Abstract
Malignant mesothelioma is a relatively rare malignancy arising in the body’s serosal surfaces, with malignant pleural mesothelioma (MPM) being the most common type. It is characterized by local spread within the thorax, poor prognosis and resistance to treatment. The development of various immunotherapeutic options has provided a new way- and hope- in treating cancer patients. Chimeric antigen receptor (CAR) T cell therapy has been proven very successful in treating hematological cancers, like leukemias and lymphomas, and its use is now being tested in solid tumors. CARs that recognize and bind to a specific tumor-associated antigen on the tumor’s cell surface, are engineered and transduced into T cells. Interaction of the CAR T cell with the tumor then results in T cell activation and subsequent tumor cell lysis. In this review, we provide a current update on our previous comprehensive study summarizing the CAR T cell preclinical studies and clinical trials in MM, and discuss the future perspectives of CAR T cell therapy in this disease.
Keywords: Chimeric antigen receptor T cells, Mesothelioma, Adoptive cell transfer, Solid tumor, Tumor microenvironment
Introduction
Malignant mesothelioma (MM) is an incurable primary tumor of the body’s serosal surfaces: the pleura, peritoneum, pericardium and the tunica vaginalis (in men). It is causally linked to occupational or environmental exposure to asbestos [1-3], a natural mineral that has been recognised as a carcinogen and its mining and use has been subsequently banned or reduced. Despite the severe health implications in using asbestos and the stringent regulations on its use imposed, the asbestos industry is still present in many countries worldwide. Given the long latency period from exposure to diagnosis [4], the incidence of MM is still increasing in some countries. The UK is currently in the midst of a ‘mesothelioma epidemic’ with the number of deaths having increased from 153 per annum in 1968 to 2,046 in 2005 and currently affecting around 3,000 people annually [5]. The incidence of MM in the United States peaked in around 2000 and it is currently estimated to be around 3300 cases per year [6].
Malignant pleural mesothelioma (MPM) arises in the parietal pleura, spreads contiguously to invade local structures and can pass through the diaphragm into the abdominal cavity. MPM accounts for around 65% to 70% of all mesotheliomas, while those arising in other mesothelial membranes (peritoneal around 30%; pericardial 1-2%) are much less common [7]. Histopathologically, there are three main subtypes of mesothelioma: epithelioid, sarcomatoid and biphasic [4]. The disease is characterised by profound resistance to therapy and poor prognosis.
There is currently no known cure for MM, therefore any treatment aims to improve quality of life and extend survival. Standard treatments may involve a combination of surgery, chemotherapy and radiotherapy (trimodality therapy). Radical surgery aiming for complete tumor resection, either by sacrificing the neighboring/affected lung (extrapleural pneumonectomy; EPP) or by sparing it (pleurectomy/decortication; P/D), can be beneficial to a small cohort of selected patients [8,9]. The mainstay of treatment in MPM, whether after surgery or in unresectable tumors, has been chemotherapy. Although the only FDA-approved frontline therapy is pemetrexed with platinum (cisplatin or carboplatin) [10], other chemotherapeutic agents including gemcitabine and vinorelbine have also been used [11]. Radiotherapy is being used to the surgical wound to prevent tumor seeding (prophylactic radiotherapy) after surgery, or in the palliative setting [12]. To date, the outcomes of multimodality therapy regimens have been disappointing with modest survival benefit of 9-17 months, emphasizing the need for more effective treatments [13,14].
Chimeric Antigen Receptors (CARs)
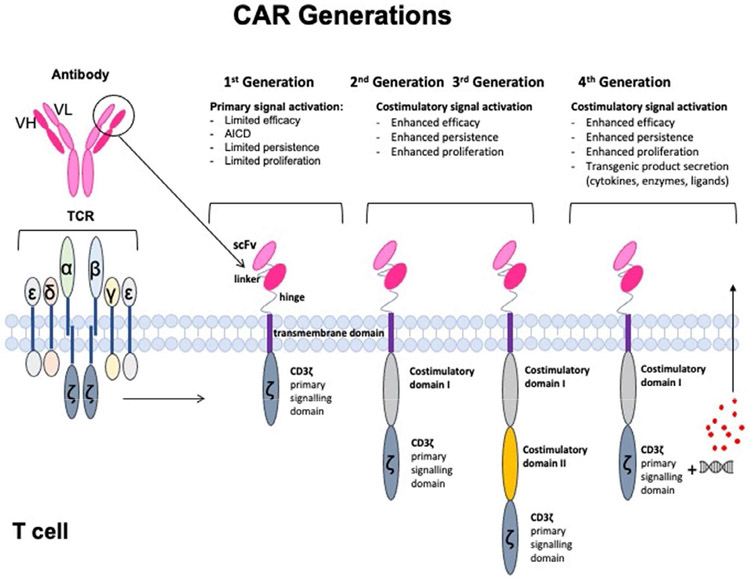
Chimeric antigen receptors (CARs) are engineered T cell receptor-like molecules whose antibody-based extracellular targeting moieties provide exquisite specificity to tumor associated antigens (TAAs) and result in T cell activation in a predictable fashion. CARs can bind directly to a variety cell surface targets (including proteins, lipids and carbohydrates), independently of MHC presentation of the antigen and provide thus a wide targeting repertoire [15]. Intracellularly, the CAR’s main component is the CD3ζ activating signaling domain, with additional costimulatory domains fused that provide lasting T cell activation and survival [15,16]. The extra- and intra-cellular domains are fused together with appropriate spacer and transmembrane molecules. The multiple design options resulted in “generations” of CARs. “First generation” CARs consist of an extracellular domain that binds to the tumor antigen via a single-chain variable antibody fragment (scFv) that is fused to a CD3ζ intracellular activating domain [17]. “Second generation” CARs have ccommonly incorporated on co-stimulatory domain, like CD28 and 4-1BB, to the primary signaling domain CD3ζ [17]. CARs with combined co-stimulatory domains are termed “third generation”. The addition of one or more co-stimulatory domains led to enhanced overall CAR T cell effector function, increased T-cell proliferation and persistence, delayed apoptosis, and markedly improved anti-tumor efficacy in vivo [18,19]. “Fourth generation” CARs, termed TRUCK (T cells Redirected for Universal Cytokine-mediated Killing) CARs have the ability to release transgenic ‘payload’ (cytokines, enzymes, co-stimulatory ligands) upon CAR T cell activation [20]. The four generations of CAR designs are drawn in Figure 1.
Figure 1:
Generations of chimeric antigen receptor (CAR). The single chain scFv- derived from the heavy (VH) and light (VL) antigen-binding domains of antibodies- is fused through a hinge and a transmembrane domain to CD3ζ, the primary signalling domain from the T-cell receptor (TCR) complex; this is the first-generation CAR. Additional intracellular domains (such as CD28 and 41BB) added for costimulatory signals, to yield second- and third- generation CARs. In addition to costimulatory signals, the fourth generation CAR (also referred to as “TRUCK”) incorporates transgenic expression of stimulatory molecules (cytokines, enzymes, or ligands).
Other CAR T cell designs include: a) Two co-expressed CARs so that two TAAs on a tumor cell can be synchronously recognized [21], b) A bispecific CAR (TanCAR) that transmits the activating signal upon engagement of either antigen 1 or antigen 2 or both [22], and c) an inhibitory CAR (iCAR) that provides a blocking signal upon antigen binding [23].
CARs can be inserted into autologous T cells using viral (lentiviral or retroviral) or non-viral (transposon) gene transfer systems to achieve permanent CAR expression or using messenger RNA (mRNA) electroporation to achieve transient expression for toxicity assessment [24,25]. Following transduction, the produced CAR T cells are expanded ex vivo in specialized gene transfer facilities and re-infused to the patient, either systemically or regionally, as a therapeutic intervention. CARs targeting the B-cell antigen CD19, have shown dramatic results in clinical trials for a number of hematologic B cell malignancies (ALL, CLL, and lymphoma) [26-28], and have provided the “proof of principal” rationale for CAR T cell development in a variety of solid tumors, including MPM [18]. A requirement for successful CAR T cell therapy, however, is a specific and highly expressed candidate TAA. In MPM, two such candidate target TAAs are currently being investigated in clinical trials: mesothelin, which is overexpressed on the tumor cells, and fibroblast activation protein (FAP) that is overexpressed on tumor stromal cells [18].
Mesothelin-targeting CARs
Mesothelin is a cell-surface glycoprotein that is expressed at low levels on normal tissues whereas it is overexpressed in the majority of MPM, as well as in lung, pancreatic, and ovarian carcinomas [29]. Mesothelin is an especially appealing target antigen since several preclinical and clinical studies found that it was involved in the malignant transformation of tumors and had a clear association with tumor aggressiveness, which led to local invasion and eventual metastasis [30,31]. Given its overexpression in MPM, versus limited expression on normal mesothelial cells of the pleura, pericardium, peritoneum, and tunica vaginalis, mesothelin-targeting CARs have been extensively studied in the preclinical and clinical setting.
Based on potent anti-tumor effects observed in preclinical studies using mRNA electroporation [32], an initial study focusing on toxicity assessment was conducted (NCT01355965) at the University of Pennsylvania, US, using T cells that only transiently expressed the second-generation murine anti-mesothelin CAR that contained the CD3ζ and 41BB signalling domains [24,33]. No patient in this phase I safety trial demonstrated “on target, off tumor” toxicity (pleuritis, peritonitis, pericarditis) from CAR mesothelin T cell infusion; however, no evident clinical responses were attained [18,24]. Interestingly, an immediate serious anaphylactic reaction was noted in one patient during a delayed mesothelin CAR T cell infusion that was attributed to the immunogenicity of the murine SS1 scFv used in the CAR construct [33]. Given safety confirmation with transient CAR mesothelin expression, a second phase 1 trial (NCT02159716) was conducted in 15 patients with mesothelioma, ovarian cancer and pancreatic cancer using a lentiviral transduction vector expressing the same murine-based anti-mesothelin second generation CAR [34]. In this trial, two different doses of T cells were administered and cyclophosphamide was added as a lymphodepletion agent in some cohorts. The mesothelin CAR T cells were well tolerated and CARexpressing cells in the blood could be detected using qPCR for about 30 days. Cyclophosphamide pre-treatment enhanced CART-meso expansion but did not improve persistence beyond 28 days [34]. Unfortunately, limited clinical activity was reported, with best overall response being stable disease in 11/15 patients [34]. A third trial has been initiated at the University of Pennsylvania using a more active, fully human anti-mesothelin CAR, with addition of cyclophosphamide and using different routes of administration (intravenous and intrapleural respectively) aiming in overall enhanced CAR T cell persistence and efficacy (NCT03054298).
Investigators at the Memorial Sloan Kettering are also conducting mesothelin-targeting CAR T cell trial for the treatment of malignant pleural disease, including MPM (NCT02414269). Their approach is based on preclinical studies in an orthotopic MPM mouse model showing potent and long-lasting antitumor efficacy of intrapleurally-administered mesothelin CAR T-cell therapy [35]. This phase I/II clinical trial uses a CAR with a human-derived anti-mesothelin scFv and a CD3Z/CD28 signalling domain transduced using a retroviral vector and is being administered intrapleurally in patients with primary or secondary pleural malignancies, with MPM being the primary target population. A subset of the MPM patient cohort is also subsequently administered Pembrolizumab, a PD-1 checkpoint inhibitor, to test whether it prolonged the activity of CAR T cell therapy. Preliminary results from 27 patients (25 of whom had MPM) were presented at the 2019 American Society of Clinical Oncology (ASCO) meeting and showed that, of the patients who received cyclophosphamide, CAR-T cell therapy, and who had at least 3 doses of Pembrolizumab, 63% of patients achieved either a partial response or a complete response [36]. Additionally, in these patients, the CAR T cells persisted in the pleural fluid for up to 42 weeks [36]. This promising trial is currently recruiting. A summary of all mesothelin-targeting CAR T cell clinical trials is summarized in Table 1 below.
Table 1:
Clinical trials of mesothelin-targeted CAR T cells in MPM.
| Clinical Trial | Phase | Setting | Intervention | Status | Location |
|---|---|---|---|---|---|
| NCT02580747 | I | Malignant Mesothelioma, Pancreatic Cancer, Ovarian Tumour, Triple Negative Breast Cancer, Endometrial Cancer, Other Mesothelin Positive Tumours | Anti-meso-CAR T cells | Unknown | Chinese PLA General Hospital, Beijing, China |
| NCT03747965 | I | Solid Tumour, Adult | Mesothelin-directed CAR-T cells | Recruiting | Chinese PLA General Hospital, Beijing, China |
| NCT03054298 | I | Lung Adenocarcinoma, Ovarian Cancer, Peritoneal Carcinoma, Fallopian Tube Cancer, Mesotheliomas Pleural, Mesothelioma Peritoneum | huCART-meso cells | Active, not recruiting | University of Pennsylvania, Philadelphia, PA, USA |
| NCT02414269 | I/II | Malignant Pleural Disease, Mesothelioma, Metastases, Lung Cancer, Breast Cancer | MSLN iCasp9M28z T cell infusions Drug: cyclophosphamide Drug: pembrolizumab |
Recruiting | Memorial Sloan Kettering, New York City, NY, USA |
| NCT02159716 | I | Metastatic Pancreatic (Ductal) Adenocarcinoma, Epithelial Ovarian Cancer, Malignant Epithelial Pleural Mesothelioma | CART-meso | Completed | University of Pennsylvania, Philadelphia, PA, USA |
| NCT01583686 | I/II | Cervical Cancer, Pancreatic Cancer, Ovarian Cancer, Mesothelioma, Lung Cancer | Anti-mesothelin chimeric T cell receptor (CAR) transduced peripheral blood lymphocytes (PBL) Drug: Cyclophosphamide Drug: Aldesleukin Drug: Fludarabine |
Terminated (Due to slow/insufficient accrual) | National Cancer Institute, Bethesda, MD, USA |
Other CARs Targeting Tumor Antigens in MPM Being Studied Preclinically
Pan-ErbB ‘T4’ CAR
Klampatsa et al. investigated the efficiency of a CAR targeting the four members of the ErbB family (EGFR, HER2, ErbB3, ErbB4) in MPM [37]. They firstly demonstrated expression of EGFR and ErbB4 in a cohort of MPM histological samples, which provided the rationale for testing this CAR in MPM [37]. To redirect T-cell specificity against the ErbB family, they engineered a second generation CAR named T1E28Z [37]. The CAR is co-expressed with a chimeric cytokine receptor named 4ab that delivers an interleukin (IL)-2/IL-15 signal upon binding of IL-4, thereby enabling the selective enrichment of CAR T-cells during ex vivo expansion [37]. The study used MPM patients’ blood and showed that successful transduction and enrichment of CAR T-cells was achieved in all patients, either at diagnosis or following chemotherapy [18]. Functionality of the expanded cells was indicated both in vitro and in vivo. These data provided support for clinical evaluation of intra-cavitary T4 immunotherapy in MPM patients in a Phase 1 clinical trial, subject to funding acquisition.
5T4 CAR
The oncofetal cell surface glycoprotein, 5T4, is overexpressed in numerous malignancies, including testicular, breast and colon cancer, while its expression in normal tissues is restricted to specialized epithelial cells [38], making it another promising immunotherapy target. Recently, it was reported that 5T4 was also expressed in a very high percentage of MPM cell lines and biopsies and pleural fluid samples [39]. Interestingly, 5T4-specific CD8+ T-cells were able to kill four out of six HLA-A2+ MPM cell lines but not an HLA-A2– cell line, demonstrating immune recognition of MPM-associated 5T4 antigen at the effector T-cell level [39]. Given that a 5T4 CAR has recently been generated and shown to efficiently kill 5T4-expressing nasopharyngeal carcinoma cells in vitro [40], 5T4 CARs represent a promising therapeutic strategy for MPM.
Chondroitin sulfate proteoglycan CARs
The cell surface proteoglycan chondroitin sulphate proteoglycan 4 (CSPG4) has been found to be overexpressed MPM where it has been reported that CSPG4 was overexpressed in 6 out of 8 MPM cell lines, and in 25 out of 41 MPM biopsies [41]. In 2014, investigators from the National Cancer Institute in the United States constructed four 2nd generation CARs, each from a different murine monoclonal antibody, linked to the CD28 co-stimulatory domain and the intracellular T cell receptor signalling chain CD3ζ [24,42]. Donor T cells transduced with these CARs demonstrated cytokine release and cytolytic function when co-cultured with several tumor cell lines, including MPM [42]. The authors concluded that CSPG4 is an attractive target for CAR T-cell therapy, yet some issues were raised about lowlevel expression of this protein in normal small bowel samples [42].
MET CAR
MET is a single pass tyrosine kinase receptor, normally expressed by cells of epithelial origin, but abnormally activated and overexpressed in a variety of cancers, including MM [43,44]. Thayaparan et al. engineered MET-specific CARs in which a CD28+CD3ζ endodomain was fused to one of three peptides from the N and K1 domains of hepatocyte growth factor (HGF), the minimum MET binding element present in HGF [45]. The developed constructs were demonstrated to show cytotoxicity against MET-expressing MPM cell lines in vitro and in an orthotopic (abdominal) tumor model in vivo, with no adverse effects.
CARs Targeting Non-Tumor Antigens in MPM
In addition to tumor cell-specific antigens, it has been proposed that CARs that target essential components of the tumor-associated stroma, such as fibroblasts or endothelial cells, might also be valuable to enhance anti-tumor activity [18]. There are multiple potential advantages to this approach: 1) stromal cells are more genetically stable and less likely to lose antigen expression, 2) attacking the stromal components may alter the tumor microenvironment to improve standard chemotherapeutic or ACT efficacy, and 3) it could be used on multiple different tumor types [18]. Two proposed stromal candidates are fibroblast activation protein (FAP) and vascular endothelial growth factor receptor 2 (VEGFR2), of which FAP is being explored in MPM.
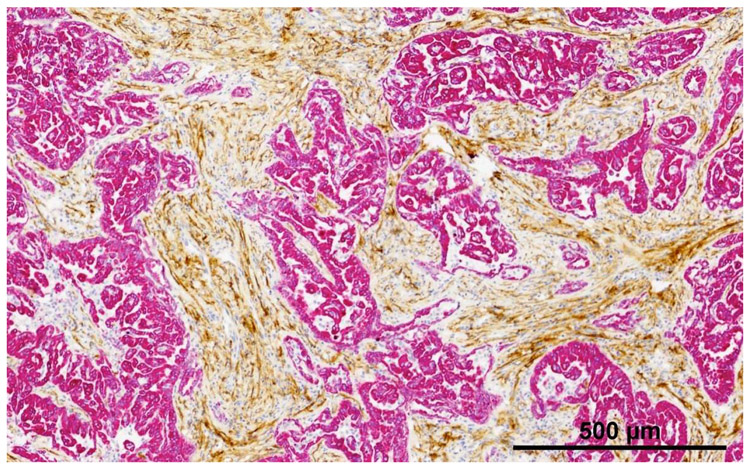
FAP is a transmembrane serine protease, which is highly expressed in the cancer-associated stromal cells (CASCs) of virtually all epithelial cancers with low expression on normal cells [46]. FAP is overexpressed in all three major MPM subtypes including epithelioid, sarcomatoid, and biphasic [47]. Figure 2 shows an example of FAP staining in two mesothelioma tumors.
Figure 2:
Fibroblast Activating Protein (FAP) immunohistochemical staining in MPM. Histological slide of an MPM tumor shows dual staining of tumor cells (red; cytokeratin) and cancer-associated fibroblasts staining (brown; FAP).
Preclinical studies have demonstrated that CAR T cells targeted to murine FAP have anti-tumor efficacy in MPM models with minimal toxicity [46]. An anti-human directed FAP CAR with the CD3ζ and CD28 signaling domains was produced at the University of Zurich and shown to induce killing of tumor cells expressing human FAP [47]. Based on these preclinical studies, this group has initiated a phase I clinical trial to evaluate the safety of administering FAP-redirected T cells intrapleurally to patients with MPM (NCT01722149) [48]. Preliminary results presented at the European Society of Molecular Oncology (ESMO) Congress in Autumn 2019 showed that, in 3 MPM patients treated, there was good tolerance of treatment and some persistence of CAR-T cells was seen. With a median follow-up of 18 months, 2 out of 3 patients were alive [49].
The Future: CAR Augmentation Strategies to Overcome the Challenges of MPM TME
To date, the success of CAR T cells seen in hematologic malignancies has not yet been reproduced in solid tumors and successful MPM treatment with CAR T cells will doubtlessly be challenging. This will be due to multiple mechanisms that include insufficient T cell trafficking to the tumor, CAR T cell suppression due to soluble mediators (like adenosine, TGFβ, and prostaglandin E2), upregulation of checkpoint inhibitors (like PD1) on the CARs, and CAR suppression due to intrinsic inhibitory T cell programs [50]. In addition, TAA expression heterogeneity and immune escape could also be potential issues.
Many groups are developing approaches aiming to overcome these hurdles. Combination of CAR T cells with systemic drugs that affect immune function in general, such as checkpoint blockade using antibodies [51], inhibitors of immunosuppressive agents, like indoleamine 2-3 dioxygenase (IDO) [52], adenosine, PGE2, or immunosuppressive cell types (like CD4 T-regulatory cells) have been explored. The Adusumilli group has investigated the effect of PD-1/PD-L1 mediated T-cell exhaustion on mesothelin CAR T cells using an orthotopic mouse model of MPM [53]. Their study demonstrated that, following repeated antigen exposure, CAR T cells become exhausted and non-functional [54]. This was attributed to PD-1 upregulation on CAR T cells, with corresponding tumor cell upregulation of PD-L1 and PD-L2 in response to effector cytokines exposure, and they demonstrated that exhausted CAR T cells can be rescued by PD-1 checkpoint blockade [54]. CARs can also be combined with other types of immunotherapy such as oncolytic viruses or whole-cell vaccines.
The ability to genetically manipulate CARs allows the possibility of generating improved CARs by inserting or removing specific genes, thus adding an additional level of opportunity and excitement. Aiming to improve CAR T cell trafficking, Moon et al. explored the efficacy of genetically enhancing a matched chemokine receptor (CCR2b) that is expressed on mesothelin CAR T cells in response to measuring elevated levels of the corresponding tumor-secreted chemokine (CCL2) by the MPM cells [55]. This preclinical in vivo study demonstrated that transduction of CCR2b onto mesothelin-targeting CAR T cells was able to significantly enhance tumor localization, infiltration, and eradication [55]. Liu et al. successfully demonstrated the utility of combining CAR T cells with a PD1/CD28 chimeric switch-receptor to overcome the suppressive effects of this inhibitory regulator [56]. This switch-receptor fuses an extracellular PD-1 domain to a cytoplasmic CD28 domain, which stimulates the T-cell upon binding to the PD-L1 ligand, thereby activating, rather than inhibiting, T-cell effector function [56]. Administration of PD1CD28 resulted in significantly increased levels of T-cell infiltration, cytokine secretion, and lytic function with long-term statistical reductions in tumor volume [56].
Other examples include: TGFβ blockade expressing a decoy receptor [57], inhibition of adenosine and PGE2 immunosuppressive effects by inserting a small peptide that prevents activation of protein kinase A [58], delivery of activating cytokines (like IL-12) [20] and improved CAR T cell function by using cytoplasmic domains derived from natural killer cells [59]. Many other approaches are actively being pursued.
Conclusion
The exciting successes seen in hematologic malignancies have prompted development of CAR T-cell therapy for solid tumors, such as MPM. Mesothelioma has two potential advantages. First, a relatively safe and specific TAA (mesothelin) has been identified. Second, mesothelioma may provide an opportunity to use local therapy by intrapleural or intratumoral injection. However, although clinical trials of CARs for use in mesothelioma are underway, clear success of CAR T cells in any solid tumor has not yet been achieved. Accordingly, the results of these trials and knowledge gained from CAR T cell trials in other solid tumors will need to be used iteratively to improve the next series of trials, hopefully eventually leading to adoptive T cell transfer as an important part of the MPM therapeutic armamentarium.
Acknowledgements
Steven M. Albelda received grant support from NCI GRANT P01-CA217805. Astero Klampatsa is supported by the CRIS Cancer Foundation.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest. Steven M. Albelda received prior research funding from Novartis, and currently receives research funding from Tmunity.
References
- 1.Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Industrial Health. 2007;45(3):379–87. [DOI] [PubMed] [Google Scholar]
- 2.Liu B, Van Gerwen M, Bonassi S, Taioli E. Epidemiology of environmental exposure and malignant mesothelioma. Journal of Thoracic Oncology. 2017. July 1;1 2(7):1031–45. [DOI] [PubMed] [Google Scholar]
- 3.Lin RT, Takahashi K, Karjalainen A, Hoshuyama T, Wilson D, Kameda T, et al. Ecological association between asbestos-related diseases and historical asbestos consumption: an international analysis. The Lancet. 2007. March 10;309(9504):844–9. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Current Treatment Options in Oncology. 2008. June 1;9(2-3):147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. British Journal of Cancer. 2005. February;92(3):587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazurek JM, Syamlal G, Wood JM, Hendricks SA, Weston A. Malignant mesothelioma mortality—United States, 1999–2015. MMWR. Morbidity and Mortality Weekly Report. 2017. March 3;66(8):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridda A, Padoan I, Mencarelli R, Frego M. Peritoneal mesothelioma: a review. Medscape General Medicine. 2007;9(2):32. [PMC free article] [PubMed] [Google Scholar]
- 8.Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Annals of Surgery. 2014. October;260(4):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang-Lazdunski L, Bille A, Lal R, Cane P, McLean E, Landau D, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. Journal of Thoracic Oncology. 2012. April 1;7(4):737–43. [DOI] [PubMed] [Google Scholar]
- 10.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. Journal of Clinical Oncology. 2003. July 15;21(14):2636–44. [DOI] [PubMed] [Google Scholar]
- 11.Zauderer MG, Kass SL, Woo K, Sima CS, Ginsberg MS, Krug LM. Vinorelbine and gemcitabine as second-or thirdline therapy for malignant pleural mesothelioma. Lung Cancer. 2014. June 1;84(3):271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baas P, Fennell D, Kerr KE, Van Schil PE, Haas RL, Peters S. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2015. September 1;26(suppl_5):v31–9. [DOI] [PubMed] [Google Scholar]
- 13.Gomez D, Tsao AS. Local and systemic therapies for malignant pleural mesothelioma. Current Treatment Options in Oncology. 2014. December 1;15(4):683–99. [DOI] [PubMed] [Google Scholar]
- 14.Steele JP, Klabatsa A. Chemotherapy options and new advances in malignant pleural mesothelioma. Annals of Oncology. 2005. March 1;16(3):345–5t. [DOI] [PubMed] [Google Scholar]
- 15.Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discovery. 2016. February 1;6(2):133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, et al. Current progress in CAR-T cell therapy for solid tumors. International Journal of Biological Sciences. 2019;i5(12):2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nature Reviews Drug Discovery. 2015. July;14(7):499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klampatsa A, Haas AR, Moon EK, Albelda SM. Chimeric antigen receptor (CAR) T cell therapy for malignant pleural mesothelioma (MPM). Cancers. 2017. September;9(9):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences. 2009. March 3;106(9):3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chmielewski M, Hombach AA, Abken H. Of CAR s and TRUCK s: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunological Reviews. 2014. January;257(1):83–90. [DOI] [PubMed] [Google Scholar]
- 21.Jena B, Moyes JS, Huls H, Cooper LJ. Driving CARbased T-cell therapy to success. Current Hematologic Malignancy Reports. 2014. March 1;9(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, et al. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Molecular Therapy-Nucleic Acids. 2013. January 1;2:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedorov VD, Themeli M, Sadelain M. PD-1-and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Science Translational Medicine. 2013. December 11;5(215):215ra172-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunology Research. 2014. February 1;2(2):112–20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Molecular Therapy-Oncolytics. 2016. January 1;3:16015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28Z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science Translational Medicine. 2014. February 19;6(224):224ra25-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nature Reviews Clinical Oncology. 2013. May;10(5):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science Translational Medicine. 2015. September 2;7(303):303ra139-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin immunotherapy for cancer: ready for prime time?. Journal of Clinical Oncology. 2016. December 1;34(34):4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori JI, Sima C, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clinical Cancer Research. 2012. May 1;18(9):2478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clinical Cancer Research. 2014. February 15;20(4):1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Research. 2010. November 15;70(22):9053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunology Research. 2013. July 1;1(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas AR, Tanyi JL, O’Hara MH, Gladney WL, Lacey SF, Torigian DA, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Molecular Therapy. 2019. November 6;27(ii):1919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Science Translational Medicine. 2014. November 5;6(261):261ra151-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adusumilli PS, Zauderer MG, Rusch VW, O’Cearbhaill R, Zhu A, Ngai D, et al. Regional delivery of mesothelin-targeted CAR T cells for pleural cancers: Safety and preliminary efficacy in combination with anti-PD-1 agent. Journal of Clinical Oncology, 2019. 37(15_suppl): p. 2511–2511.31154918 [Google Scholar]
- 37.Klampatsa A, Achkova DY, Davies DM, Parente-Pereira AC, Woodman N, Rosekilly J, et al. Intracavitary ‘T4 immunotherapy’of malignant mesothelioma using pan-ErbB re-targeted CAR T-cells. Cancer Letters. 2017. May 1;393:52–9. [DOI] [PubMed] [Google Scholar]
- 38.Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. British Journal of Cancer. 1990. January;61(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Taei S, Salimu J, Lester JF, Linnane S, Goonewardena M, Harrop R, et al. Overexpression and potential targeting of the oncofoetal antigen 5T4 in malignant pleural mesothelioma. Lung Cancer. 2012. August 1;77(2):312–8. [DOI] [PubMed] [Google Scholar]
- 40.Guo X, Zheng H, Luo W, Zhang Q, Liu J, Yao K. 5T4-specific chimeric antigen receptor modification promotes the immune efficacy of cytokine-induced killer cells against nasopharyngeal carcinoma stem cell-like cells. Scientific Reports. 2017. July 7;7(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivera Z, Ferrone S, Wang X, Jube S, Yang H, Pass HI, et al. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clinical Cancer Research. 2012. October 1;18(19):5352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beard RE, Zheng Z, Lagisetty KH, Burns WR, Tran E, Hewitt SM, et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. Journal for Immunotherapy of Cancer. 2014. December;2(1):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levallet G, Vaisse-Lesteven M, Le Stang N, Ilg AG, Brochard P, Astoul P, et al. Plasma cell membrane localization of c-MET predicts longer survival in patients with malignant mesothelioma: a series of 157 cases from the MESOPATH Group. Journal of Thoracic Oncology. 2012. March 1;7(3):599–606. [DOI] [PubMed] [Google Scholar]
- 44.Tolnay E, Kuhnen C, Wiethege T, König JE, Voss B, Muller KM. Hepatocyte growth factor/scatter factor and its receptor c-Met are overexpressed and associated with an increased microvessel density in malignant pleural mesothelioma. Journal of Cancer Research and Clinical Oncology. 1998. July 1;124(6):291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thayaparan T, Petrovic RM, Achkova DY, Zabinski T, Davies DM, Klampatsa A, et al. CAR T-cell immunotherapy of MET-expressing malignant mesothelioma. Oncoimmunology. 2017. December 2;6(12):e1303137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunology Research. 2014. February 1;2(2):154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuberth PC, Hagedorn C, Jensen SM, Gulati P, van den Broek M, Mischo A, et al. Treatment of malignant pleural mesothelioma by fibroblast activation proteinspecific re-directed T cells. Journal of Translational Medicine. 2013. December;11(1):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrausch U, Schuberth PC, Hagedorn C, Soltermann A, Tomaszek S, Stahel R, et al. Re-directed T cells for the treatment of fibroblast activation protein (FAP)-positive malignant pleural mesothelioma (FAPME-1). BMC Cancer. 2012. December 1;12(1):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curioni A, Britschgi C, Hiltbrunner S, Bankel L, Gulati P, Weder W, et al. 1226P A phase I clinical trial of malignant pleural mesothelioma treated with locally delivered autologous anti-FAP-targeted CAR T-cells. Annals of Oncology. 2019. October 1;30(Supplement_5):mdz253–052. [Google Scholar]
- 50.Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annual Review of Medicine. 2017. January 14;68:139–52. [DOI] [PubMed] [Google Scholar]
- 51.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by genemodified T cells. Clinical Cancer Research. 2013. October 15;19(20):5036–46. [DOI] [PubMed] [Google Scholar]
- 52.Ninomiya S, Narala N, Huye L, Yagyu S, Savoldo B, Dotti G, et al. Tumor indoleamine 2, 3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015. June 18;125(25):3905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-i checkpoint blockade resist tumor-mediated inhibition. The Journal of Clinical Investigation. 2016. August 1;126(8):3130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeltsman M, Dozier J, McGee E, Ngai D, Adusumilli PS. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Translational Research. 2017. September 1;187:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clinical Cancer Research. 2011. July 15;17(14):4719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Research. 2016. March 15;76(6):1578–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bollard CM, Rössig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor β-related tumor protection strategy to enhance antitumor immunity. Blood, The Journal of the American Society of Hematology. 2002. May 1;99(9):3179–87. [DOI] [PubMed] [Google Scholar]
- 58.Newick K, O’Brien S, Sun J, Kapoor V, Maceyko S, Lo A, et al. Augmentation of CAR T-cell trafficking and antitumor efficacy by blocking protein kinase A localization. Cancer immunology research. 2016. June 1;4(6):541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang E, Wang LC, Tsai CY, Bhoj V, Gershenson Z, Moon E, et al. Generation of potent T-cell immunotherapy for cancer using DAP12-based, multichain, chimeric immunoreceptors. Cancer Immunology Research. 2015. July 1;3(7):815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]