Abstract
Objectives.
Determine whether adults with childhood-onset systemic lupus erythematosus (cSLE) are at increased risk for disease- and steroid-related damage as compared to individuals with adult-onset SLE (aSLE), and whether they continue to accumulate disease damage in adulthood.
Methods.
Data derive from the 2007–2015 cycles of the Lupus Outcomes Study, a longitudinal cohort of adults with confirmed SLE. The Brief Index of Lupus Damage (BILD), a validated, patient-reported measure, was used to assess SLE-associated damage. Participants with baseline BILD were included (N=1035). Diagnosis at age <18 years was defined as cSLE (N=113). Outcome variables included BILD score at baseline and follow-up, clinically significant change in BILD score over follow-up period, and presence of steroid-related damage (cataracts, osteoporosis-related fracture, avascular necrosis or diabetes mellitus).
Results.
Mean time between baseline and follow up BILD assessment was 6.3±1.7 years. In adjusted analyses, participants with cSLE and aSLE had similar levels of disease-related damage, and accumulated damage at similar rates. Participants with cSLE were more likely to report steroid-related damage (OR 1.7, 95% CI 1.1–2.8) in the adjusted analysis as compared to those with aSLE. Likelihood of steroid-related damage increased with disease duration for both groups, but was consistently higher among cSLE participants.
Conclusion.
In this longitudinal cohort of adults with SLE, participants continued to accumulate damage at similar rates over time, regardless of age at onset or disease duration. Childhood-onset predicted increased risk of steroid-related damage. Aggressive use of steroid-sparing treatment strategies during childhood may be important to prevent steroid-related damage in adulthood.
Keywords: childhood-onset systemic lupus erythematosus, adult-onset systemic lupus erythematosus, disease related damage, steroid related damage, Brief Index of Lupus Damage (BILD) score
Introduction
Disease damage has been shown to predict future mortality in SLE, with higher Systemic Lupus Damage Index (SDI) and Brief Index of Lupus Damage (BILD) scores both correlating with increased risk of premature death (1,2). While disease-related damage is prevalent in SLE, previous studies suggest that childhood-onset SLE (cSLE) may be associated with increased disease damage as compared to adult-onset SLE (aSLE) (3,4).
Due to the difficulty of following patients from pediatric to adult care settings, little is known about adult outcomes of childhood-onset SLE (5,6). Lim et al. recently published longitudinal data on long-term outcomes of juvenile-onset SLE in a Canadian inception cohort (7), which used the SDI to assess damage. The median duration of follow up was 5.6 years (25th-75th percentile 3.0–10.8) and the median age at last follow-up was 19.7 years (25th-75th percentile 17.2–24.4). Cataracts (14%), avascular necrosis (10%), and osteoporosis (5%) were the most frequently-reported damage items. Damage continued to accrue over the entire disease course for childhood-onset SLE patients, with no plateau in the damage trajectory identified. However, many of these patients were still adolescents at final follow up, and their damage accrual was not compared to individuals with adult-onset SLE.
The purpose of the current study was to assess SLE-related damage and damage progression over time among adults with cSLE versus aSLE using the BILD. In addition, since individuals with cSLE demonstrate a higher incidence of renal and CNS disease requiring aggressive treatment (5,8–10), we sought to determine whether childhood-onset disease increases the risk of steroid-related damage.
Patients and Methods
Data sources:
Data derive from the 2007–2015 cycles of Lupus Outcome Study (LOS). The LOS is a longitudinal, U.S.-based cohort of over 1200 adults with SLE, 10% of whom had disease onset in childhood (defined as age at diagnosis <18 years). Details regarding LOS eligibility and enrollment are described elsewhere (11). Briefly, participants were recruited from community (70%) and clinical (30%) sources, with data collected annually via telephone by trained interviewers. All participants had a confirmed diagnosis of SLE according to chart review supervised by a rheumatologist, using the American College of Rheumatology (ACR) classification criteria for SLE (12).
Measures:
Primary outcome measures were 1) total damage scores at baseline and follow-up, 2) the presence of a clinically significant change in damage (≥2) over the follow-up period, defined as the minimum clinically important difference (MCID) in damage (13); and 3) the presence of steroid-related damage, reported as a component of the BILD assessments.
Damage was assessed using the BILD, a validated, patient-reported measure of lupus disease damage. Studies examining disease damage in SLE have traditionally relied on a physician-reported measure, the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) (14). The SDI requires a trained physician with either comprehensive knowledge of the patient or access to comprehensive medical records to complete a 41-item questionnaire. The BILD was developed as a proxy for the SDI, in order to address the need for a patient-reported damage measure in research, (15) particularly when physician assessment is not feasible. The BILD captures information on 26 of the original 41 SDI items, with a score range of 0–28 (Supplement 1). Disease manifestations that were not included in the BILD score are either considered to be too rare to be likely to contribute meaningfully to the score (e.g., shrinking lung), or not likely to be interpreted with enough specificity to capture the concept of damage (e.g., alopecia). The observed agreement between BILD items and the SDI was shown to range from 75–100%, with moderately high Spearman rank correlation of 0.64 (p<0.001) (15). The BILD has been shown to differentiate between high and low levels of SLE damage, with higher levels of damage predicting mortality (16). The MCID was previously defined as an increase of 2 or more points in the BILD score, which has been shown to predict an elevated risk of mortality (16).
Authors have previously categorized items from the SDI damage score into 3 groups in accordance with their likely relationship to steroids: definitely steroid related, possibly steroid related, and not steroid related (17). Cataracts and musculoskeletal items (osteoporosis with fracture and avascular necrosis) were defined as “definitely steroid related,” and diabetes was defined as “possibly steroid related”. In our study, steroid-related damage was defined as ocular, metabolic or skeletal complications of steroid use as identified by the BILD assessment: cataracts, osteoporosis resulting in fracture, avascular necrosis (AVN) or diabetes mellitus (DM). Previous work has found high levels of agreement between these four BILD items and the SDI, with percent agreement and adjusted kappa scores of 93% (K=0.85) for cataracts, 95% (K=0.9) for osteoporosis, 96% (K=0.93) for AVN and 90% (K=0.8) for DM (15).
The primary predictor variable was cSLE, defined as age <18 years at diagnosis. Demographic predictors included sex, race/ethnicity and age at baseline damage assessment. Race/ethnicity was categorized as White, Hispanic, African American, Asian and other. To account for the fact that the incidence of cataracts, osteoporosis and diabetes increase with age, we adjusted all multivariable analyses to age at baseline assessment. SLE-related predictors included disease duration, history of steroid use ever, and history of cyclophosphamide use. Cyclophosphamide use was included as a proxy for disease severity. Disease duration was categorized (0–10 years, 11–20 years, and >20 years) to account for the collinear relationship among current age, age at SLE onset and disease duration in statistical analyses.
Statistical analysis:
First, baseline characteristics of the cSLE and aSLE groups were summarized and compared using bivariate statistics (Student’s t-test, rank sum and chi-square test), as appropriate. Second, mean BILD scores at baseline and at follow-up among participants with cSLE and aSLE were compared using adjusted negative binomial regression, controlling for sex and ethnicity, as well as age and disease duration at baseline BILD score. Third, multivariable logistic regression was used to compare unadjusted and adjusted odds of a clinically significant change in BILD score between baseline and follow-up assessment, adjusting the covariates above plus time between baseline and follow-up assessments. Fourth, multivariable logistic regression was used to compare unadjusted and adjusted odds of steroid-related damage among participants with cSLE and aSLE, reported as part of either the BILD score assessments. We developed two multivariable models, with the first model examining the association between cSLE and steroid-related damage, adjusting for age at baseline BILD assessment and history of steroid use. The second model is built on the previous model, including additional demographic factors (sex and ethnicity), as well as disease duration and cyclophosphamide use (SLE-related predictors that demonstrated p<0.2 in univariate analysis). Using this final model, we also estimated the adjusted prevalence of steroid-related damage for cSLE and aSLE by categories of disease duration. Statistical analyses were performed using STATA 13.0 (StataCorp, College Station, TX.)
Results
Demographics:
The study population consisted of 1035 participants with SLE, including 113 (11%) with cSLE. The baseline characteristics of the cSLE and aSLE subgroups are described in Table 1. As compared to participants with aSLE, those with cSLE were younger at the time of baseline BILD score (mean age 32±10 versus 50±13 years; p<0.001), more likely to be male (12% versus 7%; p=0.1) and less likely to be white (41% versus 60%; p<0.001). Mean age at diagnosis was 14±3 years (range 2–17) versus 36±12 years (range 18–75). Participants with cSLE were more likely to have ever required dialysis (19% versus 8%; p<0.001), to have undergone kidney transplantation (17% versus 5%; p<0.001) and to have been diagnosed with lupus nephritis (59% versus 25%; p<0.001). Participants with cSLE were also more likely to have been treated with cyclophosphamide (25% versus 16%; p=0.01). Nearly all cSLE and aSLE participants reported a history of steroid use (99% versus 95%; p=0.02).
Table 1:
Baseline characteristics of 1,035 childhood- and adult- onset SLE patients enrolled in Lupus Outcome Study
| cSLE(N=113) | aSLE(N=922) | P | |
|---|---|---|---|
| Sociodemographics | N (%) or Mean ± SD or median (range) | ||
| Age at baseline BILDa score, years | 32±10 | 50±13 | <0.001 |
| Age at diagnosis, years | 14±3 | 36±12 | <0.001 |
| Sex, female | 100 (88%) | 855 (93%) | 0.1 |
| Ethnicity, non white | 67 (59%) | 367 (40%) | 0.001 |
| SLE Characteristics | |||
| Disease duration at baseline BILD, years | 16 (4,50) | 13 (0,49) | <0.001 |
| Dialysis ever | 22 (19%) | 73 (8%) | <0.001 |
| Renal transplant ever | 19 (17%) | 44 (5%) | <0.001 |
| History of lupus nephritisb | 67 (59%) | 232 (25%) | <0.001 |
| Cyclophosphamide use ever | 28 (25%) | 144 (16%) | 0.01 |
| Steroid use ever | 112 (99%) | 749 (95%) | 0.07 |
| Steroid-associated variables | |||
| Cataract | 28 (25%) | 404 (44%) | <0.001 |
| Osteoporosis with Fracture | 13 (12%) | 169 (18%) | 0.07 |
| Avascular necrosis | 16 (14%) | 72 (8%) | 0.02 |
| Diabetes | 9 (8%) | 123 (13%) | 0.1 |
BILD= Brief Index of Lupus Damage
Met ACR SLE criteria for nephritis at study entry
Disease-related damage
Baseline BILD scores were similar between groups, ranging from 0 – 13 with a median of 1 in the cSLE group, and 0–12 with a median of 2 in the aSLE group (N=1035). When negative binomial regression was used to control for sex, race/ethnicity, baseline age and baseline disease duration, the baseline damage score did not significantly differ between groups, with adjusted mean of 2.4 (95% CI 1.9, 2.9) among participants with cSLE versus mean of 2.1 (95% CI 2.0, 2.2) among those with aSLE (Figure 1A).
Figure 1: Adjusted mean SLE-related damage at baseline (N=1035) and follow up (N=729) assessments, by age at disease onset.
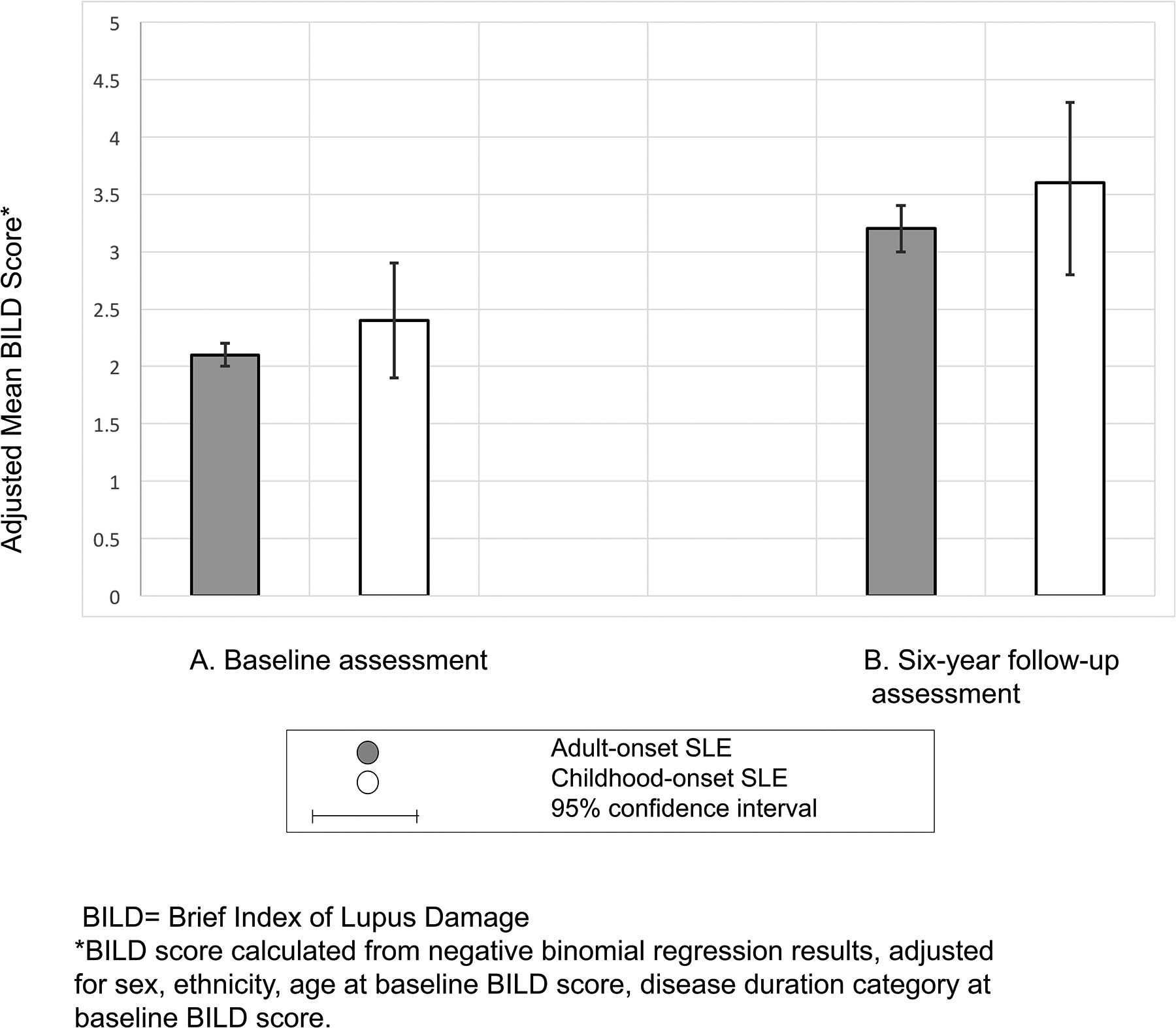
Adjusted for sex, ethnicity, age at baseline and disease duration at baseline. The BILD damage score was used as a damage measure.
Follow-up BILD scores, assessed 6.3±1.7 years after baseline, were available for 729 (70.4%) of the cohort. Follow-up scores ranged from 0 – 11 with a median of 2 among the cSLE group and 0–18 with a median of 2 for the aSLE group (N=729). Mean BILD scores were again similar in the two groups when controlling for sex, race/ethnicity, age at baseline assessment and disease duration at baseline. Mean BILD score was 3.6 (95% CI 2.8, 4.3) for the cSLE group versus 3.2 (95% CI 3, 3.4) for the aSLE group (Figure 1B).
While cSLE did not independently predict total disease damage, disease duration did predict total damage (Figure 2). At baseline assessment, participants with less than 10 years of disease duration had a mean BILD score of 1.7 (95%CI 1.5, 1.9), while those with 10–20 years of disease duration had a mean score of 1.9 (95%CI 1.7, 2.0, p=0.2). Participants with more than 20 years of disease duration at baseline assessment had a mean BILD score of 3.0 (95% CI 2.6, 3.3), which differed significantly from those with less than 10 years of disease (p<0.001) (Figure 2A). A similar pattern was observed at follow-up BILD assessment, with mean a BILD score 4.1 (95%CI 3.6, 4.7) after 20 years of disease, as compared to mean BILD score of 2.7 (95%CI 2.4, 3) among participants with less than 10 years of disease (p<0.001) (Figure 2B). Mean BILD score among those with disease duration of 10–20 years at follow-up was 3.0 (95%CI 2.8, 3.4).
Figure 2: Adjusted mean SLE-related damage at baseline (N=1035) and follow up (N=729) assessments, by disease duration.
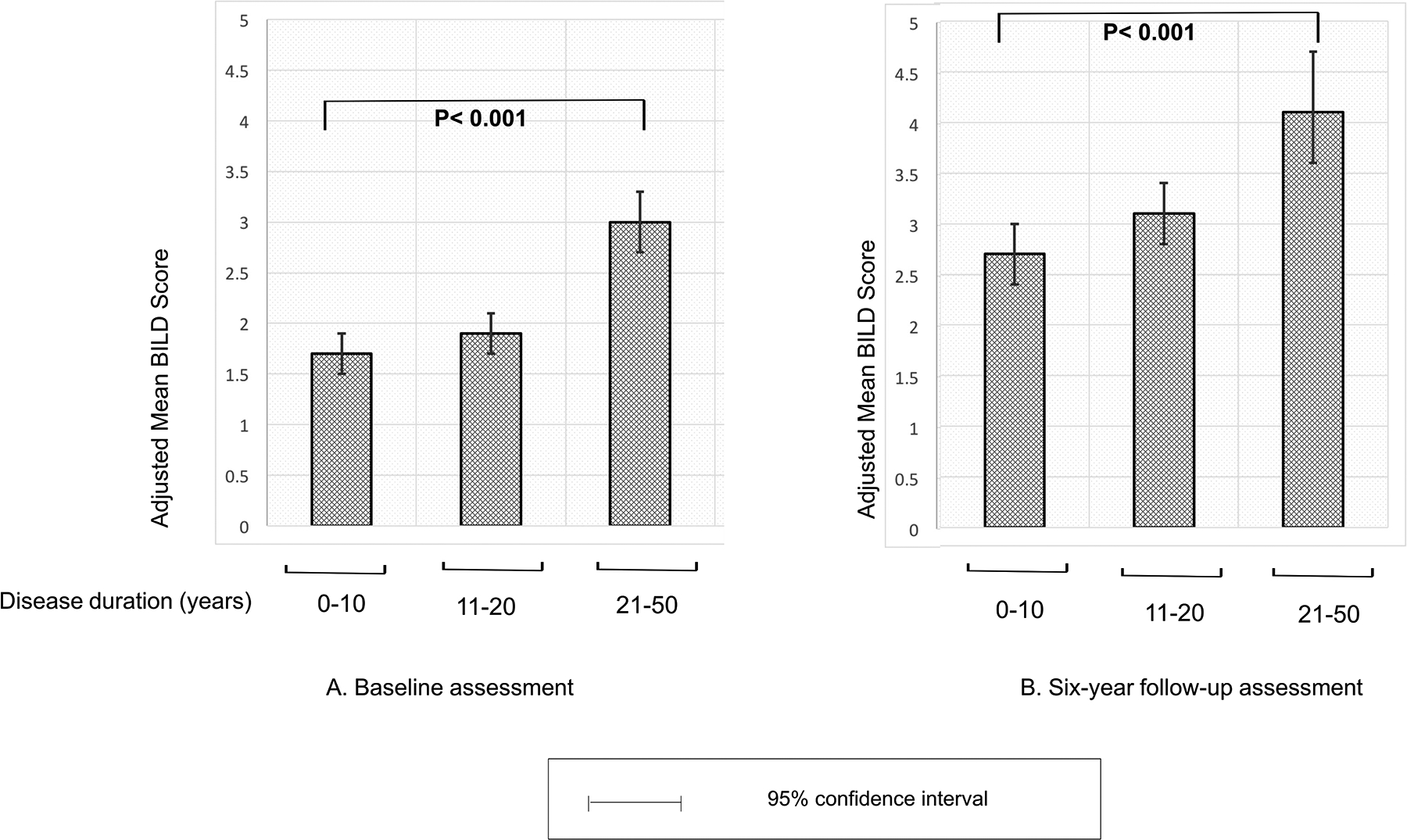
2A. Mean BILD score at baseline, among 1,035 participants, by disease duration category: Participants with longer disease duration (>20 years) had significantly higher mean damage scores as compared to those with shorter disease duration. 2B. Mean BILD scores at 6 years follow up, among 729 participants, by disease duration category: Overall higher BILD scores as compared to baseline, with significantly higher mean damage scores for patients with disease duration of >20 years as compared to shorter disease duration (p<0.001).
Minimum clinically important difference (MCID) in damage score
Finally, we compared the proportion of participants with cSLE and aSLE who experienced a clinically significant change in BILD damage score over the follow-up period (N=729). In bivariate analysis, the proportion of participants with an increase in 2 or more points did not differ between groups (49% for cSLE vs. 53% for aSLE, p=0.4). In multivariable logistic regression analyses controlling for sex, ethnicity/race, age at baseline damage score, baseline disease duration and time between the two damage assessments, participants with cSLE still did not differ in the likelihood of a clinically significant increase in damage when compared to those with aSLE (OR 1.1, 95% 0.6, 2.1, p=0.3). Significant predictors of clinically significant increase in damage score included disease duration of greater than 20 years (OR 1.9, 95% CI 1.2–3.0, p=0.007), and age at baseline BILD assessment (OR 1.02, 95% CI 1.01–1.04, p=0.005).
Steroid-related damage:
The unadjusted frequency of the four steroid-related items for the whole lupus cohort were 42% for cataracts, 18% for osteoporosis resulting in fracture, 8.5% for avascular necrosis and 13% for diabetes. Multivariable analyses predicting the presence of steroid-related damage are presented in Table 2. In model 1, adjusting only for basic factors that are fundamental to adjust for (age at baseline BILD score and steroid exposure, i.e. history of steroid use ever), participants with cSLE had twice the odds of steroid-related damage when compared to participants with aSLE (OR 2.0, 95% CI 1.3–3.3). After further adjustment for demographic factors and SLE-related predictors in model 2 (sex, race/ethnicity, disease duration, cyclophosphamide use), participants with cSLE continued to have significantly higher odds of steroid-related damage as compared to participants with aSLE (OR 1.7, 95% CI 1.1–2.8). In this final model, history of cyclophosphamide use was associated with increased risk of steroid-related damage (OR 2.3, 95% CI 1.5–3.4). As expected, history of steroid use ever also predicted steroid-related damage (OR 2.9, 95% CI 1.4, 5.8). While disease duration predicted steroid-related damage in the model, cSLE also remained an independent predictor of steroid-related damage (Figure 3). Of note, the adjusted frequency of steroid-related damage was high in the entire cohort, with 78% of cSLE participants and 69% of aSLE participants reporting steroid-related damage after 20 or more years of disease duration (p=0.004).
Table 2:
Predictors of Steroid-related Damage^ in cSLE as compared to aSLE among 1035 participants in Lupus Outcomes Study
| Multivariate Analyses | ||
|---|---|---|
| Model 1 | Model 2 | |
| OR (95% Cl) | OR (95% Cl) | |
| cSLE | 2.04 (1.30,3.30)* | 1.70 (1.10,2.80)* |
| Demographics | ||
| Age at baseline | 1.08 (1.06,1.09)* | 1.07 (1.06,1.09)* |
| Sex | ||
| Male (reference) | ||
| Female | 1.10 (0.70,1.90) | |
| Ethnicity | ||
| Caucasian (reference) | ||
| Hispanic | 0.90 (0.60,1.40) | |
| African American | 1.80 (1.1,2.9)* | |
| Asian | 1.30 (0.80,2.00) | |
| Other | 1 (0.50,1.80) | |
| SLE-related characteristics | ||
| Disease duration category | ||
| 0–10 yrs (reference) | ||
| 10–20 years | 1.70 (1.20,2.30)* | |
| >20 years | 1.80 (1.20, 2.70)* | |
| Cyclophosphamide use | 2.30 (1.50,3.40)* | |
| Steroid use | 3.62 (1.80,7.25)* | 2.90 (1.40,5.80)* |
Steroid-related damage is defined as one of the following: cataract, osteoporosis resulting fracture, avascular necrosis, diabetes.
OR=odds ratio
P<0.05
Model 1: calculated from logistic regression results, adjusted for age at baseline BILD score and steroid use.
Model 2: calculated from logistic regression results, adjusted for baseline age, steroid use ever, demographics (sex, ethnicity) and SLE-related predictors (disease duration, cyclophosphamide use ever).
Figure 3: Adjusted frequency of steroid-related damage among adults with aSLE vs. cSLE by disease duration (N=1035).
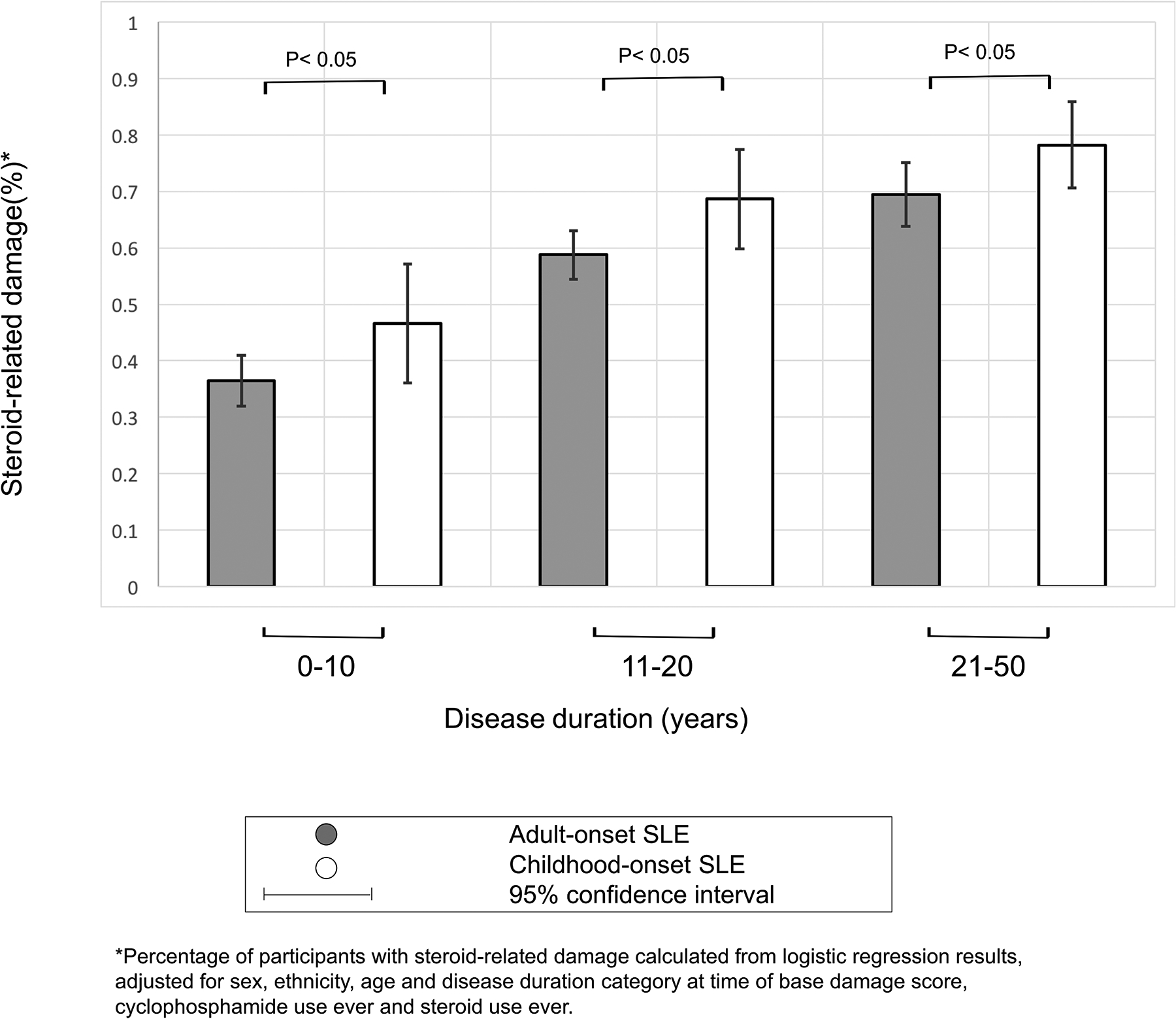
Adjusted frequency of steroid-related damage is high in the entire cohort, but significantly higher in the childhood-onset group across all disease duration categories, with 78% of cSLE participants and 69% of aSLE participants reporting steroid-related damage after 20 years of disease duration (p=0.004).
Discussion
In this large cohort of adults with SLE, we found that childhood-onset disease predicts increased risk of steroid-related damage, defined as cataracts, avascular necrosis, osteoporosis resulting in fracture, and diabetes. The risk of steroid-related damage increased with disease duration among all participants, though a higher proportion of childhood-onset participants reported steroid-related damage across disease duration categories.
It is important to note that our aSLE cohort is significantly older than our cSLE cohort. Since three of the four steroid-related damage items (diabetes, cataracts and osteoporosis-related fracture) are highly correlated with age in the general population, it is not surprising that a higher percentage of the aSLE cohort reported steroid-related damage as compared to the cSLE cohort in unadjusted analyses. However, after controlling for history of steroid use and age at baseline damage score, the cSLE cohort demonstrated a significantly greater odds of steroid-related damage. This increased risk of steroid-related damage in the cSLE cohort persisted after controlling for additional demographic and disease-related factors.
With respect to overall disease-related damage, we demonstrated that adults with cSLE and aSLE both continue to accumulate damage at similar rates over time. However, since SLE patients with childhood-onset disease have the potential to live with SLE for many more years, they may be at risk to ultimately accumulate more disease damage than aSLE patients. Our results suggest that greater disease duration may in large part account for the higher damage scores noted among adults with cSLE as compared to those with aSLE, rather than unique features of childhood-onset SLE (i.e. more aggressive disease). The observation that about 50% of both cSLE and aSLE participants had a clinically significant increase in their BILD score over 6 years of follow up underscores the impact of damage progression over time, as clinically significant increase in BILD score has been shown to correlate with future mortality.
Importantly, we did not see evidence of a plateau in damage accumulation among adults with cSLE, in spite of decades of disease duration. Our results are consistent with the recent Canadian longitudinal study that showed that damage, as measured by the SDI, continues to accrue over the entire disease course for childhood-onset SLE patients (7). The Canadian study also concluded that higher doses of prednisone predicted a subsequent increase in the damage trajectory.
While authors have previously categorized items from the SDI damage score into 3 groups in accordance with their likely relationship to steroids (definitely steroid related, possibly steroid related, and not steroid related (17)), it is important to note that the attribution of damage items to steroid exposure may be confounded by indication, as patients with severe SLE (who may be more likely to have cSLE), are also more likely to receive high-dose and/or prolonged courses of corticosteroids, and to accumulate disease-related damage. With regard to avascular necrosis, active SLE has been identified in the past as a risk factor independent of steroids (18). However, a recent study comparing SLE outcomes in steroid-exposed versus steroid-naïve adult-onset SLE identified avascular exclusively among corticosteroid-exposed participants (19). Cataracts have been seen in individuals taking low-dose steroids or who were steroid-naïve, but to a lesser extent (19,20).
Our study is the first to compare damage accrual over time in adults with cSLE and aSLE. Our results suggest that damage accrual in cSLE continues in adulthood, and that rates of damage accrual do not differ significantly when compared to those who developed SLE later in life. Our findings have two main clinical applications for rheumatologists. First, strategies to minimize damage progression, i.e. minimizing steroid exposure while maximizing use of steroid-sparing medications to achieve tight disease control, are particularly critical for patients with cSLE, who may require lupus treatment for many decades and are at risk for ongoing accumulation of damage. Second, adult rheumatologists should be aware that their cSLE patients may be at higher risk for steroid-related damage, and consider screening for it as clinically indicated.
Our study is also the first to use the BILD damage score to evaluate damage in adults with cSLE. The consistency of our results with the Canadian cohort utilizing the SDI, which is to the best of our knowledge the only other longitudinal study examining damage accrual during adulthood in cSLE, further supports the validity of the self-reported BILD damage score. These results suggest that the BILD is a useful tool for studies that utilize patient-reported data, generating results similar to the SDI.
There are several limitations to our study. First, since our data was obtained via participant self-report, inaccuracies may occur. This limitation has been addressed in part by the fact that our primary outcome measure, a participant-reported damage index (BILD), has been well-validated and correlates closely to the physician-reported SDI (15). Second, detailed information regarding early disease course, such as steroid dosing, cumulative steroid exposure and disease activity, is not available, since the LOS is not an inception cohort study. We have attempted to address this limitation by using history of cyclophosphamide use as a covariate to predict history of intensive SLE treatment. Patients treated with cyclophosphamide are likely to have also received high-dose steroids. It is also important to note though that there was likely diversity in disease management strategies in our cohort, given the diversity of our recruitment sources, which included both academic and community-based practices. Third, this study may be subject to survivorship bias. Patients with more severe cSLE may not have survived to participate in the LOS as adults, therefore our cSLE participants may represent a healthier cohort when compared to all individuals with cSLE.
Conclusion
In conclusion, this study is among the first to assess long-term disease- and treatment-related damage accumulation among adults with cSLE, and the first to compare damage scores between adults with childhood- and adult-onset SLE. Our results demonstrate that childhood-onset SLE may predict increased risk of steroid-related damage independent of disease duration. However, total disease-related damage may be more strongly predicted by disease duration rather than childhood-onset of SLE, with ongoing damage accrual occurring at similar rates among adults with cSLE and aSLE. More aggressive use of steroid-sparing management strategies during childhood may be important to prevent steroid-related damage in adulthood.
Supplementary Material
Significance and Innovations:
Due to the difficulty of following patients from pediatric to adult care settings, little is known about adult outcomes of childhood-onset SLE.
This study is among the first to assess long-term disease- and treatment-related damage accumulation among adults with cSLE, and the first to compare damage scores between adults with childhood- and adult-onset SLE.
This study demonstrates that childhood-onset SLE may predict increased risk of steroid-related damage independent of disease duration. However, total disease-related damage may be more strongly predicted by disease duration rather than childhood-onset of SLE, with ongoing damage accrual occurring at similar rates among adults with cSLE and aSLE.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- BILD
Brief Index of Lupus Damage
- SDI
Systemic Lupus Erythematosus Damage Index
- cSLE
childhood-onset Systemic Lupus Erythematosus
- aSLE
adult-onset Systemic Lupus Erythematosus
Contributor Information
Merav Heshin-Bekenstein, Division of Pediatric Rheumatology, University of California San Francisco. Address: 550 16th Street, 5th Floor, San Francisco, CA 94143-0632.; Pediatric Rheumatology, Tel Aviv Medical Center, Tel Aviv University, Israel.
Laura Trupin, Rosalind Russell/Ephraim Engleman Rheumatology Research Center, University of California, San Francisco. Address: 513 Parnassus Avenue, Box 0500..
Ed Yelin, Rosalind Russell/Ephraim Engleman Rheumatology Research Center, University of California San Francisco. Address: UCSF San Francisco, CA 94143-0920..
Emily von Scheven, Division of Pediatric Rheumatology, University of California San Francisco. Address: 550 16th Street, 5th Floor, San Francisco, CA 94143-0632..
Jinoos Yazdany, Division of Rheumatology, University of California San Francisco, 1001 Potrero Avenue, Building 30, San Francisco, CA 94110..
Erica F Lawson, Division of Pediatric Rheumatology, University of California San Francisco, 550 16th Street, 5th Floor, San Francisco, CA 94143-0632..
REFERENCES
- 1.Legge A, Doucette S, Hanly JG. Predictors of Organ Damage Progression and Effect on Health-related Quality of Life in Systemic Lupus Erythematosus. J Rheumatol 2016;43:1050–6. [DOI] [PubMed] [Google Scholar]
- 2.Yelin E, Yazdany J, Trupin L. The Relationship between Poverty and Mortality in Systemic Lupus Erythematosus. Arthritis Care Res 2018; 70:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson L, Leone V, Pilkington C, Tullus K, Rangaraj S, McDonagh JE, et al. Disease activity, severity, and damage in the UK Juvenile-Onset Systemic Lupus Erythematosus Cohort. Arthritis Rheum 2012;64:2356–65. [DOI] [PubMed] [Google Scholar]
- 4.Brunner HI, Gladman DD, Ibañez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 2008;58:556–62. [DOI] [PubMed] [Google Scholar]
- 5.Hersh AO, von Scheven E, Yazdany J, Panopalis P, Trupin L, Julian L, et al. Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum 2009;61:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersh A, von Scheven E, Yelin E. Adult outcomes of childhood-onset rheumatic diseases. Nat Rev Rheumatol 2011;7:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim LSH, Pullenayegum E, Lim L, Gladman D, Feldman B, Silverman E. From Childhood to Adulthood: The Trajectory of Damage in Patients With Juvenile-Onset Systemic Lupus Erythematosus. Arthritis Care Res 2017;69:1627–1635. [DOI] [PubMed] [Google Scholar]
- 8.Sousa S, Gonçalves MJ, Inês LS, Eugénio G, Jesus D, Fernandes S, et al. Clinical features and long-term outcomes of systemic lupus erythematosus: comparative data of childhood, adult and late-onset disease in a national register. Rheumatol Int 2016;36:955–960. [DOI] [PubMed] [Google Scholar]
- 9.Brunner HI, Gladman DD, Ibañez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 2008;58:556–62. [DOI] [PubMed] [Google Scholar]
- 10.Tucker LB, Uribe AG, Fernandez M, Vila LM, McGwin G, Apte M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case—control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 2010;17:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum 2007;57:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 13.Yelin E, Trupin L, Yazdany J. A Prospective Study of the Impact of Current Poverty, History of Poverty, and Exiting Poverty on Accumulation of Disease Damage in Systemic Lupus Erythematosus. Arthritis Rheumatol 2017;69:1612–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 15.Yazdany J, Trupin L, Gansky SA, Dall’era M, Yelin EH, Criswell LA, et al. Brief index of lupus damage: a patient-reported measure of damage in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz P, Trupin L, Rush S, Yazdany J. Longitudinal validation of the Brief Index of Lupus Damage. Arthritis Care Res (Hoboken) 2014;66:1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladman DD, Urowitz MB, Rahman P, Ibañez D, Tam L-S. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955–9. [PubMed] [Google Scholar]
- 18.DUBOIS EL, COZEN L. Avascular (aseptic) bone necrosis associated with systemic lupus erythematosus. JAMA 1960;174:966–71. [DOI] [PubMed] [Google Scholar]
- 19.Sheane BJ, Gladman DD, Su J, Urowitz MB. Disease Outcomes in Glucocorticosteroid-Naive Patients With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2017;69:252–256. [DOI] [PubMed] [Google Scholar]
- 20.Alderaan K, Sekicki V, Magder LS, Petri M. Risk factors for cataracts in systemic lupus erythematosus (SLE). Rheumatol Int 2015;35:701–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


