Highlights
-
•
Revised UK suspected-cancer guidance liberalised investigation of patients.
-
•
Diagnostic interval was longer for patients with newly introduced referral criteria.
-
•
Scope remains to reduce diagnostic interval further.
Keywords: Neoplasms, Early detection of cancer, Clinical decision rules, Health care reform, Primary health care, Diagnostic interval, Time to diagnosis, Semiparametric varying-coefficient model
Abstract
Background
UK primary-care referral guidance describes the signs, symptoms, and test results (“features”) of undiagnosed cancer. Guidance revision in 2015 liberalised investigation by introducing more low-risk features. We studied adults with cancer whose features were in the 2005 guidance (“Old-NICE”) or were introduced in the revision (“New-NICE”). We compared time to diagnosis between the groups, and its trend over 2006—2017.
Methods
Clinical Practice Research Datalink records were analysed for adults with incident myeloma, breast, bladder, colorectal, lung, oesophageal, ovarian, pancreatic, prostate, stomach or uterine cancers in 1/1/2006–31/12/2017. Cancer-specific features in the year before diagnosis were used to create New-NICE and Old-NICE groups. Diagnostic interval was time between the index feature and diagnosis. Semiparametric varying-coefficient analyses compared diagnostic intervals between New-NICE and Old-NICE groups over 1/1/2006–31/12/2017.
Results
Over all cancers (N = 83,935), median (interquartile range) Old-NICE diagnostic interval rose over 2006–2017, from 51 (20–132) to 64 (30–148) days, with increases in breast (15 vs 25 days), lung (103 vs 135 days), ovarian (65·5 vs 100 days), prostate (80 vs 93 days) and stomach (72·5 vs 102 days) cancers. Median New-NICE values were consistently longer (99, 40–212 in 2006 vs 103, 42–236 days in 2017) than Old-NICE values over all cancers. After guidance revision, New-NICE diagnostic intervals became shorter than Old-NICE values for colorectal cancer.
Conclusions
Despite improvements for colorectal cancer, scope remains to reduce diagnostic intervals for most cancers. Liberalised investigation requires protecting and enhancing cancer-diagnostic services to avoid their becoming a rate-limiting step in the diagnostic pathway.
1. Introduction
Early cancer detection is central to improving outcomes [1]. Most early-detection strategies focus on the timely recognition and investigation of people likely to have undiagnosed cancer [[2], [3], [4]]. As screening detects <6 % of cancer [5], UK strategies focus on promptly recognising the symptoms, signs or test results associated with undiagnosed cancer (“features of possible cancer”, or simply “features”) [6]. In 2005, UK suspected-cancer guidance was published, listing features warranting cancer testing or investigation [7].
The guidance was revised in 2011 for ovarian cancer [8], and in 2015 for remaining cancers [2]. The aim was to expedite cancer diagnosis by lowering the risk of undiagnosed cancer warranting clinical action from ≥5 % to 3 % [2], which was achieved by introducing more vague features into the guidance [2,9]. The revised guidance is officially applicable in England, and endorsed in Wales and Northern Ireland [10].
Our objective was to explore the timeliness of cancer diagnosis in England, Wales and Northern Ireland in 2006–2017 for 11 common internal cancers. We compared time from first feature to diagnosis between two groups: “Old-NICE” (with features of possible cancer in the original 2005 guidance) and “New-NICE” (only participants with features introduced during guidance revision). We hypothesised that times to diagnosis would be longer for New-NICE than for Old-NICE participants, because diagnosing cancer is more challenging and may take longer when symptoms are vague [9,[11], [12], [13], [14]]. We also hypothesised that the difference in time to diagnosis between New-NICE and Old-NICE groups would reduce over time, as evidence on vague cancer features emerged and was translated into practice by guidance revision [2,15].
2. Methods
2.1. Study setting and design
This serial, cross-sectional, primary-care study used UK Clinical Practice Research Datalink (CPRD GOLD) with linked National Cancer Registration and Analysis Service (NCRAS, Set 15) data. CPRD GOLD comprises prospective, coded, and anonymised medical records from >600 UK general practices, with 389 having NCRAS linkage [16]. The study examined participants in the year before their cancer diagnosis between 2006 and 2017.
2.2. Inclusion and exclusion criteria
Inclusion criteria:
-
•
Age ≥18 years
-
•
An incident diagnostic code recorded between 1st January 2006 and 31st December 2017 for myeloma(ICD10 C90), breast(C50), bladder(C67), colorectal(C18–C20), lung(C34), oesophageal(C15), ovarian(C56), pancreatic(C25), prostate(C61), stomach(C16), or uterine(C54) cancer.
-
•
Practice registration ≥1 year before cancer diagnosis.
These sites were selected because the revised guidance introduced new features of possible cancer for them, allowing participant grouping into “Old-NICE” and “New-NICE” categories (see Section 2.3.3).
Exclusion criteria:
-
•
Scotland, where separate guidance applies [17].
-
•
Multiple primary cancers.
-
•
Cancer typical of the opposite sex; e.g. male breast cancer.
-
•
Screen-detected cancer, identified from NCRAS or by CPRD screening codes in the year before diagnosis.
-
•
No primary care attendance or no recorded feature of the participant’s cancer in the year before diagnosis.
2.3. Variables and outcome measures
2.3.1. Features of possible cancer
CPRD codes for features of possible cancer were collated [18], based on the symptoms, signs or blood test results in the original or revised guidance (Table 1) [2,7,8]. Occurrences of these codes, restricted to the relevant cancer site, identified participants presenting with these features in the year before diagnosis. Separate generic “suspected-cancer” codes were identified to explore for changing recording practices.
Table 1.
Cancer features sought in participants' medical records in the year before diagnosis.
| Cancer site | Features listed in NICE 2005 (“Old NICE”) | Features added in NICE 2015 (“New NICE”) |
|---|---|---|
| Bladder | Haematuria, visible | Dysuria |
| Haematuria, non-visible | Raised white cell count | |
| Urinary tract infection | ||
| Abdominal mass | ||
| Breast | Breast lump | Breast pain |
| Nipple discharge | Lump in axilla | |
| Nipple retraction | Other changes of concern, such as distorted breast contour | |
| Skin changes | ||
| Colorectal | Rectal bleeding | Abdominal pain |
| Iron-deficiency anaemia | Faecal occult blood | |
| Change in bowel habit | Weight loss | |
| Rectal mass | ||
| Abdominal mass | ||
| Lung | X-ray findings suggestive of lung cancer | Fatigue |
| Haemoptysis | Appetite loss | |
| Cough | Chest infection | |
| Dyspnoea | Thrombocytosis | |
| Chest pain | ||
| Weight loss | ||
| Finger clubbing | ||
| Lymphadenopathy (supraclavicular, cervical) | ||
| Hoarseness | ||
| Features suggestive of lung metastases | ||
| Signs of superior vena cava obstruction | ||
| Stridor | ||
| Shoulder pain | ||
| Chest signs consistent with lung cancer | ||
| Oesophagus and stomach | Dysphagia | Reflux |
| Weight loss | Haematemesis | |
| Upper abdominal pain | Thrombocytosis | |
| Low haemoglobin/chronic gastrointestinal bleeding | ||
| Dyspepsia | ||
| Back pain | ||
| Upper abdominal mass | ||
| Suspicious barium meal results | ||
| Nausea and/or vomiting | ||
| Pancreas | Jaundice | Weight loss |
| Diarrhoea | ||
| Back pain | ||
| Abdominal pain | ||
| Nausea and/or vomiting | ||
| Constipation | ||
| New-onset diabetes | ||
| Ovary | Abdominal distension/bloating | Early satiety/loss of appetite |
| Abdominal pain | Pelvic pain | |
| Urinary urgency/frequency | Weight loss | |
| Abdominal/pelvic mass | Fatigue | |
| Constipation | Change in bowel habit | |
| Back pain | Raised Ca125 | |
| Ascites | ||
| Uterus | Postmenopausal bleeding | High blood glucose |
| Abdominal or pelvic mass | Low haemoglobin | |
| Gynaecological symptoms, such as altered menstrual cycle, intermenstrual bleeding, and post-coital bleeding | Reported haematuria | |
| Thrombocytosis | ||
| Vaginal discharge | ||
| Prostate | Abnormal digital rectal examination | Erectile dysfunction |
| Nocturia | Haematuria, visible | |
| Urinary frequency | ||
| Urinary hesitancy | ||
| Urinary urgency | ||
| Urinary retention | ||
| Raised PSA above age-specific value | ||
| Myeloma | Spinal cord compression suspected of being caused by myeloma | Bone pain |
| Renal failure suspected of being caused by myeloma | Back pain | |
| Unexplained fracture | ||
| Hypercalcaemia | ||
| Leukopenia | ||
| Plasma viscosity consistent with myeloma | ||
| Erythrocyte sedimentation rate consistent with myeloma | ||
| Protein electrophoresis suggesting myeloma | ||
| Bence-Jones protein urine test suggesting myeloma |
2.3.2. Milestone dates and diagnostic interval
The cancer diagnosis date was the earliest CPRD or NCRAS diagnostic code. The first recorded feature of possible cancer (index feature) was identified, along with the index date. Our outcome variable was “diagnostic interval”: days from index date to diagnosis [19].
2.3.3. NICE grouping
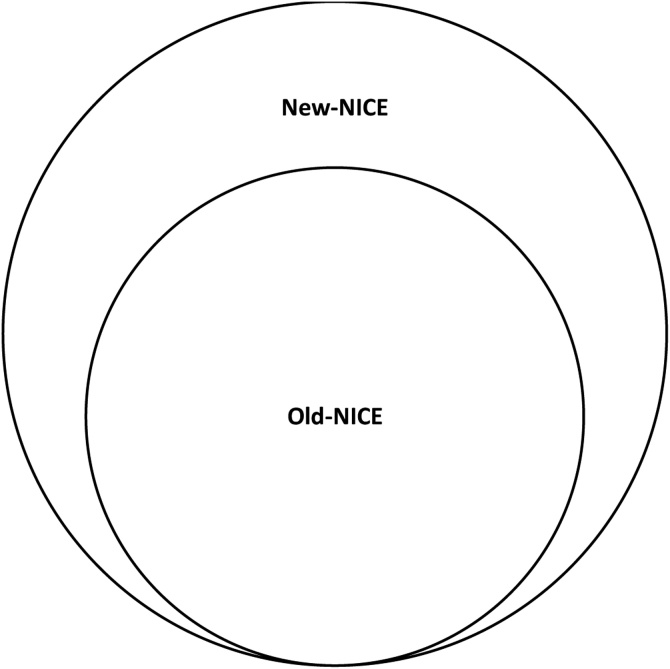
Participants were grouped by their index feature(s) (Fig. 1, Table 1):
-
•
Old-NICE: participants with ≥1 index feature from the 2005 guidance [7].
-
•
New-NICE: limited to participants who only had index feature(s) introduced during guidance revision [2,8].
Fig. 1.
Schematic to show participant grouping. "Old-NICE": participants with a first feature of possible cancer listed in NICE 2005 (including those that have a first feature listed in both NICE 2005 and NICE 2015); “New-NICE”: participants whose first possible feature(s) of cancer is listed solely in NICE 2015 (or in NICE 2011 for ovarian cancer).
Participants whose only index feature was a generic “suspected-cancer” code were omitted from analyses.
2.3.4. Other variables
Age and sex were identified from the CPRD year of birth, assigning a birthday of 1st July.
2.4. Analyses
Simple descriptive statistics summarised age (mean and standard deviation), sex (male, n, %), NICE grouping (New-NICE group, n, %), and the index feature(s) (n, % of all index features). We summarised diagnostic interval using mean (standard deviation) and the 25th, 50th, 75th, and 90th centiles. Diagnostic interval has a skewed distribution and was log-transformed for analyses [13].
Semiparametric varying-coefficient methods estimated coefficients representing the percentage difference in mean log-transformed diagnostic interval between New-NICE and Old-NICE groups (see accompanying methodological paper [20]). A coefficient of 0 represents no difference between the NICE groups. Positive coefficients indicate that diagnostic intervals are longer for the New-NICE than the Old-NICE group; negative coefficients, that they are shorter. The coefficients are estimated on a daily basis, so cannot be reported using a single summary statistic, and are plotted (with 95 % confidence intervals, using bootstrapping, n = 1000 replications [21]) to allow visualisation over 2006—17. The models adjusted for age and sex. Analyses examined each cancer site separately, sample size permitting (package “np” in R) [22].
2.5. Study size
For the descriptive statistics, we included all CPRD participants meeting our inclusion criteria. Semiparametric varying-coefficient analyses were limited to cancer sites with participant numbers providing ≥90 % power at the 5 % level to detect a 14-day difference in diagnostic interval between New-NICE and Old-NICE groups. Assuming mean diagnostic intervals of 114 and 100 days, respectively, for the Old-NICE and New-NICE groups, a common standard deviation of 100 days and 10 % of participants classified as New-NICE requires 5980 total participants. An effect size of 14 days matches the two-week-wait target for urgent investigation. We assessed uncertainty in the estimates by confidence interval width.
2.6. Missing data and bias
To explore for potential bias associated with changing coding practice, we identified, for annual cohorts: (a) the percentages of participants excluded for having no coded features or only suspected-cancer codes; (b) the proportions of Old-NICE and New-NICE participants; (c) demographic characteristics of participants excluded because they lacked coded features.
3. Results
3.1. Participants
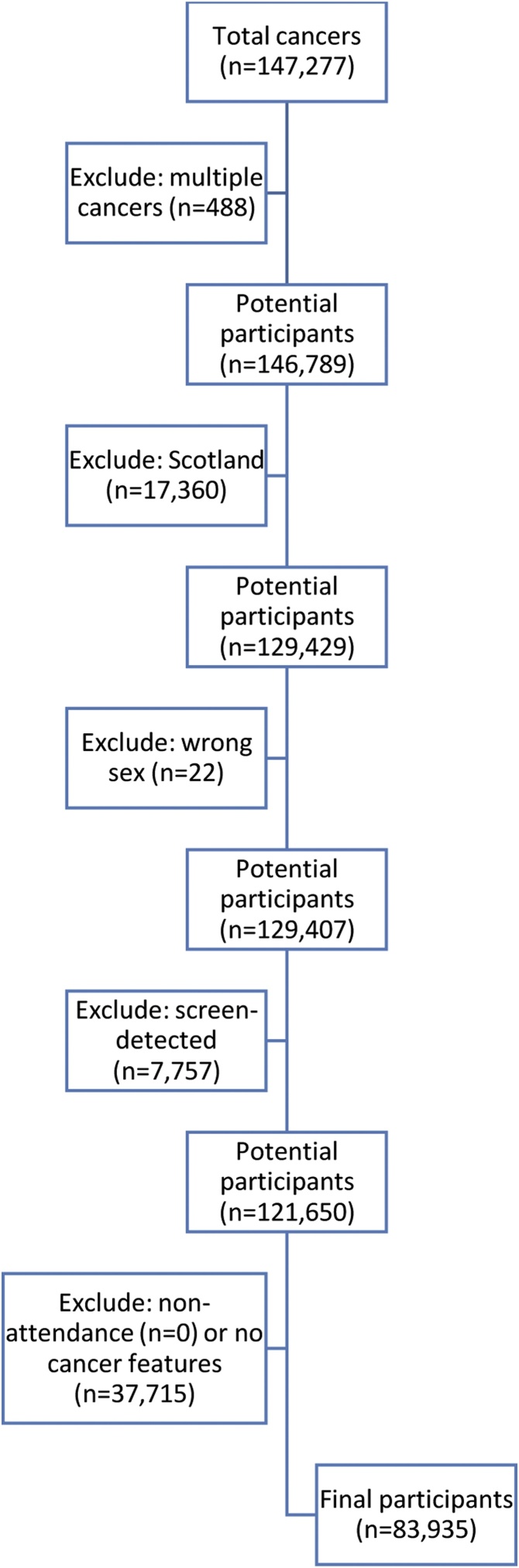
The CPRD provided 147,106 participants, of whom 63,171 (42·9%) were excluded, leaving 83,935 (57·1%) entering the analyses, from 603 practices, of which 384 (63·7%) had NCRAS linkage (Table 2). The main reasons for exclusion were lack of recorded features (n = 37,715), Scottish residence (n = 17,360) and detection following screening (n = 7757) (Fig. 2).
Table 2.
Numbers of potential inclusions (individual diagnoses), with Cancer Registry linkage, and exclusions, to give final sample sizes by cancer site. The final sample is described in terms of size (N), age (mean, SD), number (%) who are male, and number (%) with an index cancer feature introduced during guidance revision.
| Cancer site | Potential inclusions | No. (%) with NCRS linkage | Exclusions | Final sample |
|||
|---|---|---|---|---|---|---|---|
| N | Age, mean (SD) | No. (%) male | No. (%) in New-NICE group | ||||
| Bladder | 9030 | 2583 (28·6) | 3787 | 5243 | 73·0 (11·5) | 3870 (73·8) | 799 (15·2) |
| Breast | 37,369 | 17,452 (46·7) | 21,827 | 15,542 | 62·9 (16·7) | 0 (0) | 858 (5·5) |
| Colorectal | 25,011 | 11,786 (47·1) | 13,169 | 11,842 | 70·2 (12·6) | 6477 (54·7) | 5017 (42·4) |
| Lung | 20,033 | 9080 (45·3) | 6926 | 13,107 | 71·9 (10·6) | 7175 (54·7) | 3384 (25·8) |
| Myeloma | 2758 | 1257 (45·6) | 1224 | 1534 | 71·0 (11·5) | 818 (53·3) | 1529 (99·7) |
| Oesophagus | 6041 | 2710 (44·9) | 1769 | 4272 | 71·3 (11·8) | 2900 (67·9) | 451 (10·6) |
| Ovary | 3887 | 1672 (43·0) | 1406 | 2481 | 65·5 (13·8) | 0 (0) | 614 (24·7) |
| Pancreas | 4844 | 2292 (47·3) | 1677 | 3167 | 71·7 (11·5) | 1580 (49·9) | 2672 (84·4) |
| Prostate | 30,083 | 14,488 (48·2) | 8630 | 21,453 | 71·6 (9·3) | 21,453 (100) | 1662 (7·7) |
| Stomach | 3839 | 1930 (50·3) | 1051 | 2788 | 73·4 (12·2) | 1823 (65·4) | 294 (10·5) |
| Uterus | 4382 | 2124 (48·5) | 1876 | 2506 | 67·1 (11·3) | 0 (0) | 713 (28·5) |
| Total | 147,277a | 67,374 (45·7) | 63,342b | 83,935 | 69·6 (12·8) | 46,096 (54·9) | 17,993 (21·4) |
147,277 cancers in 147,106 participants (of whom 317 had multiple index cancers, including cancer types not in this study).
63,342 exclusions in 63,171 patients.
Fig. 2.
Application of exclusion criteria.
The sex distributions indicate male dominance in bladder (3870/5243, 73·8 %), oesophageal (2900/4272, 67·9%) and stomach (1823/2788, 65·4 %) cancers (Table 2). The overall mean (SD) age at diagnosis (n = 83,935) was 69·6 years (12·8), ranging from 62·9 years (16·7) for breast to 73·4 years (12·2) for stomach (Table 2).
3.2. NICE grouping
The percentage of participants whose index feature was introduced during guidance revision (New-NICE group) varied by cancer, ranging from 1529/1534 (99·7 %) for myeloma to 858/15,542 (5·5%) for breast. More even distributions were observed for colorectal (5017/11,842, 42·4%), lung (3384/13,107, 25·8 %), ovarian (614/2481, 24·8%), and uterine (713/2506, 28·5%) cancers (Table 2).
3.3. Index features of cancer
Breast, bladder, and prostate cancers were dominated by one index feature: lump (14,200/15,662, 91·0%), raised prostate-specific antigen (14,473/22,270, 65·0%), and visible haematuria (3435/5346, 64·3%), respectively (Table 3). The remaining sites showed more heterogeneity. Colorectal cancer was characterised by abdominal pain (4291/12,084, 35·5%) and rectal bleeding (3913/12,084, 32·4%). For lung, cough (4005/13,913, 28·8%), dyspnoea (2876/13,913, 20·7%), and chest infection (2072/13,913, 14·9%) were most frequent. Approximately half of all index features were accounted for by dysphagia (1466/4521, 32·4%) and low haemoglobin (745/4521, 16·5%) in oesophageal cancer, and by low haemoglobin (943/3077, 30·6%), upper abdominal pain (479/3077, 15·6%), and dyspepsia (361/3077, 11·7%) in stomach cancer. Abdominal pain (925/2669, 34·7%) was most common in ovarian cancer, whereas ascites was uncommon (67/2669, 2·5%). Pancreatic cancer was characterised by abdominal pain (1068/3259, 32·8%), diabetes (717/3259, 22·0%), and less commonly by jaundice (495/2669, 15·2%). Postmenopausal bleeding accounted for nearly half of all index features of uterine cancer (1305/2619, 49·8%), with lower frequencies for high blood glucose (300/2619, 11·5%) and low haemoglobin (275/2619, 10·5%).
Table 3.
Coded index features of cancer (n, % of total index features presenteda). Features are listed in order of frequency within cancer site.
| Site | Feature | n (% of all index features) |
|---|---|---|
| Bladder | Haematuria, visible | 3435 (64·5) |
| Urinary tract infection | 847 (15·9) | |
| Dysuria | 426 (8·0) | |
| Raised white cell count | 427 (8·0) | |
| Haematuria, non-visible | 180 (3·4) | |
| Abdominal mass | 13 (0·2) | |
| Total | 5328 (100) | |
| Breast | Lump | 14,200 (91·0) |
| Breast pain | 845 (5·4) | |
| Nipple discharge | 253 (1·6) | |
| Nipple retraction | 225 (1·4) | |
| Other changes of concern | 65 (0·4) | |
| Breast skin changes | 44 (0·3) | |
| Axillary lymph nodes | 30 (0·2) | |
| Total | 15,662 (100) | |
| Colorectal | Abdominal pain | 4291 (35·5) |
| Rectal bleed | 3913 (32·4) | |
| Change in bowel habit | 1940 (16·1) | |
| Iron-deficiency anaemia | 1013 (8·4) | |
| Weight loss | 574 (4·8) | |
| Abdominal mass | 195 (1·6) | |
| Faecal occult blood | 136 (1·1) | |
| Rectal mass | 22 (0·2) | |
| Total | 12,084 (100) | |
| Lung | Cough | 4005 (28·8) |
| Dyspnoea | 2876 (20·7) | |
| Chest infection | 2072 (14·9) | |
| Chest pain | 1189 (8·5) | |
| Thrombocytosis | 965 (6·9) | |
| Fatigue | 558 (4·0) | |
| Shoulder pain | 520 (3·7) | |
| Weight loss | 485 (3·5) | |
| Haemoptysis | 472 (3·4) | |
| Signs of lung metastases | 270 (1·9) | |
| Hoarseness | 158 (1·1) | |
| Chest signs consistent with lung cancer | 125 (0·9) | |
| Appetite loss | 110 (0·8) | |
| X-ray findings suggestive of lung cancer | 59 (0·4) | |
| Lymphadenopathy (supraclavicular, cervical) | 16 (0·1) | |
| Finger clubbing | 19 (0·1) | |
| Signs of superior vena cava obstruction | 12 (0·1) | |
| Stridor | 2 (0·01) | |
| Total | 13,913 (100) | |
| Myeloma | Back pain | 735 (44·5) |
| Abnormal erythrocyte sedimentation rate | 426 (25·8) | |
| Abnormal white cell count | 189 (11·5) | |
| Hypercalcaemia | 140 (8·5) | |
| Plasma viscosity consistent with myeloma | 71 (4·3) | |
| Bone pain | 51 (3·1) | |
| Pathological fracture | 11 (0·7) | |
| Bence Jones protein | 11 (0·7) | |
| Paraprotein | 11 (0·7) | |
| Spinal cord compression suspected of being caused by myeloma | 5 (0·3) | |
| Total | 1650 (100) | |
| Oesophagus | Dysphagia | 1466 (32·4) |
| Low haemoglobin/chronic gastrointestinal bleeding | 745 (16·5) | |
| Dyspepsia | 597 (13·2) | |
| Upper abdominal pain | 402 (8·9) | |
| Reflux | 357 (7·9) | |
| Back pain | 345 (7·6) | |
| Thrombocytosis | 208 (4·6) | |
| Weight loss | 160 (3·5) | |
| Vomiting | 152 (3·4) | |
| Nausea | 61 (1·3) | |
| Haematemesis | 26 (0·6) | |
| Upper abdominal mass | 2 (0·04) | |
| Total | 4521 (100) | |
| Ovary | Abdominal pain | 925 (34·7) |
| Raised Ca125 | 345 (12·9) | |
| Abdominal distension/bloating | 267 (10·0) | |
| Abdominal/pelvic mass | 254 (9·5) | |
| Back pain | 219 (8·2) | |
| Constipation | 201 (7·5) | |
| Fatigue | 132 (4·9) | |
| Change in bowel habit | 83 (3·1) | |
| Ascites | 67 (2·5) | |
| Pelvic pain | 55 (2·1) | |
| Frequency | 53 (2·0) | |
| Weight loss | 48 (1·8) | |
| Early satiety/appetite loss | 14 (0·5) | |
| Urgency | 6 (0·2) | |
| Total | 2669 (100) | |
| Pancreas | Abdominal pain | 1068 (32·8) |
| Diabetes | 717 (22·0) | |
| Jaundice | 495 (15·2) | |
| Back pain | 373 (11·4) | |
| Constipation | 236 (7·2) | |
| Weight loss | 164 (5·0) | |
| Nausea | 112 (3·4) | |
| Vomiting | 82 (2·5) | |
| Diarrhoea | 12 (0·4) | |
| Total | 3259 (100) | |
| Prostate | Raised PSA | 14,473 (65·0) |
| Lower urinary tract symptoms | 5649 (25·4) | |
| Erectile dysfunction | 933 (4·2) | |
| Haematuria, visible | 927 (4·2) | |
| Abnormal digital rectal exam | 288 (1·3) | |
| Total | 22,270 (100) | |
| Stomach` | Low haemoglobin/chronic gastrointestinal bleeding | 943 (30·6) |
| Upper abdominal pain | 479 (15·6) | |
| Dyspepsia | 361 (11·7) | |
| Dysphagia | 260 (8·4) | |
| Thrombocytosis | 241 (7·8) | |
| Back pain | 201 (6·5) | |
| Reflux | 198 (6·4) | |
| Weight loss | 133 (4·3) | |
| Vomit | 130 (4·2) | |
| Nausea | 68 (2·2) | |
| Haematemesis | 57 (1·9) | |
| Upper abdominal mass | 6 (0·2) | |
| Total | 3077 (100) | |
| Uterus | Postmenopausal bleeding | 1305 (49·8) |
| High blood glucose | 300 (11·5) | |
| Low haemoglobin | 275 (10·5) | |
| General gynaecological symptoms | 247 (9·4) | |
| Vaginal discharge | 218 (8·3) | |
| Reported haematuria | 129 (4·9) | |
| Thrombocytosis | 114 (4·4) | |
| Abdominal or pelvic mass | 31 (1·2) | |
| Total | 2619 (100) |
Note: Some participants presented with multiple index features; hence, the totals are greater than the final sample sizes.
3.4. Diagnostic interval
Overall, the median diagnostic interval was 58 days (interquartile range (IQR) 23–158, N = 83,935). By cancer site, the shortest diagnostic interval was in breast (median, IQR: 20, 10–30 days, N = 15,542) and the longest in lung (median, IQR: 129, 46–263 days, N = 13,107) (Table 4).
Table 4.
Diagnostic interval (25th, 50th, 75th, and 90th centiles, mean and standard deviation) by cancer site.
| Cancer site | Group | N | Diagnostic interval (days) |
|||||
|---|---|---|---|---|---|---|---|---|
| Centile |
Mean | SD | ||||||
| 25th | 50th | 75th | 90th | |||||
| Bladder | New-NICE | 799 | 61 | 133 | 239 | 322 | 153·2 | 106·4 |
| Old-NICE | 4444 | 32 | 58 | 113 | 226 | 89·1 | 84·2 | |
| Total | 5243 | 34 | 64 | 135 | 253 | 98·9 | 90·9 | |
| Breast | New-NICE | 858 | 17 | 44 | 138 | 272 | 92·4 | 101·4 |
| Old-NICE | 14,684 | 10 | 15 | 28 | 53 | 28·5 | 43·7 | |
| Total | 15,542 | 10 | 16 | 30 | 62 | 32·0 | 50·8 | |
| Colorectal | New-NICE | 5017 | 29 | 70 | 159 | 270 | 105·4 | 97·4 |
| Old-NICE | 6825 | 25 | 51 | 105 | 208 | 80·7 | 80·2 | |
| Total | 11,842 | 27 | 57 | 126 | 237 | 91·2 | 88·7 | |
| Lung | New-NICE | 3384 | 51 | 139·5 | 270 | 336 | 160·6 | 116·9 |
| Old-NICE | 9723 | 44 | 124 | 260 | 331 | 152·4 | 116·9 | |
| Total | 13,107 | 46 | 129 | 263 | 332 | 154·5 | 117·0 | |
| Myeloma | New-NICE | 1529 | 37 | 97 | 216 | 307 | 131·5 | 109·1 |
| Old-NICE | 5 | 0·5 | 4 | 10 | 338 | 70·6 | 149·5 | |
| Total | 1534 | 37 | 97 | 216 | 307 | 131·3 | 109·2 | |
| Oesophagus | New-NICE | 451 | 38 | 77 | 161 | 280 | 112·1 | 96·7 |
| Old-NICE | 3821 | 21 | 55 | 167 | 293 | 104·2 | 106·8 | |
| Total | 4272 | 23 | 57 | 166 | 292 | 105·1 | 105·8 | |
| Ovary | New-NICE | 614 | 26 | 56 | 133 | 281 | 95·9 | 99·0 |
| Old-NICE | 1867 | 34 | 72 | 170 | 283 | 110·9 | 100·5 | |
| Total | 2481 | 31 | 67 | 160 | 283 | 107·2 | 100·4 | |
| Pancreas | New-NICE | 2672 | 49 | 126 | 258·5 | 329 | 154·0 | 114·6 |
| Old-NICE | 495 | 11 | 23 | 48 | 91 | 40·5 | 53·5 | |
| Total | 3167 | 35 | 97 | 232 | 321 | 136·3 | 115·0 | |
| Prostate | New-NICE | 1662 | 56 | 123 | 240 | 321 | 151·0 | 107·9 |
| Old-NICE | 19,791 | 37 | 77 | 174 | 287 | 115·1 | 99·7 | |
| Total | 21,453 | 38 | 80 | 181 | 291 | 117·9 | 100·8 | |
| Stomach | New-NICE | 294 | 41 | 94·5 | 219 | 315 | 133·4 | 112·0 |
| Old-NICE | 2494 | 32 | 88 | 216 | 314 | 127·9 | 111·7 | |
| Total | 2788 | 33 | 88·5 | 216 | 314 | 128·5 | 111·7 | |
| Uterus | New-NICE | 713 | 76 | 174 | 284 | 337 | 179·1 | 112·5 |
| Old-NICE | 1793 | 25 | 50 | 108 | 206 | 80·8 | 80·9 | |
| Total | 2506 | 30 | 67·5 | 167 | 285 | 108·7 | 101·2 | |
| Total | New-NICE | 17,993 | 39 | 98 | 222 | 315 | 134·2 | 110·5 |
| Old-NICE | 65,942 | 21 | 51 | 135 | 271 | 94·2 | 99·4 | |
| Total | 83,935 | 23 | 58 | 158 | 285 | 102·8 | 103·2 | |
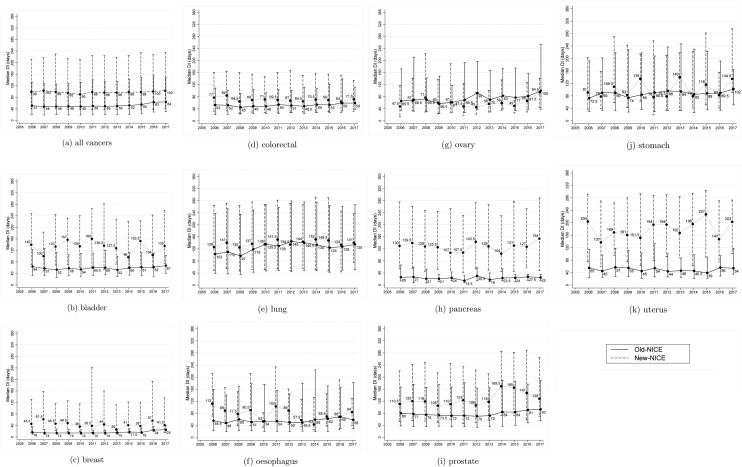
Median (interquartile range) diagnostic intervals by year and by NICE grouping are plotted in Fig. 3. For all cancers combined, median Old-NICE diagnostic interval was 51 (interquartile range 20–132) days in 2006, compared with 64 (30–148) days in 2017. Median New-NICE diagnostic interval was longer, at 99 (40–212) days in 2006 vs 103 (42–236) days in 2017.
Fig. 3.
Median (interquartile range) diagnostic interval (days) by year of diagnosis (2006 to 2017), and by NICE grouping: New-NICE (dashed) and Old-NICE (solid).
New-NICE diagnostic intervals were considerably and consistently longer than Old-NICE values in bladder (133 vs 58 days), breast (44 vs 15 days), pancreatic (126 vs 23 days), prostate (123 vs 77 days), and uterine (174 vs 50 days) cancers (Table 4, Fig. 3). Median diagnostic intervals were longer for New-NICE than for Old-NICE participants for colorectal (70 vs 51 days), oesophageal (77 vs 55 days), and lung (139·5 vs 124 days) cancers; however, this difference tended to decrease or disappear over time (Fig. 3). In ovarian cancer, diagnostic intervals were shorter in the New-NICE than in the Old-NICE group overall (56 vs 72 days), notably in 2010—16 (Fig. 3).
For bladder, colorectal, oesophageal, pancreatic and uterine cancers, median Old-NICE diagnostic intervals remained constant over 2006–2017. They were longer in 2017 compared with 2006 for breast (25 vs 16 days), lung (135 vs 103 days), ovarian (100 vs 65·5 days), prostate (93 vs 80 days) and stomach (102 vs 72·5 days) cancers (Fig. 3).
3.5. Semiparametric varying-coefficient analyses
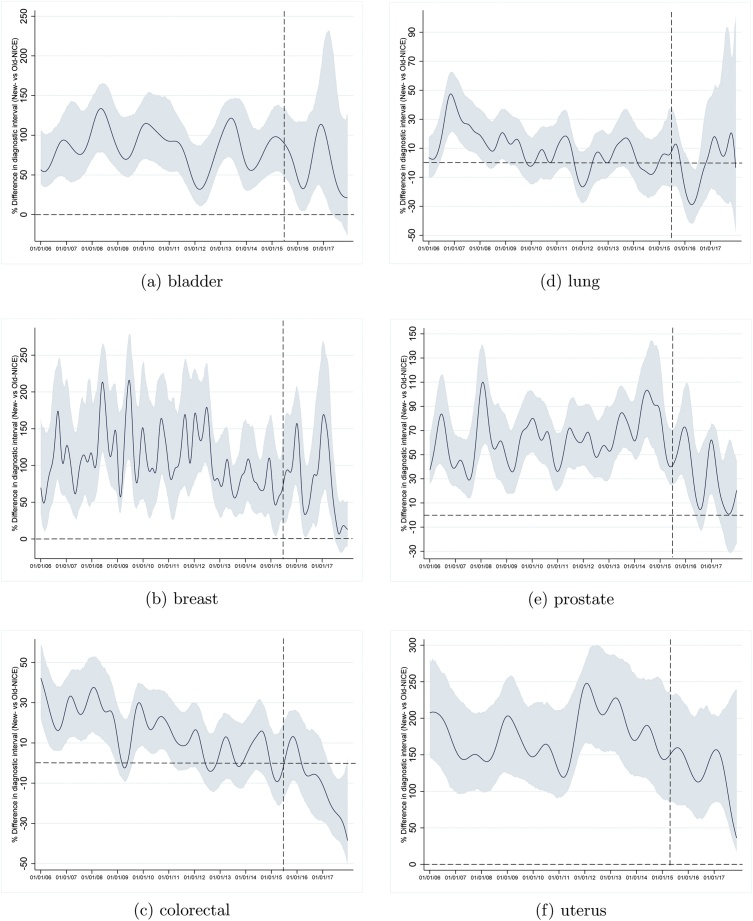
Semiparametric varying-coefficient analyses were powered for bladder, breast, colorectal, lung, prostate and uterine cancers. The percentage differences (with 95 % confidence intervals) in mean log-transformed diagnostic interval between New-NICE and Old-NICE groups over time are plotted in Fig. 4.
Fig. 4.
Percentage change in diagnostic interval in New-NICE vs Old-NICE groups, by year of diagnosis (2006 to 2017) for cancers of the bladder, breast, colorectal, lung, prostate, and uterus.
After guidance revision on 23rd June 2015, New-NICE diagnostic intervals tended to shorten relative to those of the Old-NICE group in prostate (Fig. 4e) and uterine (Fig. 4f) cancers (note the downward trajectory towards the horizontal dashed line).
For colorectal cancer, the difference in diagnostic interval between the New-NICE and Old-NICE groups reduced over time. After guidance revision, New-NICE diagnostic intervals were shorter than Old-NICE intervals, as indicated by the trend dropping below the horizontal dashed line (Fig. 4c).
For lung cancer, New-NICE were longer than Old-NICE diagnostic intervals in the years 2006–10. In 2010–15, there was no difference between the groups. In 2016 (post guidance revision), New-NICE diagnostic intervals shortened relative to Old-NICE diagnostic intervals, but this was not sustained into 2017—18 (Fig. 4d).
3.6. Missing data and bias
The proportions of eligible participants excluded for lack of coded features increased over time for bladder, colorectal, lung, oesophageal, ovarian, pancreatic, stomach, and uterine cancers. This coincided with increased use of suspected-cancer codes (Fig. S1). The demographic details of excluded and included participants were similar (Table S1 and Table 2). The proportions of Old-NICE and New-NICE participants were largely similar across time within cancer sites (Fig. S2).
4. Discussion
4.1. Findings
This study examined diagnostic intervals for 11 cancers in England, Wales and Northern Ireland over 2006–2017, a period including major revision of national suspected-cancer referral guidance. As hypothesised, times to diagnosis were generally longer for “New-NICE” participants (with index feature(s) of cancer introduced during guidance revision) than for “Old-NICE” participants (with feature(s) in the original guidance). Importantly, for colorectal cancer, New-NICE diagnostic intervals were shorter than Old-NICE diagnostic intervals after guidance revision. The gap between New- and Old-NICE groups decreased for prostate and uterine cancers over time, consistent with decreasing New-NICE diagnostic intervals aided by increasing Old-NICE diagnostic intervals for prostate cancer. The revised national guidance and GP responses to its preceding evidence base may have contributed to these changes, along with other early-diagnosis initiatives. In conclusion, scope remains to reduce time to diagnosis for symptomatic cancers in England, Wales and Northern Ireland.
4.2. Strengths and limitations
A considerable strength is the study’s primary-care setting, where suspected-cancer guidance is implemented. The CPRD is the largest primary-care database worldwide and is recognised for its high-quality data [23]. We used established methods for case identification [18], with validation of cancer diagnosis by NCRAS where linkage was available. NCRAS data completeness improved in 2013 [24]. Pre-2013 studies report a concordance rate of 83·3% between CPRD and cancer registry information [25]. The CPRD diagnosis date was a median of 11 days (interquartile range –6 to 30 days) later than the registry date pre-2013 for colorectal, lung, gastrointestinal, and urological cancers [26]. Thus pre-2013 diagnostic intervals may be overestimated compared with post-2013 values. Reassuringly, no step-change in New- or Old-NICE diagnostic intervals were observed around 2013, suggesting that any associated bias is small.
We studied diagnostic interval rather than the primary care (time from index date to referral) or secondary care (time from referral to treatment) interval to avoid restricting analyses to participants referred to secondary care [19]. A limitation was the inability to analyse diagnostic intervals separately for participants referred via the two-week-wait pathway [27] because robust data sources for identifying them were unavailable to us.
We found conflicting evidence of changes in GP recording practice over time. The proportion excluded for lack of coded features increased over time for some cancers, often coinciding with increased use of “suspected-cancer” codes. The proportions of Old- and New-NICE groups over time were constant and the similar demographic details for included and excluded participants suggests no marked selection bias. We excluded approximately 26 % of participants for lack of coded features, a proportion consistent with evidence that coded CPRD data identifies 80 % of visible haematuria or jaundice events, and 60–70 % of abdominal pain in patients with pancreatic or bladder cancers [28]. Of participants without recorded features, some will have presented at Emergency Departments without prior primary-care consultations [5,29,30], some will had the information recorded in “free text” [28], and others may have presented with features outside NICE guidance. Such features were deliberately omitted from our study, as irrelevant to our focus on guidance revision.
Our analytical method allowed us to explore trends in the difference in diagnostic interval between groups aligned by their index feature(s) to the revised (New-NICE) or original (Old-NICE) guidance [20]. The method was derived to explore the time-varying and gradual impact of emerging clinical evidence that is legitimised into clinical practice by official guidance revision and implementation [20].
4.3. Comparison with existing literature
Our findings build on previous analysis of the original 2005 NICE guideline’s impact on diagnostic interval [13]. Mean diagnostic interval for 15 UK cancers reduced between 2001–2 and 2007–8 by 5·4 days (95 % CI: 2·4–8·5 days) from an initial value of 125·8 days. Similar to our study, median diagnostic intervals were shortest for cancers commonly presenting with lumps/masses (e.g. 26 days for breast) and longest for cancers often presenting with symptoms shared with other diseases (e.g. 112 days in lung cancer) [13]. Our estimates of diagnostic interval for colorectal cancer are similar to those obtained by the International Cancer Benchmarking Partnership using different data sources [31]. Our findings are consistent with the taxonomy of cancer symptom “signatures” and diagnostic difficulty [9]. Breast cancer had a narrow signature of a single alarm feature (breast lump) highly predictive of undiagnosed cancer plus the shortest diagnostic interval. In contrast, lung cancer had a very broad signature and the longest diagnostic interval.
Jensen et al. [27] investigated the impact of implementing a standardised cancer patient pathway in Denmark in 2007–2009. Post-implementation diagnostic intervals were 15 (12–17) days shorter than peri-implementation values for the 37 % of patients actually referred via a cancer pathway, but were 4 (1–7) days longer for the 63 % of patients diagnosed via other routes. The authors concluded that the cancer pathways expedited diagnosis for a minority of patients.
4.4. Clinical interpretation and policy implications of the findings
The relationship between diagnostic interval and mortality (and stage) is U-shaped, reflecting confounding by indication [[32], [33], [34]]. Patients with advanced tumours generally receive an expedited diagnosis (possibly as an emergency) and have poor outcomes because of their high inherent mortality: the so-called “sick-quick”. Conversely, patients presenting with vague symptoms usually have longer diagnostic intervals, and higher mortality – thought to reflect the impact of diagnostic delay, particularly between referral and diagnosis [[32], [33], [34], [35]]. The revised guidance aimed to benefit patients by legitimising doctors to investigate at a lower risk of undiagnosed cancer. This change can reduce both diagnostic delay and emergency presentation. In this study, for colorectal cancer, New-NICE diagnostic intervals reduced relative to Old-NICE interval after guidance revision. This is consistent with general practitioners acting on the vague (“New-NICE”) features introduced during guidance revision. Indeed, the proportion diagnosed via the urgent cancer referral pathway increased from 30 % (95 %CI 29 %–30 %) in 2013 to 33 % (33 %–34 %) in 2016, spanning the period of guidance revision [36].
Our findings of increasing Old-NICE diagnostic intervals over time may reflect growing strain on NHS diagnostic-endoscopy and imaging services [37], as demand for all indications (not just cancer) rises [38], particularly if CT-based targeted screening for lung cancer is introduced [39]. In 2018, inadequate diagnostic capacity was considered a rate-limiting step in the diagnostic pathway [40], and a negative impact of Covid-19 on diagnostic services is already becoming apparent [41].
5. Conclusions
We conclude that scope remains to reduce time to cancer diagnosis. The revised colorectal cancer diagnostic guidance may be exerting a downward pressure on time to diagnosis of this cancer, through impacts on the vague features of cancer introduced during guidance revision. Future studies using causal analysis should examine the impact of guidance revision on staging at diagnosis and survival for all cancers, and the possible downstream effects on investigative services. Policy-makers are urged to enhance cancer diagnostic services so that they do not pose a rate-limiting step in the diagnostic pathway, and to protect them from the pressures of Covid-19.
Funding
This study was funded by Cancer Research UK [C56843/A21550], who were not involved in any aspect of the conduct of the study, in writing the manuscript or in the decision to submit for publication. This research is also linked to the CanTest Collaborative, which is funded by Cancer Research UK [C8640/A23385], of which WH is co-Director, GL is Associate Director, AS is Senior Faculty, and SP is an affiliated Research Fellow.
SB and OU were supported by the National Institute for Health Research (NIHR)Applied Research Collaboration (ARC) South West Peninsula. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. GL is supported by a Cancer Research UK Advanced Clinician Scientist Fellowship Award [C18081/A18180].
CRediT authorship contribution statement
Sarah Price: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Anne Spencer: Conceptualization, Methodology, Writing - review & editing, Supervision, Project administration, Funding acquisition. Xiaohui Zhang: Conceptualization, Methodology, Software, Writing - review & editing, Supervision. Susan Ball: Methodology, Writing - review & editing. Georgios Lyratzopoulos: Conceptualization, Writing - review & editing, Supervision, Funding acquisition. Ruben Mujica-Mota: Conceptualization, Writing - review & editing, Supervision, Funding acquisition. Sal Stapley: Conceptualization, Writing - review & editing, Supervision, Funding acquisition. Obioha C Ukoumunne: Conceptualization, Writing - review & editing, Supervision, Funding acquisition. Willie Hamilton: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
WH was clinical lead of the guideline development group which formulated the revised NICE suspected-cancer guidelines (NG12). This paper is written in a personal capacity and is not to be interpreted as representing the views of the Group or of NICE. The remaining authors report no declarations of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.canep.2020.101805.
Contributor Information
Sarah Price, Email: S.J.Price@exeter.ac.uk.
Anne Spencer, Email: a.e.spencer@exeter.ac.uk.
Xiaohui Zhang, Email: X.Zhang1@exeter.ac.uk.
Susan Ball, Email: S.Ball3@exeter.ac.uk.
Georgios Lyratzopoulos, Email: y.lyratzopoulos@ucl.ac.uk.
Ruben Mujica-Mota, Email: r.e.mujica-mota@leeds.ac.uk.
Sal Stapley, Email: S.A.Stapley@exeter.ac.uk.
Obioha C Ukoumunne, Email: O.C.Ukoumunne@exeter.ac.uk.
Willie Hamilton, Email: W.Hamilton@exeter.ac.uk.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Independent Cancer Taskforce . NHS England; London: 2016. Achieving World-Class Cancer Outcomes: Taking the Strategy Forward. [Google Scholar]
- 2.National Institute for Health and Care Excellence . NICE; London: 2015. Suspected Cancer: Recognition and Referral [NG12] [PubMed] [Google Scholar]
- 3.Probst H.B., Hussain Z.B., Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—a national Danish project. Health Policy. 2012;105(1):65–70. doi: 10.1016/j.healthpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Wilkens J., Thulesius H., Schmidt I., Carlsson C. The 2015 National Cancer Program in Sweden: Introducing standardized care pathways in a decentralized system. Health Policy. 2016;120(12):1378–1382. doi: 10.1016/j.healthpol.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 5.2015. Public Health England, Routes to Diagnosis 2006-2013.https://www.cancerdata.nhs.uk/routestodiagnosis (Accessed 24 July 2020) [Google Scholar]
- 6.Rubin G., Berendsen A., Crawford S.M., Dommett R., Earle C., Emery J., Fahey T., Grassi L., Grunfeld E., Gupta S., Hamilton W., Hiom S., Hunter D., Lyratzopoulos G., Macleod U., Mason R., Mitchell G., Neal R.D., Peake M., Roland M., Seifert B., Sisler J., Sussman J., Taplin S., Vedsted P., Voruganti T., Walter F., Wardle J., Watson E., Weller D., Wender R., Whelan J., Whitlock J., Wilkinson C., de Wit N., Zimmermann C. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16(12):1231–1272. doi: 10.1016/S1470-2045(15)00205-3. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Clinical Excellence . NICE; London: 2005. Referral Guidelines for Suspected Cancer. [Google Scholar]
- 8.National Institute for Health and Care Excellence . NICE; London: 2011. Ovarian Cancer: Initial Recognition and Management. [PubMed] [Google Scholar]
- 9.Koo M.M., Hamilton W., Walter F.M., Rubin G.P., Lyratzopoulos G. Symptom signatures and diagnostic timeliness in cancer patients: a review of current evidence. Neoplasia. 2018;20(2):165–174. doi: 10.1016/j.neo.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Health . Department of Health; London: 2012. Northern Ireland Referral Guidance for Suspected Cancer. [Google Scholar]
- 11.Jensen H., Tørring M.L., Olesen F., Overgaard J., Vedsted P. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14(1):636. doi: 10.1186/1471-2407-14-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Din N.U., Ukoumunne O.C., Rubin G., Hamilton W., Carter B., Stapley S., Neal R.D. Age and gender variations in cancer diagnostic intervals in 15 cancers: analysis of data from the UK clinical practice research datalink. PLoS One. 2015;10(5):e0127717. doi: 10.1371/journal.pone.0127717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neal R.D., Din N.U., Hamilton W., Ukoumunne O.C., Carter B., Stapley S., Rubin G. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br. J. Cancer. 2014;110(3):584–592. doi: 10.1038/bjc.2013.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen H., Tørring M.L., Olesen F., Overgaard J., Vedsted P. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14:636. doi: 10.1186/1471-2407-14-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenhalgh T., Robert G., MacFarlane F., Bate P., Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br. J. Cancer. 2009;101(Suppl):S80–6. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHS Scotland . 2014. Scottish Referral Guidelines for Suspected Cancer. [DOI] [PubMed] [Google Scholar]
- 18.Watson J., Nicholson B.D., Hamilton W., Price S. Identifying clinical features in primary care electronic health record studies: methods for codelist development. BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-019637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weller D., Vedsted P., Rubin G., Walter F.M., Emery J., Scott S., Campbell C., Andersen R.S., Hamilton W., Olesen F., Rose P., Nafees S., van Rijswijk E., Hiom S., Muth C., Beyer M., Neal R.D. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer. 2012;106(7):1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price S.J., Zhang X., Spencer A.E. Measuring the impact of national guidelines: what methods can be used to uncover time-varying effects for healthcare evaluations? Soc. Sci. Med. 2020;258 doi: 10.1016/j.socscimed.2020.113021. [DOI] [PubMed] [Google Scholar]
- 21.Feng G., Gao J., Peng B., Zhang X. A varying-coefficient panel data model with fixed effects: theory and an application to US commercial banks. J. Econometr. 2017;196(1):68–82. [Google Scholar]
- 22.Li Q., Ouyang D., Racine J.S. Categorical semiparametric varying-coefficient models. J. Appl. Econometr. 2013;28(4):551–579. [Google Scholar]
- 23.Herrett E., Gallagher A.M., Bhaskaran K., Forbes H., Mathur R., van Staa T., Smeeth L. Data resource profile: Clinical Practice Research Datalink (CPRD) Int. J. Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henson K.E., Elliss-Brookes L., Coupland V.H., Payne E., Vernon S., Rous B., Rashbass J. Data resource profile: National Cancer Registration Dataset in England. Int. J. Epidemiol. 2019;49(1):16. doi: 10.1093/ije/dyz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boggon R., van Staa T.P., Chapman M., Gallagher A.M., Hammad T.A., Richards M.A. Cancer recording and mortality in the General Practice Research Database and linked cancer registries. Pharmacoepidemiol. Drug Saf. 2013;22(2):168–175. doi: 10.1002/pds.3374. [DOI] [PubMed] [Google Scholar]
- 26.Dregan A., Moller H., Murray-Thomas T., Gulliford M.C. Validity of cancer diagnosis in a primary care database compared with linked cancer registrations in England. Population-based cohort study. Cancer Epidemiol. 2012;36(5):425–429. doi: 10.1016/j.canep.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Jensen H., Tørring M.L., Olesen F., Overgaard J., Fenger-Grøn M., Vedsted P. Diagnostic intervals before and after implementation of cancer patient pathways - a GP survey and registry based comparison of three cohorts of cancer patients. BMC Cancer. 2015;15(308) doi: 10.1186/s12885-015-1317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price S.J., Stapley S.A., Shephard E., Barraclough K., Hamilton W.T. Is omission of free text records a possible source of data loss and bias in Clinical Practice Research Datalink studies? A case–control study. BMJ Open. 2016;6(5) doi: 10.1136/bmjopen-2016-011664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel G.A., Mendonca S.C., McPhail S., Zhou Y., Elliss-Brookes L., Lyratzopoulos G. Emergency diagnosis of cancer and previous general practice consultations: insights from linked patient survey data. Br. J. Gen. Pract. 2017;67(659):e377–e387. doi: 10.3399/bjgp17X690869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murchie P., Smith S.M., Yule M.S., Adam R., Turner M.E., Lee A.J., Fielding S. Does emergency presentation of cancer represent poor performance in primary care? Insights from a novel analysis of linked primary and secondary care data. Br. J. Cancer. 2017;116(9):1148–1158. doi: 10.1038/bjc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weller D., Menon U., Zalounina Falborg A., Jensen H., Barisic A., Knudsen A.K., Bergin R.J., Brewster D.H., Cairnduff V., Gavin A.T., Grunfeld E., Harland E., Lambe M., Law R.J., Lin Y., Malmberg M., Turner D., Neal R.D., White V., Harrison S., Reguilon I., Vedsted P. Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP) BMJ Open. 2018;8(11) doi: 10.1136/bmjopen-2018-023870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadpara P., Madhavan S.S., Tworek C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: a population-based study. Cancer Epidemiol. 2015;39(6):1136–1144. doi: 10.1016/j.canep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tørring M.L., Frydenberg M., Hamilton W., Hansen R.P., Lautrup M.D., Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J. Clin. Epidemiol. 2012;65(6):669–678. doi: 10.1016/j.jclinepi.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y., Mendonca S.C., Abel G.A., Hamilton W., Walter F.M., Johnson S., Shelton J., Elliss-Brookes L., McPhail S., Lyratzopoulos G. Variation in 'fast-track' referrals for suspected cancer by patient characteristic and cancer diagnosis: evidence from 670 000 patients with cancers of 35 different sites. Br. J. Cancer. 2018;118(1):24–31. doi: 10.1038/bjc.2017.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tørring M.L., Falborg A.Z., Jensen H., Neal R.D., Weller D., Reguilon I., Menon U., Vedsted P. Advanced-stage cancer and time to diagnosis: an International Cancer Benchmarking Partnership (ICBP) cross-sectional study. Eur. J. Cancer Care. 2019;28(5) doi: 10.1111/ecc.13100. [DOI] [PubMed] [Google Scholar]
- 36.Public Health England . 2019. National Cancer Registration and Analysis Service: Routes to Diagnosis.http://www.ncin.org.uk/publications/routes_to_diagnosis (Accessed 6 February 2019) [Google Scholar]
- 37.NHS Improvement . Department of Health; London: 2012. Rapid Review of Endoscopy Services. [Google Scholar]
- 38.Brown H., Wyatt S., Croft S., Gale N., Turner A., Mulla A. Cancer Research UK; London: 2015. Scoping the Future: An Evaluation of Endoscopy Capacity Across the NHS in England. [Google Scholar]
- 39.NHS England - National Cancer Programme . National Cancer Programme; London: 2019. Targeted Screening for Lung Cancer with Low Radiation Dose Computed Tomography. [Google Scholar]
- 40.Richards M., Thorlby R., Fisher R., Turton C. The Health Foundation; London: 2018. Unfinished Business: An Assessment of the National Approach to Improving Cancer Services in England 1995-2015. [Google Scholar]
- 41.Weller D. Cancer diagnosis and treatment in the COVID-19 era. Eur. J. Cancer care. 2020;29(3):e13265. doi: 10.1111/ecc.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.