of drugs can be achieved in the tumor with intratumoral injection [17] and cytotoxic drugs directly injected into a tumor can also be transported through lymphatic vessels to the regional lymph nodes [10], we believe that the tumor remission, excellent local control and absence of regional recurrence seen in our series is attributable entirely to direct intratumoral effects of the chemotherapeutic and not systemic effects of the injected agent..
As previously noted, comparisons between treatment modalities cannot be made. On the basis of this study, we are unable to identify variables that would predict superior response to intratumoral cisplatin, but did note a small trend toward inverse correlation between response and lesion size. Our study, however, is too small to establish a prediction model for determining which patients will respond to ITC. This case series, nonetheless, contributes additional information to support this approach for management of IMHR in patients with lung cancer previously treated with mediastinal radiation. Ours is the largest reported series published on bronchoscopic treatment with ITC in this group of patients with very limited treatment options. We therefore believe that based on outcomes of our study, intratumoral EBUS guided injection of cisplatin can represent a new management paradigm for these patients with isolated mediastinal and hilar lung cancer recurrence who have received full-dose EBRT to those sites in the past.
6. Conclusion
ITC appears to be a promising treatment modality for patients with lung cancer recurrence and otherwise limited therapeutic options. Multicenter randomized trials could elucidate the efficacy of this approach and determine prognostic factors as to the characteristics of responders vs. non-responders.
Acknowledgements
This work was partially supported by NIH grant 1UL1TR000064 from the National Center for Advancing Translational Sciences. Authors would like to thank Dr. Eugene Goldberg from the University of Florida for his pioneering work in the field of intratumoral chemotherapy injections and for providing information on this technique.
Abbreviations:
- ITC
intratumoral chemotherapy
- IMHR
isolated mediastinal and hilar recurrence
- EBRT
External beam radiation therapy
- PFS
Progression-free survival
- CT
computed tomography
- PET
positron emission tomography
- EBUS
Endobronchial ultrasound
Footnotes
Disclosures
No potential conflicts of interest exist with any companies/organizations whose products or services are discussed in this article.
References
- [1].International Agency for Research on Cancer (IARC). 2012. [Google Scholar]
- [2].Siegel R, et al. , Cancer statistics, CA Cancer J. Clin 64 (1) (2014) 9–29. [DOI] [PubMed] [Google Scholar]
- [3].Jeremic B, Videtic GM, Chest reirradiation with external beam radiotherapy for locally recurrent non-small-cell lung cancer: a review, Int. J. Radiat. Oncol. Biol. Phys 80 (4) (2011) 969–977. [DOI] [PubMed] [Google Scholar]
- [4].Perez CA, et al. , Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group, Cancer 50 (6) (1982) 1091–1099. [DOI] [PubMed] [Google Scholar]
- [5].Blanke C, et al. , Phase III trial of thoracic irradiation with or without cisplatin for locally advanced unresectable non-small-cell lung cancer: a Hoosier Oncology Group protocol, J. Clin. Oncol 13 (5) (2015) 1425–1429. [DOI] [PubMed] [Google Scholar]
- [6].Schaake-Koning C, et al. , Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer, N. Engl. J. Med 326 (8) (1992) 524–530. [DOI] [PubMed] [Google Scholar]
- [7].Hainsworth JD, et al. , Weekly docetaxel with either gemcitabine or vinorelbine as second-line treatment in patients with advanced nonsmall cell lung carcinoma: Phase II trials of the Minnie Pearl Cancer Research Network, Cancer 92 (9) (2001) 2391–2398. [DOI] [PubMed] [Google Scholar]
- [8].Grossi F, et al. , Phase II study of irinotecan and docetaxel in patients with previously treated non-small cell lung cancer: an Alpe-Adria thoracic oncology multidisciplinary group study (ATOM 007), Lung Cancer 52 (1) (2006) 89–92. [DOI] [PubMed] [Google Scholar]
- [9].Celikoglu SI, et al. , Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction, Postgrad. Med. J 73 (857) (1997) 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Celikoglu F, Celikoglu SI, Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction, J. Pharm. Pharmacol 55 (10) (2003) 1441–1448. [DOI] [PubMed] [Google Scholar]
- [11].Hohenforst-Schmidt W, et al. , Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies, Drug Des. Dev. Ther 7 (2013) 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1), Eur. J. Cancer 45 (2) (2009) 228–247. [DOI] [PubMed] [Google Scholar]
- [13].Celikoglu F, et al. , Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer, Lung Cancer 51 (2) (2006) 225–236. [DOI] [PubMed] [Google Scholar]
- [14].Celikoglu SI, Celikoglu F, Goldberg EP, Endobronchial intratumoral chemotherapy (EITC) followed by surgery in early non-small cell lung cancer with polypoid growth causing erroneous impression of advanced disease, Lung Cancer 54 (3) (2006) 339–346. [DOI] [PubMed] [Google Scholar]
- [15].Kilburn JM, et al. , Management of mediastinal relapse after treatment with stereotactic body radiotherapy or accelerated hypofractionated radiotherapy for stage I/II non-small-cell lung cancer, J. Thorac. Oncol 9 (4) (2014) 572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Noble J, et al. , Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline, J. Thorac. Oncol 1 (9) (2006) 1042–1058. [PubMed] [Google Scholar]
- [17].Goldberg EP, et al. , Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery, J. Pharm. Pharmacol 54 (2) (2002) 159–180. [DOI] [PubMed] [Google Scholar]
- [18].Sandler AB, et al. , Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer, J. Clin. Oncol 18 (1) (2000) 122–130. [DOI] [PubMed] [Google Scholar]
- [19].Ciotti R, et al. , Prospective evaluation of anthracycline-related early cardiac damage: how do we monitor it? J. Clin. Oncol 19 (22) (2001) 4269–4270. [DOI] [PubMed] [Google Scholar]
- [20].Burris HA 3rd, et al. , Intratumoral cisplatin/epinephrine-injectable gel as a palliative treatment for accessible solid tumors: a multicenter pilot study, Otolaryngol. Head Neck Surg 118 (4) (1998) 496–503. [DOI] [PubMed] [Google Scholar]
- [21].Vogl TJ, et al. , CT-guided intratumoural administration of cisplatin/epinephrine gel for treatment of malignant liver tumours, Br. J. Cancer 86 (4) (2002) 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Monga SP, et al. , Endoscopic treatment of gastric cancer with intratumoral cisplatin/epinephrine injectable gel: a case report, Gastrointest. Endosc 48 (4) (1998) 415–417. [DOI] [PubMed] [Google Scholar]
- [23].Monga SP, et al. , Intratumoral therapy of cisplatin/epinephrine injectable gel for palliation in patients with obstructive esophageal cancer, Am. J. Clin. Oncol 23 (4) (2000) 386–392. [DOI] [PubMed] [Google Scholar]
- [24].Shepherd FA, et al. , Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy, J. Clin. Oncol 18 (10) (2000) 2095–2103. [DOI] [PubMed] [Google Scholar]
- [25].Hanna N, et al. , Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy, J. Clin. Oncol 22 (9) (2004) 1589–1597. [DOI] [PubMed] [Google Scholar]


