Abstract
Background:
Bullous pemphigoid (BP), the most common autoimmune blistering disease, may be diagnostically challenging. Direct immunofluorescence (DIF), indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and recently, C3d immunohistochemistry (IHC), are used as adjuncts to diagnosis.
Objective:
To compare C3d IHC to DIF, IIF, and ELISA testing in BP diagnosis.
Methods:
C3d IHC was performed on skin biopsy specimens from 51 patients (27 with BP and 24 with other blistering diseases) and compared to DIF and IIF, with anti-BP180 or anti-BP230 ELISA results used as the gold standard.
Results:
We found C3d IHC, DIF, and IIF had similar sensitivity (74.1%, 63.1%, and 70.4%), specificity (95.8%, 100%, and 100%), positive predictive value (95.2%, 100%, and 100%), and negative predictive value (76.7%, 70.6%, and 75%) for BP. Cases with positive C3d IHC, DIF, and IIF had significantly higher anti-BP180 and anti-BP230 by ELISA than cases with negative testing (P < .0001). False-negative IIF results were associated with lower BP230 compared with true-positive results (P = .03).
Limitations:
This was a single-center, retrospective study.
Conclusion:
Our study compared C3d IHC to DIF and IIF in BP diagnosis, demonstrating C3d IHC on fixed tissue provides similar diagnostic utility to immunofluorescence and ELISA.
Keywords: bullous pemphigoid, C3d immunohistochemistry, diagnosis, direct immunofluorescence, ELISA, indirect immunofluorescence
Bullous pemphigoid (BP) is the most common autoimmune blistering disease, with an annual incidence of 4.28 per 100,000 person-years.1 BP is caused by autoantibodies that target hemidesmosomal proteins BP antigen 2 (BP180, BPAG2) or BP antigen 1 (BP230, BPAG1) at the dermal-epidermal junction.2 BP180 is thought to be the primary target of pathogenic autoantibodies, whereas antibodies to BP230 may be secondarily produced due to tissue damage and inflammation. The subsequent loss of hemidesmosomal adhesion at the basement membrane zone (BMZ) caused by immunologic sequelae, including complement deposition, leads to blistering between the epidermis and dermis.2
An understanding of these immunologic mechanisms of disease has enabled improved diagnosis of BP. Direct immunofluorescence (DIF) and indirect immunofluorescence (IIF) assays are both performed for diagnosis of BP. DIF on frozen perilesional biopsy specimens shows linear C3 or IgG deposits along the BMZ in BP and is considered by many to be the gold standard when combined with a suggestive clinical picture.3,4 However, DIF can be positive at the BMZ in other autoimmune blistering diseases, including epidermolysis bullosa acquisita or p200 or laminin 332 pemphigoid, among others.
IIF is also routinely performed, in which animal or human donor tissues are incubated with patient serum to detect the presence of circulating autoantibodies that target the BMZ. Salt-split human skin can also be used as a substrate to increase the sensitivity of IIF.5
More recently, enzyme-linked immunosorbent assays (ELISAs), which more precisely define disease based on autoantigenic targets, have proven to be a reliable diagnostic test to quantify the presence of BP180 or BP230 autoantibodies in serum.6–8
The ability to use immunohistochemistry (IHC) on paraffin-fixed tissue has additional advantages in the diagnosis of BP, particularly when frozen tissue for DIF is unavailable or a second biopsy specimen cannot be obtained. Interpreting IHC staining using a standard light microscope allows simultaneous visualization of tissue and cellular morphologies, and chromogenic IHC detection methods overcome many issues with fluorescent imaging, including autofluorescence, photobleaching, and signal decay.
Several studies have looked at the use of complement IHC in the diagnosis of BP, with conflicting results.3,9,10 C3d, in particular, is formed from inactivated C3b and remains deposited after complement activation. C3d IHC can thus be performed on formalin-fixed tissue. The largest study of C3d IHC in BP looked at 38 patients with DIF-positive BP, where 14 patients (37%) had C3d at the BMZ,2 whereas 2 other smaller studies found 31 of 32 (97%) and 17 of 17 DIF-positive patients with BP (100%) had C3d BMZ deposition.11,12 Other studies have also corroborated C3d staining by IHC in DIF-positive BP, with conflicting data on its reliability.9,10
Previous analyses of C3d IHC in BP have used DIF as the gold standard to which C3d IHC was compared. DIF, however, may have shortcomings as a diagnostic method, including low sensitivity, which can decrease further depending on the transport medium.13 Thus, we believe it is useful to use ELISA as the gold standard for BP diagnosis, which is a more quantitative measure with reported sensitivities and specificities of as high as 89% and 98%, respectively, compared with DIF.6–8 Because ELISA testing is a newer diagnostic tool, routine access to it may be limited, but it has effectively been used as a gold standard against which ancillary diagnostic tests for BP can be compared.14–17
In this study, we compared the utility of C3d IHC to DIF and IIF techniques for diagnosis of BP, using ELISA for BP180 and BP230 antigens as the gold standard.
METHODS AND MATERIALS
Case selection
Use of specimens in this study was provided through a protocol approved by University of Pennsylvania Institutional Review Board (#820338). Patients with at least 1 positive BP180 or BP230 ELISA, or both, in the setting of clinical concern for BP (based on clinical documentation) and a skin biopsy specimen within 1 year of ELISA testing, between 2010 and 2014, were identified from our dermatopathology database NewPath. This search yielded 110 patients. We considered positive ELISA testing in the setting of clinical concern for BP to be the gold standard for diagnosis. We identified 51 patients with concomitant C3d, DIF, and IIF testing. Patients without all 4 tests or with indeterminate results were not included in our analysis.
C3d immunohistochemical staining
C3d (cat# 403A-78; Cell Marque, Rocklin, CA) IHC staining was performed by first incubating 5-µm formalin-fixed paraffin embedded tissue sections from lesional skin at 58°C for 1 hour. Sections were placed in xylene for 3 changes of 5 minutes each, and were subsequently placed in absolute ethanol for 3 changes, and then rinsed with deionized water. The slides were placed in methanol hydrogen peroxide for 5 minutes, rinsed with water, and then placed in Tris-buffered saline (TBS) for 3 changes. The slides were incubated with a rabbit polyclonal C3d antibody (cat# 403A-78, Cell Marque) for 30 to 60 minutes and then washed in TBS for 3 changes. The slides were incubated with the biotinylated secondary antisera (Red Refine Detection Kit, DS9390; Leica Biosystems, Buffalo Grove, IL) for 30 minutes at 37°C. The slides were washed in TBS for 3 changes, incubated with tertiary solution for 30 minutes at 37°C, and then rinsed in TBS and then in deionized water. These slides were incubated with 3-amino-9-ethylcarbazole and rinsed with deionized water. Gill’s I hematoxylin was applied for 5 minutes as a counterstain, and the slides were washed in tap water and placed in aqueous mounting medium with a coverslip.
C3d stains were examined by a dermatopathologist (E.C.) blinded to the ELISA result and categorized as positive, negative, or indeterminate for linear C3d BMZ staining. Indeterminate specimens were defined as sections with poor staining or a complete subepidermal split that rendered the results uninterpretable.
ELISA testing for BP180 and BP230
The BP180 ELISA Kit (cat #7612E) and BP230 ELISA Kit (cat #RG-M7613-D) purchased from Medical and Biological Laboratories (Nagoya, Aichi, Japan) was used. A dilute wash solution (100 mL concentrate into 900 mL distilled water) was prepared, and 100 µL of each calibrator and diluted sample was transferred to each appropriate well. The plates were covered and incubated at room temperature for 1 hour. Plate covers were removed and wells washed with wash buffer (100 µL, 4 changes). Next, 100 µL of conjugated reagent and 100 µL of substrate was applied to each well, and these were covered and incubated for 30 minutes, with subsequent application of stop solution. The results were read on a spectrophotometer, calculated based on the following formula:
| Index value = (1:100 sample − negative sample)/(positive sample − negative sample) × 100 |
Anti-BP180 or anti-BP230 values ≥9 U/mL were interpreted as positive ELISA results, according to the Medical and Biological Laboratories kit package insert.
Immunofluorescence testing
Samples were submitted in Michel’s solution or saline. For DIF, 3-µm sections of tissue were cut and fixed in 95% ethanol and then rinsed in phosphate-buffered saline. Solutions (1 mL in phosphate-buffered saline) of immunoglobulin M (1:40), IgG (1:66), IgA (1:40), C3 (1:40), and fibrinogen (1:100) antibodies were placed on the tissues for 1 hour, and the slides were washed twice with phosphate-buffered saline. The slides were mounted with Glycergel mounting medium (Dako, Carpinteria, CA) and stored at 4°C.
For IIF, whole blood was centrifuged at 1000 rpm for 30 minutes, with serum removed and placed in cryogen tubes. Monkey esophagus substrate slides (cat #5308; Scimedx, Dover, NJ) were washed in TBS-calcium chloride wash solution twice for 5 minutes and placed in blocking buffer for 30 minutes. Patient serum at appropriate dilutions was placed in sample wells. After 1 hour, the slides were washed twice in TBS-CaCl, and the secondary antibody (polyclonal fluorescein isothiocyanate-conjugated; MP Biomedical Research Products, Irvine, CA) was applied to the slide wells for 30 minutes, with 2 subsequent washes of TBS-CaCl. Slides were then covered with Glycergel and a coverslip.
Statistical analysis
All statistical analysis was performed in Stata 13 software (StataCorp, College Station, TX). Continuous variables were compared using Wilcoxon rank sum, and categorical variables were compared using the Fisher exact test. The discriminative ability of each test (C3d, DIF, and IIF) using ELISA as the gold standard was evaluated and compared to one another using the area under the receiver operating characteristic curve. Statistical significance was determined by 2-sided P values at P < .05.
RESULTS
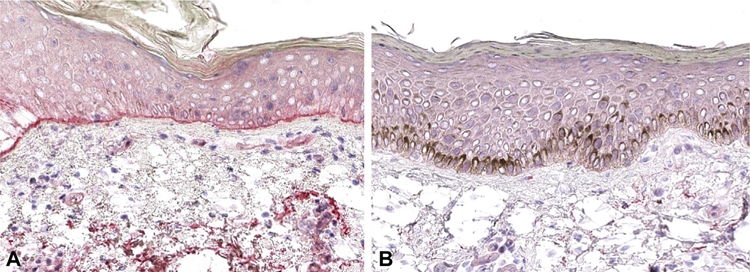
We identified 110 patients in our dermatopathology database for whom ELISA testing was performed in addition to a skin biopsy for routine histopathology with C3d. Of the 110 patients identified, 51 also had DIF and IIF testing. Representative interpretations of positive and negative C3d staining results are provided for reference (Fig 1). No significant differences in age or sex were identified between the 24 patients who tested BP negative by ELISA compared with the 27 who tested BP positive (Table I).
Fig 1.
Representative C3d (A) positive and (B) negative immunohistochemistry. (B) Positive C3d testing is indicated by uniform red linear staining along the dermal-epidermal junction.
Table I.
Baseline demographics and bullous pemphigoid (BP) diagnosis
| Variable | No BP (n = 24) | BP (n = 27) | P value |
|---|---|---|---|
| Age, median (IQR), y | 68.5 (56.5, 84) | 72 (56, 80) | .97* |
| Male sex, No. (%) | 10 (41.6) | 17 (62.9) | .17† |
IQR, Interquartile range; No., number.
Wilcoxon rank sum test.
Fisher exact test.
The sensitivity of C3d IHC was 74.1% (95% confidence interval [CI], 53.7%−88.9%), and the specificity was 95.8% (95% CI, 78.9%−99.9%). The positive predictive value of C3d IHC was 95.2% (95% CI, 76.2%−99.9%), and the negative predictive value was 76.7% (95% CI, 57.7%−90.1%) (Table II).
Table II.
C3d immunohistochemistry (IHC ), direct immunofluorescence (DIF), and indirect immunofluorescence (IIF) test characteristics for bullous pemphigoid (BP) diagnosis*
| BP diagnosis |
||||
|---|---|---|---|---|
| Test | Positive | Negative | Total | |
| C3d diagnosis | ||||
| Positive | 20 | 1 | 21 | PPV: 95.2% (76.2–99.9%) |
| Negative | 7 | 23 | 30 | NPV: 76.7% (57.7–90.1%) |
| Total | 27 | 24 | 51 | |
| Sensitivity: 74.1% (53.7%−88.9%) | Specificity: 95.8% (95.8%−99.9%) | |||
| BP diagnosis |
||||
| Positive | Negative | Total | ||
| DIF diagnosis | ||||
| Positive | 17 | 0 | 17 | PPV: 100% (80.5%−100%) |
| Negative | 10 | 24 | 34 | NPV: 70.6% (52.5%−84.9%) |
| Total | 27 | 24 | 51 | |
| Sensitivity: 63.1% (42.4%−80.6%) | Specificity: 100.0% (85.8%−100%) | |||
| IIF diagnosis |
||||
| Positive | Negative | Total | ||
| BP diagnosis | ||||
| Positive | 19 | 0 | 19 | PPV: 100% (82.4%−100%) |
| Negative | 8 | 24 | 32 | NPV: 75% (56.6%−88.5%) |
| Total | 27 | 24 | 51 | |
| Sensitivity: 70.4% (49.8%−86.2%) | Specificity: 100% (85.8%−100%) | |||
NPV, Negative predictive value; PPV, positive predictive value.
The values for PPV, NPV, sensitivity, and specificity are shown with the 95% confidence interval, and the other values are the number of patients.
For DIF, we found a sensitivity of 63.1% (95% CI, 42.4%−80.6%), specificity of 100% (95% CI, 85.8%−100%), positive predictive value of 100% (95% CI, 80.5%−100%), and negative predictive value of 70.6% (95% CI, 52.5%−84.9%) (Table II). For IIF, sensitivity was 70.4% (95% CI, 49.8%−86.2%), specificity was 100% (95% CI, 85.8%−100%). Positive predictive value was 100% (95% CI, 82.4%−100%), and negative predictive value was 75% (95% CI, 56.8%−88.5%) (Table II). Areas under the receiver operating characteristic curves for C3d (0.85; 95% CI, 0.76–0.94), DIF (0.82; 95% CI, 0.72–0.91), and IIF (0.85; 95% CI, 0.76–0.94) were not statistically different among the 3 diagnostic tests (P = .76) (Table III).
Table III.
Comparison of C3d, direct immunofluorescence (DIF), and indirect immunofluorescence receiver operating characteristic (ROC) curve area
| Diagnostic test | ROC curve area | 95% CI | P value* |
|---|---|---|---|
| C3d | 0.85 | 0.76–0.94 | .76 |
| DIF | 0.81 | 0.72–0.91 | |
| IIF | 0.85 | 0.76–0.94 |
CI, Confidence interval.
Comparison among the areas under the ROC curve using enzyme-linked immunosorbent assay as the gold standard
We next assessed the relationship between C3d IHC results and ELISA values for BP180 and BP230. Patients who were positive for C3d by IHC also had significantly higher BP180 and BP230 values compared with patients who were negative for C3d, suggesting a relationship between C3d IHC and BP180 or BP230 ELISA (Table IV). These results are consistent with the ability of IgG1 autoantibodies to BP180 and BP230 to fix complement at the BMZ.18
Table IV.
BP180 and BP230 levels via enzyme-linked immunosorbent assay testing compared with C3d, direct immunofluorescent (DIF), and indirect immunofluorescent (IIF) results
| Variable* | C3d (+) (n = 21)) | C3d (−) (n = 30) | P value* | C3d true positive (N = 20) | C3d false negative (n = 7) | P value† |
|---|---|---|---|---|---|---|
| BP180 | 74.9 (34.5, 121.2) | 2.8 (1.6, 5.6) | <.0001 | 76.6 (40.1, 131.1) | 5.6 (3.0, 139.7) | .06 |
| BP230 | 43.3 (6.7, 75.6) | 3.4 (1.5, 5.9) | <.0001 | 51.5 (10.1, 78.4) | 12.6 (4.3, 71.8) | .38 |
| DIF (+) (n = 17) | DIF (−) (n = 34) | DIF true positive (n = 17) | DIF false negative (n = 10) | |||
| BP180 | 86.9 (34.5, 141.0) | 3.3 (1.6, 8.8) | <.0001 | 86.9 (34.5, 141.0) | 35.0 (5.6, 73.9) | .08 |
| BP230 | 59.7 (6.3,75.6) | 4.1 (1.5,7.0) | .0021 | 59.7 (6.3, 75.6) | 26.4 (12.6, 60.9) | .76 |
| IIF (+) (N = 19) | IIF (−)(N=32) | IIF true positive (n = 19) | IIF false negative (n = 8) | |||
| BP180 | 74.9 (22.5, 139.7) | 2.8 (1.6, 9.8) | <.0001 | 74.9 (22.5, 139.7) | 32.5 (17.6, 150.1) | .56 |
| BP230 | 60.9 (12.6, 105.9) | 3.5 (1.5, 6.3) | <.0001 | 60.9 (12.6, 105.9) | 12.8 (5.5, 26.4) | .03 |
Data are presented as the median (interquartile range).
Wilcoxon rank sum test.
In the assessment of specific ELISA results compared with C3d results, true positives (C3d positive, ELISA positive) had higher anti-BP180 ELISA results than false negatives (C3d negative, ELISA positive), although this value bordered on statistical significance (P = .06). There was no difference in BP230 ELISAs between true positives and false negatives (Table IV).
On one hand, patients who had positive DIF testing also had significantly higher BP180 and BP230 ELISA results (Table IV), but there was no significant difference in BP180 or BP230 levels between true positives and false negatives. On the other hand, although patients positive by IIF also had significantly higher BP180 and BP230 levels by ELISA (Table IV), true positives by IIF were associated with significantly higher BP230 levels compared with false negatives (Table IV), indicating that positive IIF most closely relates to anti-BP230.
DISCUSSION
In this study, we found that C3d IHC, DIF, and IIF had similar test characteristics for identifying BP using ELISA for anti-BP180 or anti-BP230 as the diagnostic gold standard. Notably, the areas under the receiver operating characteristic curve for each test were not significantly different, suggesting similar discriminative abilities for BP diagnosis. Together, these data suggest that C3d may be used to support a diagnosis of pemphigoid, particularly when fluorescence microscopy may be unavailable or equivocal or frozen tissue sections have not been collected.
We further found that patients with positive C3d IHC, DIF, and IIF had significantly higher levels of BP180 and BP230 on ELISA than those with negative values, confirming the correlation between positive staining and antibody levels. In particular, false-negative C3d IHC may be closely associated with lower anti-BP180, although this effect bordered on significance in our study. On one hand, this difference may be explained with the suggested mechanism that anti-BP180 is actually directly responsible for disease pathogenesis and that the false-negative C3d IHC cohort may have lower BP180 levels that do not activate complement to detectable levels by IHC. On the other hand, false-negative IIF was associated with significantly lower anti-BP230 but not anti-BP180 ELISA. Anti-BP180 IgG does not bind well to monkey esophagus as a substrate compared with normal human skin, which can cause false-negative IIF results in the setting of elevated anti-BP180.19
As previously discussed, prior studies have shown C3d IHC has sensitivities of 97%, 100%, and 90%.10–12 A meta-analysis assessing the utility of IHC for C3d and C4d in patients with BP across 7 studies found that 125 of 134 (93%) stained positive for C3d or C4d, although C3d and C4d may represent different pathways in complement activation, whereby C3d is deposited during the alternative pathway and C4d is deposited from the classical pathway.20 Their results are in line with our findings and suggest the sensitivities of C3d are high, supporting their use as a diagnostic tool. Moreover, our finding that C3d positivity correlates with ELISA, particularly with anti-BP180 levels, is further suggestive of its utility. C3d deposition may provide insight into the pathogenesis of BP, given its reflection of the autoimmune response to the BP180 antigen more than the BP230 antigen.
Although many other studies have compared C3d IHC and DIF, the latter has shortcomings. ELISA is quantitative and reproducible, performs well compared with DIF in many diagnostic studies, and has been used as an acceptable gold standard.14–17,21–23 For our study, we believe ELISA provides an improved BP gold standard for comparison with the C3d IHC testing. Illustrating this point, C3d IHC had a sensitivity of only 40% compared with positive DIF but had a sensitivity of 80% compared with positive ELISA.3 This suggests that the BP gold standard to which C3d IHC is compared directly affects the sensitivity of C3d IHC and prompts the question of whether ELISA may be preferred to DIF in these instances.
In this study, we used samples that were collected for the purposes of histologic diagnosis, which were a mixture of lesional blistered and lesional non-blistered skin, and sensitivity and specificity were calculated in this context. Although our study was not designed to determine the ideal location of the biopsy for histology and C3d IHC, this should be investigated in future studies.
A major limitation of our study is that it is a single-center, retrospective study. Other shortcomings include the lack of identification of antibody isotypes, as it affects their ability to fix complement. Positive linear C3d staining is not entirely specific and could be seen in a mixed IgA/IgG linear IgA disease or epidermolysis bullosa acquisita, both of which may show linear complement.
As mentioned, the use of monkey esophagus compared with human skin increases false-negative IIF rates. Salt-split IIF could also be used as a more specific comparator for our studies instead of IIF alone.
Finally, our study only included patients for whom C3d IHC, ELISA, DIF, and IIF testing were performed, potentially biasing our study towards cases of more diagnostic uncertainty. Moreover, our analysis did not include patients with indeterminate results from the above studies, and further investigation of C3d staining in this population is warranted.
CONCLUSION
This study is unique in that it examines a cohort of patients who were diagnosed with the aid of ELISA testing but corroborates previous reports that have suggested that C3d IHC may be helpful in diagnosing BP. The ability to use IHC on fixed tissue adds to the diagnostic toolkit for BP, potentially reducing the need for additional biopsy specimens or ancillary tests if positive. At institutions where immunofluorescence or ELISA may not be readily available, C3d IHC may be incorporated into a diagnostic pipeline and performed immediately on fixed tissue after biopsy for rapid diagnosis of BP.
CAPSULE SUMMARY.
When enzyme-linked immunosorbent assay is used as the gold standard, C3d immunohistochemistry has a sensitivity and specificity comparable to direct immunofluorescence and indirect immunofluorescence in diagnosing bullous pemphigoid.
C3d immunohistochemistry may be used to support a diagnosis of pemphigoid, particularly if direct immunofluorescence, indirect immunofluorescence, or enzyme-linked immunosorbent assay are not available.
Acknowledgments
Funding sources: Dr Simpson is supported by a Dermatology Foundation Physician-Scientist Career Development Award. Dr Takeshita is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grant K23-AR-068433. This study was supported by a Dermatology Foundation Dermatopathology Career Development Award to Dr Chu.
Abbreviations used:
- BMZ
basement membrane zone
- BP
bullous pemphigoid
- CI
confidence interval
- DIF
direct immunofluorescence
- ELISA
enzyme-linked immunosorbent assay
- IHC
immunohistochemistry
- IIF
indirect immunofluorescence
- ROC
receiver operating characteristic
- TBS
Tris-buffered saline
Footnotes
Conflicts of interest: Dr Novoa has served as consultant for Enspectra Health and received speaker’s honoraria from Novartis Argentina and HealthCert for work unrelated to this manuscript. Dr Takeshita receives a research grant to the Trustees of the University of Pennsylvania from Pfizer Inc for work that is unrelated to this manuscript and received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly. Dr Payne has served as a consultant for SyntImmune Inc, holds equity in Cabaletta Bio, Inc, and has patents licensed by Novartis and Cabaletta Bio, Inc, focused on cellular therapies for autoimmune diseases. Drs Wang, Moshiri, Simpson, and Chu have no conflicts of interest to declare.
IRB approval status: Use of human specimens in this study was provided through University of Pennsylvania Institutional Review Board-approved protocol #820338.
REFERENCES
- 1.Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18(4):513–528. [DOI] [PubMed] [Google Scholar]
- 2.Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol Mech Dis. 2016; 11(1):175–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glauser S, Rutz M, Cazzaniga S, Hegyi I, Borradori L, Beltraminelli H. Diagnostic value of immunohistochemistry on formalin-fixed, paraffin-embedded skin biopsy specimens for bullous pemphigoid. Br J Dermatol. 2016;175(5): 988–993. [DOI] [PubMed] [Google Scholar]
- 4.Kassaby SS, Hicks A, Leicht S, Youngberg GA. Bullous pemphigoid: use of C4d immunofluorescent staining in a case with repeated negative conventional direct immunofluorescence studies. Am J Dermatopathol. 2017;39(12):932–934. [DOI] [PubMed] [Google Scholar]
- 5.Satyapal S, Amladi S, Jerajani HR. Evaluation of salt split technique of immunofluorescence in bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2002;68(6):330–333. [PubMed] [Google Scholar]
- 6.Sakuma-Oyama Y, Powell AM, Oyama N, Albert S, Bhogal BS, Black MM. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151(1):126–131. [DOI] [PubMed] [Google Scholar]
- 7.Roussel A, Benichou J, Randriamanantany ZA, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147(3):293. [DOI] [PubMed] [Google Scholar]
- 8.Muglia C, Bronsnick T, Kirkorian AY, Cha J. Questioning the specificity and sensitivity of ELISA for bullous pemphigoid diagnosis. Cutis. 2017;99(1):E27–E30. [PubMed] [Google Scholar]
- 9.Velez AMA, Googe PB, Howard MS. Immunohistochemistry versus immunofluorescence in the diagnosis of autoimmune blistering diseases. Our Dermatol Online. 2013;4(Suppl 3):585–595. [Google Scholar]
- 10.Al-Shenawy HA-S. Can immunohistochemistry replace immunofluorescence in diagnosis of skin bullous diseases? APMIS. 2017;125(2):114–121. [DOI] [PubMed] [Google Scholar]
- 11.Pfaltz K, Mertz K, Rose C, Scheidegger P, Pfaltz M, Kempf W. C3d immunohistochemistry on formalin-fixed tissue is a valuable tool in the diagnosis of bullous pemphigoid of the skin. J Cutan Pathol. 2010;37(6):654–658. [DOI] [PubMed] [Google Scholar]
- 12.Magro CM, Dyrsen ME. The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59(5):822–833. [DOI] [PubMed] [Google Scholar]
- 13.Chandler W, Zone J, Florell S. C4d immunohistochemical stain is a sensitive method to confirm immunoreactant deposition in formalin-fixed paraffin-embedded tissue in bullous pemphigoid. J Cutan Pathol. 2009;36(6):655–659. [DOI] [PubMed] [Google Scholar]
- 14.van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarian H, Saponeri A, Michelotto A, Zattra E, Belloni-Fortina A, Alaibac M. Biochip technology for the serological diagnosis of bullous pemphigoid. ISRN Dermatol. 2012;2012:237802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirotsu K, Chiou AS, Chiang A, Kim J, Kwong BY, Pugliese S. Localized bullous pemphigoid in a melanoma patient with dual exposure to PD-1 checkpoint inhibition and radiation therapy. JAAD Case Rep. 2017;3(5):404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao CY, Murrell DF. Advances in understanding and managing bullous pemphigoid. F1000Res. 2015;4 10.12688/f1000research.6896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Li L, Xia Y. BP180 is critical in the autoimmunity of bullous pemphigoid. Front Immunol. 2017;8:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emtenani S, Yuan H, Lin C, et al. Normal human skin is superior to monkey oesophagus substrate for detection of circulating BP180-NC16A-specific IgG antibodies in bullous pemphigoid. Br J Dermatol. 2019;180(5):1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akbari Oryani M, Safaei M, Farzam F, Razmara H, Fathi N. C4d and C3d immunohistochemical evaluation on formalin-fixed paraffin-embedded tissue for the diagnosis of bullous pemphigoid: systematic review of the literatures. Rev Clin Med. 2017;4(1):26–30. [Google Scholar]
- 21.Eckardt J, Eberle FC, Ghoreschi K. Diagnostic value of autoantibody titres in patients with bullous pemphigoid. Eur J Dermatol. 2018;28(1):3–12. [DOI] [PubMed] [Google Scholar]
- 22.Horváth ON, Varga R, Kaneda M, Schmidt E, Ruzicka T, Sárdy M. Diagnostic performance of the ‘‘MESACUP anti-Skin profile TEST’’. Eur J Dermatol. 2016;26(1):56–63. [DOI] [PubMed] [Google Scholar]
- 23.van Beek N, Dähnrich C, Johannsen N, et al. Prospective studies on the routine use of a novel multivariant enzyme-linked immunosorbent assay for the diagnosis of autoimmune bullous diseases. J Am Acad Dermatol. 2017;76(5):889–894.e5. [DOI] [PubMed] [Google Scholar]