Abstract
We compared GABAergic function and neuronal excitability in the hippocampal tissue of seven sporadic MTLE patients with a patient carrying a SCN1A loss‐of‐function mutation. All had excellent outcome from anterior temporal lobectomy, and neuropathological study always showed characteristic hippocampal sclerosis (Hs). Compared to MTLE patients, there was a more severe impairment of GABAergic transmission, due to the lower GABAergic activity related to the NaV1.1 loss‐of‐function, in addition to the typical GABA‐current rundown, a hallmark of sporadic MTLE. Our results give evidence that a pharmacological rescuing of the GABAergic dysfunction may represent a promising strategy for the treatment of these patients.
Introduction
Mesial temporal lobe epilepsy (MTLE) with hippocampal sclerosis (Hs) represents the most common type of focal drug‐resistant epilepsy. 1 Although MTLE is now routinely treated with neurosurgery, over a third of MTLE patients do not achieve seizure freedom, 2 and surgery can have important adverse consequences. Better treatment options, or even prevention, of MTLE and Hs are therefore needed, but rational therapy remains elusive because their causes remain unclear. A large body of evidence indicates the biologic processes that are relevant in the pathogenesis of Hs include glial activation, immune response, synaptic transmission, signal transduction, ions transport, and synaptic plasticity. 3 , 4 In the hippocampus, GABAergic inhibitory dysfunction mostly contributes to hyperexcitability in MTLE with Hs. 5 , 6 , 7
Of interest, recent studies illustrate SCN1A involvement in the epileptogenic neuronal network underlying MTLE associated with Hs. 8 Accordingly, we already illustrated that MTLE with febrile seizures may be part of the epileptic phenotype encountered in a large family carrying a SCN1A mutation. 9 , 10 This M145T mutation of SCN1A cosegregated in all affected individuals of a large family affected by simple febrile seizures (FS), three of whom later developed MTLE. 10 Most important, the M145T mutation is located in a highly conserved amino acid in the first transmembrane segment of domain I of the SCN1A, and functional studies in mammalian cells demonstrated that the M145T mutation causes a 60% reduction of current density and a 10 mV positive shift of the activation curve. 9 More recently, one family member with refractory MTLE and Hs has benefited from surgery, and now we wish to report the extensive neurophysiological study from the resected hippocampal tissue of this patient, in comparison with seven sporadic patients with refractory MTLE and Hs. We believe that this study may provide important new directions to the physiopathological comprehension of MTLE with Hs and FS, and ultimately may pave the road to develop new therapeutic approaches in MTLE.
Patients and Methods
Patients
The clinical data of seven sporadic MTLE patients with Hs, whose hippocampal tissues were used for electrophysiological recordings, are described in detail in Supplemental Material and summarized in Table S1. The patient (mutated, mNaV1.1) carrying a loss‐of‐function missense mutation (M145T) of SCN1A is a 31‐year‐old man with MTLE, whose electroclinical data were reported in detail elsewhere. 9 , 10 He had simple FS every 3–6 months from the age of 8 months until 70 months. At the age of 13 years, he began to have afebrile seizures characterized by behavioral arrest, some lip smacking and gestural automatisms, or nocturnal secondary generalized motor seizures. Over the years, he also began to experience epigastric auras with some experiential phenomena prior to the loss of awareness. Since seizures became refractory to antiepileptic drugs (AEDs) that included oxcarbazepine, phenobarbital, topiramate, and valproate, and he underwent an extensive presurgical evaluation. Interictal awake and sleep EEG recording showed bilateral mesiotemporal epileptiform spikes, which strongly predominated (>80%) on the right side. On intensive video‐polygraphic monitoring, several typical seizures with onset in the right midinferomesial temporal region were recorded. Brain MRI revealed right Hs and, at the age of 27, he underwent surgery, in the form of right anterio‐temporal lobectomy. Histopathological findings disclosed type 2 Hs with the characteristic pattern of predominant neuronal loss in area CA1. 3 , 4 Since surgery, in the last 50 months, he has only had non‐disabling auras (Engel’s outcome classification Class 1B), and he has been taking a dual therapy with carbamazepine plus valproate.
Electrophysiology
See Supplemental Material for details. The study was approved by the local Ethics Committees.
Results
Patch‐clamp experiments in human slices
To highlight the functional implications of mNaV 1.1 expression, patch‐clamp experiments were performed on pyramidal cells and interneurons of the resected hippocampus of the mNaV1.1 patient, comparing the results with those obtained from sporadic MTLE patients (patients, # 4‐8, Table S1). Current steps (100–150 pA, 500 ms duration) applied to the cells were able to elicit one or more action potentials (APs), with pyramidal neurons from all patients exhibiting APs with similar characteristics, in particular with no change in AP threshold (MTLE: −53 ± 3 mV; n = 10; # 4‐8, Table S1, mNaV 1.1 patient: −53 ± 3 mV; n = 5; P = 0.64; Fig. 1A,B). By contrast, three interneurons from the mNaV1.1 patient showed a significantly depolarized AP threshold (−40 ± 3 mV) in comparison to interneurons from MTLE patients (−58 ± 12 mV, n = 5; # 4‐8, Table S1; P = 0.04; Fig. 1A,B, see Table S2 for electrophysiological parameters). These data suggest that mNaV1.1 expressing interneurons may have a reduced intrinsic excitability as expected from M145T mutation of SCN1A. 9
Figure 1.
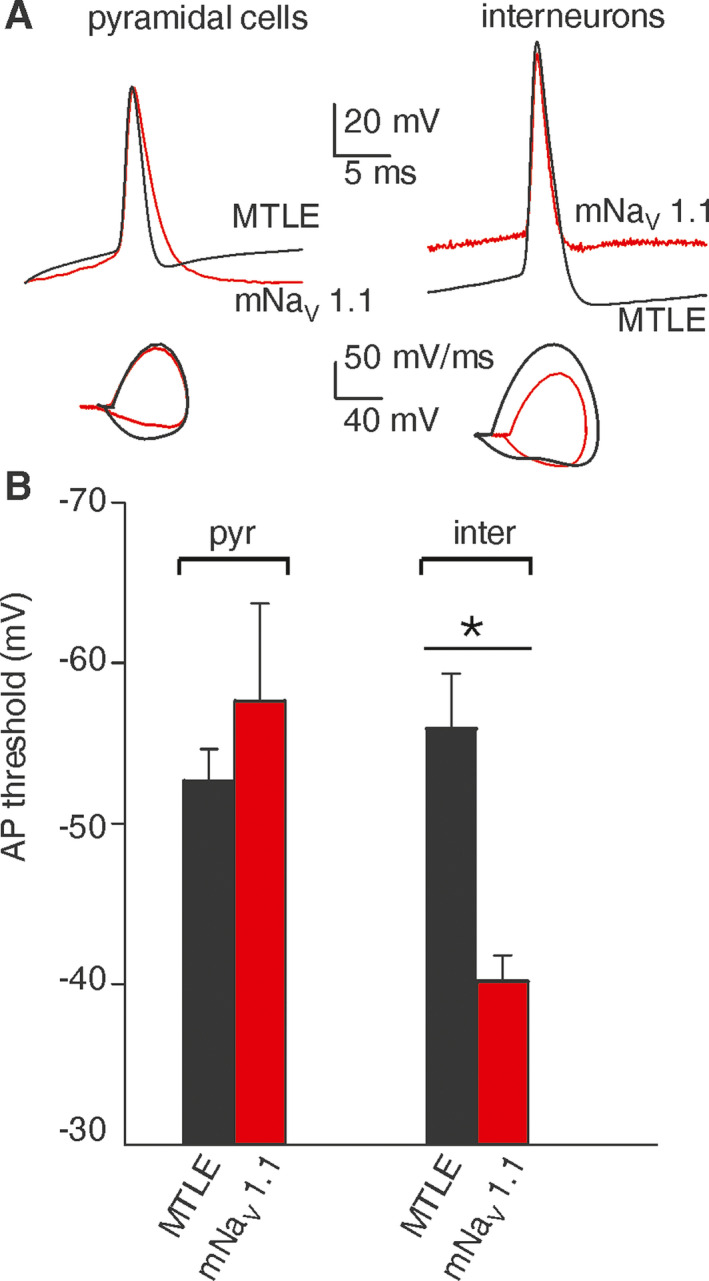
mNaV 1.1 patient exhibits depolarized action potential (AP) threshold in hippocampal interneurons but not in pyramidal cells. (A) Left top, superimposed AP traces recorded from one pyramidal neuron of the mNaV 1.1 patient (red trace) and from one MTLE patient (black trace, #5); left bottom, superimposed phase‐plane plot obtained from APs showed on top. Right top, superimposed AP traces recorded from one interneuron of mNaV 1.1 patient (red trace) and from the same MTLE patient (black trace); right bottom, superimposed phase‐plane plot obtained from APs showed on top. (B) Bar graph representing the mean action potential threshold values recorded from pyramidal neurons (pyr; n = 10) and interneurons (inter; n = 5) in resected hippocampi of patients with MTLE (black bars, #4‐8, Table S1) and from pyramidal cells (pyr; n = 5) and interneurons (inter; n = 3) of mNaV 1.1 patient (red bars). *P = 0.044; One‐way ANOVA Test, and post hoc Holm–Sidak test.
GABA currents rundown
To investigate how GABA currents respond in mNaV1.1 patient’s hippocampus, we performed microtransplantation experiments injecting oocytes with hippocampal tissues from sporadic MTLE patients (without known genetic mutations and with Hs, Fig. 2A), one control individual (Fig. 2B) and mNaV 1.1 patient (Fig. 2C‐E). The results confirmed that a strong, pathological GABA‐current rundown is present in drug‐resistant MTLE hippocampi (49.0 ± 9.1%; n = 33; patients #1,2,4,5 Table S1; Fig. 2A), whereas the same phenomenon was absent in the control (75.8 ± 7.0 %; n = 10; # 3, Table S1; Fig. 2B) as previously shown. 5 , 6 , 7
Figure 2.
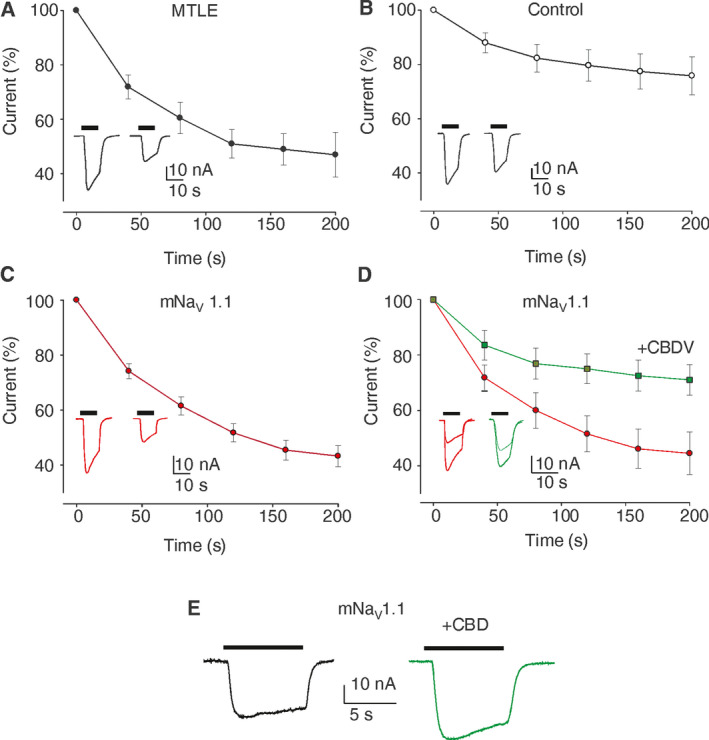
mNaV 1.1 patient exhibits a GABA current rundown similar to MTLE hippocampal tissues. (A) Oocytes injected with hippocampal membranes of MTLE patients (n = 33; Imax = 40.5 ± 20 nA; #1,2, 4,5 Table S1). Black dots (●) represent the amplitude of consecutive GABA currents as % of the first response (GABA 500 µM). Data points represent means ± SEM. In this and following panels all the currents are normalized to the first current (Imax) and black horizontal bars represent the timing of GABA application. In all experiments, the holding potential was −60 mV. Inset: Representative current traces elicited by the first and sixth GABA application (500 µM, horizontal bar); (B) Oocytes injected with hippocampal membranes from a non‐epileptic control (n = 10; Imax = 49.5 ± 17.3 nA). White dots (○) represent the amplitude of consecutive GABA currents as % of the first response (GABA 500 µM). Inset: Representative current traces as in (A). (C) Oocytes injected with hippocampal membranes from mNaV 1.1 patient (n = 19; Imax = 30.8 ± 5.7 nA). Red dots ( ) represent the amplitude of consecutive GABA currents as % of the first response (GABA 500 µM). (Inset) Representative current traces as in (A). Note the current rundown similar to MTLE patients. (D) Oocytes injected with hippocampal membranes from the mNaV 1.1 patient before and after CBDV treatment. Red dots (
) represent the amplitude of consecutive GABA currents as % of the first response (GABA 500 µM). (Inset) Representative current traces as in (A). Note the current rundown similar to MTLE patients. (D) Oocytes injected with hippocampal membranes from the mNaV 1.1 patient before and after CBDV treatment. Red dots ( ) represent the amplitude of consecutive GABA currents as % of the first response (GABA 500 µM) while the green squares
) represent the amplitude of consecutive GABA currents as % of the first response (GABA 500 µM) while the green squares  ) represent the amplitude of consecutive GABA currents on the same cells after 2 hours of incubation with CBDV 50 nM. Data points represent means ± SEM [
) represent the amplitude of consecutive GABA currents on the same cells after 2 hours of incubation with CBDV 50 nM. Data points represent means ± SEM [ ) Imax = 30.7 ± 8.0 nA; (
) Imax = 30.7 ± 8.0 nA; ( ) Imax = 27.8 ± 7 nA)]. Inset: Representative GABA current traces as in (A), before (red traces) and after 50 nM CBDV application (green traces) as indicated. P = 0.002, n = 19 by Shapiro–Wilk test and Student's t‐test. (E) Representative GABA current traces evoked by 50 µM GABA in one oocyte of 13 injected with membranes of mNaV 1.1 before (black trace) and after CBD co‐application (5 µM, green trace).
) Imax = 27.8 ± 7 nA)]. Inset: Representative GABA current traces as in (A), before (red traces) and after 50 nM CBDV application (green traces) as indicated. P = 0.002, n = 19 by Shapiro–Wilk test and Student's t‐test. (E) Representative GABA current traces evoked by 50 µM GABA in one oocyte of 13 injected with membranes of mNaV 1.1 before (black trace) and after CBD co‐application (5 µM, green trace).
Interestingly, the sclerotic hippocampus of mNaV 1.1 patient also showed a GABAergic rundown (43.2 ± 3.1 %; n = 19; Fig. 2C) similar to sporadic MTLE patients. This current rundown was mitigated by pretreatment of 2 hours with endogenous factors, as BDNF, or exogenous agents, as the cannabis derivative cannabidivarine (CBDV, Fig. 2D; Table S3) showing again a strong similarity with data previously published for MTLE patients. 5 , 11
Interestingly, we found that acute co‐application of cannabidiol (CBD, 5 µM, GABA 50 µM), which has been reported to be clinically effective in Dravet syndrome, 12 potentiated both GABA‐current amplitude from mNaV 1.1 patient (IGABA; +29.8 ± 4.1 %; n = 13; Fig. 2E, Table S3) and from sporadic MTLE patients (IGABA; +27.5 ± 8.8 %; n = 22; #1,2, Table S3). 13
Discussion
The novelty of our study is that we performed electrophysiological experiments from an exclusive hippocampal tissue obtained from a patient with MTLE and Hs, who also carried the M145T SCN1A loss‐of‐function mutation. Compared to hippocampal tissue from MTLE patients, there was a more severe impairment of GABAergic transmission, due to the lower GABAergic activity related to the NaV1.1 loss‐of‐function, in addition to the characteristic GABA‐current rundown, a hallmark of MTLE. 5 , 6 , 7 , 11
We recorded valuable APs on both pyramidal neurons and interneurons from mNaV 1.1 and five (of seven) sporadic MTLE patients. In all of them, the histopathological study showed the features of Hs related to MTLE, with the characteristic pattern of neuronal loss in CA1 (see Table S1). 3 , 4 Nonetheless, only in hippocampal mNaV 1.1 slices, AP threshold was more depolarized in interneurons than in pyramidal neurons, reflecting a reduced excitability of GABAergic interneurons related to SCN1A mutation. 14 , 15 In spite of the limited number of recordings on mNav 1.1 interneurons, this type of AP responses paralleled the functional data obtained from cells transfected with M145T mutated cDNA. 9
Because of the limited quantity of fresh tissue from the patient and technical difficulty of recordings from acute hippocampal slices, part of the tissue was snap‐frozen and used for recordings in microtransplanted oocytes. 6 , 16 Notably, we found a GABA current rundown that was very similar to the one recorded from hippocampi of sporadic MTLE patients. 5 , 6 , 7 , 11 The GABA current rundown is considered an electrophysiological dysfunction of refractory human MTLE, 5 , 6 , 7 which may explain, at least in part, the hyperexcitability underlying the ictogenesis in these patients. Interestingly, we previously showed in epileptic rats that GABA current rundown arises from the hippocampal region (during status epilepticus) then spreading to temporal cortex in the chronic phase of seizures. 7 , 17
We already emphasized that the pharmacological modulation of the GABAergic impairment (i.e., GABA current rundown) underlying MTLE may represent an interesting therapeutic approach in pharmacoresistant MTLE. 17 In this way, it is not surprising that BDNF could ameliorate the GABA current rundown both in the mNaV 1.1 and sporadic MTLE patients, as previously reported for both refractory human MTLE 5 and preclinical animal models of MTLE. 7 Notably, CBDV continues to show its potential in recovering the mNav 1.1 GABAergic rundown as previously shown for MTLE. 11 Furthermore, CBD was capable to increase GABA current amplitude in mNaV 1.1 and MTLE patients, as reported in Dravet syndrome and tuberous sclerosis complex. 12 , 13 , 18 Altogether our results emphasize the role of cannabis derivatives as treatment for refractory epilepsies.
In our patient, the overall clinical, pharmacological, and neuropathological findings were consistent with those observed in sporadic MTLE with Hs, including the excellent outcome from anterior temporal lobectomy. In this way, our findings reinforce the belief that known genetic defect, which is present diffusely in the brain, do not necessarily preclude a good prognosis following epilepsy surgery, if surgery is a reasonable option based on the concordance of other data during presurgical evaluation. It cannot be excluded, however, that the milder loss‐of‐function impairment of SCN1A related to the M145T mutation could also influence the better postsurgical outcome.
Important, as previously reported in Dravet syndrome and tuberous sclerosis complex, 13 , 18 CBD was capable to increase GABA current amplitude in mNaV 1.1 and MTLE patients, that was recently approved as treatment for Dravet and Lennox–Gastaut syndromes. 12 Thus, in accordance with the “interneuron hypothesis,” 14 our findings support the assumption that seizures in mNaV 1.1 patient could arise from a minor GABA release from interneurons together with a reduction of GABAergic postsynaptic efficacy after repetitive stimulation (i.e., rundown). Major therapeutic implication of these findings is that by rescuing the GABAergic inhibitory activity it is possible to improve the clinical outcome of this kind of patients. In this way, this study ultimately may pave the road to develop new antiepileptic approaches in epileptic patients with Hs.
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Supporting information
MTLE Patients. Detailed description of patients.
Table S1. Clinical characteristics and neurophysiological findings of MTLE patients.
Electrophysiology. Patch‐clamp in human slices; Membrane Preparation, Injection Procedure, and voltage‐clamp Recordings in Oocytes.
Table S2. Electrophysiological parameters in patch‐clamped human neurons.
Table S3. Effects of pharmacological agents on mNaV1.1 and MTLE patients.
Figure S1. Firing of hippocampal interneurons.
Statistics. Detailed description of the statistical analysis.
Acknowledgments
The work was supported by grants from AICE‐FIRE; UCB Pharma, Brussels, Belgium (IIS‐2014‐101617, E.P.); “Bando di Ateneo (Sapienza University),” grant no RM1181642D8BD87B. G.R. was supported by a fellowship from “Istituto Pasteur Italia – Fondazione Cenci Bolognetti.” We are grateful to all the families and patients who were recruited in the studies.
Funding Statement
This work was funded by AICE‐FIRE grant ; UCB grant IIS‐2014‐101617; Sapienza University grant RM1181642D8BD87B; Istituto Pasteur Italia – Fondazione Cenci Bolognetti grant .
Contributor Information
Eleonora Palma, Email: eleonora.palma@uniroma1.it.
Antonio Gambardella, Email: a.gambardella@unicz.it.
References
- 1. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685. [DOI] [PubMed] [Google Scholar]
- 2. De Tisi J, Bell GS, Peacock JL, et al. The long‐term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 2011;378:1388–1395. [DOI] [PubMed] [Google Scholar]
- 3. Blumcke I, Thom M, Aronica E, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013;54:1315–1329. [DOI] [PubMed] [Google Scholar]
- 4. Thom M. Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol 2014;40:520–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ragozzino D, Palma E, Di Angelantonio S, et al. Rundown of GABA type A receptors is a dysfunction associated with human drug‐resistant mesial temporal lobe epilepsy. Proc Natl Acad Sci USA 2005;102:15219–15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palma E, Esposito V, Mileo AM, et al. Expression of human epileptic temporal lobe neurotransmitter receptors in Xenopus oocytes: an innovative approach to study epilepsy. Proc Natl Acad Sci USA 2002;99:15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cifelli P, Palma E, Roseti C, et al. Changes in the sensitivity of GABAA current rundown to drug treatments in a model of temporal lobe epilepsy. Front Cell Neurosci 2013;7:108 DOI: 10.3389/fncel.2013.00108. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasperaviciute D, Catarino CB, Matarin M, et al. Epilepsy, hippocampal sclerosis and febrile seizures linked by common genetic variation around SCN1A. Brain 2013;136:3140–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mantegazza M, Gambardella A, Rusconi R, et al. Identification of an Nav1.1 sodium channel (SCN1A) loss‐of‐function mutation associated with familial simple febrile seizures. Proc Natl Acad Sci USA 2005;102:18177–18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colosimo E, Gambardella A, Mantegazza M, et al. Electroclinical features of a family with simple febrile seizures and temporal lobe epilepsy associated with SCN1A loss‐of‐function mutation. Epilepsia 2007;48:1691–1696. [DOI] [PubMed] [Google Scholar]
- 11. Morano A, Cifelli P, Nencini P, et al. Cannabis in epilepsy: from clinical practice to basic research focusing on the possible role of cannabidivarin. Epilepsia Open 2016;1:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arzimanoglou A, Brandl U, Cross JH, Other members of The Cannabinoids International Experts Panel . Epilepsy and cannabidiol: a guide to treatment. Epileptic Disord 2020;22(1):1–14. 10.1684/epd.2020.1141. [DOI] [PubMed] [Google Scholar]
- 13. Ruffolo G, Cifelli P, Roseti C, et al. A novel GABAergic dysfunction in human Dravet syndrome. Epilepsia 2018;59:2106–2117. [DOI] [PubMed] [Google Scholar]
- 14. Chopra R, Isom LL. Untangling the Dravet syndrome seizure network: the changing face of a rare genetic epilepsy. Epilepsy Curr. 2014;14(2):86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheah CS, Yu FH, Westenbroek RE, et al. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA 2012;109(36):14646–14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eusebi F, Palma E, Amici M, et al. Microtransplantation of ligand‐gated receptor‐channels from fresh or frozen nervous tissue into Xenopus oocytes: a potent tool for expanding functional information. Prog Neurogibol 2009;88:32–40. [DOI] [PubMed] [Google Scholar]
- 17. Gambardella A, Labate A, Cifelli P, et al. Pharmacological modulation in temporal lobe epilepsy: current status and future perspectives. Pharmacol Res 2016;113:421–425. [DOI] [PubMed] [Google Scholar]
- 18. Palma E, Ruffolo G, Cifelli P, et al. Modulation of GABAA Receptors in the Treatment of Epilepsy. Curr Pharm Des 2017:23(37), 5563–5568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MTLE Patients. Detailed description of patients.
Table S1. Clinical characteristics and neurophysiological findings of MTLE patients.
Electrophysiology. Patch‐clamp in human slices; Membrane Preparation, Injection Procedure, and voltage‐clamp Recordings in Oocytes.
Table S2. Electrophysiological parameters in patch‐clamped human neurons.
Table S3. Effects of pharmacological agents on mNaV1.1 and MTLE patients.
Figure S1. Firing of hippocampal interneurons.
Statistics. Detailed description of the statistical analysis.


