Summary.
Background:
Incidental pulmonary embolism (IPE) is defined as pulmonary embolism (PE) diagnosed on computed tomography scanning not performed for suspected PE. IPE has been estimated to occur in 3.1% of all cancer patients and is a growing challenge for clinicians and patients. Nevertheless, knowledge about the treatment and prognosis of cancer-associated IPE is scarce. We aimed to provide the best available evidence on IPE management.
Methods:
Incidence rates of symptomatic recurrent venous thromboembolism (VTE), major hemorrhage, and mortality during 6-month follow-up were pooled using individual patient data from studies identified by a systematic literature search. Subgroup analyses based on cancer stage, thrombus localization, and management were performed.
Results:
In 926 cancer patients with IPE from 11 cohorts, weighted pooled 6-month risks of recurrent VTE, major hemorrhage and mortality were 5.8% (95% confidence interval [CI] 3.7–8.3%), 4.7% (95% CI 3.0–6.8%), and 37% (95% CI 28–47%). VTE recurrence risk was comparable under low molecular weight heparins (LMWH) and vitamin K antagonists (VKAs) (6.2% vs. 6.4%; hazard ratio [HR] 0.9; 95% CI 0.3–3.1), while 12% in untreated patients (HR 2.6; 95% CI 0.91–7.3). Risk of major hemorrhage was higher under VKAs than under LMWH (13% vs. 3.9%; HR 3.9; 95% CI 1.6–10). VTE recurrence risk was comparable in patients with an subsegmental IPE and those with a more proximally localized IPE (HR 1.1; 95% CI 0.50–2.4).
Conclusion:
These results support the current recommendation to anticoagulate cancer-associated IPE with LMWH and argue against different management of subsegmental IPE.
Keywords: hemorrhage, incidental finding, prognosis, pulmonary embolism, venous thromboembolism
Introduction
Incidental pulmonary embolism (IPE) is defined as pulmonary embolism diagnosed on a computed tomography (CT) scan performed for reasons other than a clinical suspicion of pulmonary embolism (PE). In cancer patients, IPE has been estimated to occur in 2.2% to 4.1% [1]. Knowledge of the clinical implications of cancer-associated IPE is scarce and almost entirely based on small observational studies. Key finding of these studies was the similar prognosis with regard to recurrence risk, major hemorrhage, and mortality in cancer patients with IPE compared with those with proven symptomatic PE (SPE) [2–4]. Based on these observations, international guidelines recommend an identical anticoagulant treatment regimen for cancer-associated IPE and SPE, and consequently, almost all patients with IPE receive anticoagulant treatment (ACCP level of Evidence 2B) [5,6].
However, it should be noted that the supporting evidence for this recommendation is limited by the small size of the studies. In addition, essential clinical questions on the subject of IPE management remain unanswered, namely (i) the risks of recurrent venous thrombolembolism (VTE) if left untreated, (ii) the risks of hemorrhage and its dependence on the type of anticoagulation, and (iii) the relevance of subsegmental IPE vs. more centrally located IPE. To provide the best available evidence on the management of IPE, we pooled individual patient data from 11 observational studies and ongoing registries, which were identified by a systematic literature search.
Methods
Data sources, searches, and study selection
We searched PubMed, MEDLINE, EMBASE, Web of Science, Academic Search Premier, Science Direct, and the Cochrane Database of systematic reviews for publications concerning cancer patients with IPE from inception to November 2013. The search strategy is available in the Supplementary Data. The electronic search was complemented by a manual review of reference lists of relevant articles, and we contacted experts to ask about the existence of unpublished cohorts.
References were screened for relevance by two independent reviewers based on the title and abstract (T.v.d.H. and P.d.E.). Discrepancies were resolved by consensus after contacting a third reviewer (F.K.). Abstracts or full-text articles identified by either reviewer as potentially relevant were retrieved for further evaluation. Predefined inclusion criteria for eligible cohorts were: (i) ≥ 20 consecutive patients with IPE; (ii) patients with a concomitant active cancer (both solid and hematologic cancer), defined as cancer diagnosed within 6 months before IPE, recurrence or progressive cancer or any cancer that necessitated curative or palliative treatment within the previous 6 months; (iii) at least 6 months of follow-up; (iv) information about the management of the IPE; and (v) reporting at least one of the predefined primary and/or secondary study end points. Completed studies and ongoing patient registries were eligible. An invitation, and study proposal were sent to the authors of the selected references as well as at least one reminder.
Patients and clinical data collection
IPE was defined as PE detected on a CT scan ordered for reasons other than a clinical suspicion of PE [7]. Patients were managed according to local practices. International guidelines available during the study periods recommended anticoagulant treatment for a period of at least 6 months with prolongation for as long as the cancer was active [5,8,9]. Low molecular weight heparin (LMWH) was the treatment of choice for cancer-associated incidental VTE from 2004 on.
Individual patient-level data were collected, consisting of general baseline characteristics, the location of IPE and the applied anticoagulant treatment regimen. The primary end point was the occurrence of symptomatic recurrent VTE, defined as a positive finding of the diagnostic workup of suspected acute PE or DVT of the lower or upper extremities [10]. Incidental VTE events were not adjudicated as recurrent events. Secondary end points included major hemorrhage, fatal hemorrhage, and mortality. The duration of follow-up was 6 months. DVT of the lower extremities was diagnosed in case of non-compressibility by compression ultrasonography at the trifurcation of the popliteal vein or above or, in case of an intraluminal filling defect above the trifurcation of the popliteal vein, by CT or venography [5,10]. Recurrent PE was diagnosed in case of a new intraluminal filling defect in a subsegmental or larger pulmonary artery, in case of a ventilation/perfusion scanning with a high probability of PE in a new lung segment unaffected by the index IPE, or in case of a new intraluminal filling defect by pulmonary angiography [6]. Major hemorrhage was defined as overt and associated with a decrease in the hemoglobin level of ≥ 2 g dL−1, requiring transfusion of ≥ 2 units of blood, occurring in a critical site (intracranial, intraspinal, intraocular, pericardial, intra-articular intramuscular with compartment syndrome, retroperitoneal), or contributing to death [11].
Statistical analysis
The end points were defined and all statistical analyses were performed according to a predefined statistical protocol, agreed on by all authors. Baseline characteristics were reported for the combined cohorts and for subgroups based on the management of the IPE. All outcomes were pooled using the DerSimonian–Laird weights in a random-effects model. Additionally, baseline characteristics and outcomes were reported for the individual cohorts (see Supplementary Tables).
For the subgroup analyses, outcomes were pooled using the DerSimonian–Laird weights in a random effects model. To calculate hazard ratios (HRs), all cohorts and registries were combined and considered as one cohort. Subgroups analyses were performed for: (i) patients treated with LMWH, patients treated with vitamin K antagonists (VKAs) after an initial course of LMWH, and those who were left untreated; (ii) patients with metastatic cancer and non-metastatic cancer; and (iii) patients with centrally located thrombi (defined as a central or lobar thrombus location) and more peripherally located thrombi (defined as a segmental or subsegmental thrombus location). Additionally, outcomes for patients with isolated subsegmental IPE were reported separately. The HRs were calculated using Cox regression analysis. Regarding the subgroup analysis based on management, an intention-to-treat analysis was used for which patients were classified according to the initial management even when anticoagulant treatment was prematurely discontinued. Additionally, per-treatment analysis was performed for which outcomes were related to the management at the time the outcome occurred. A competing risk model was used for the survival tables for recurrent VTE and major bleeding with death as competing risk. SPSS, version 20 (SPSS Inc, Chicago, IL, USA) and StatsDirect software (StatsDirect Ltd, Cheshire, UK) were used for all analyses.
Results
Identification of cohorts and registries
The initial search identified 106 records in PubMed, 61 unique references in MEDLINE, 153 unique references in EMBASE, 28 unique references in Web of Science, 12 unique references in the Cochrane Database of Systematic Reviews, and two unique references in Academic Search Premier, resulting in a total of 362 references. Based on screening titles and abstracts, 44 references were extensively studied and, when available, read in full text. Of these 44 references, 11 references were excluded because no or only limited follow-up was reported, 12 because they concerned < 20 patients, one because IPE were diagnosed on additionally performed CT pulmonary angiography after the initial CT scan, and one because it did not meet the definition of IPE (see Supplementary Data for excluded references). Finally, 19 references from the literature search and one unpublished registry that met our inclusion criteria were included. Patients of the unpublished registry were collected in the Ramón y Cajal Hospital in Madrid, Spain. Of these 20 cohorts, the authors of four references refrained from participating [12–15] and the authors of five references [16–20] did not respond to repeated invitations, resulting in the inclusion of 11 cohorts and registries [2,4,21–28] (Fig. 1).
Fig. 1.
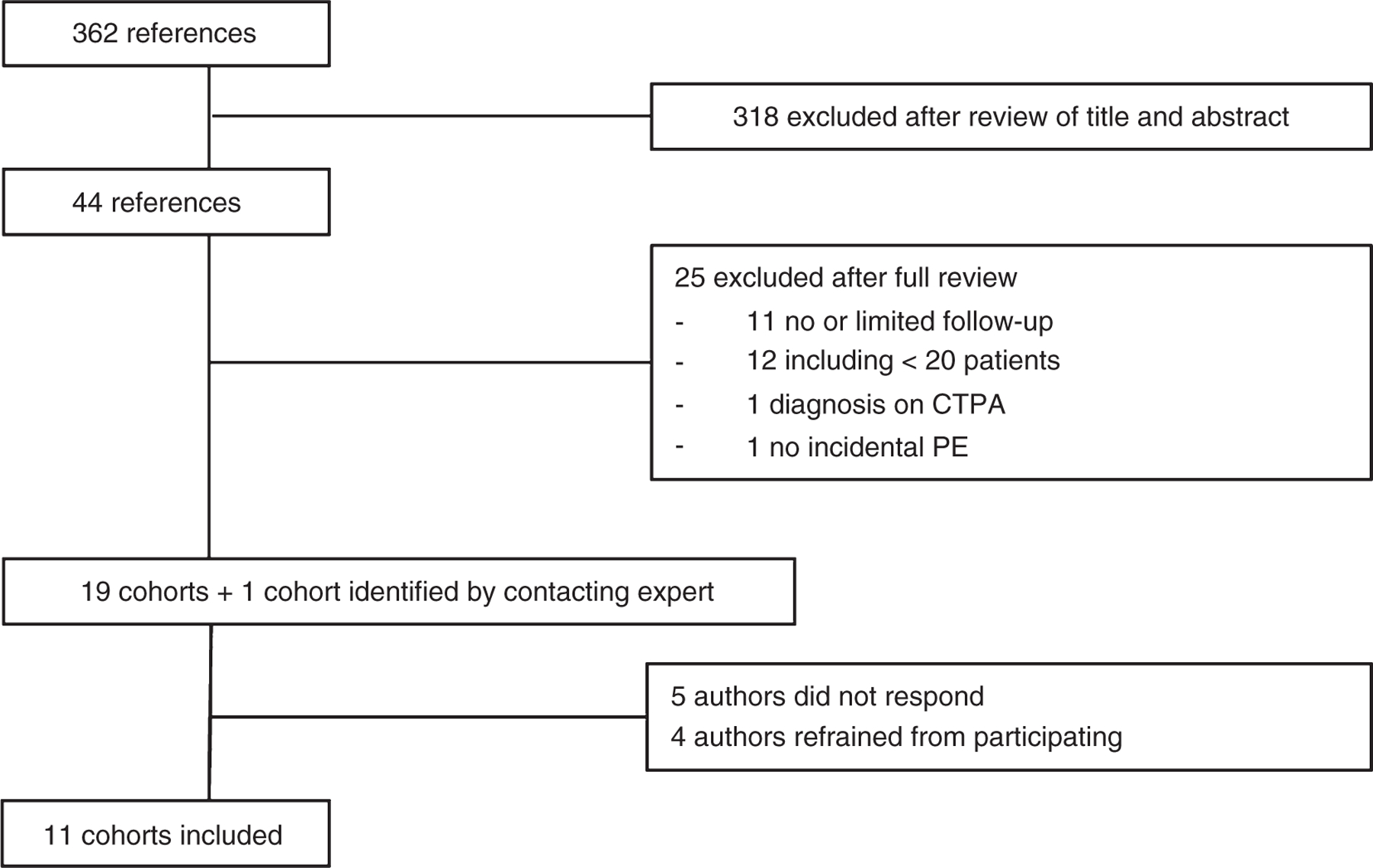
Flow chart selection of cohorts.
Baseline characteristics
The number of patients of the 11 included individual cohorts and registries varied from 21 to 204 patients (Table S1). All cohorts and registries were collected from 2001 and 2013. Nine of the 11 cohorts and registries were retrospectively collected and two were prospectively collected. In total, individual patient data of 945 patients were available, of which 6-month follow-up data were complete for 926 patients (98%) and these comprised the study patients for whom baseline characteristics are shown in Table 1. A total of 732 (79%) patients were treated with prolonged therapeutic LMWH, 100 (11%) patients were treated with VKAs, 41 (4.4%) patients received another treatment (ie, inferior vena cava filter or unfractionated heparin), and 53 (5.7%) patients received no treatment.
Table 1.
Baseline characteristics of total cohort and stratified by management
| Total cohort | LMWH | VKA | Other | None | |
|---|---|---|---|---|---|
| Treatment | n = 926 (100%) | n = 732 (79%) | n = 100 (11%) | n = 41 (4.4%) | n = 53 (5.7%) |
| Mean age (SD; range) | 65 (12; 19–94) | 64 (12; 19–94) | 68 (12; 20–91) | 68 (13; 28–90) | 65 (14; 27–91) |
| Male sex, n (%) | 491 (53) | 378 (52) | 60 (60) | 22 (54) | 31 (58) |
| Heart failure, n (%) | 27/470 (5.7) | 19/382 (5.0) | 4/56 (7.1) | 1/10 (10) | 3/22 (14) |
| COPD, n (%) | 35/471 (7.4) | 25/383 (6.5) | 7/56 (13) | 0/10 (0) | 3/22 (14) |
| Previous VTE, n (%) | 47/566 (8.3) | 32/435 (7.4) | 10/86 (12) | 1/13 (7.7) | 4/32 (13) |
| Stage of malignancy, n (%) | |||||
| Metastatic cancer | 501 (54) | 400 (55) | 56 (56) | 12 (29) | 33 (62) |
| Non-metastatic cancer | 192 (21) | 143 (20) | 34 (34) | 3 (7.3) | 12 (23) |
| Unspecified | 233 (25) | 189 (26) | 10 (10) | 26 (63) | 8 (15) |
| Type of malignancy, n (%) | |||||
| Lung | 176 (19) | 135 (18) | 16 (16) | 7 (17) | 18 (34) |
| Colorectal | 185 (20) | 150 (20) | 20 (20) | 6 (15) | 9 (17) |
| Other gastrointestinal | 187 (20) | 147 (20) | 15 (15) | 12 (29) | 13 (25) |
| Breast | 65 (7.0) | 52 (7.1) | 10 (10) | 2 (4.9) | 1 (1.9) |
| Gynecological | 64 (6.9) | 56 (7.7) | 5 (5.0) | 3 (7.3) | 0 (0) |
| Other or unknown | 206 (22) | 155 (21) | 31 (31) | 10 (24) | 10 (19) |
| HVKAsmatological | 43 (4.6) | 37 (5.1) | 3 (3.0) | 1 (2.4) | 2 (3.8) |
| Largest artery involved, n (%) | |||||
| Central | 292 (32) | 230 (31) | 30 (30) | 21 (51) | 11 (21) |
| Segmental | 301 (33) | 238 (33) | 35 (35) | 7 (17) | 21 (40) |
| Subsegmental | 193 (21) | 156 (21) | 27 (27) | 2 (4.9) | 8 (15) |
| Unspecified | 140 (15) | 108 (15) | 8 (8.0) | 11 (27) | 13 (25) |
COPD, chronic obstructive pulmonary disease; VTE, venous thromboembolism; LMWH, low molecular weight heparins; VKA, vitamin K antagonist.
Symptomatic recurrent VTE
Data regarding the occurrence of recurrent VTE were available from 10 of the 11 cohorts that included 857 patients, of whom 19 developed an objectively proven DVT and 22 recurrent PEs (with or without DVT), and for three patients, the type of the recurrent VTE was unspecified. Nine (20%) of these 44 recurrent VTEs occurred while anticoagulant treatment was discontinued: four events during temporary discontinuation of LMWH and five after treatment with LMWH was permanently stopped. Outcomes of the individual cohorts are avaliable in Table S2.
Based on the intention-to-treat analysis, the weighted pooled 6-month risk of recurrent VTE was 6.2% (95% CI 3.5–12%) in patients treated with LMWH and 6.4% (95% CI 2.2–12%) in those who received VKAs (Table 2A, Fig. 2), with an HR adjusted for sex, age, type of cancer, and cancer stage of 0.92 (95% CI 0.3–3.1). In the 10 cohorts that reported data on recurrent VTE, a total of 42 (4.9%) patients did not receive any anticoagulant treatment, of whom four developed symptomatic VTE, resulting in a weighted pooled 6-month risk of 12% (95% CI 4.7–23%). Of these 42 patients, seven had a centrally located IPE, 18 had a segmental IPE, four had a subsegmental IPE, and in 13 patients, the thrombus location was unspecified. Of the four patients who did not receive anticoagulant treatment and developed a recurrent VTE, two had a subsegmentally located IPE and the other two had a segmentally located IPE. Compared with patients who were treated with either LMWH or VKAs, the HR of symptomatic recurrent VTE in patients who did not receive anticoagulant treatment was 2.0 (95% CI 0.65–5.9) adjusted for age, sex, type of cancer, and cancer stage. Outcomes stratified for cancer type are avaliable in Table S3.
Table 2.
Primary and secondary outcomes for total cohort and stratified by management
| Outcome | Weight pooled risk in % (95% CI) |
||||
|---|---|---|---|---|---|
| Total cohort | LMWH | VKA | Other | None | |
| (A) Pooled outcomes after 6 months of follow-up and stratified by initial management | |||||
| Recurrent VTE | 5.8 (3.7–8.3) | 6.2 (3.5–9.6) | 6.4 (2.2–12) | 4.3 (3.3–12) | 12 (4.7–23) |
| Major hemorrhage | 4.7 (3.0–6.8) | 3.9 (2.3–5.9) | 13 (6.4–20) | 6.4 (0.2–20) | 6.4 (1.3–15) |
| Mortality | 37 (28–47) | 37 (29–44) | 28 (18–40) | 58 (38–77) | 47 (28–66) |
| Outcome | Incidence rate per 100 patient-years (95% CI) |
|||
|---|---|---|---|---|
| LMWH | VKA | Other | None | |
| (B) Incidence rates per 100 patient-years and stratified by management based on a per-protocol analysis | ||||
| Recurrent VTE | 12 (8.3–17) | 9.8 (2.0–29) | 9.5 (0.24–53) | 30 (8.2–77) |
| Major hemorrhage | 10 (6.6–15) | 26 (11–52) | 18 (2.2–66) | 4.6 (0.55–17) |
VTE, venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism; CI, confidence interval.
Fig. 2.
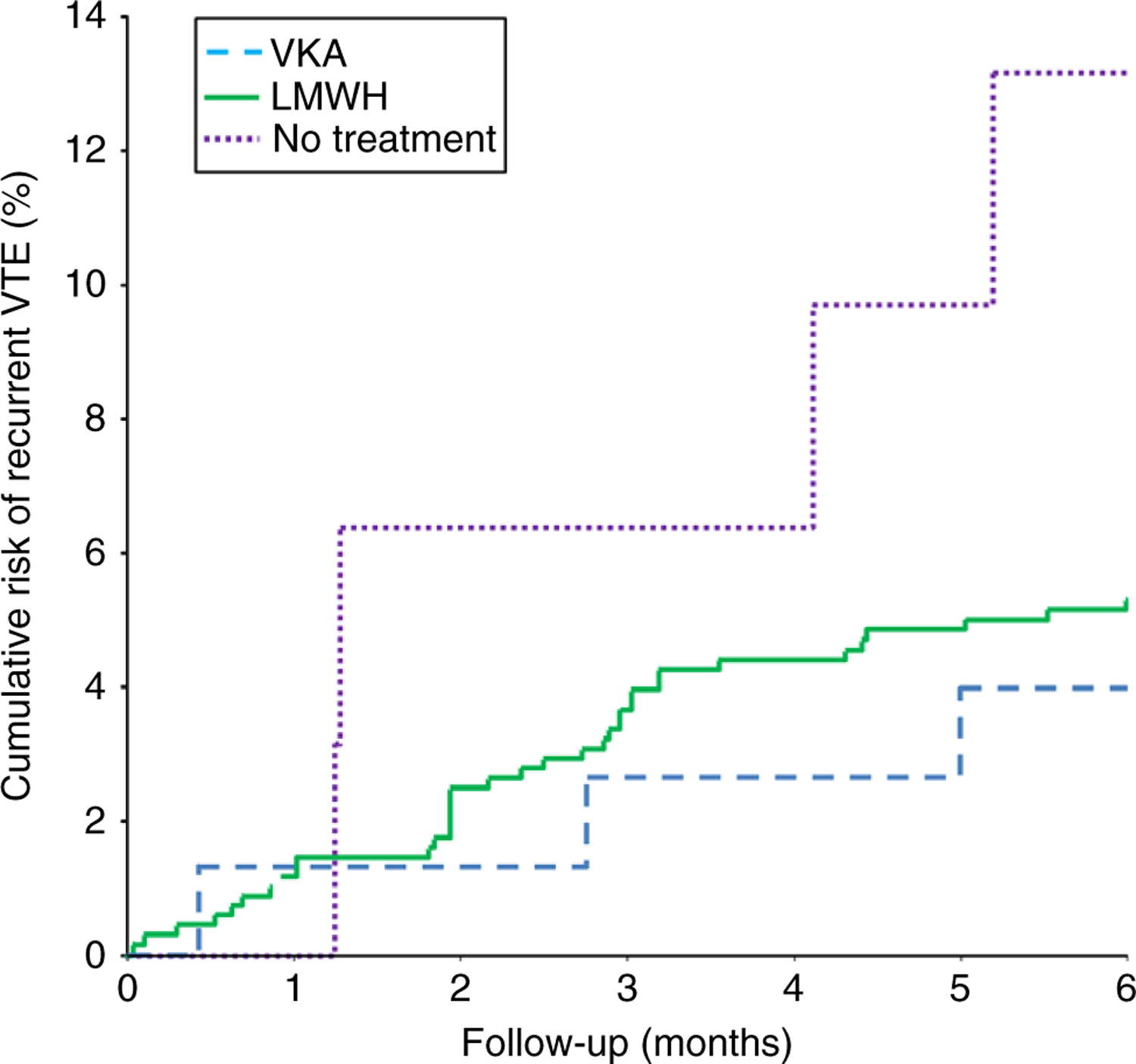
Cumulative risk of recurrent venous thromboembolism related to management. VKA, vitamin K antagonist; LMWH, low molecular weight heparins. Based on a competing risk analysis.
The risk of recurrent VTE was non-significantly higher in patients with metastatic cancer at time of diagnosing IPE compared with those with non-metastatic cancer with an HR of 1.4 (95% CI 0.59–3.2) adjusted for age, sex, type of cancer, and management (Table S4). Regarding the location of the IPE, the weighted pooled 6-month risk of recurrent VTE was comparable in patients with a centrally located IPE compared with those with peripherally located IPE, 5.6% (95% CI 3.1–8.7%) and 6.6% (95% CI 3.5–11%), respectively, with an HR of 0.65 (95% CI 0.22–1.9) adjusted for age, sex, type of cancer, cancer stage, and management (Table S5). When patients with a subsegmental IPE were compared with those with a more centrally located IPE, incidence rates were 7.8% (95% CI 2.8–14.9%) and 5.5% (95% CI 2.9–8.8%), respectively, with an HR of 1.3 (95% CI 0.57–3.0) adjusted for age, sex, type of cancer, cancer stage, and management.
Based on the per-treatment analysis, the incidence rates of recurrent VTE were 12 per 100 patient years (PY) (31 events during 252 years of treatment; 95% CI 8.3–17) and 9.8 per 100 PY (3 events during 31 years of treatment; 95% CI 2.0–29) while receiving LMWH and VKAs, respectively. For patients who did not receive anticoagulant treatment, either from the initial diagnosis or after LMWH or VKAs were stopped within 6 months for reasons other than death, the incidence rate was 20 per 100 PY (nine events during 45 years of treatment; 95% CI 9.2–38) (Table 2B).
Major hemorrhage
Information regarding major hemorrhage was available for 10 cohorts concerning a total of 857 patients of whom 38 patients experienced a major hemorrhage. Overall weighed pooled incidence rates are provided in Table 2. The risk of major hemorrhage was comparable in patients with metastatic and non-metastatic cancer and in patients with a centrally located IPE compared with those with a peripherally located IPE, with an HR of 1.8 (95% CI 0.68–4.8) adjusted for age, sex, type of cancer, and management and 1.0 (95% CI 0.31–3.0), adjusted for age, sex, type of cancer, cancer stage, and management (Tables S4 and S5).
Based on the intention-to-treat analysis, the weighted pooled 6-month risk of major hemorrhage was significantly higher in patients treated with VKAs compared with those treated with LMWH, 13% (95% CI 6.4–20%) vs. 3.9% (95% CI 2.3–5.9%) with an HR of 4.0 (95% CI 1.5–10) adjusted for age, sex, type of cancer, and cancer stage (Table 2A, Fig. 3). The weighted 6-month pooled risk of major hemorrhage in patients who were left untreated was 6.4% (95% CI 1.3–15%). Based on the per-treatment analysis, the incidence rate of major hemorrhage while receiving VKA treatment was 26 per 100 PY (eight events during 30 years of treatment; 95% CI 11–52), and while receiving LMWH treatment, the incidence rate was 10 per 100 PY (26 events during 257 years of treatment; 95% CI 6.6–15) (Table 2B).
Fig. 3.
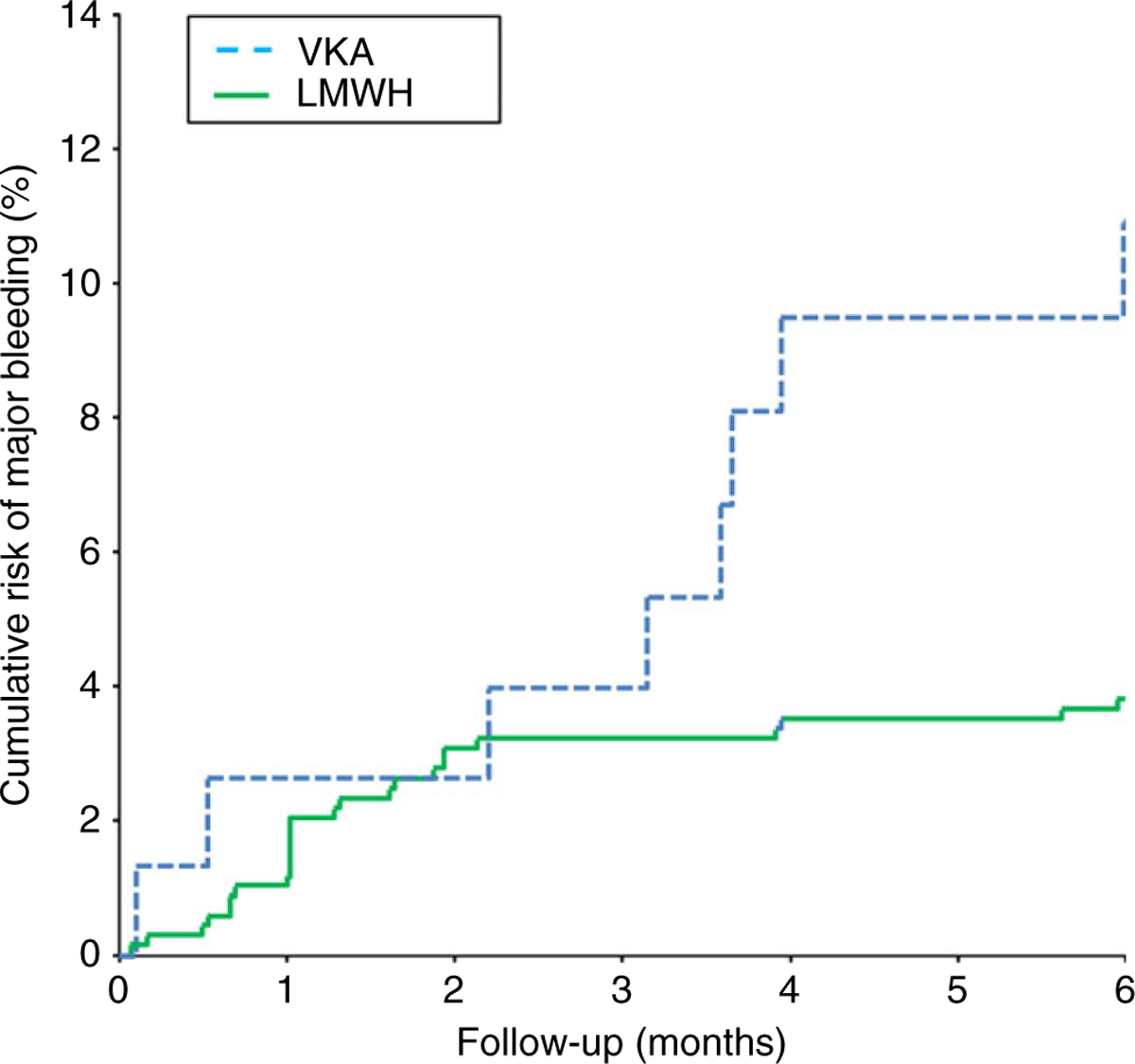
Cumulative risk of major hemorrhage complications according to anticoagulant treatment. VKA, vitamin K antagonist; LMWH, low molecular weight heparins. Based on a competing risk analysis.
Mortality
Of the 926 patients, 331 died during follow-up, resulting in a weighted pooled 6-month mortality of 37% (95% CI 28–47%; Table 2A). Mortality varied between cancer type and cancer stage (Tables S3 and S4). The weighted pooled 6-month mortality was higher in patients with a centrally located IPE compared with those with a peripherally located IPE: 42% (95% CI 33–52%) vs. 30% (95% CI 25–36%) with an HR of 1.5 (95% CI 1.1–2.0) adjusted for age, sex, type of cancer, cancer stage, and management. Patients with a centrally located IPE more frequently had metastatic cancer (79%) compared with those with a more peripherally located IPE (67%) (χ2 test: P < 0.01).
The weighted pooled 6-month mortality was 37% (95% CI 29–44%) in patients treated with LMWH and 28% (95% CI 18–40%) in those treated with VKAs (HR 1.1; 95% CI 0.70–1.6 adjusted for age, sex, cancer type, and cancer stage). In patients who did not receive any treatment, the weighted pooled 6-month mortality was 47% (95% CI 28–66%).
Discussion
This study of individual patient data of 926 patients from 11 registries is the largest study on cancer-associated IPE thus far and provides several important new findings.
First, this study demonstrates a 6-month VTE recurrence risk of 12% (95% CI 4.7–23%) in patients who were left untreated. Although it is possible that these patients were left untreated for a specific reason, that is, a high risk of hemorrhage, a poor overall prognosis, or a supposed low risk of recurrent VTE, the patient’s characteristics did not differ greatly from those of treated patients. Importantly, the higher mortality in the untreated patients may even have resulted in an underestimation of the pooled 6-month VTE recurrence risk due to significant competing risk of death. In the per-treatment analysis, the incidence rate of recurrent VTE in patients who did not receive anticoagulant treatment was even 30 per 100 PY (95% CI 8.2–77). Thus, this observation emphasizes the high risk of symptomatic recurrent VTE in cancer patients with IPE and recalls the effect size of anticoagulants used in SPE, thereby supporting the initiation of anticoagulation in cancer-associated IPE [5,6].
Second, we observed a comparable efficacy of VKAs and LMWH with a significantly higher risk of major hemorrhage in patients who were treated with VKAs. Although these findings should be interpreted with caution due to the observational study design and the lack of information about the quality of anticoagulant treatment, it seems unlikely that patients with a high risk of major hemorrhage were predominantly assigned to receive VKAs. This is reflected by the comparable baseline characteristics of both groups and by the non-significantly lower mortality in patients treated with VKAs. Notably, a comparable risk of major hemorrhage between oral and parenteral anticoagulants has been demonstrated in cancer patients with proven clinically suspected PE, while the recurrence risk was lower in those treated with LMWH [29]. This notable difference between the efficacy and safety of oral vs. parenteral anticoagulants in IPE and SPE may be caused by the observational design of our study in which all cancer patients with IPE were included, whereas patients with a high risk of major hemorrhage were excluded from the trials in cancer patients with SPE. A second explanation could be poor quality of anticoagulant treatment, on which information was unfortunately unavailable for our study subjects. However, the comparable risk of recurrent VTE in patients treated with VKAs and LMWH argues against a poor quality of anticoagulant management. Regardless, the observations from the current study supports LMWH as treatment of choice for cancer-associated VTE [5,6].
Given the debate regarding the clinical relevance of isolated subsegmental SPE, the clinical significance and management of subsegmental IPE may be even less clear [30,31]. Therefore, the comparable risk of recurrent VTE in cancer patients with a subsegmental IPE vs. more centrally located IPE and the observation of recurrent events in untreated patients with subsegmental IPE are further key findings of this study. Both observations argue against subsegmental IPE as a distinct disease entity and support an identical management. Since the presence of (asymptomatic) DVT in patients with a subsegmental IPE was not investigated in the cohorts, conclusions regarding the clinical relevance of isolated subsegmental IPE can not be drawn. The finding that subsegmental PE is not associated with a more favorable prognosis with regard to VTE recurrences was recently described in non-cancer patients with SPE as well [32]. Notably, in line with the observation of O’Connell and colleagues, the current analysis centrally located IPE was associated with a higher mortality than distally located PE [24]. Two likely explanations for this phenomenon could be a higher mortality directly related to VTE, as observed for SPE, or a higher cancer-related mortality [33].
Strengths of this study are the systematic literature search for potential studies and ongoing registries; the high number of included patients, far exceeding previously published cohorts; the strict and identical diagnostic criteria for IPE among the included studies and registries; the reporting of objectively established outcomes; and the use of patient-level data.
The most relevant limitation of this study is related to the observational and predominantly retrospective designs of the individual registries and the unavailability of results from nine identified cohorts that may have introduced selection bias. Since four cohorts were only described in a meeting abstract and the risk of recurrent VTE is only described for one of five cohorts published in a peer-reviewed journal, our study seems to be a good representation of existing literature. It should be mentioned that patients were not randomly assigned to treatment and no uniform management protocol was applied, and it is unknown whether the presence of asymptomatic DVT had been investigated and influenced management decisions. Also, initial CT results and outcomes were not adjudicated by an independent committee. The impact of ongoing oncologic management (e.g., systemic chemotherapy) and its potential contribution to the risk of recurrent VTE and/or cancer related prognosis are additional confounding factors that cannot fully be accounted for in this study. Due to the study design, we were unable to provide a reliable estimation of the burden of recurrent VTE on mortality. Ideally, a randomized clinical trial should be performed to provide more definite answers. However, given all available evidence to date, we consider conducting a randomized clinical trial allocating patients with cancer-associated IPE to placebo or anticoagulant treatment as ethically very challenging. This is supported by the results of the enquiry among physicians, of whom 89–100% judged treatment of cancer-associated IPE to be necessary [34].
In conclusion, this study demonstrates a substantial risk of symptomatic recurrent VTE in cancer patients with IPE and suggests an even higher recurrence risk when anticoagulant treatment is withheld. An LMWH-based treatment regimen was associated with a lower risk of major hemorrhage than treatment with VKAs. These observations support the current guideline recommendations to initiate anticoagulant treatment with LMWH for cancer-associated VTE. Finally, our data argue against different management of subsegmental cancer-associated IPE.
Supplementary Material
Essentials.
We performed a pooled analysis of 926 patients with cancer-associated incidental pulmonary embolism (IPE).
Vitamin K antagonists (VKA) are associated with a higher risk of major hemorrhage.
Recurrence risk is comparable after subsegmental and more proximally localized IPE.
Our results support low molecular weight heparins over VKA and similar management of subsegmental IPE.
Acknowledgements
M. Nishino was supported by a National Institutes of Health grant (1K23CA157631).
Footnotes
Disclosure of Conflict of Interests
M. Nishino served as a consultant to Bristol-Myers Squibb. The other authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Baseline characteristics of the individual cohorts.
Table S2. Outcomes of the individual cohorts.
Table S3. Outcomes stratified for cancer type.
Table S4. Outcomes in patients with metastatic cancer and non-metastatic cancer.
Table S5. Outcomes related to thrombus location.
Data S1. Search strategy.
References
- 1.Dentali F, Ageno W, Becattini C, Galli L, Gianni M, Riva N, Imberti D, Squizzato A, Venco A, Agnelli G. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysis. Thromb Res 2010; 125: 518–22. [DOI] [PubMed] [Google Scholar]
- 2.den Exter PL, Hooijer J, Dekkers OM, Huisman MV. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol 2011; 29: 2405–9. [DOI] [PubMed] [Google Scholar]
- 3.Shinagare AB, Okajima Y, Oxnard GR, Dipiro PJ, Johnson BE, Hatabu H, Nishino M. Unsuspected pulmonary embolism in lung cancer patients: comparison of clinical characteristics and outcome with suspected pulmonary embolism. Lung Cancer 2012; 78: 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahut DM, Caumont PA, Planquette B, Revel MP, Avillach P, Chatellier G, Sanchez O, Meyer G. Risk factors and clinical outcome of unsuspected pulmonary embolism in cancer patients: a case–control study. J Thromb Haemost 2012; 10: 2032–8. [DOI] [PubMed] [Google Scholar]
- 5.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e419S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman M, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014; 35: 3033–80..25173341 [Google Scholar]
- 7.Khorana AA, O’Connell C, Agnelli G, Liebman HA, Lee AY. Incidental venous thromboembolism in oncology patients. J Thromb Haemost 2012; 10: 2602–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133: 454S–545S. [DOI] [PubMed] [Google Scholar]
- 9.Buller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 401S–28S. [DOI] [PubMed] [Google Scholar]
- 10.Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost 2013; 11: 412–22. [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010; 8: 202–4. [DOI] [PubMed] [Google Scholar]
- 12.Deng K, Parameswaran R, Soff BP, Soff GA. Incidental versus symptomatic pulmonary embolism in cancer patients: a multi-variate analysis of recurrent VTE and mortality. ASH Annual Meeting Abstracts 2013; 120: 2257. [Google Scholar]
- 13.Dentali F, Ageno W, Pierfranceschi MG, Imberti D, Malato A, Nitti C, Salvi A, Siragusa S, Squizzato A, Vitale J, Agnelli G. Prognostic relevance of an asymptomatic venous thromboembolism in patients with cancer. J Thromb Haemost 2011; 9: 1081–3. [DOI] [PubMed] [Google Scholar]
- 14.Font C, Farrus B, Vidal L, Caralt TM, Visa L, Mellado B, Tassies D, Monteagudo J, Reverter JC, Gascon P. Incidental versus symptomatic venous thrombosis in cancer: a prospective observational study of 340 consecutive patients. Ann Oncol 2011; 22: 2101–6. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez Otero P, Lecumberri R, Garcia Munoz R, Ruiz de Gaona E, Rocha E, Paramo JA. Unsuspected pulmonary embolism: impact on cancer patients’ survival. Haematol Hematol J 2007; 92: 305. [Google Scholar]
- 16.Engelke C, Rummeny EJ, Marten K. Pulmonary embolism at multi-detector row CT of chest: one-year survival of treated and untreated patients. Radiology 2006; 239: 563–75. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Escobar I, Rossi M, Gravalos CC, Sepulveda SJ, Castellanos D, Aix SP, Nunez JA, Cortes-Funes H. Pulmonary embolism: unsuspected finding in cancer patients. Ann Oncol 2010; Conference: viii384.
- 18.Piacentini G, Fregoni V, Rizzo G, Da Prada GA, Lorenzetti IT, Gallizzi G, Pavesi L, Riccardi A. Incidental pulmonary embolism in cancer patients: clinical features and outcome. ASH Annual Meeting Abstracts 2013; 120: 2243. [Google Scholar]
- 19.Savla GV, Gladish G, Zhou X, Vadhan-Raj S. Differences in the clinical presentation, severity, and treatment outcome for symptomatic versus asymptomatic pulmonary embolism in cancer patients. J Clin Oncol 2008; 26, 20688. [Google Scholar]
- 20.Sun JM, Kim TS, Lee J, Park YH, Ahn JS, Kim H, Kwon OJ, Lee KS, Park K, Ahn MJ. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer 2010; 69: 330–6. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Razeq HN, Mansour AH, Ismael YM. Incidental pulmonary embolism in cancer patients: clinical characteristics and outcome – a comprehensive cancer center experience. Vasc Health Risk Manag 2011; 7: 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozas G, Ramasamy S, Avery G, Maraveyas A. Pulmonary embolism as an incidental finding in ambulatory cancer outpatients. Characteristics and outcome. Thromb Res 2010; 125: S168. [Google Scholar]
- 23.Donnelly OG, Jones J, Carey B, Swinson D, Radhakrishna G. Incidental pulmonary emboli in cancer patients – a single centre experience. Thromb Res 2010; 125: S167. [Google Scholar]
- 24.O’Connell C, Razavi P, Ghalichi M, Boyle S, Vasan S, Mark L, Caton A, Duddalwar V, Boswell W, Grabow K, Liebman HA. Unsuspected pulmonary emboli adversely impact survival in patients with cancer undergoing routine staging multi-row detector computed tomography scanning. J Thromb Haemost 2011; 9: 305–11. [DOI] [PubMed] [Google Scholar]
- 25.Shteinberg M, Segal-Trabelsy M, Adir Y, Laor A, Vardi M, Bitterman H. Clinical characteristics and outcomes of patients with clinically unsuspected pulmonary embolism versus patients with clinically suspected pulmonary embolism. Respiration 2012; 84: 492–500. [DOI] [PubMed] [Google Scholar]
- 26.Soler S, Delgado C, Ballaz A, Cisneros E, Maly R, Babalis D, Monreal M. Unsuspected pulmonary embolism in patients with cancer. Thromb Res 2012; 129(Suppl. 1): S16–9. [DOI] [PubMed] [Google Scholar]
- 27.Tiseo M, Bersanelli M, Pesenti BM, Bartolotti M, De LG, Gelsomino F, Camisa R, Cademartiri F, Ardizzoni A. Asymptomatic pulmonary embolism in lung cancer: prevalence and analysis of clinical and radiological characteristics in 141 outpatients. Tumori 2012; 98: 594–600. [DOI] [PubMed] [Google Scholar]
- 28.Shinagare AB, Guo M, Hatabu H, Krajewski KM, Andriole K, Van den Abbeele AD, Dipiro PJ, Nishino M. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer 2011; 117: 3860–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akl EA, Kahale L, Barba M, Neumann I, Labedi N, Terrenato I, Sperati F, Muti P, Schunemann H. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev 2014; 7: CD006650. [DOI] [PubMed] [Google Scholar]
- 30.Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, Pleasance S, Le Gal G. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost 2010; 8: 1716–22. [DOI] [PubMed] [Google Scholar]
- 31.Carrier M, Righini M, Le Gal G. Symptomatic subsegmental pulmonary embolism: what is the next step? J Thromb Haemost 2012; 10: 1486–90. [DOI] [PubMed] [Google Scholar]
- 32.den Exter PL, van Es J, Klok FA, Kroft LJ, Kruip MJ, Kamphuisen PW, Buller HR, Huisman MV. Risk profile and clinical Outcomes in cancer-associated incidental PE 113 outcome of symptomatic subsegmental acute pulmonary embolism. Blood 2013; 122: 1144–9. [DOI] [PubMed] [Google Scholar]
- 33.Klok FA, Djurabi RK, Nijkeuter M, Eikenboom HC, Leebeek FW, Kramer MH, Kaasjager K, Kamphuisen PW, Buller HR, Huisman MV. High D-dimer level is associated with increased 15-d and 3 months mortality through a more central localization of pulmonary emboli and serious comorbidity. Br J Haematol 2008; 140: 218–22. [DOI] [PubMed] [Google Scholar]
- 34.den Exter PL, van Roosmalen MJ, van den Hoven P, Klok FA, Monreal M, Jimenez D, Huisman MV. Physicians’ management approach to an incidental pulmonary embolism: an international survey. J Thromb Haemost 2013; 11: 208–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


