Summary
Background
The ELIANA trial showed that 61 (81%) of 75 paediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukaemia achieved overall remission after treatment with tisagenlecleucel, a chimeric antigen receptor targeted against the CD19 antigen. We aimed to evaluate patient-reported quality of life in these patients before and after tisagenlecleucel infusion.
Methods
ELIANA, a global, single-arm, open-label, phase 2 trial, was done in 25 hospitals across Australia, Austria, Belgium, Canada, France, Germany, Italy, Japan, Norway, Spain, and the USA. Patients with B-cell acute lymphoblastic leukaemia aged at least 3 years at the time of screening and 21 years or younger at the time of initial diagnosis who were in second or greater bone marrow relapse, chemorefractory, relapsed after allogeneic stem-cell transplantation, or were otherwise ineligible for allogeneic stem-cell transplantation were enrolled. Patients received a single intravenous administration of a target dose of 0·2–5 × 106 transduced viable T cells per kg for patients weighing 50 kg or less or 0·1–2·5 × 108 transduced viable T cells for patients weighing more than 50 kg. The primary outcome, reported previously, was the proportion of patients who achieved remission. A prespecified secondary endpoint, reported here, was patient-reported quality of life measured with the Pediatric Quality of Life Inventory (PedsQL) and European Quality of Life-5 Dimensions questionnaire (EQ-5D). Patients completed the questionnaires at baseline, day 28, and months 3, 6, 9, and 12 after treatment. The data collected were summarised using descriptive statistics and post-hoc mixed models for repeated measures. Change from baseline response profiles were illustrated with cumulative distribution function plots. The proportion of patients achieving the minimal clinically important difference and normative mean value were reported. Analysis was per protocol. This study is registered with ClinicalTrials.gov, NCT02435849.
Findings
Between April 8, 2015, and April 25, 2017, 107 patients were screened, 92 were enrolled, and 75 received tisagenlecleucel. 58 patients aged 8–23 years were included in the analysis of quality of life. At baseline, 50 (86%) patients had completed the PedsQL questionnaire and 48 (83%) had completed the EQ-5D VAS. Improvements in patient-reported quality-of-life scores were observed for all measures at month 3 after tisagenlecleucel infusion (mean change from baseline to month 3 was 13·3 [95% CI 8·9–17·6] for the PedsQL total score and 16·8 [9·4–24·3] for the EQ-5D visual analogue scale). 30 (81%) of 37 patients achieved the minimal clinically important difference at month 3 for the PedsQL total score and 24 (67%) of 36 patients achieved this for the EQ-5D visual analogue scale.
Interpretation
These findings, along with the activity and safety results of ELIANA, suggest a favourable benefit–risk profile of tisagenlecleucel in the treatment of paediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukaemia.
Funding
Novartis.
Introduction
Acute lymphoblastic leukaemia represents 75–80% of acute leukaemias in children and is the most common paediatric cancer in the USA.1,2 Approximately 85% of children diagnosed with acute lymphoblastic leukaemia have B-cell lineage.3 With current multiagent chemotherapy, the survival at 5 years for paediatric acute lymphoblastic leukaemia is almost 90%, but is lower in adolescents and young adults.4 However, outcomes are poor for children who relapse or are refractory to standard treatments.5,6
Patient-reported quality of life is an increasingly important component in the assessment of new oncology therapies. Certain symptoms and functional losses in patients with cancer might not be measurable with clinical tests or procedures.7–11 Previous studies in children with acute lymphoblastic leukaemia have generally reported a substantial decline in patient-reported quality of life during active therapy (usually lasting years) followed by improvements in patients who achieved remission.12–18 Quality of life in children with acute lymphoblastic leukaemia treated with chimeric antigen receptor-expressing T cells (CAR T cells) has not been previously reported and might be different from that in children given cytotoxic chemotherapy, given the distinct toxicity profiles and different durations of therapy.19
Tisagenlecleucel (approved by the US Food and Drug Administration) is an autologous immunocellular therapy consisting of CD3 T cells that have undergone ex-vivo T-cell activation, gene modification, expansion, and formulation in infusible cryomedia.20 The transgene expressed via lentiviral vector transduction is a CAR targeted against the CD19 antigen.
The ELIANA trial assessed the efficacy and safety of tisagenlecleucel in children and young adult with relapsed or refractory B-cell acute lymphoblastic leukaemia. Efficacy analyses demonstrated that 61 (81%) of 75 patients who received the infusion achieved complete remission with or without complete blood count recovery within 3 months. Safety analyses showed that 52 (69%) of 75 patients had treatment-related grade 3–4 adverse events within 8 weeks of tisagenlecleucel infusion, which decreased to 12 (17%) of 75 after 8 weeks.20 In this study, we aimed to evaluate the impact of a single one-time infusion of tisagenlecleucel on patient-reported quality of life.
Methods
Study design and participants
ELIANA, a global, single-arm, open-label, phase 2 trial, was done in 25 hospitals across Australia, Austria, Belgium, Canada, France, Germany, Italy, Japan, Norway, Spain, and the USA (appendix p 7). The entry criteria of this trial have previously been described.20 Briefly, the treatment population consisted of paediatric and young adult patients aged at least 3 years at the time of screening and 21 years or younger at the time of initial diagnosis with B-cell acute lymphoblastic leukaemia who were in second or greater bone marrow relapse, chemorefractory, relapsed after allogeneic stem-cell transplantation, or were otherwise ineligible for allogeneic stem-cell transplantation and had 5% or more lymphoblasts in the bone marrow during screening. Patients were required to have adequate renal, hepatic, pulmonary, and cardiac function, and could not have received previous CD19-directed therapy. Patients were also required to have a Karnofsky (ages 16 years and older) or Lansky (ages younger than 16 years) performance status of 50% or greater at screening. Full eligibility criteria are listed in the protocol (appendix pp 61–65). Although trial recruitment is not active, follow-up is ongoing among enrolled patients.
The study was approved by the institutional review board at each participating institution, and patients or their guardians provided written, informed consent or assent.
Procedures
All patients included in this quality-of-life study were given a single one-time tisagenlecleucel intravenous infusion of a target dose of 0·2–5 x 106 transduced viable T cells per kg for patients weighing 50 kg or less and 0·1–2·5 x 108 transduced viable T cells for patients weighing more than 50 kg. Baseline clinical characteristics were collected at the time patients met study enrolment criteria. Tumour response was assessed from day 28, every month, to month 6, then every 3 months until month 12, and is ongoing. Patients are being followed-up for 5 years for efficacy and safety unless withdrawn from study by patient or investigator choice or lost to follow-up.
Patient-reported quality of life was assessed before and after infusion of tisagenlecleucel. No disease-specific instruments were available to assess the burden and the effect of symptoms associated with B-cell acute lymphoblastic leukaemia; thus, the impact of the treatment was assessed in broader concepts associated with patients’ overall quality of life using two validated instruments: the Pediatric Quality of Life Inventory (PedsQL) and the European Quality Of Life-5 Dimensions (EQ-5D) questionnaire. Self-reported data were collected on paper.
Both instruments are widely used and have been previously described and evaluated.21–24 Briefly, the PedsQL is a standardised, generic assessment of health-related perceptions of quality of life in paediatric patients. The PedsQL consists of emotional, social, and school functioning subscale scores (five items each); physical (eight items) and psychosocial health summary scores; and a total score (sum of all the items over the number of items answered on all the scales). All PedsQL scores range from 0 to 100, with higher scores indicating better quality of life. The EQ-5D questionnaire is a standardised measure of health status applicable to a wide range of health conditions and treatments. The EQ-5D questionnaire consists of a descriptive system comprising five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and a visual analogue scale (VAS) that records self-rated overall health state (range 0 to 100, with higher scores indicating better quality of life).
Patients completed the PedsQL and EQ-5D questionnaires at baseline, day 28, and months 3, 6, 9, and 12 after treatment, with data collection ongoing. The results presented in this analysis include data from baseline to month 12 of follow-up. For the PedsQL, patients aged 8–12 years completed the Children version, patients aged 13–17 years completed the Teens version, and patients aged 18 years and older completed the Adults version. The youth-friendly version of the EQ-5D (EQ-5D-Y) was completed by patients aged 8–12 years, whereas the standard 3-level EQ-5D (EQ-5D-3L) questionnaire was completed by patients aged 13 years or older.
Outcomes
The primary endpoint was the proportion of patients who achieved overall remission, and secondary endpoints included the proportion of patients who achieved complete remission or complete remission with incomplete haematological recovery with undetectable minimal residual disease, duration of remission, event-free survival, overall survival, cellular kinetics, and safety. Results for these aforementioned endpoints have been reported.20 Patient-reported quality of life assessed by PedsQL and the EQ-5D questionnaire was a prespecified secondary endpoint. A comprehensive list with detailed definitions of all trial endpoints is provided in the appendix (pp 54–57).
Statistical analysis
The enrolment target of the ELIANA trial was 95 patients to allow infusion in 50 patients younger than 18 years at the time of screening. Based on the null hypothesis of 20% or less patients achieving overall remission, at least 76 patients were required to provide 95% power to reject the null hypothesis at an overall one-sided 2·5% level of significance, if the underlying proportion of patients achieving overall remission was 45% or greater. The study was not powered for the analysis of quality of life, which was added as a secondary objective by protocol amendment on May 22, 2015. All p values in this analysis were generated in a post-hoc exploratory manner and should be interpreted as descriptive statistics. Eligible patients for the current quality-of-life study were limited per protocol to the subset of patients who were aged 8 years and older at enrolment to restrict the data to self-reported outcomes. We tabulated the numbers of patients with available patient-reported quality-of-life data at each study visit for all patients and responders only (ie, patients who achieved either complete remission or complete remission with incomplete haematological recovery). Patients without baseline data were excluded from analyses at postbaseline visits.
We used descriptive statistics to summarise demographic and baseline characteristics of infused patients who did and did not complete at least one patient-reported instrument. The proportion of patients reporting no problems (level 1) on each dimension of the EQ-5D questionnaire was summarised at each study visit.
We characterised mean quality-of-life scores and changes from baseline in PedsQL scores and the EQ-5D VAS with descriptive statistics among patients with non-missing data at baseline and each relevant postbaseline visit. We used a post-hoc mixed models for repeated measures analysis to evaluate the magnitudes of changes from baseline for the PedsQL total score and EQ-5D VAS.
For the PedsQL instrument and EQ-5D VAS score, minimal clinically important differences (MCIDs)23,24 and normative means for healthy children25,26 from literature point estimates were used. We based our MCID analysis of the EQ-5D VAS on the range reported by Pickard and colleagues.23 In a post-hoc analysis, we computed the proportion of these patients with a change from baseline greater than or equal to the MCID for each instrument on the basis of observed data, in which the number of patients with a score greater than or equal to the MCID was divided by the number of patients with non-missing data at the relevant study visit. Additionally, we used the worst-case scenario approach to address potential bias of missing patient-reported quality-of-life data in which it was assumed that all patients with missing data at postbaseline visits did not achieve the MCID. We generated cumulative distribution function plots to show the quality-of-life response profiles for the PedsQL total score and EQ-5D VAS score. These plots depict the proportion of patients achieving a change from baseline in patient-reported quality-of-life score by at least a specific amount.
We computed the proportion of patients achieving normative mean values in the PedsQL and EQ-5D VAS using the worst-case scenario approach and on the basis of observed data.
Post-hoc subgroup analyses of change in quality of life were based on severe (grade 3 or 4) cytokine release syndrome and neurotoxicity status.
All 95% CIs were derived from observed standard errors based on the t distribution. All statistical analyses were done with SAS (version 9.4).
This study is registered with ClinicalTrials.gov, number NCT02435849.
Role of the funding source
The study was designed by representatives of the funder, in conjunction with the lead investigators. The funder had a role in data collection, data analysis, data interpretation, and writing of the report in collaboration with the authors. The corresponding author had full access to all data and the final responsibility for the decision to submit for publication.
Results
Between April 8, 2015, and April 25, 2017, 107 patients were screened for the ELIANA trial, 92 were enrolled, 75 received tisagenlecleucel, and 58 were aged 8–23 years and included in this analysis (figure 1). 50 (86%) of 58 patients had completed PedsQL data and 48 (83%) had completed EQ-5D VAS data at baseline, and, of 48 (83%) of 58 patients with treatment response, 43 (90%) had PedsQL data and 40 (83%) had EQ-5D VAS data at baseline (table 1). The median follow-up of this group of patients was 9·9 months (IQR 5·3–13·5). The number of patients who remained on study and thus eligible to submit patient-reported quality-of-life data decreased at each successive study visit.
Figure 1:
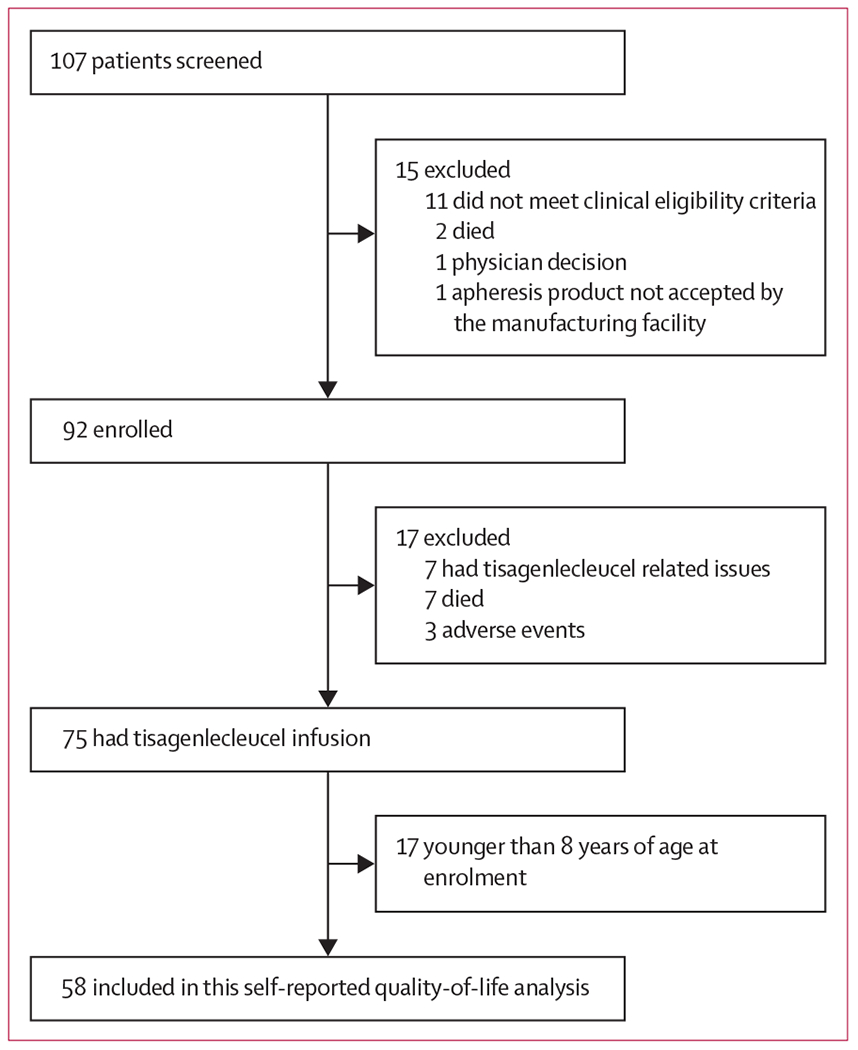
Trial profile
Table 1:
Overall compliance with patient-reported quality-of-life assessments by responder status
| Baseline (n=58) |
Day 28 (n=57) |
Month 3 (n=47) |
Month 6 (n=34) |
Month 9 (n=25) |
Month 12 (n=14) |
|
|---|---|---|---|---|---|---|
| Pediatric Quality of Life Inventory | ||||||
| Treatment responders | 43 | 37 | 36 | 30 | 21 | 14 |
| All patients | 50 | 43 | 38 | 32 | 21 | 14 |
| EQ-5D visual analogue scale | ||||||
| Treatment responders | 40 | 37 | 36 | 29 | 21 | 14 |
| All patients | 48 | 44 | 39 | 31 | 21 | 14 |
Treatment responders (n=48) defined as patients with complete remission with or without complete blood count recovery. Patients without baseline data were excluded from analyses at postbaseline visits. EQ-5D=European Quality of Life-5 Dimensions questionnaire.
Of the 58 patients aged 8 years and older at baseline, 48 (83%) had an assessment of the PedsQL or EQ-5D VAS at baseline and at least one postbaseline visit (table 2). Patients who completed a baseline and at least one subsequent patient-reported quality-of-life instrument were similar in their distributions of sex, age, race, Karnofsky or Lansky performance status, previous haemopoietic stem-cell transplantation, and disease status compared with patients who did not (table 2).
Table 2:
Demographic and baseline characteristics according to data availability at baseline and postbaseline visits
| Patients with quality-of-life assessment at baseline and at least one postbaseline (n=48) | Patients without quality-of-life assessment at baseline and at least one postbaseline (n=10) | |
|---|---|---|
| Sex | ||
| Female | 19 (40%) | 6 (60%) |
| Male | 29 (60%) | 4 (40%) |
| Age, years | ||
| Mean (SD) | 14·3 (4·5) | 11·9 (3·6) |
| Median (IQR) | 14·0 (10·0–17·5) | 11·5 (9·0–14·0) |
| Race | ||
| White | 38 (79%) | 8 (80%) |
| Other | 10 (21%) | 2 (20%) |
| Karnofsky or Lansky performance status | ||
| 90–100% | 28 (58%) | 5 (50%) |
| <90% | 20 (42%) | 5 (50%) |
| Previous haemopoietic stem-cell transplantation | ||
| 0 | 18 (38%) | 5 (50%) |
| 1 or 2 | 30 (62%) | 5 (50%) |
| Disease status | ||
| Primary refractory | 5 (10%) | 0 |
| Relapsed disease | 43 (90%) | 10 (100%) |
Data are n (%) unless otherwise stated. Quality-of-life assessment defined non-missing data for either the European Quality of Life-5 Dimensions visual analogue scale or Pediatric Quality of Life Inventory total score.
At day 28, quality-of-life data was submitted by 37 (77%) of 48 responders for each measure, and six (60%) of ten patients with no response submitted PedsQL data and seven (70%) submitted EQ-5D VAS data. Although the protocol allowed patients with no response to continue to contribute quality-of-life data, few patient-reported quality-of-life instruments were completed by non-responders beyond day 28, because most of these patients discontinued study, were lost to follow-up, or died shortly thereafter. Among the 48 treatment responders, seven (15%) patients underwent stem-cell transplantation while in remission.
Mean baseline values for all patient-reported quality-of-life scores were less than the normative means, ranging from a relative 43% reduction for the physical health summary score (mean baseline score 48·4 [SD 27· 1]; normative mean 84 ·4) to a relative 17% reduction for social functioning (mean baseline score 72 ·4 [20 ·3]; normative mean 87·4; table 3). For the EQ-5D VAS, the mean baseline score (66·8 [SD 21·8]) was a relative 23% less than the normative mean (86–2). The proportion of patients reporting no problems (level 1) on each dimension of the EQ-5D questionnaire at baseline ranged from 16 (33%) of 49 patients for pain/discomfort to 32 (65%) of 49 patients for self-care (appendix p 1).
Table 3:
Normative means, minimal clinically important differences, and baseline scores for patient-reported quality-of-life outcomes
| Baseline score mean (SD) | Normative mean* | Absolute difference | Relative difference | Minimal clinically important difference* | |
|---|---|---|---|---|---|
| Pediatric Quality of Life Inventory | 58·0 (20·1) | 83·0 | −25·0 | −30% | 4·36 |
| Emotional functioning | 61·0 (20·5) | 80·9 | −19·9 | −25% | 8·94 |
| Social functioning | 72·4 (20·3) | 87·4 | −15·0 | −17% | 8·36 |
| School functioning | 57·6 (22·7) | 78·6 | −21·0 | −27% | 9·12 |
| Physical functioning | 48·4 (27·1) | 84·4 | −36·0 | −43% | 6·66 |
| Psychosocial health summary score | 63·3 (18·6) | 82·8 | −19·5 | −24% | 5·30 |
| Total score | 58·0 (20·1) | 83·0 | −25·0 | −30% | 4·36 |
| EQ-5D visual analogue scale | 66·8 (21·8) | 86·2 | −19·4 | −23% | 7–10 |
Absolute differences were calculated as the difference between the mean baseline score and the normative mean. Relative differences were calculated as the absolute difference divided by the normative mean. EQ-5D=European Quality of Life-5 Dimensions questionnaire.
From literature point estimates.
For the EQ-5D questionnaire, the proportions of patients reporting no problems were greater at day 28 and months 3, 6, 9, and 12 than at baseline for all dimensions except self-care, for which the day 28 proportion was lower than the baseline proportion (appendix p 1).
Mean change from baseline to month 3 in the PedsQL total score was 13·3 (95% CI 8·9-17·6). Mean change from baseline in PedsQL scores at day 28 was greatest for emotional functioning (7·3 [95% CI 1·6–13·1]) and least for school functioning (0·2 [−8·3 to 8·8]; figure 2). Between months 3 and 12, improvements from baseline in patient-reported quality of life were observed for all measures, which increased over time. Generally, these improvements from baseline were smallest for social functioning and greatest for physical functioning (figure 2). For the EQ-5D VAS, mean change scores increased from baseline and were 16·8 (95% CI 9·4–24·3) at month 3, 17·4 (9·0–25·7) at month 6, 18·8 (7·8–29·9) at month 9, and 24·7 (13·5–35·9) at month 12. Results from post-hoc mixed models for repeated measure analyses supported these observations of improvements in scores from baseline (appendix p 4).
Figure 2: Change from baseline in PedsQL and EQ-5D VAS.
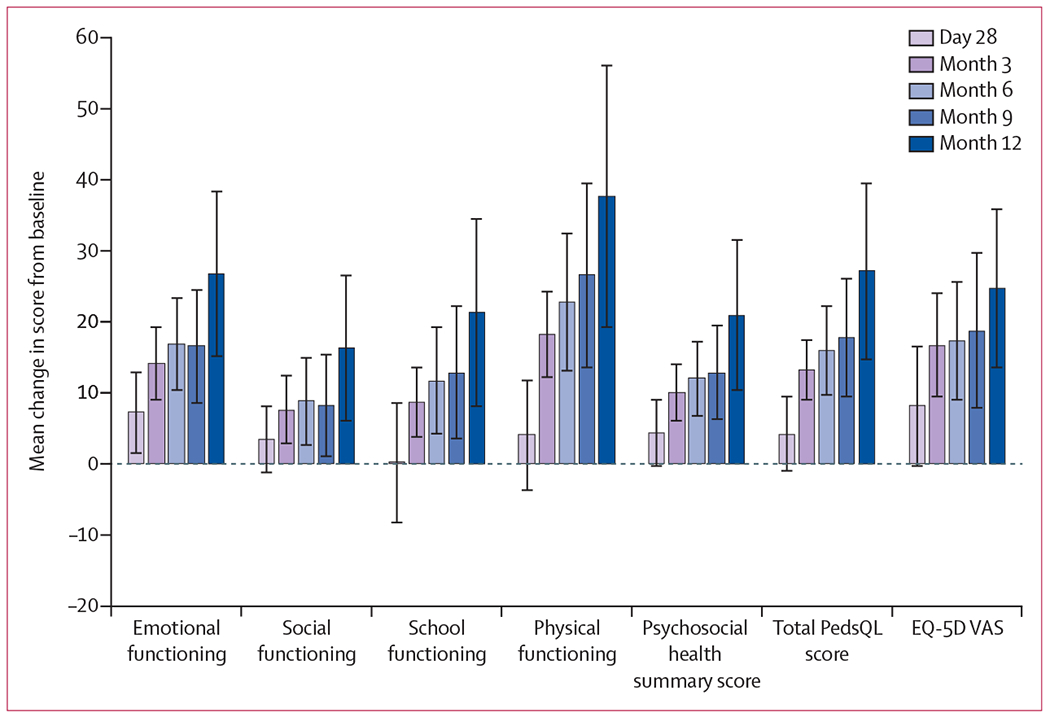
Error bars represent 95% CIs, which were derived from the observed standard errors assuming a t distribution. Analysis was based on patients with non-missing data at both baseline and the postbaseline study assessment of interest. EQ-5D VAS=European Quality of Life-5 Dimensions questionnaire visual analogue scale. PedsQL=Pediatric Quality of Life Inventory.
Improvements in mean change score values at months 3, 6, 9, and 12 were greater than the PedsQL MCIDs for the total score, psychosocial health summary score, and physical and emotional functioning subscales, and mean change score values were positive and near the MCIDs for social and school functioning subscales. Similarly, improvement in mean change score values for the EQ-5D VAS at months 3 to 12 was greater than the MCID (figure 2).
On the basis of observed data, 18 (46%) of 39 patients achieved the MCID for the PedsQL total score at day 28, 30 (81%) of 37 at month 3, and 23 (72%) of 32 at month 6. With the worst-case scenario approach, 18 (36%) of 50 patients achieved the MCID for the PedsQL total score at day 28, 30 (60%) of 50 at month 3, and 23 (46%) of 50 at month 6. Assuming a conservative MCID of 10, 21 (53%) of 40 patients achieved the MCID for the EQ-5D VAS on the basis of observed data at day 28, 24 (67%) of 36 at month 3, and 18 (62%) of 29 at month 6. With the worst-case scenario approach, 21 (44%) of 48 patients achieved the MCID for the EQ-5D VAS at day 28, 24 (50%) of 48 at month 3, and 18 (38%) of 48 at month 6. 21 (70%) of the 30 patients who achieved the MCID at month 3 sustained that achievement at month 6 for the PedsQL total score and 15 (54%) of 28 for the EQ-5D VAS. As a supportive approach to allow assessment of different threshold values, illustrations of the change from baseline response profiles of the PedsQL total score and EQ-5D VAS at months 3 and 6 are presented as cumulative distribution function plots (appendix pp 2–3).
For each patient-reported quality-of-life measure except social functioning, proportions of patients achieving the normative mean at all postbaseline study visits were greater than those at baseline even when all patients with missing data were assumed to not achieve the normative mean (figure 3A). Across all postbaseline visits, proportions were smallest in physical functioning and greatest in emotional functioning for the PedsQL. For the EQ-5D VAS, the proportions of patients achieving the normative mean value were 17 (39%) of 44 patients at day 28, 21 (54%) of 39 patients at month 3, 15 (48%) of 31 patients at month 6, 13 (62%) of 21 patients at month 9, and nine (64%) of 14 patients at month 12 (figure 3B).
Figure 3: Proportion of patients reaching normative mean values in PedsQL and EQ-5D VAS.
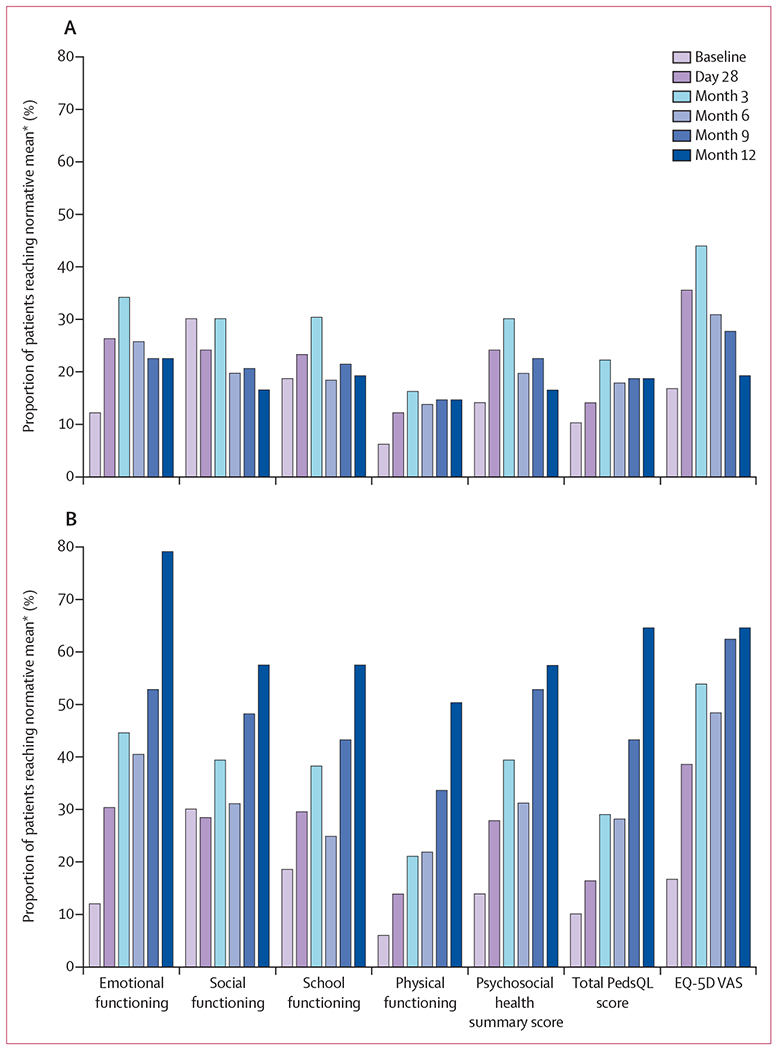
(A) Analyses assumed that all patients who had missing data at each assessment did not achieve the normative mean value for the patient-reported quality-of-life scale of interest (ie, patients were counted in the denominator but not in the numerator). (B) Analyses were limited to patients who had non-missing data at each study assessment. EQ-5D VAS=European Quality of Life-5 Dimensions questionnaire visual analogue scale. PedsQL=Pediatric Quality of Life Inventory. *From literature point estimates.
As expected, the proportion of patients achieving the normative mean at postbaseline visits were greater when based on the observed data than in the worst-case scenario (figure 3). However, overall results were similar between the two methods. Thus, notable global increases in quality-of-life scores were observed regardless of treatment of missing data.
Post-hoc subgroup analyses suggested that patients with severe cytokine release syndrome (23 patients for the PedsQL and 22 for the EQ-5D VAS) had lower mean improvement in quality of life at day 28 than patients without severe cytokine release syndrome (27 patients for the PedsQL and 26 for the EQ-5D VAS; mean of 0·3 [SD 20·8] vs 7·1 [13·0] for PedsQL total score and 3·3 [27·5] vs 12·2 [26·4] for EQ-5D VAS), but that improvements were similarly observed in both groups by months 3 and 6 and sustained at month 12 (appendix p 5). Similar results were observed by severe neurotoxicity status (appendix p 6).
Discussion
The findings from this study suggest that rapid improvements in broad aspects of patient-reported quality of life occurred after one-time treatment with tisagenlecleucel. To our knowledge, this is the first report on patient-reported quality of life after CAR T-cell therapy for patients with relapsed or refractory B-cell acute lymphoblastic leukaemia. Patients began to report improvements in patient-reported quality-of-life scores at day 28 after tisagenlecleucel therapy across most domains. By month 3, observed increases in score were clinically meaningful (greater than or equal to the MCIDs), which persisted at months 6, 9, and 12. Some delay in quality-of-life improvement was noted in patients who had severe cytokine release syndrome or neurotoxicity, but meaningful improvements in quality of life were still evident in these groups of patients by months 3–6. This temporal pattern of improvement is consistent with the temporal pattern of common side-effects related to tisagenlecleucel. Cytokine release syndrome occurs a median of 3 days (range 1–22) after infusion and resolves after a median of 8 days (1–30),20 and most neurological adverse events occur during cytokine release syndrome or shortly after its resolution. These findings, taken together with the preliminary activity and safety results,20 suggest a favourable benefit-risk profile of tisagenlecleucel in the treatment of paediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukaemia.
This timeframe of improvement in quality of life is shorter than that of traditional therapy for relapsed or refractory B-cell acute lymphoblastic leukaemia, which might include months of chemotherapy followed by stem-cell transplantation and the potential for graft-versus-host disease and other life-threatening toxicities. A recent systematic review27 on health-related quality of life in children on treatment for acute lymphoblastic leukaemia revealed substantial reductions in quality of life during treatment that were especially associated with intensive phases of chemotherapy and corticosteroid therapy. Furthermore, although quality of life improved over time in longitudinal studies, it remained lower than that of the general population after treatment, with lasting effects observed particularly in the social and emotional quality-of-life domains. Similarly, a long-term follow-up study28 of childhood survivors of acute lymphoblastic leukaemia reported that both medical and psychosocial conditions related to their original diagnosis persist into adulthood more than 20 years from diagnosis.
Patient-reported quality-of-life outcomes in adults with relapsed and refractory acute lymphoblastic leukaemia treated with other immunotherapies have recently been reported. In the phase 3 TOWER study,11 blinatumomab delayed deterioration in quality of life compared with standard therapy, although minimal improvement in quality of life was seen with blinatumomab. In the phase 3 INO-VATE study,10 inotuzumab ozogamicin resulted in improved physical, role, and social functioning compared with chemotherapy, with some improvement over baseline quality of life noted during the first cycle of therapy. It is important to note that when given with curative intent in the relapsed setting, blinatumomab and inotuzumab are frequently followed by allogeneic transplantation, and future studies evaluating quality of life following stem-cell transplantation in this patient population are necessary.
Notably, the greatest improvements from baseline occurred for physical functioning, for which patients had the lowest mean baseline score. Despite this, only 50% of patients who submitted data achieved the physical functioning normative mean score at 12 months. The contribution of tisagenlecleucel versus pre-existing toxicity in these patients who have been heavily pretreated remains to be elucidated.
A main limitation of this trial is its single-arm study design. As a result, change in patient-reported quality of life before and after an intervention was evaluated without the presence of a comparison group consisting of patients who received standard therapies. Rapid declines in quality of life have been noted in adults with relapsed or refractory leukaemia treated with standard chemotherapy in control groups in both the TOWER and INO-VATE studies.10,11 As our results are not directly comparable to these studies in adults, cautious interpretation is necessary; however, the remarkable activity of tisagenlecleucel and lack of an acceptable comparator group means that a randomised study in this patient population is not feasible.
Additional limitations of this study include the secondary nature of this objective on the ELIANA trial. Patients who completed patient-reported quality-of-life instruments represented a subset of the overall infused population based on age criteria to allow self-reporting. However, the majority of infused patients (83%) completed patient-reported quality-of-life instruments, and baseline characteristics were similar between patients who completed and did not complete the instruments.
Additionally, patients who are sicker and those who did not respond to treatment were less likely to complete patient-reported quality-of-life instruments, especially at the later postbaseline visits. Although common to oncology trials with patient-reported components, the missing data probably reflect patients with worse quality of life than those who submitted data. To address this potential bias, a worst-case scenario was included in the analysis in which patients with missing patient-reported quality-of-life data were assumed not to have achieved the normative mean values. Similar results were observed with this approach. Even in this worst case, improvements in the proportion of patients achieving normative mean values were observed. Because of the small sample size of the trial, it was not meaningful to apply more sophisticated statistical methods (eg, pattern mixture models) to explore further the potential effect of missing data on study findings. Future studies to evaluate the impact of baseline patient characteristics, including age, and side-effects beyond cytokine release syndrome and neurotoxicity will be of interest.
In this study, patient-reported quality-of-life scores were compared with universal normative scores rather than those from any age distribution profile, which could be a potential limitation in their interpretation.29 The MCID values used in these analyses were not developed specifically for the population under examination. The MCID values for the PedsQL were based on published studies establishing important differences in quality of life, and the MCID value for the EQ-5D VAS was based on a young adult rather than paediatric population. Although these values were not ideal, they represented the best estimates available from the literature. To help overcome this limitation, cumulative distribution function plots were presented for the PedsQL total score and EQ-5D VAS, which provided a comprehensive illustration of patient-reported quality-of-life response in this population over all specified values, allowing the reader to interpret change from baseline at any threshold values of interest. The increase in patient-reported quality-of-life scores, along with the activity and safety results of the ELIANA study, suggest a favourable benefit-risk profile of tisagenlecleucel in the treatment of paediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukaemia.
Supplementary Material
Research in context.
Evidence before this study
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor therapy that induced complete remission in 81% of children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia treated in the ELIANA trial, and had a manageable safety profile. Before our study, there was a paucity of information about quality of life in patients treated with chimeric antigen receptor therapy. We searched PubMed for articles published in English between inception and March 20, 2019, with the following search terms: “quality of life” or “patient reported outcomes” and “chimeric antigen receptor” or “tisagenlecleucel.” The search yielded one publication providing an overview of the opportunities and challenges of incorporation of patient-reported outcomes in clinical trials of chimeric antigen receptor cell therapy. We did not identify any papers reporting the results of patient-reported outcomes or quality of life in patients receiving this therapy.
Added value of this study
We present patient-reported quality-of-life data that were prospectively collected and analysed in the global, single-arm, open-label, phase 2 ELIANA trial of tisagenlecleucel for children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia. Our study findings suggest that broad-based clinically meaningful improvements in patient-reported quality of life occur as soon as 1–3 months after treatment with tisagenlecleucel and persist for 12 months. To our knowledge, these results represent the first report of patient-reported quality of life associated with a novel treatment that has demonstrated remarkable efficacy in children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia.
Implications of all the available evidence
Tisagenlecleucel was approved by the US Food and Drug Administration in 2017 and European Medicines Agency in 2018 for children and young adults up to age 25 years with B-cell acute lymphoblastic leukaemia that is refractory or in second or greater relapse. The rapid improvement in patient-reported quality of life scores presented here suggest that tisagenlecleucel is a promising treatment option for this patient population. Clinical trials of tisagenlecleucel in earlier lines of therapy are warranted.
Acknowledgments
This study was funded by Novartis. We thank the research staff at all participating hospitals who collected this time-intensive data and the patients and their families who participated in the trial.
Declaration of interests
TWL reports grants, personal fees, and non-financial support from Novartis (during the conduct of the study); personal fees from Loxo Oncology, Bayer, and Eli Lilly (outside the submitted work); and grants from Pfizer (outside the submitted work). GDM reports personal fees and consulting fees for work on trial from Novartis (during the conduct of the study), and non-financial support, advisory board honorarium, and institutional research support for trial from Novartis (outside the submitted work). AB reports personal fees and non-financial support from Novartis (during the conduct of the study); personal fees from Servier, Celgene, and Jazz (outside the submitted work); non-financial support from Kite Gilead (outside the submitted work); and grants and personal fees from Shire (outside the submitted work). ACD is now employed by bluebird bio, which had no oversight or support of the submitted work. MAP reports personal fees from Novartis (outside the submitted work). HB reports consulting fees from Novartis Oncology, and personal fees and non-financial support from Jazz (outside the submitted work). JB reports personal fees, advisory board honorarium and non-financial support from Novartis (during the conduct of the study) and personal fees advisory board honorarium from Pfizer (outside the submitted work). BDM reports grants from Novartis and non-financial support from Articulate Science (during the conduct of the study), and non-financial support from Jazz Pharmaceuticals (outside the submitted work). KLD reports advisory board honorarium from Novartis, from advisory board participation during the conduct of the study. FM reports personal fees and travel support from Novartis (during the conduct of the study). NB reports personal fees from Novartis (outside the submitted work). SR reports personal fees, advisory board honorarium, speaker fees, and travel and accommodation support from Novartis (during the conduct of the study); personal fees, advisory board honorarium, speaker fees, and travel and accommodation support from Jazz Pharma and Shire/Servier (outside the submitted work); personal fees, advisory board honorarium, and travel and accommodation support from Amgen (outside the submitted work). PB reports personal fees from Novartis (during the conduct of the study); reports advisory board honorarium from Novartis and Servier (outside the submitted work); reports speaker bureau support from Amgen (outside the submitted work); is on advisory boards for Amgen, Medac, Servier, Celgene, and Novartis; and reports institutional grants from Riemser, Neovii, and Medac. CP reports personal fees from Novartis (during the conduct of the study). SAG reports grants and personal fees from Novartis (during the conduct of the study); reports grants from Kite and Servier (outside the submitted work); reports personal fees from and is on advisory boards for Cellectis, Adaptimmune, Eureka, TCR2, Juno, GlaxoSmithKline, Vertex, Cure Genetics, Humanigen, Roche, and Cellular Biomedicine Group (outside the submitted work); and has a patent of Toxicity management for anti-tumor activity of CARs (WO 2014011984 A1) issued. HES reports personal fees from Novartis (during the conduct of the study). LR is an employee of RTI Health Solutions, a company that received funding from Novartis for statistical support. LY, SS, and JZ are Novartis employees. ACH reports non-financial support from Articulate Science (during the conduct of the study). All other authors declare no competing interests.
Footnotes
Data sharing
Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymised to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
References
- 1.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet 2013; 381: 1943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics. CA Cancer J Clin 2014; 64: 83–103. [DOI] [PubMed] [Google Scholar]
- 3.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med 2015; 373: 1541–52. [DOI] [PubMed] [Google Scholar]
- 4.Pulte D, Jansen L, Gondos A, et al. Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PLoS One 2014; 9: e85554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: therapeutic advances in childhood leukemia consortium study. J Clin Oncol 2010; 28: 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol 2010; 28: 2339–47 [DOI] [PubMed] [Google Scholar]
- 7.Osoba D Health-related quality of life and cancer clinical trials. Ther Adv Med Oncol 2011; 3: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016; 34: 557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017; 35: 96–112. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Su Y, Jabbour EJ, et al. Patient-reported outcomes from a phase 3 randomized controlled trial of inotuzumab ozogamicin versus standard therapy for relapsed/refractory acute lymphoblastic leukemia. Cancer 2018; 124: 2151–60. [DOI] [PubMed] [Google Scholar]
- 11.Topp MS, Zimmerman Z, Cannell P, et al. Health-related quality of life in adults with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab. Blood 2018; 131: 2906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furlong W, Rae C, Feeny D, et al. Health-related quality of life among children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2012; 59: 717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage E, Riordan AO, Hughes M. Quality of life in children with acute lymphoblastic leukaemia: a systematic review. Eur J Oncol Nurs 2009; 13: 36–48. [DOI] [PubMed] [Google Scholar]
- 14.Sung L, Yanofsky R, Klaassen RJ, et al. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. Int J Cancer 2011; 128: 1213–20. [DOI] [PubMed] [Google Scholar]
- 15.Castillo-Martínez D, Juárez-Villegas LE, Palomo-Colli MA, Medina-Sanson A, Zapata-Tarrés M. Quality of life in children with acute lymphoblastic leukemia during induction therapy with PedsQL Cancer Module. Bol Med Hosp Infant Mex 2009; 66: 410–18. [Google Scholar]
- 16.Yahia S, El-Hadidy MA, El-Gilany AH, et al. Cognitive function and quality of life in Egyptian children with acute lymphoblastic leukemia. J Blood Disord Transfus 2015; 6: 291. [Google Scholar]
- 17.Mitchell HR, Lu X, Myers RM, et al. Prospective, longitudinal assessment of quality of life in children from diagnosis to 3 months off treatment for standard risk acute lymphoblastic leukemia: results of Children’s Oncology Group study AALL0331. Int J Cancer 2016; 138: 332–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal M, Sharma KK, Vatsa M, Bakhshi S. Comparison of health-related quality of life of children during maintenance therapy with acute lymphoblastic leukemia versus siblings and healthy children in India. Leuk Lymphoma 2013; 54: 1036–41. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty R, Sidana S, Shah GL, Scordo M, Hamilton BK, Majhail NS. Patient-reported outcomes with chimeric antigen receptor t cell therapy: challenges and opportunities. Biol Blood Marrow Transplant 2018; 25: e155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378: 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002; 94: 2090–106. [DOI] [PubMed] [Google Scholar]
- 22.Szende A, Williams A, eds. Measuring self-reported population health: an international perspective based on EQ-5D. Budepest: SpringMed Publishing, 2004. [PubMed] [Google Scholar]
- 23.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3: 329–41. [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Seid M, Smith Knight T, Burwinkle T, Brown J, Szer IS. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory Generic Core Scales and Rheumatology Module. Arthritis Rheum 2002; 46: 714–25. [DOI] [PubMed] [Google Scholar]
- 26.Janssen B, Szende A. Population norms for the EQ-5D In Szende A, Janssen B, Cabases J, eds. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer, 2014. [PubMed] [Google Scholar]
- 27.Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer 2017; 64: e26489. [DOI] [PubMed] [Google Scholar]
- 28.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood 2008; 111: 5515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life? An analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


