Background:
Giant-cell tumor of bone (GCTB) is a locally aggressive intermediate bone tumor with a rarely metastasizing disposition. Standard surgical treatment consists of curettage, adjuvant treatment, and augmentation with allograft, autograft, or synthetics. Polymethylmethacrylate (PMMA) has been widely used for augmentation of the bone defect; however, the hyperthermic polymerization of PMMA may cause damage to articular cartilage, and the stiffness of the material may decrease the ability of the joint to absorb shock. These properties were reported to result in secondary osteoarthritis. Calcium phosphate cement has a low degree of thermal reaction and a strength that is similar to cortical bone. The aim of the present study was to investigate the incidence of secondary osteoarthritis around the knee joint following augmentation with calcium phosphate cement.
Methods:
We retrospectively evaluated 19 patients with primary GCTB from 2003 to 2012. Curettage, high-speed burring, phenolization, and filling with calcium phosphate cement were performed in all patients. Radiographic evidence of osteoarthritis progression was evaluated with use of the Kellgren-Lawrence grade; the postoperative grade was compared with both the preoperative grade and the grade of the nonoperative contralateral knee at the time of the latest follow-up. The Musculoskeletal Tumor Society score and oncological outcomes at the time of the latest follow-up were evaluated.
Results:
At a median follow-up period of 131 months, osteoarthritic progression was observed in 5 patients (26%), of which 2 were classified as Kellgren-Lawrence grade 3 and 1 was classified as Kellgren-Lawrence grade 4. The patient with grade-4 osteoarthritis underwent total knee arthroplasty, and 1 of the patients with grade-3 osteoarthritis underwent open-wedge high tibial osteotomy. The 10-year survival rate of joint cartilage with a Kellgren-Lawrence grade of <3 was 83%. The average Musculoskeletal Tumor Society score was 29 points. GCTB recurred in 2 patients, and 1 of these patients developed pulmonary metastasis.
Conclusions:
The incidence of secondary osteoarthritis was low, despite the long follow-up period. Prospective investigation comparing PMMA and calcium phosphate cement is warranted to determine the relative rate of secondary osteoarthritis and the outcomes associated with the 2 different types of augmentation.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Giant-cell tumor of bone (GCTB) is a locally aggressive intermediate bone tumor, with a rarely metastasizing disposition, that usually affects the ends of long bones; approximately half of these tumors are localized around the knee joint1,2. GCTB usually arises in adolescents and young adults from 20 to 40 years old1-3. Recurrence following surgical treatment is observed in 12% to 27% of patients, and 1% to 4% of patients develop pulmonary metastasis1,2,4-11. Pathological fracture sometimes occurs12.
The standard surgical treatment of GCTB consists of curettage, adjuvant treatment, and bone-grafting of the bone defect3,13-17. High-speed burring or chemical adjuvants are usually utilized as adjuvant treatments. Chemical adjuvants include phenol, alcohol, hydrogen peroxide, and liquid nitrogen18. There are many options of materials for augmentation: allograft, autograft, and synthetics. Of these, polymethylmethacrylate (PMMA) is most commonly utilized to fill the cavity. PMMA is easily moldable and provides immediate mechanical support following the surgical procedure; however, the hyperthermic polymerization of PMMA may cause damage to articular cartilage, and the stiffness of the material may decrease the ability of the joint to absorb shock15,19-21. In previous studies, degenerative change of the articular cartilage was reported in 30% to 33% of patients with GCTB at 5 years after curettage and bone-grafting with PMMA22,23. Osteoarthritis is one of the most notable complications, especially among young patients.
Calcium phosphate cement (CPC) mainly consists of alpha-tricalcium phosphate. The material is sometimes utilized as a scaffold for bone cavities in trauma cases; however, compared with its use in the fields of craniofacial or dental surgery, its use in orthopaedic surgery is less common24-27. CPC has little thermal reaction at hardening, has strength similar to cortical bone, is osteoconductive, and achieves good long-lasting stability28-30. To our knowledge, there have been no reports regarding secondary osteoarthritis in the knee joint following curettage and subsequent augmentation with CPC instead of PMMA.
We evaluated the prevalence of radiographic findings of secondary osteoarthritis following curettage and augmentation with CPC, as well as the clinical outcomes of the procedure.
Materials and Methods
We retrospectively evaluated 19 patients who underwent curettage and augmentation with CPC for primary GCTB in the distal aspect of the femur or the proximal aspect of the tibia with a minimum follow-up of 5 years. These patients were selected from a total of 37 consecutive patients identified with use of a pathological database between 2003 and 2012. The cases with a history of treatment for GCTB (n = 3), cases with insufficient follow-up duration (n = 6), and cases in which the tumor was located on the humerus, radius, pelvis, or fibula (n = 9) were excluded. The study population included 12 women and 7 men. The median follow-up was 131 months (range, 66 to 205 months). The median age at the time of the index procedure was 39 years (range, 20 to 63 years).
The index procedure was performed by oncological orthopaedic surgeons in all cases. Curettage and intralesional treatment were preferred for GCTB at our institution, even in the presence of soft-tissue extension. En bloc excision was not performed as a primary treatment for GCTB. Internal fixation was sometimes performed in cases involving pathological fracture. The standard surgical treatment consisted of curettage, adjuvant treatment of high-speed burring, and phenolization with rinsing of the cavity with ethanol (performed 3 times), followed by filling with CPC (BIOPEX or BIOPEX-R; HOYA Technosurgical). The cost of CPC in U.S. dollars is approximately $150/mL, which is expensive, but is all covered by the social insurance system in Japan. Cementation was performed under tourniquet control. Autologous bone graft was considered after curettage when the cortical bone defect was large enough to leak into the intra-articular space. The cement volume depended on the size of the lesion; the average volume (and standard deviation) was 58 ± 22 mL. Postoperative rehabilitation consisted of functional mobilization and immediate weight-bearing for most patients. Patients with a pathological fracture were permitted only partial weight-bearing for 6 to 12 weeks. There were no limitations on sports or activities beginning 6 months after the surgical procedure. The follow-up protocol consisted of conventional radiography and magnetic resonance imaging (MRI) every 4 to 6 months until 5 years after the procedure, and every 6 months or 1 year thereafter to detect recurrence or complications. Chest radiographs were also made at the same intervals to detect lung metastasis.
Medical records and radiographs were reviewed to determine age, sex, tumor size, tumor location, Campanacci classification, tumor-cartilage distance, exposed articular surface, involvement of subchondral bone, pathological fracture, usage of bone-modifying agents such as bisphosphonate or denosumab, recurrence, metastasis, complications, preoperative and postoperative Kellgren-Lawrence (KL) grade, primary and additional surgical procedures, and the duration of follow-up.
Osteoarthritis was assessed according to the KL grade on preoperative and postoperative weight-bearing radiographs in both the operative and contralateral knees31. The assessment was performed by 2 independent observers. The KL grade of the nonoperative contralateral knee was utilized as a control to reduce the impact of age. The time of secondary osteoarthritis following the surgical procedure was defined as the time at which signs of osteoarthritis appeared to have progressed more than both the preoperative KL grade and the KL grade of the nonoperative contralateral side at the time of the latest follow-up. We evaluated the overall joint cartilage survival and the joint cartilage survival until progression to KL grade 3 or 4. The osteoarthritis evaluation in reoperation cases, such as total knee arthroplasty and high tibial osteotomy, was performed prior to the time of reoperation.
The amount of subchondral bone involvement was defined as the area of the affected knee compartment in which ≤3 mm of subchondral bone thickness remained, as assessed by radiography or computed tomography (CT), and was calculated as a percentage with the method described by Chen et al.32. Tumor-cartilage distance and exposed articular surface were assessed on preoperative CT or MRI.
The functional outcome was evaluated with use of the Musculoskeletal Tumor Society (MSTS) score, and the oncological outcome and disease-free survival were assessed at the time of the latest follow-up. The MSTS score in reoperation cases, such as total knee arthroplasty and high tibial osteotomy, was evaluated prior to the time of reoperation.
This retrospective study of patients was approved by the ethical committee of Kanazawa University Hospital (Institutional Review Board Number 1804) in compliance with the guidelines of the Helsinki Declaration of 1975.
Statistical Analysis
The Kaplan-Meier method was utilized to assess the probability of disease-free survival and osteoarthritis progression (joint cartilage survival). The Cohen kappa statistic was utilized to determine an interobserver agreement for osteoarthritic progression. Excellent agreement was defined as κ ≥ 0.80, substantial agreement as κ = 0.60 to 0.79, moderate agreement as κ = 0.40 to 0.59, and poor agreement as κ < 0.40. All statistical analyses were performed with use of EZR (Saitama Medical Center, Jichi Medical University)33.
Results
The tumors were located at the distal aspect of the femur in 12 patients and at the proximal aspect of the tibia in 7 patients. The Campanacci classification was grade I in 3 patients, grade II in 12 patients, and grade III in 4 patients. The average tumor size was 58 ± 22 cm3. The average tumor-cartilage distance was 3.4 ± 3.0 mm, and the average amount of subchondral bone involvement was 19% ± 13%. One patient had a pathological fracture that was detected preoperatively; that patient underwent additional screw fixation and autologous bone graft with bone taken from the ilium in addition to CPC augmentation and curettage. The other 18 cases were all just filled with CPC after curettage, without any additional internal fixation. There were no complications, including infection, postoperative fracture, instability, and restricted range of motion.
Secondary osteoarthritis of the knee joint was detected in a total of 5 patients (26%) at an average of 131 months of follow-up (Table I). None of the other 14 patients experienced progression of osteoarthritis secondary to the surgical procedure, with 12 patients maintaining the same KL grade as measured both preoperatively and in the nonoperative contralateral knee (Fig. 1). For the other 2 patients, although progressive osteoarthritis was observed, it was observed bilaterally, with the nonoperative contralateral knee having the same KL grade as the operative knee; for this reason, these 2 cases were considered to be age-related osteoarthritis, rather than secondary to the surgical procedure. Three of the 5 patients with secondary osteoarthritis progressed to KL grade 3 (2 patients) or grade 4 (1 patient). The Cohen kappa statistic showed excellent agreement (κ = 0.83) between the 2 independent observers.
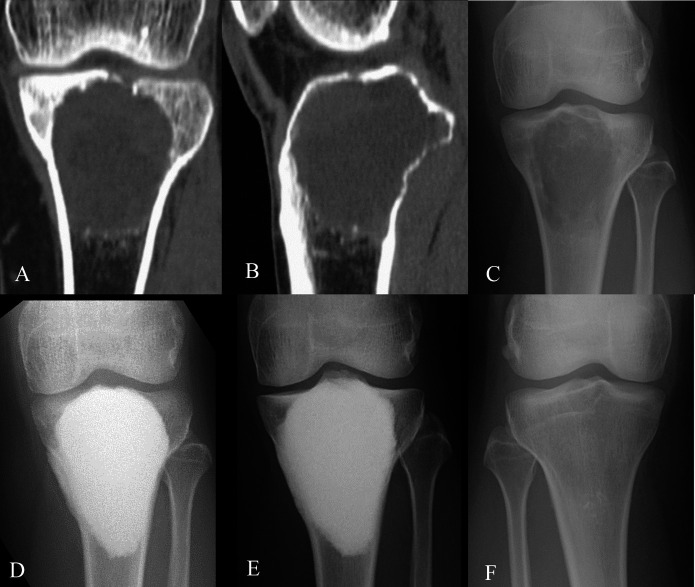
Fig. 1.
Figs. 1-A through 1-F Preoperative CT and anteroposterior radiographs showing change in the knee joint of a 31-year-old woman with GCTB at the proximal aspect of the left tibia (Case 12, Table I). Fig. 1-A Coronal plane of the lesion with partial subchondral bone loss. Fig. 1-B Sagittal plane of the lesion with partial subchondral bone loss. Fig. 1-C Preoperative state. Fig. 1-D State at 3 months postoperatively. Fig. 1-E State at about 12 years postoperatively. Fig. 1-F State of the nonoperative contralateral knee at about 12 years postoperatively.
TABLE I.
Patient Characteristics*
| Case No. | Age in Years, Sex | Location | Size (cm3) | Pathological Fracture | ASB | Dimensions of Exposed Articular Cartilage (mm) | Preop. KL Grade | KL Grade at Latest Follow-up | Contralateral KL Grade | Postop. Osteoarthritis | Bone-Modifying Agents | Follow-up Period (mo) | Additional Operation |
| 1 | 29, M | DF | 45 | No | 7% | 2 × 7 | 0 | 0 | 0 | – | – | 98 | Re-curettage |
| 2 | 30, M | DF | 36 | No | 3% | 3 × 6 | 0 | 3 | 0 | Secondary | – | 111 | OWHTO |
| 3 | 20, F | DF | 75 | Yes | 38% | 3 × 3 | 0 | 1 | 0 | Secondary | – | 102 | Hardware removal |
| 4 | 41, F | DF | 40 | No | 10% | 5 × 4 | 1 | 1 | 1 | – | – | 112 | – |
| 5 | 60, F | PT | 84 | No | 11% | 11 × 6 | 2 | 4 | 2 | Secondary | Denosumab | 112 | TKA |
| 6 | 33, F | PT | 90 | No | 27% | 2 × 4 | 1 | 1 | 1 | – | – | 117 | – |
| 7 | 35, F | DF | 66 | No | 17% | NA | 1 | 2 | 1 | Secondary | – | 128 | – |
| 8 | 43, M | PT | 39 | No | 10% | 0 | 1 | 1 | 1 | – | – | 139 | – |
| 9 | 28, M | DF | 57 | No | 17% | 5 × 6 | 0 | 0 | 0 | – | – | 66 | – |
| 10 | 59, F | DF | 96 | No | 21% | 8 × 9 | 1 | 3 | 1 | Secondary | Bisphosphonate | 69 | – |
| 11 | 27, M | DF | 66 | No | 8% | 0 | 0 | 0 | 0 | – | – | 140 | – |
| 12 | 31, F | PT | 66 | No | 42% | 5 × 6 | 0 | 0 | 0 | – | – | 141 | – |
| 13 | 63, F | PT | 15 | No | 13% | NA | 1 | 2 | 2 | Age-related | Bisphosphonate | 155 | – |
| 14 | 58, M | DF | 80 | No | 12% | NA | 1 | 1 | 1 | – | – | 165 | – |
| 15 | 26, F | PT | 42 | No | 18% | 0 | 0 | 0 | 0 | – | – | 172 | – |
| 16 | 33, F | DF | 32 | No | 10% | NA | 0 | 0 | 0 | – | – | 169 | – |
| 17 | 61, F | DF | 60 | No | 17% | NA | 1 | 2 | 2 | Age-related | – | 193 | – |
| 18 | 28, F | DF | 54 | No | 53% | 7 ×9 | 0 | 0 | 0 | – | – | 205 | – |
| 19 | 20, M | PT | 36 | No | 19% | 9 × 9 | 0 | 0 | 0 | – | Denosumab | 104 | – |
ASB = amount of subchondral bone involvement, preop. = preoperative, postop. = postoperative, DF = distal aspect of the femur, OWHTO = open-wedge high tibial osteotomy, PT = proximal aspect of the tibia, TKA = total knee arthroplasty, and NA = not applicable.
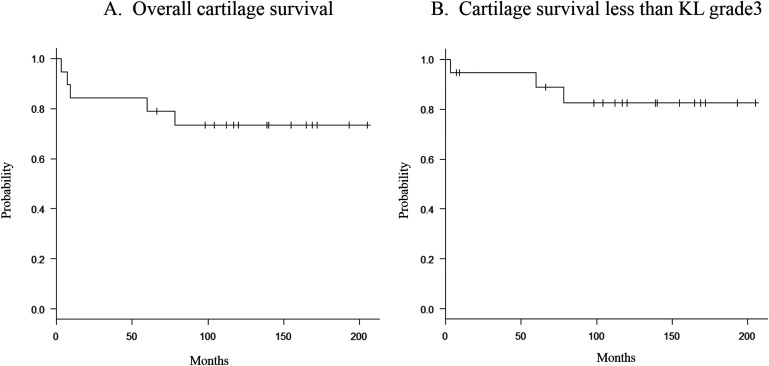
The overall survivorship of joint cartilage was 84% at 5 years and 73% at 10 years (Fig. 2-A), and the survivorship of joint cartilage with a KL grade of <3 was 95% at 5 years and 83% at 10 years (Fig. 2-B). Total knee arthroplasty was performed in 1 patient with progression to KL grade 4 at 79 months because the arthritis-related pain could not be managed nonoperatively. Following the total knee arthroplasty, the patient underwent denosumab therapy for prevention of recurrence and treatment of osteoporosis. In another patient, open-wedge high tibial osteotomy was performed for progression to KL grade 3 at 89 months because the arthritis-related pain could not be managed nonoperatively. The other patient with progression to KL grade 3 was observed and nonoperatively managed because the osteoarthritis was not symptomatic (Fig. 3). Two patients with secondary osteoarthritis with progression to KL grade 1 and grade 2, respectively, were only followed at an outpatient clinic because they experienced no arthritis-related pain.
Fig. 2.
Kaplan-Meier curve for overall cartilage survival (Fig. 2-A) and cartilage survival with KL grade <3 (Fig. 2-B).
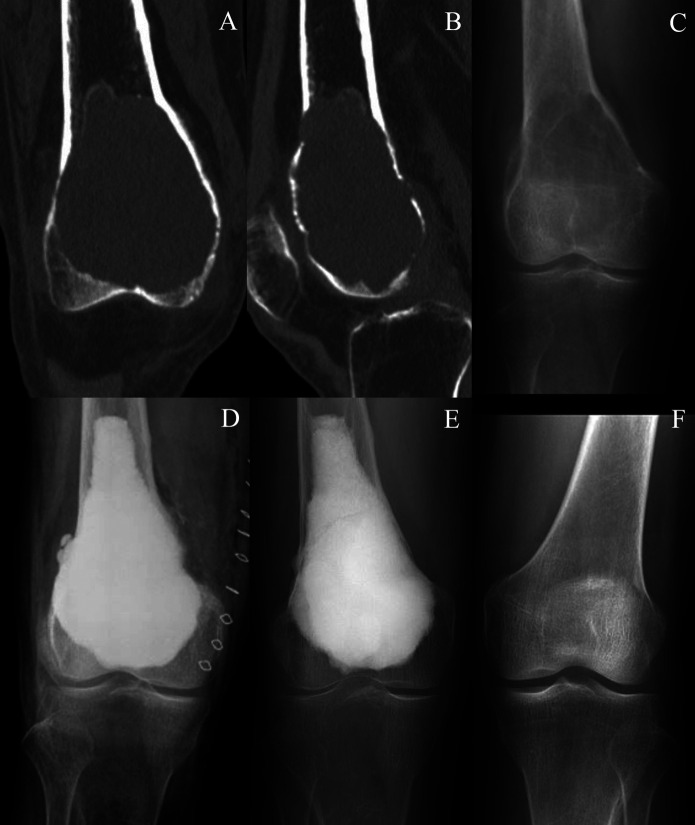
Fig. 3.
Figs. 3-A through 3-F Preoperative CT and anteroposterior radiographs showing change in the knee joint of a 59-year-old woman with GCTB at the distal aspect of the right femur (Case 10, Table I). Fig. 3-A Coronal plane of the lesion. Fig. 3-B Sagittal plane of the lesion. Fig. 3-C Preoperative state. Fig. 3-D Immediate postoperative state. Fig. 3-E State at about 6 years postoperatively. Fig. 3-F State of the nonoperative contralateral knee at about 6 years postoperatively.
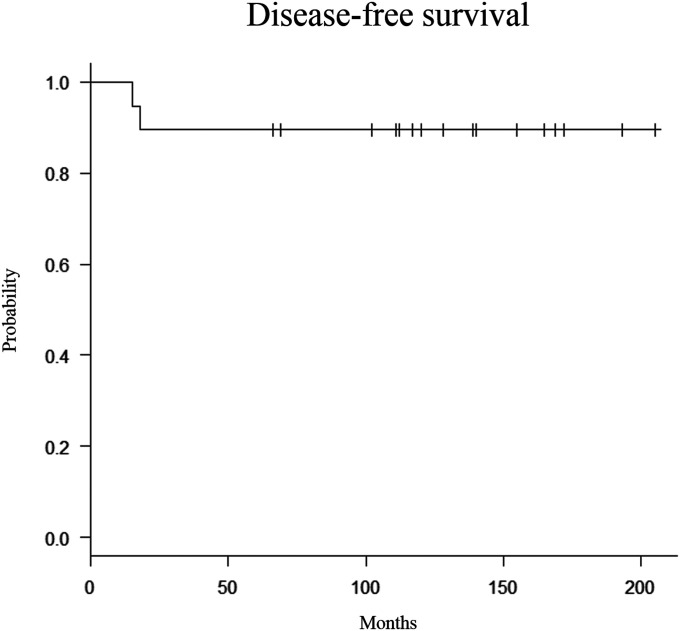
Oncological assessment revealed that 17 patients were free of oncological disease; there was no evidence of disease after re-curettage for the recurrence in 1 additional patient, and the remaining patient was alive with disease. The 10-year survivorship free of oncological disease was 90% (Fig. 4). One patient developed a recurrent tumor at 18 months postoperatively and underwent re-curettage and additional augmentation with CPC. One patient experienced recurrent GCTB and pulmonary metastasis at 16 months and was treated with denosumab only for 6 years. Even at 95 months after the primary surgical procedure, the patient remained stable and alive with disease; neither progression of the disease nor secondary osteoarthritis was observed. Bisphosphonate was utilized for the other 2 patients for both prevention of recurrent GCTB and treatment of osteoporosis.
Fig. 4.
Kaplan-Meier curve for disease-free survival.
Functional outcomes were excellent in all cases, with an average MSTS score of 29 points (range, 23 to 30 points). The lowest MSTS score (23 points) was observed in the patient who eventually underwent total knee arthroplasty for symptomatic progression of osteoarthritis to KL grade 3, with MSTS scoring having occurred prior to the arthroplasty procedure.
Discussion
Previous studies of GCTB have reported secondary osteoarthritis in 30% to 33% of patients who undergo curettage, adjuvant treatment, and augmentation with PMMA with mean follow-ups of 25 to 57 months22,23, compared with 5 (26%) of 19 patients at a mean follow-up of 131 months in the present study. Previous studies have also reported a progression of osteoarthritis to KL grade 3 or 4 in 17% to 26% of patients with mean follow-ups of 86 to 120 months5,6, compared with 3 patients (16%) at a mean follow-up of 131 months in the present study (Table II).
TABLE II.
Previous Studies on the Incidence of Secondary Osteoarthritis and Recurrence Rate Following Curettage for GCTB*
| Author, Year | Cases | Average Follow-up (mo) | Bone Graft | Osteoarthritis Progression | Osteoarthritis Progression to KL 3-4 | Recurrence Rate |
| Suzuki,22 | 30 | 57 | PMMA | 10 (33%) | NA | 10 (33%) |
| Xu23 | 76 | 35 | PMMA | 23 (30%) | NA | 3 (6%) |
| van der Heijden5 | 53 | 86 | PMMA | NA | 8 (17%) | 15 (28%) |
| Caubère6 | 19 | 120 | PMMA | NA | 5 (26%) | 8 (42%) |
| Present study | 19 | 131 | CPC | 5 (26%) | 3 (16%) | 2 (11%) |
NA = not applicable
PMMA has a number of advantages, including ease of use, cytotoxic or necrotic effects on the tumor remnants, and the fact that it supports full weight-bearing immediately after the surgical procedure3,5,13,15,19. PMMA is widely recommended to occupy the cavity left after curettage, although its hyperthermic polymerization and its stiffness may cause degenerative change of the joint cartilage15,19-21. CPC mainly consists of alpha-tricalcium phosphate and is just as moldable as PMMA for augmentation of the bone cavity, but the thermal reaction during hardening is not as strong as that of PMMA34. This thermal property might have contributed to the low rate of secondary osteoarthritis in the present study. The strength of CPC peaked at 1 week, and patients were able to walk with full weight-bearing at postoperative day 3, which is similar to patients who undergo this procedure with PMMA. The compressive stress of the CPC is approximately 65 MPa at postoperative day 3 and reaches >80 MPa (final strength) at 1 week after mixing35. The strength of CPC was less than PMMA but similar to the cortical bone, which might also contribute to the low rate of secondary osteoarthritis. Other studies have also reported the osteoconductivity and long-lasting stability of CPC28-30. With the exception of the 1 patient who underwent total knee arthroplasty, all patients in the present study showed excellent function scores, as assessed with use of the MSTS score, and some of the patients could participate in sports or squat deeply without any limitations.
To our knowledge, no previous study has assessed secondary osteoarthritis by comparison of the KL grade of the operative and nonoperative contralateral knees. Previous studies have only compared the preoperative and postoperative KL grade of the operative knee5,6,22,23. The previous methods possibly underestimate the progression of age-related osteoarthritis. We therefore compared the result to not only the preoperative KL grade of the operative knee, but also the KL grade of the nonoperative contralateral knee as a control in order to decrease the impact of age-related osteoarthritis. Actually, 2 patients in this study were not considered secondary osteoarthritis, but rather age-related osteoarthritis. One of these patients was a 63-year-old woman with GCTB of the proximal aspect of the left tibia; KL grade-2 osteoarthritis was observed in both sides of the knee joint at 155 months postoperatively, despite a preoperative KL grade of 1 in both sides of the knee joint. The patient complained of slight pain during walking at the time of the latest follow-up. The other patient was a 61-year-old woman with GCTB of the distal aspect of the left femur; KL grade-2 osteoarthritis was observed in both sides of the knee joint at 193 months postoperatively, despite a preoperative KL grade of 0 in both sides of the knee joint. The patient was asymptomatic and is being followed at an outpatient clinic.
Bone remodeling ability gradually declines with age, and osteoporosis induces articular osteoarthritic change36,37. The curettage procedure for GCTB might cause the cartilage damage when performed in deteriorated bone. The surgeon must pay close attention not to break the joint cartilage during the curettage. It is also important to inform elderly patients of the possibility of reoperation for secondary osteoarthritis.
The combination of PMMA and subchondral bone-grafting has been reported to reduce the risk of osteoarthritis or mechanical failure38,39. Wu et al. reported that PMMA and subchondral cancellous bone graft with supplemental cortical bone graft reduced the risk of progression of osteoarthritis to 11% at an average of 33 months38. Similarly, Teng et al. suggested that extensive curettage with bone-grafting and cementing reduced the risk of mechanical failure in the knee at a mean of 33 months39. However, the follow-ups of these studies were not long enough to evaluate the occurrence of osteoarthritis.
Recurrence rates among patients who undergo curettage for GCTB are not low5,6,12-14,19,22, with rates ranging from 27% to 65% when the remaining cavity is not filled following curettage or when filling is performed with cancellous bone graft1,2,4. On the other hand, recurrence rates ranging from 12% to 42% have been reported among patients who undergo filling with PMMA4,6,13,40. In recent years, those recurrence rates have decreased to 12% to 27% as a result of adjuvant treatment or advanced operative techniques10,11. However, among studies with a long-term follow-up, Caubère et al.6 reported a recurrence rate of 42% at 120 months, whereas van der Heijden et al.5 reported a rate of 28% at 86 months (Table I). In the present study, only 2 patients (11%) experienced recurrence at 131 months. This rate was much lower compared with those reported in previous studies (Table I).
Some studies have reported that the residual tumor cells that remain following curettage and adjuvant treatment can be killed by the hyperthermic reaction of PMMA, and that this effect reduced the rate of recurrence5,13,19; however, the recurrence rate in the present study was lower despite the low thermal reaction of CPC. Recently, denosumab therapy was reported to be effective for advanced GCTB41. However, it has been reported that the risk of local recurrence might be increased in patients with GCTB who are receiving denosumab therapy42. This therapy is currently controversial; however, in the present study, neither of the 2 patients who received postoperative denosumab therapy had progression of disease or secondary osteoarthritis.
This study had several limitations. First, this was a retrospective study that included a relatively small study population in a single institution. Second, the residual amount of cartilage could have been measured more accurately by MRI for the evaluation of osteoarthritis; however, MRI assessment was not performed at every outpatient clinic. Third, there were no cases in which PMMA was utilized; ideally, this study would have compared the results to cases in which PMMA was utilized. Fourth, the high cost of CPC was not considered in this study. There was some variability in cost depending on the size of the tumor; however, the cost may be a problem in the countries in which the social insurance system is not well developed.
In conclusion, when GCTB around the knee joint was treated with curettage and augmentation with CPC, the incidence of secondary osteoarthritis was comparatively low, even with a long-term follow-up. Secondary osteoarthritis occurred at a rate of 26% at a mean follow-up of 131 months in patients who underwent augmentation with CPC after curettage. Prospective investigation comparing PMMA and CPC is warranted to determine the relative rate of secondary osteoarthritis and the outcome associated with the 2 different types of augmentation.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, Graduate School of Medical Sciences, Kanazawa University, Kanazawa, Japan
Disclosure: The authors indicated that no external funding was received for any aspect of this work. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A195).
References
- 1.van der Heijden L. Giant cell tumor of bone and tenosynovial tissue: surgical outcome [PhD dissertation]. Leiden: Leiden University; 2014. Accessed 2020 Jun 16. https://openaccess.leidenuniv.nl/handle/1887/28526 [Google Scholar]
- 2.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987. January;69(1):106-14. [PubMed] [Google Scholar]
- 3.Fraquet N, Faizon G, Rosset P, Phillipeau JM, Waast D, Gouin F. Long bones giant cells tumors: treatment by curretage and cavity filling cementation. Orthop Traumatol Surg Res. 2009. October;95(6):402-6. Epub 2009 Sep 19. [DOI] [PubMed] [Google Scholar]
- 4.Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, Hardes J, Gosheger G. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008. September;134(9):969-78. Epub 2008 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Heijden L, van de Sande MA, Heineken AC, Fiocco M, Nelissen RG, Dijkstra PD. Mid-term outcome after curettage with polymethylmethacrylate for giant cell tumor around the knee: higher risk of radiographic osteoarthritis? J Bone Joint Surg Am. 2013. November 6;95(21):e159. [DOI] [PubMed] [Google Scholar]
- 6.Caubère A, Harrosch S, Fioravanti M, Curvale G, Rochwerger A, Mattei JC. Does curettage-cement packing for treating giant cell tumors at the knee lead to osteoarthritis? Orthop Traumatol Surg Res. 2017. November;103(7):1075-9. Epub 2017 Aug 3. [DOI] [PubMed] [Google Scholar]
- 7.Siebenrock KA, Unni KK, Rock MG. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J Bone Joint Surg Br. 1998. January;80(1):43-7. [DOI] [PubMed] [Google Scholar]
- 8.Rock MG, Pritchard DJ, Unni KK. Metastases from histologically benign giant-cell tumor of bone. J Bone Joint Surg Am. 1984. February;66(2):269-74. [PubMed] [Google Scholar]
- 9.Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994. May;302:219-30. [PubMed] [Google Scholar]
- 10.van der Heijden L, Dijkstra PDS, Blay JY, Gelderblom H. Giant cell tumour of bone in the denosumab era. Eur J Cancer. 2017. May;77:75-83. Epub 2017 Mar 30. [DOI] [PubMed] [Google Scholar]
- 11.Hasan O, Ali M, Mustafa M, Ali A, Umer M. Treatment and recurrence of giant cell tumors of bone- a retrospective cohort from a developing country. Ann Med Surg (Lond). 2019. December;15(48):29-34. Epub 2019 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto S, Mavrogenis AF, Tanzi P, Leone G, Righi A, Akahane M, Kido A, Honoki K, Tanaka Y, Donati DM, Errani C. Similar local recurrence but better function with curettage versus resection for bone giant cell tumor and pathological fracture at presentation. J Surg Oncol. 2019. June;119(7):864-72. Epub 2019 Feb 7. [DOI] [PubMed] [Google Scholar]
- 13.Becker WT Dohle J Bernd L Braun A Cserhati M Enderle A Hovy L Matejovsky Z Szendroi M Trieb K Tunn PU; Knochentumoren Arbeitsgemeinschaft. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008. May;90(5):1060-7. [DOI] [PubMed] [Google Scholar]
- 14.Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011. February;469(2):591-9. Epub 2010 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bini SA, Gill K, Johnston JO. Giant cell tumor of bone. Curettage and cement reconstruction. Clin Orthop Relat Res. 1995. December;321:245-50. [PubMed] [Google Scholar]
- 16.Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, Rossi G, Longhi A, Mercuri M. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010. February;36(1):1-7. Epub 2009 Oct 30. [DOI] [PubMed] [Google Scholar]
- 17.Zheng K, Yu XC, Hu YC, Wang Z, Wu SJ, Ye ZM; Giant Cell Tumor Group of China (GTOC). How to fill the cavity after curettage of giant cell tumors around the knee? A multicenter analysis. Chin Med J (Engl). 2017. November 5;130(21):2541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heijden L, van der Geest IC, Schreuder HW, van de Sande MA, Dijkstra PD. Liquid nitrogen or phenolization for giant cell tumor of bone?: a comparative cohort study of various standard treatments at two tertiary referral centers. J Bone Joint Surg Am. 2014. March 5;96(5):e35. [DOI] [PubMed] [Google Scholar]
- 19.Gaston CL, Bhumbra R, Watanuki M, Abudu AT, Carter SR, Jeys LM, Tillman RM, Grimer RJ. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J Bone Joint Surg Br. 2011. December;93(12):1665-9. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DA, Barker ME, Hamlin BH. Thermal effects of acrylic cementation at bone tumour sites. Int J Hyperthermia. 1997. May-Jun;13(3):287-306. [DOI] [PubMed] [Google Scholar]
- 21.Radev BR, Kase JA, Askew MJ, Weiner SD. Potential for thermal damage to articular cartilage by PMMA reconstruction of a bone cavity following tumor excision: a finite element study. J Biomech. 2009. May 29;42(8):1120-6. Epub 2009 Apr 2. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Nishida Y, Yamada Y, Tsukushi S, Sugiura H, Nakashima H, Ishiguro N. Re-operation results in osteoarthritic change of knee joints in patients with giant cell tumor of bone. Knee. 2007. October;14(5):369-74. Epub 2007 Jun 29. [DOI] [PubMed] [Google Scholar]
- 23.Xu HR, Niu XH, Zhang Q, Hao L, Ding Y, Li Y. Subchondral bone grafting reduces degenerative change of knee joint in patients of giant cell tumor of bone. Chin Med J (Engl). 2013. August;126(16):3053-6. [PubMed] [Google Scholar]
- 24.Zhang L, Weir MD, Chow LC, Reynolds MA, Xu HH. Rechargeable calcium phosphate orthodontic cement with sustained ion release and rerelease. Sci Rep. 2016. November 3;6:26476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Huang S, Zou R, Gao X, Ruan J, Weir MD, Reynolds MA, Qin W, Chang X, Fu H, Xu HHK. Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering. Dent Mater. 2019. July;35(7):1031-41. Epub 2019 May 7. [DOI] [PubMed] [Google Scholar]
- 26.Winge MI, Røkkum M. CaP cement is equivalent to iliac bone graft in filling of large metaphyseal defects: 2 year prospective randomised study on distal radius osteotomies. Injury. 2018. March;49(3):636-43. Epub 2017 Nov 22. [DOI] [PubMed] [Google Scholar]
- 27.Ho S, Nallathamby V, Ng H, Ho M, Wong M. A novel application of calcium phosphate-based bone cement as an adjunct procedure in adult craniofacial reconstruction. Craniomaxillofac Trauma Reconstr. 2011. December;4(4):235-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi A, Suwanpramote P, Yamamoto N, Shirai T, Hayashi K, Kimura H, Miwa S, Higuchi T, Abe K, Tsuchiya H. Mid- to long-term clinical outcome of giant cell tumor of bone treated with calcium phosphate cement following thorough curettage and phenolization. J Surg Oncol. 2018. May;117(6):1232-8. Epub 2018 Jan 8. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi T, Yamamoto N, Hayashi K, Takeuchi A, Kimura H, Miwa S, Igarashi K, Abe K, Taniguchi Y, Aiba H, Tsuchiya H. Calcium phosphate cement in the surgical management of benign bone tumors. Anticancer Res. 2018. May;38(5):3031-5. [DOI] [PubMed] [Google Scholar]
- 30.Jeong J, Kim JH, Shim JH, Hwang NS, Heo CY. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019. January 14;23:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957. December;16(4):494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen TH, Su YP, Chen WM. Giant cell tumors of the knee: subchondral bone integrity affects the outcome. Int Orthop. 2005. February;29(1):30-4. Epub 2005 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013. March;48(3):452-8. Epub 2012 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, Gai W, Pan S, Liu Z. The exothermal behavior in the hydration process of calcium phosphate cement. Biomaterials. 2003. August;24(18):2995-3003. [DOI] [PubMed] [Google Scholar]
- 35.Matsumine A, Kusuzaki K, Matsubara T, Okamura A, Okuyama N, Miyazaki S, Shintani K, Uchida A. Calcium phosphate cement in musculoskeletal tumor surgery. J Surg Oncol. 2006. March 1;93(3):212-20. [DOI] [PubMed] [Google Scholar]
- 36.Wen L, Shin MH, Kang JH, Yim YR, Kim JE, Lee JW, Lee KE, Park DJ, Kim TJ, Park YW, Kweon SS, Lee YH, Yun YW, Lee SS. The relationships between bone mineral density and radiographic features of hand or knee osteoarthritis in older adults: data from the Dong-gu Study. Rheumatology (Oxford). 2016. March;55(3):495-503. Epub 2015 Oct 13. [DOI] [PubMed] [Google Scholar]
- 37.Linde KN, Puhakka KB, Langdahl BL, Søballe K, Krog-Mikkelsen I, Madsen F, Stilling M. Bone mineral density is lower in patients with severe knee osteoarthritis and attrition. Calcif Tissue Int. 2017. December;101(6):593-601. Epub 2017 Aug 24. [DOI] [PubMed] [Google Scholar]
- 38.Wu M, Yao S, Xie Y, Yan F, Deng Z, Lei J, Cai L. A novel subchondral bone-grafting procedure for the treatment of giant-cell tumor around the knee: a retrospective study of 27 cases. Medicine (Baltimore). 2018. November;97(45):e13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teng W, Lin P, Li Y, Yan X, Li H, Li B, Wang Z, Wu Y, Wang S, Zhou X, Wang Z, Ye Z. Bone combined cement grafting in giant cell tumor around the knee reduces mechanical failure. Int Orthop. 2019. February;43(2):475-82. Epub 2018 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo D, Zheng L, Sun W, Fu D, Hua Y, Cai Z. Contemporary adjuvant polymethyl methacrylate cementation optimally limits recurrence in primary giant cell tumor of bone patients compared to bone grafting: a systematic review and meta-analysis. World J Surg Oncol. 2013. July 16;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal MG, Gundavda MK, Gupta R, Reddy R. Does denosumab change the giant cell tumor treatment strategy? lessons learned from early experience. Clin Orthop Relat Res. 2018. September;476(9):1773-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, Donati DM. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018. March 21;100(6):496-504. [DOI] [PubMed] [Google Scholar]