Abstract
Advances in nanotechnology have enabled the design of nanotherapeutic platforms that could address the challenges of targeted delivery of active therapeutic agents to the central nervous system (CNS). While the majority of previous research studies on CNS nanotherapeutics have focused on neurons and endothelial cells, the predominant resident immune cells of the CNS, microglia, are also emerging as a promising cellular target for neurodegeneration considering their prominent role in neuroinflammation. Under normal physiological conditions, microglia protect neurons by removing pathological agents. However, long-term exposure of microglia to stimulants will cause sustained activation and lead to neuronal damage due to the release of pro-inflammatory agents, resulting in neuroinflammation and neurodegeneration. This Perspective highlights criteria to be considered when designing microglia-targeting nanotherapeutics for the treatment of neurodegenerative disorders. These criteria include conjugating specific microglial receptor-targeting ligands or peptides to the nanoparticle surface to achieve targeted delivery, leveraging microglial phagocytic properties, and utilizing biocompatible and biodegradable nanomaterials with low immune reactivity and neurotoxicity. In addition, certain therapeutic agents for the controlled inhibition of toxic protein aggregation and for modulation of microglial activation pathways can also be incorporated within the nanoparticle structure without compromising stability. Overall, considering the multifaceted disease mechanisms of neurodegeneration, microglia-targeted nanodrugs and nanotherapeutic particles may have the potential to resolve multiple pathological determinants of the disease and to guide a shift in the microglial phenotype spectrum toward a more neuroprotective state.
NOMENCLATURE
- AD
Alzheimer's disease
- AJs
adherens junctions
- ALS
amyotrophic lateral sclerosis
- AM
amphiphilic macromolecules
- ApoE
apolipoprotein E
- ApoE3-rHDL
apolipoprotein E3-reconstituted high density lipoprotein
- ASYN
alpha synuclein
- Aβ
amyloid beta
- BBB
blood-brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CTE
chronic traumatic encephalopathy
- CZ NPs
ceria-zirconia nanoparticles
- DA
dopaminergic
- DAM
disease-associated microglia
- DAMP
damage-associated molecular patterns
- DLB
dementia with Lewy bodies
- EGCG
epigallocatechin gallate
- FDA
Food and Drug Administration
- FTD
frontotemporal dementia
- HIV-1
human immunodeficiency virus type-1
- IN
intranasal
- iNOS
inducible nitric oxide synthase
- LincRNA-Cox2
long intergenic non-coding RNA-cyclooxygenase-2
- LPS
lipopolysaccharide
- MS
multiple sclerosis
- MSC
mesenchymal stem cell
- NAMP
neurodegeneration-associated molecular patterns
- NO
nitric oxide
- NPs
nanoparticles
- NSAIDS
non-steroidal anti-inflammatory drugs
- PAMP
pathogen-associated molecular patterns
- PCL
poly-ε-caprolactone
- PD
Parkinson's disease
- PDD
Parkinson's disease dementia
- PEG
polyethylene glycol
- PEI
polyethylenimine
- PGE2
prostaglandin E2
- PHOX
phagocyte oxidase
- PMMA
polymethylmethacrylate
- PRRs
pattern recognition receptors
- RAGE
receptor for advanced glycation end products
- RIPK1
receptor-interacting serine/threonine-protein kinase 1
- ROS
reactive oxygen species
- SOD1
superoxide dismutase
- TAT
transactivator of transcription
- TfR
transferrin receptor
- TJs
tight junctions
- TLR
toll-like receptor
- TREM2
triggering receptor expressed on myeloid cells 2
THE ROLE OF MICROGLIA IN NEURODEGENERATIVE DISEASES
Microglial functions in healthy vs diseased brain
Microglia are the resident immune cells of the brain and are derived from primitive myeloid progenitors that arise during embryonic development.1 They represent 5%–12% of cells in the healthy CNS, with different brain regions possessing different microglial subpopulations.2 Microglia serve as the initial mode of defense by generating both innate and adaptive immune responses upon disturbance to homeostasis. In the healthy brain, microglia possess a surveillance phenotype, consisting of a ramified morphology with long cytoplasmic protrusions that allow them to survey their environment from a resting state.3 By continuously monitoring changes in the brain, microglia eliminate pathogens and preserve the health of different cell types of the CNS.4 Microglia display various signaling immunoreceptors to interact with extracellular species, including the TLR2 and TLR4 toll-like receptors, CR3 and CR4 phagocytic receptors, and CD36 and CD204 scavenger receptors.2 Because of their high degree of phenotypic and functional plasticity, microglia exhibit robust responses to changes in their microenvironment.5 These responses can be neurotoxic or neuroprotective because microglia are involved in both physiological and pathological conditions, protecting the CNS in physiological conditions and enhancing disease progression in pathological conditions. Their resting state morphology also allows them to physically interact with the synapses of neurons to regulate neuronal activity.6 They play important roles in mediating neuronal activity by preserving the neural environment, responding to injury, and facilitating repair.3 Specifically, microglia help promote neurogenesis, reshape neuronal circuitry, mediate neuronal transmission, and regulate synaptic pruning and apoptosis.2,4 Overall, there is a high degree of crosstalk between microglia and neurons under normal physiological conditions.
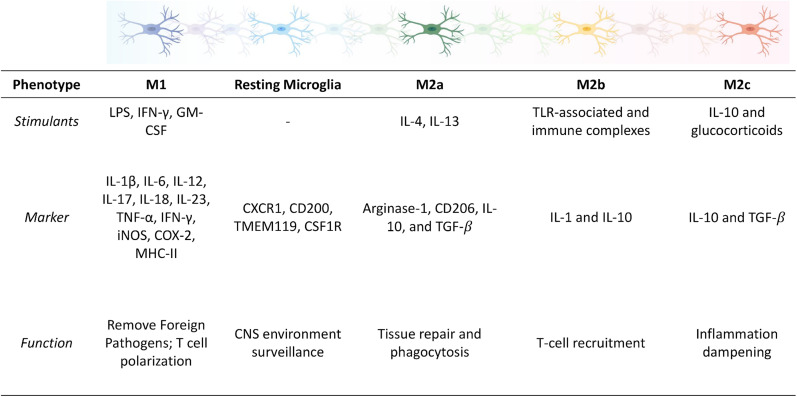
When homeostasis is compromised, microglia respond to changes in their microenvironment by transforming into a reactive phenotype marked by an ameboid morphology and the contraction of processes.2 Microglia in a resting state display a low-level expression of genes that contribute to the CNS inflammatory response, and this response is further dampened by neurotrophic factors released by neurons.7 Upon detection of injury or damage, microglial activation results in the upregulation of many cell surface receptors, the release of various complement factors, and changes to the cytokine profile.2 This activation is a plastic and dynamic process that has been shown to be brain region-specific.6 Microglial activation is often an early and sustained response in neurodegenerative diseases, leading to oxidative stress and neuroinflammation as a result of systemic inflammation. Microglial activation states have historically been described in terms of an “M1” pro-inflammatory phenotype and an “M2” anti-inflammatory phenotype although this distinction oversimplifies the dynamic range of phenotypes that microglia can possess.
The classically activated M1 phenotype is associated with disruptions in homeostasis and pro-killing functions, resulting in the release of pro-inflammatory cytokines as a first line of defense against infection or injury. The following phenotypic markers are involved in the M1 immune response: IL-1β, IL-6, IL-12, IL-17, IL-18, IL-23, TNF-α, IFN-γ, iNOS, COX-2, MHC-II, ROS, reactive nitrogen species, and prostaglandin E2 (PGE2).4 The upregulation of these markers is associated with increased oxidative stress, neuroinflammation, and ultimately exacerbated neurodegeneration. The alternatively activated M2 phenotype is associated with sustained homeostasis and inflammation dampening, resulting in the release of neurotrophic factors and anti-inflammatory cytokines to promote healing and tissue repair. Microglial M2 phenotypes can be further divided into the M2a, M2b, and M2c subtypes. The M2a subtype involves tissue repair and phagocytosis and is activated by IL-4 and IL-13, resulting in the upregulation of arginase-1, CD206, IL-10, and TGF-β.3,4 The M2b subtype involves T-cell recruitment and is activated by TLRs and immune complexes, resulting in the upregulation of IL-1 and IL-10. The M2c subtype is involved in inflammation dampening and healing and is activated by IL-10 and glucocorticoids, resulting in the upregulation of IL-10 and TGF-β.3,4 Due to the high degree of crosstalk between microglia and neurons, activation of a pro-inflammatory microglial phenotype can disrupt normal neuron-microglia communication, resulting in aberrant neuronal signaling, neuronal dysfunction, and neuronal loss that contributes to pathogenesis in neurodegenerative diseases.2,7 The phenotypes of microglia mentioned above are summarized and illustrated in Fig. 1.
FIG. 1.
Spectrum of microglial phenotypes upon stimulation. Depending on the type of stimulant, the microglial phenotype can be roughly categorized into pro-inflammatory (M1) or anti-inflammatory (M2×).2–4,7 While simply categorizing microglia into only two main categories does not do justice to their diverse function in the CNS hemostasis, the corresponding phenotypic markers listed here are only meant to guide the characterization of microglia but not to restrict the interpretation of diverse functional roles that different microglial phenotypes play in the CNS.
Many research groups now define context-specific microglial activation and phenotype as a measure of the diversity of microglial functions.8 Single-cell RNA sequencing analysis has revealed the existence of a subset of microglia that display a unique transcriptional and functional signature in neurodegenerative conditions, termed disease-associated microglia (DAM).9,10 Induction of this DAM phenotype is initiated by the recognition of neurodegeneration-associated molecular patterns (NAMPs) by receptors expressed on microglia, triggering their transition into DAM.9,10 This phenotype is shared across various neurodegenerative diseases, such as Alzheimer's Disease (AD), Amyotrophic Lateral Sclerosis (ALS), and Multiple Sclerosis (MS), and in response to aging.10,11 Researchers have also distinguished multiple disease stage-specific cell states within DAM populations, which also suggests that these DAM phenotypes may occur along the transition from early stage (i.e., protective/beneficial functions) to late stage (neurotoxic) microglia.12,13
Mechanisms of how microglia kill neurons
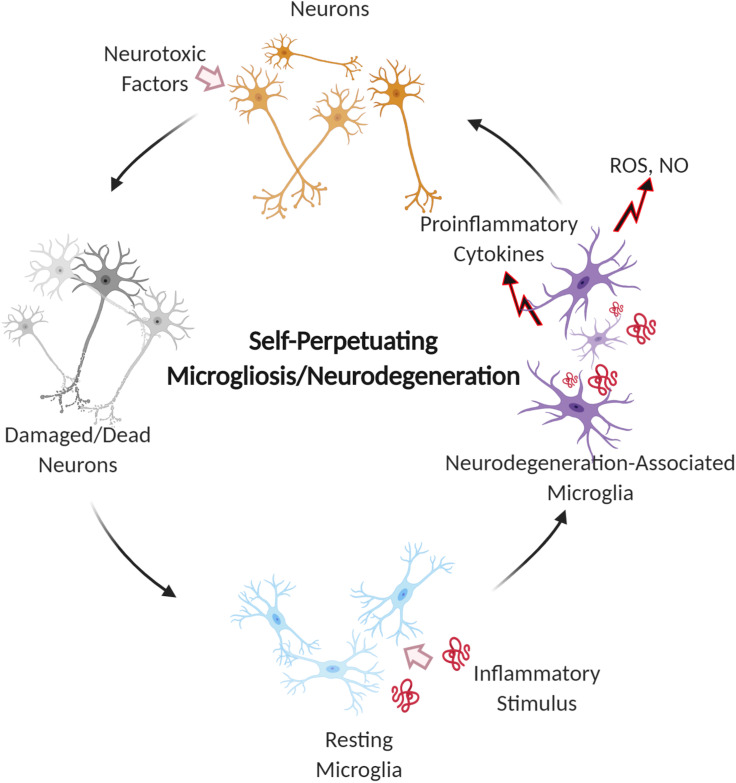
Sustained microglial activation, known as microgliosis, is believed to play a role in exacerbating neuronal loss in neurodegenerative diseases due to oxidative stress and neuroinflammation.14,15 Research has shown that activated microglia coincide with regions of neuronal cell death and phagocytose dying neurons.6 Microglia are part of a self-propelling cycle where microgliosis causes an inflammatory response that leads to neuronal death, and this neuronal death promotes further microgliosis3 (Fig. 2). Thus, microglia help amplify the progressive neurodegeneration in diseases by contributing to neuronal dysfunction. It is believed that although microglia are associated with neuronal loss, they are likely involved in the escalation of neuronal loss rather than the initial cause.6 In AD and Parkinson's Disease (PD), microglia have been shown to generate neurotoxic species following the internalization of amyloid-β (Aβ) and α-synuclein (ASYN), respectively, leading to neuronal damage.2 As neurodegenerative diseases progress, the communication between microglia and neurons is further disrupted, resulting in deregulation and abnormal activation that leads to greater neuronal loss.
FIG. 2.
Self-perpetuating cycle of microglial activation and neuron damage during neurodegeneration. Microglia recognize, uptake, and phagocytose inflammatory stimuli, the prolonged exposure to which results in chronic microglial activation. The activation of microglia initiates the release of neurotoxic free radicals and pro-inflammatory cytokines, which, in turn, leads to neuronal damage and further stress on microglia, leading to microgliosis.
There are several specific mechanisms by which activated microglia cause neuronal dysfunction and death. Inflammatory stimuli released by activated microglia increase the expression of phagocyte NADPH oxidase (PHOX) to produce superoxide and other reactive oxygen species (ROS) that stress or kill neurons.16 Increased PHOX expression also promotes microglial proliferation, leading to an increase in the production of ROS. Another mechanism involves the expression of inducible nitric oxide synthase (iNOS) in activated microglia, which results in the production of nitric oxide (NO) and other ROS. High levels of NO lead to neuronal death via inhibition of mitochondrial cytochrome oxidase in neurons, which results in glutamate release and excitotoxicity.17 Pro-inflammatory cytokines released by activated microglia, such as TNF-α and IL-1β, can induce apoptosis and result in neuronal death. Microglial phagocytosis of stressed neurons may also accelerate cell death. Reactive oxygen and nitrogen species produced by activated microglia contribute to neuronal stress and trigger exposure of phosphatidylserine on the surface of neurons, which signals for microglia to phagocytose the neurons.18
Effects of aging on microglia
Neurodegenerative diseases such as AD and PD are age-related, and the prevalence of these diseases is expected to increase over time due to higher life expectancies. Microglia play a significant role in age-related changes. Aging is associated with an increase in oxidative stress, disrupting the normal protective role of microglia in preserving neural integrity.7 Aging also leads to microglial activation, which results in a shift in the cytokine profile toward the pro-inflammatory phenotype with decreased phagocytosis and increased production of ROS.5 Activated microglia are less effective at clearing neurotoxic protein aggregates such as Aβ and ASYN. Due to the chronic nature of aging, microglia eventually cease proliferation and become senescent, which drives the progression of age-related neurodegenerative diseases. Microglia are normally able to regenerate, but during aging, telomere shortening occurs, which results in cellular dystrophy and senescence.19 The morphological characteristics of senescent microglia include the elimination of processes, formation of spheroids, and fragmentation of the cytoplasm.20 Unlike highly proliferative cells, microglia have limited telomerase activity and, thus, limited replication potential. The degeneration of senescent microglia results in a loss of their neuroprotective functions, leaving neurons vulnerable to damage. Senescent microglia are also more reactive to stimuli, referred to as microglial priming. Primed microglia exhibit a more sustained inflammatory response, contributing to the impairment of neuronal function. Given the role of microglia in aging and neurodegeneration, microglial-targeted therapies could serve as an additional approach to de-escalate neurodegenerative diseases.
Effects of neuroinflammation in various neurodegenerative diseases
Neuroinflammation is a common feature of various neurodegenerative diseases and is characterized by microglial and astroglial activation and the secretion of pro-inflammatory mediators such as ROS, NO, and various pro-inflammatory cytokines and chemokines including TNF-α, IL-1β, and IL-6.21 Several stimuli may initiate neuroinflammation, including Pathogen-Associated Molecular Patterns (PAMPs), typically found in infected tissues, and Damage-Associated Molecular Patterns (DAMPs), such as misfolded or aggregated proteins or mislocalized nucleic acids.22 These stimuli are recognized by cells in the CNS, which express pattern recognition receptors (PRRs), not only mainly comprising microglia but also including perivascular and peripheral macrophages and other glial cells and neurons to a lesser extent.21,23,24 These PRRs include various TLRs and scavenger receptors, which can also form receptor complexes in the form of heterodimers or trimers, initiating a pro-inflammatory signaling cascade that leads to microglial activation and recruitment and generates production of neurotoxic molecules that contribute to neurodegeneration.25–28 When there is a persistent presence of DAMPs, as in neurodegenerative diseases, the neuroinflammatory response becomes chronic, leading to morphological, phenotypical, and functional changes in microglia and astrocytes, and the sustained release of pro-inflammatory mediators that exacerbate neurodegeneration.23 This chronic neuroinflammation is a critical part of almost all neurodegenerative diseases. Interfering with the initial binding of DAMPs to PRRs is a potential therapeutic strategy for various neurodegenerative diseases. Acute neuroinflammation can be beneficial in certain circumstances, leading to the stimulation of myelin repair, removal of toxic aggregated proteins and cell debris, or the secretion of protective and reparative neurotrophic factors as a causative or exacerbating factor or as a secondary component.23,29
AD is a progressive neurodegenerative disorder and the most common cause of dementia in older adults.30 The hallmark pathologies of AD are the accumulation of intracellular neurofibrillary tangles of the protein tau and extracellular plaque deposits of the Aβ peptide, the deposition of which could be attributed to the dysfunction of certain transporter molecules such as P-glycoprotein or the changes in expression levels of relevant receptor proteins such as TLRs.31,32 Fibrillar forms of Aβ found in these plaques have neuroinflammatory effects, triggering the accumulation and activation of microglia that surround the plaques.26,33 Microglia play an important role in Aβ clearance, by endocytosing and degrading both soluble and fibrillar Aβ.34 The chronic accumulation of Aβ and its interaction with microglial receptors such as scavenger receptor A1, CD36, CD47, CD14, and various TLRs activate microglia and initiate the aforementioned pro-inflammatory signaling cascade.28,33 Dysregulated Aβ clearance has been identified as a major pathway in the pathology of sporadic AD cases, particularly in aged microglia that are more prone to impaired lysosomal function.35,36 In later stages of AD, the pro-inflammatory cytokines produced by activated microglia downregulate genes involved in Aβ clearance, thereby enabling Aβ accumulation.37 Microglia can also degrade and clear the aggregated tau present in AD and other tauopathies such as progressive supranuclear palsy, frontotemporal dementia (FTD), and chronic traumatic encephalopathy (CTE).38 Activation of pro-inflammatory microglia contributes to the progression of tau pathology and increases tau phosphorylation, which is associated with synaptic dysfunction and cell death.39,40
Another major class of neurodegenerative diseases is known as synucleinopathies, which are characterized by the abnormal accumulation of the protein ASYN. These diseases include PD, the associated Parkinson's disease dementia (PDD), and dementia with Lewy bodies (DLBs).41 A common pathologic feature of these diseases is the onset of neuroinflammation in the areas corresponding to ASYN deposition and neurodegeneration, particularly within the substantia nigra and striatum.42–44 Aβ deposition has also been associated with PDD and DLP, with its presence accelerating the dementia process.45 In PD, which is characterized by the loss of dopaminergic (DA) neurons in the substantia nigra, extracellular accumulation and aggregation of ASYN have been shown to cause microglial activation, triggering the release of inflammatory cytokines and other neurotoxic molecules, which cause additional neurodegeneration.46–48 Sustained microglial activation is believed to play a prominent role in exacerbating DA neuronal loss, as the substantia nigra contains 4.5 fold larger microglial population than other brain regions, and DA neurons have reduced antioxidant capacity, rendering them susceptible to oxidative stress to a greater degree relative to other cell types within the brain.49,50 Chronic activation of microglia also slows the degradation of ASYN and increases its intracellular accumulation, suggesting that activated microglia are less efficient at clearing ASYN and are a critical trigger to exacerbating ASYN pathology and neurodegeneration.51 Cell debris from dead neurons can further attract and activate microglia, resulting in a self-perpetuating cycle of inflammation and neurotoxicity.
ALS is a progressive neurodegenerative disease characterized by the loss of motor neurons.52 A common hallmark of the disease shared by sporadic and familial ALS patients is the strong activation and proliferation of microglia found at sites of motor neuron loss.53 Microglial activation may be triggered by the accumulation of aggregates of mutant superoxide dismutase (SOD1).22 These activated microglia demonstrate neuroprotective properties during the early pre-symptomatic stages of ALS but shift to a more pro-inflammatory phenotype as the disease progresses, promoting neurodegeneration.54,55
Targeting microglial activation states by suppressing pro-inflammatory neurotoxic effects of the classically activated phenotype and/or simultaneously enhancing the anti-inflammatory, neuroprotective functions of the alternately activated phenotype are gaining promise as a therapeutic approach for neurodegenerative diseases.56 A rational approach to inhibiting microglial activation induced by the accumulation of disordered proteins would rely on interrupting the key steps via which these proteins interact with and activate microglia, while, at the same time, maintaining protein clearance by microglia through inhibition of aggregation in extracellular spaces.
NANOTHERAPEUTICS IN THE CNS
CNS drug delivery crossing the blood-brain barrier
In order for a therapeutic agent to be effective, it needs to reach the site of pathology, i.e., the CNS, where the degeneration of neurons and neuroinflammation take place. The discovery of treatments for CNS disease has been challenged by the existence of the blood-brain barrier (BBB), which is a highly selective barrier that isolates the CNS from systemic circulation, protecting the brain from pathogens and maintaining CNS homeostasis to allow proper neuronal function. The BBB is mainly made up of brain capillary endothelial cells (BCECs), which are connected to each other by tight junctions (TJs) and adherens junctions (AJs).57 Lacking fenestration, these BCECs are tightly packed, restricting the paracellular diffusion of hydrophilic small molecules.58 The transport of necessary nutrients and certain drugs across the BBB is regulated by a series of specific transport mechanisms, which can generally be classified into the following categories: passive diffusion, carrier-mediated transport, and vesicular trafficking, such as receptor-mediated transcytosis and adsorptive-mediated transcytosis.59 Small molecule drugs make up a large majority of available CNS therapeutics, most of which penetrate the BBB via passive diffusion, while only a small number penetrate via carrier-mediated mechanisms.60 One of the challenges in designing BBB-crossing small molecules is to maintain molecules' high lipid solubility while enabling reasonable solubility in aqueous brain interstitial fluid to reach target cells. This property requirement screens out more than 98% of all US Food and Drug Administration (FDA)-approved small molecule drugs to be used in the CNS.59,60 Compared to small molecule therapeutics, biologic drugs, such as recombinant proteins, antibodies, or nucleic acid drugs, are larger in size and generally do not cross the BBB via passive diffusion.61 Proper delivery vehicles needs to be tailored to carry biologic and small molecule drugs that cannot penetrate the BBB, facilitating their entry into the CNS via receptor-mediated transcytosis.62
Without proper drug delivery platforms to facilitate BBB crossing, CNS drugs may be delivered via alternative routes such as cerebrospinal fluid (CSF) injection and intra-cerebroventricular delivery to bypass the BBB.61 However, drugs delivered via these administration routes have rarely obtained FDA approval and concerns have been raised regarding the limited drug penetration into brain parenchyma from the CSF, which results in the exponential drug concentration decrease in the CNS following injection.63 To date, the majority of CNS-targeted pharmacological interventions have focused on administering therapeutics systemically via intravenous injection or oral administration due to the relative non-invasiveness of these delivery methods compared to local administration.64–67 For systemic drug delivery, the therapeutic efficacy of a drug is strongly associated with the time window in which the drug concentration is maintained above its therapeutic level without systemic toxicity.68 The design of a controlled drug delivery system offers an alternative strategy to maximize drug action with minimized toxicity utilizing existing therapeutic molecules.
Additional challenges in CNS drug delivery
In addition to overcoming the BBB, other two aspects challenging the discovery of CNS therapeutics are1 maintaining stability of drugs in their active form before reaching the site of pathology and2 designing molecules targeted toward surface receptors and associated pathways of interest to reduce off-target effects.69 While either aspect could be addressed via structural modifications of the drugs, such as designing prodrugs that can be metabolized into a pharmacologically active drug after administration or drugs that target certain domains on receptors of interest, overcoming both obstacles presents major challenges in CNS drug design.70,71
Considering the difficulties in tackling all the challenges mentioned above with single therapeutic agent molecules for CNS drug delivery, it is critical to engineer drug delivery systems that could be tailored to facilitate BBB crossing of therapeutics, control the sustained release of active agents at the site of pathology, and maintain the chemical and physical stabilities of the drug. These criteria for designing drug delivery systems can all be fulfilled with nanoparticle formulations, which are designed to deliver therapeutics to the site of pathology in a targeted manner, while maintaining the unmodified structure of the active agent in an extended time-window with minimized toxicity and side effects.72 Nanoparticle-based drug delivery systems present great potential and offer a unique solution to the challenge of BBB penetration owing to their flexibly manipulated physical and chemical properties. Nanoparticles can be engineered to overcome the challenges that small molecule drugs face through surface functionalization with BBB-targeting transporters, loading of drugs that either cannot cross the BBB or lack structural stability and enabling controlled release at the site of pathology.73,74
Overcoming the CNS drug delivery challenges with nanoparticles
Nanoparticles (NPs) refer to particles for which one or more external dimensions are in the size range of 1–100 nm for at least 50% of the particles according to the European Commission's Recommendation.74,75 Compared to bulk materials, nanoparticles' high surface area to volume ratio enables not only increased cellular interaction and reactivity but also concentrated loading of large amounts of therapeutic agents with minimized toxicity. Key advantages of using NP formulations for CNS delivery include an extended half-life, enhanced deposition of drug within a targeted region, and reduced side effects.76,77 The functionality of NPs can be tailored by modifying various characteristics such as charge, size, and surface chemistry, in addition to encapsulation of a desired drug payload.
Based on their material composition, NPs can be roughly categorized into two classes: organic (including polymeric NPs and liposomes) and inorganic (including metal NPs and carbon-based NPs).72 In addition to NP formulations' usage as drug delivery vehicles, the nano-scale packing of material also imposes unique physical properties compared to bulk material. One example is NanoTherm®, an iron oxide NP-based therapeutic for intratumoral thermotherapy in glioblastoma patients, which has superparamagnetic properties that are used for local heat generation in combination with chemotherapy to prevent tumor growth.78 More recent research investigations have illustrated the use of cell-derived NPs, specifically the engineering and re-engineering of exosomes, a group of extracellular vesicles, as potential drug delivery platforms.79 Exosomes offer unique characteristics including low immunogenicity, biodegradability, and the ability to cross many biological barriers.80
NPs can be engineered to target BBB transport mechanisms mentioned before in order to efficiently cross the BBB, while carrying a therapeutic drug payload. The physicochemical properties of these NPs determine the specific mode of transport across the BBB. While the vast majority of NPs are unable to cross the BBB without functionalization, there are some exceptions such as gold NPs, which have been shown to cross the BBB via passive diffusion through ion channels, and silver and titanium dioxide NPs, which can travel into the brain by decreasing transendothelial electrical resistance and disrupting the tight junctions between BCECs.81,82 Crossing the BBB in this manner is size dependent, with NPs being less than 10 nm in diameter. There are also various methods of temporarily disrupting the permeability of the BBB to enable NP delivery, such as through the administration of ultrasound energy or hyperosmotic agents.83,84 Although these methods can improve the delivery of various therapeutics into the brain, these temporary disruptions in BBB integrity could allow the passage of toxic substances into the brain, which could affect the normal functions of the CNS.
Several types of cationic NPs have been reported to interact with the negatively charged surface of the BCECs and cross the BBB via adsorptive-mediated transcytosis. There are different methods of conferring a positive charge on the surface of NPs. One such method is by fabricating NPs from multiple components that have a positive charge at physiological pH. Single component NPs have also been synthesized using cationic polymers such as chitosan or polyethylenimine (PEI) and successfully used for brain delivery.85,86 Positively charged polymers are well suited for delivery of negatively charged nucleic acids since NPs can easily be assembled using these components via polyelectrolyte complex formation or controlled coacervation.87,88 The NP surface can be functionalized with positively charged molecules such as PEI or cell-penetrating cationic peptides, i.e., TAT peptides [transduction domain of human immunodeficiency virus type-1 (HIV-1)].89,90 Although cationic NPs can improve transport across the BBB, such NPs can have toxic effects.
NPs can be modified or conjugated to ligands that will bind to receptors on BCECs, acting as a “molecular Trojan horse” and resulting in transport across the BBB via receptor-mediated transcytosis.91 This approach is acknowledged as having the most likely chance of successfully crossing the BBB.92 Coating the surface of NPs with polysorbate 80 facilitates adsorption of apolipoprotein E (ApoE) from blood plasma, causing the coated NPs to bind to low density lipoprotein (LDL) receptors on the endothelial cells and cross the BBB via receptor-mediated transcytosis.93,94 ApoE itself has been covalently attached to human serum albumin NPs, promoting the rapid uptake of these NPs into the brain.95 Insulin and antibodies against the insulin receptor have also been used as BBB-targeting ligands for NPs.96 The transferrin receptor (TfR) is the most widely studied receptor for targeting the BBB for receptor-mediated transcytosis. NPs decorated with transferrin or lactoferrin, a protein within the transferrin family, have been successfully delivered to the brain after intravenous injection.97,98 In order to avoid competition with the abundant amount of endogenous transferrin circulating in the bloodstream, monoclonal antibodies against transferrin receptors have also been used as targeting ligands. The antibodies OX26, 8D3, and RI7217 have been used for successful TfR-targeted brain delivery in rodents.99,100
In order to increase transport efficiency across the BBB, it can be advantageous to target multiple transport mechanisms when designing NPs. Several dual mechanism-targeting NPs have been used for delivery across the BBB, such as magnetic NPs embedded with transferrin, which demonstrated a synergistic effect resulting in a 50%–100% increase in BBB crossing compared to NPs targeting only one transport mechanism.101 NPs can also be conjugated with ligands targeting multiple receptors on the BCECs, such as the example of chitosan NPs covalently conjugated to transferrin and bradykinin B2 antibodies.102 These NPs were amenable to uptake via adsorptive-mediated transcytosis due to the cationic chitosan and could also bind to the TfR and bradykinin B2 receptor for uptake via receptor-mediated transcytosis.
Another non-invasive NP delivery route is intranasal (IN) administration, which is a method that bypasses systemic circulation and the BBB to achieve direct brain delivery of therapeutics, leading to high CNS concentrations of therapeutics and low systemic accumulation.103–106 Nanotherapeutics can pass across the nasal epithelium into the brain via two different pathways. The extracellular pathway, which is the primary mechanism for brain delivery of therapeutics, involves passive transport across the nasal epithelium.107 The intracellular pathway involves endocytosis into the olfactory and trigeminal nerve branches and subsequent axonal transport into the brain.107 Although there are some limitations to this drug delivery route such as low absorption of therapeutics, high mucociliary clearance, and enzymatic degradation within the nasal cavity or during passage across the epithelial barrier, NPs can be used as delivery vehicles to protect encapsulated therapeutics, facilitating uptake and passage into the CNS.23,108
To improve the NPs' serum stability without compromising the drug loading capacity, the surface of NPs can be functionalized with additional polymers such as polyethylene glycol (PEG), the hydrophilic property of which can not only improve the bioavailability of the drug but also maintain therapeutic agent's stability before reaching cellular targets of interest by protecting it from serum proteins.109 The sustained release of loaded active agents from the nanoparticle structure can be a result of exposure to the physiological environment, in which case the dominant interaction force enabling the formation of NPs and encapsulation of active agents could be outcompeted by the change in pH, ion concentrations, or the presence of cleavage enzymes.110,111
TARGETING MICROGLIA USING NANOTHERAPEUTICS
Current therapeutic strategies for targeting microglial mediated inflammation
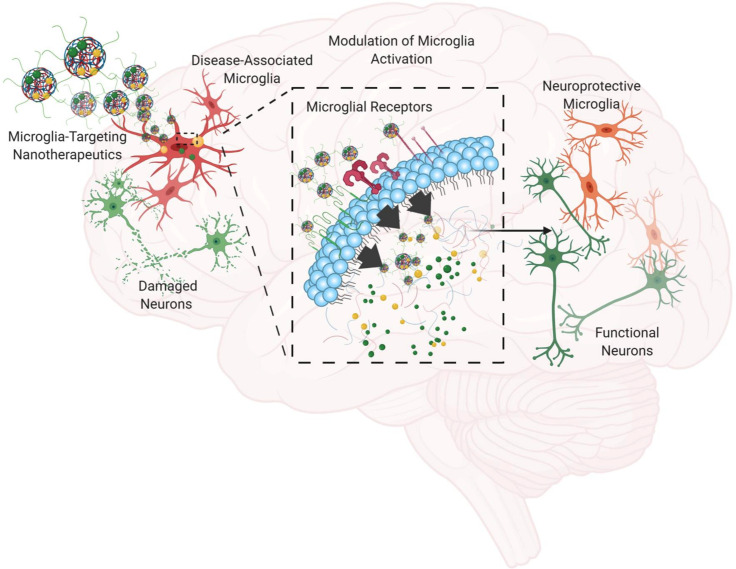
The advances in nanomaterial-based pharmacological platforms are particularly beneficial for developing efficacious therapeutic approaches for neurodegenerative diseases, with improved cellular targeting and controlled drug release properties. Current pharmacological therapies have primarily focused on neurons as the primary targets for neurodegeneration, since the loss and dysfunction of neurons, particularly DA neurons in PD and cholinergic neurons in AD, have been found to be the primary contributor to major motor and cognitive symptoms.66,67 Alternatively, microglia are a promising therapeutic target, as they are an essential cell type involved heavily in protein trafficking, aggregation, and clearance and are closely associated with the neuroinflammation process.6 As the resident immune cell in the CNS, microglia are the first responders to changes in the tissue environment and can generate adaptive or innate immune responses upon detection of invading pathogens.112 From the NP therapeutic perspective, microglia can be targeted conveniently with different types of NPs due to their intrinsic phagocytic nature as immune cells. However, microglial activation has been a concern regarding the use of NPs in the CNS.113,114 In order to utilize nanotherapeutics to target microglia with enhanced therapeutic efficacy, more intricate design of NPs is needed for enhanced cellular targeting to deliver active agents and modulate microglial activation as a result of NP administration while minimizing off-target toxicity (Fig. 3).
FIG. 3.
Utilizing microglia-targeting nanotherapeutics for modulation of neuroinflammation and neurodegeneration. Microglia will be chronically activated upon prolonged exposure to stimulants, and the subsequent release of neurotoxic factors from microglia will result in neuron damage. By introducing nanotherapeutics that are specifically designed to target microglia while delivering anti-inflammatory agents and protein aggregation inhibitors, microglia-associated neuroinflammation can be modulated and neurodegeneration can be slowed down.
Due to the involvement of microglia in neurodegeneration, potential avenues have been explored to modulate microglial pathology by either preventing early stage microglial activation or correcting pathological microglial function upon occurrence via cellular or system level intervention. Microglia participate in pathological protein processing and assist in maintaining the balance of various protein conformers and their oligomers in the intracellular and extracellular space within the CNS. To prevent toxic protein aggregate accumulation in the CNS, researchers have investigated the use of small molecules, peptides, and antibodies as inhibitors of protein aggregation, one group of which are antioxidant molecules.115–117 For example, epigallocatechin gallate (EGCG) belongs to a class of polyphenolics from plant extracts and has been shown to inhibit in vitro Aβ, tau, and ASYN aggregation.118–121 Its ability to prevent aggregation also translates to reducing toxic protein aggregate-induced cytotoxicity in neuroblastoma cells in vitro, by interfering with the membrane association of protein aggregates in order to maintain cell membrane integrity.122
In addition to protein aggregation, antioxidant molecules can also decrease cellular oxidative damage via active scavenging of free radicals and modulating the activity of enzymes involved in free radical production.123,124 Some examples are resveratrol, curcumin, and ferulic acid, all of which quench free radicals via electron transfer and protect cellular components such as DNA, RNA, and cell membranes from ROS/RNS-induced oxidative damage.123,125 In addition to chemical reactions, certain antioxidants such as resveratrol and EGCG have also demonstrated direct control in free radical-producing enzyme expression including NADPH oxidases, which has been shown to be responsible for microglial ROS-induced neuron death in PD.123,126 Besides antioxidants, non-steroidal anti-inflammatory drugs (NSAIDs), a group of compounds known for their inhibitory activity on inflammation-related enzymes including prostaglandin and cyclooxygenase, have been investigated for their ability to reduce microglial activation and protect neurodegeneration.127 While clinical investigation demonstrated that NSAIDs do not counteract AD, the early stage and chronic use of NSAIDs may inhibit build-up of Aβ and reduce the risk of developing PD.128–130 However, the use of NSAIDs after neurodegeneration takes place may accelerate disease progression as NSAIDs may interfere with microglial activity in toxic protein clearance.128
Specific microglial receptors have also been associated with the regulation of microglial neuroinflammation and neurodegeneration. For example, triggering receptor expressed on myeloid cells 2 (TREM2) has been shown to have increased expression in Aβ plaque-associated microglia, and the microglial response to aggregated protein can be remodeled via manipulation of TREM2 expression.131,132 A soluble form of TREM2 receptor protein (sTREM2) was found in the cerebrospinal fluid, and in vivo research in the AD mice model has shown that sTREM2 can modulate pathological microglial phenotypes.133,134 Modulation of microglial function by sTREM2 could be due to the competition of sTREM2 with its insoluble membrane-bound form to interfere with TREM2 involvement in protein aggregation clearance or the unknown biological function of sTREM2, which partially blocks the TREM2 signaling pathway.133 Another example is TLR4, whose involvement in the uptake and intracellular aggregation of Aβ and ASYN potentially initiates microglial activation during neurodegeneration, which has drawn great attention in the past decade.135 In vivo research in a mouse model of motor neuron degeneration has shown that chronic treatment with the TLR4 antagonist VB3323 can decrease microglial activation and improve motor function.136
Mechanisms of NP uptake by microglia
Understanding the mechanisms of NP uptake by microglia can aid in the design of appropriate nanomedicines for use within the CNS. NPs interact with the microglial cell membrane and are internalized mainly through endocytosis, which is an active transport mechanism that facilitates the uptake of extracellular materials via membrane invagination.137 Endocytosis can be broadly divided into phagocytosis, the process by which larger particles are internalized, and pinocytosis, which can be further subdivided into the categories of clathrin-mediated endocytosis, caveolin-mediated endocytosis, clathrin- and caveolin-independent endocytosis, and micropinocytosis.138 In addition to these active internalization processes, NPs can also enter cells through passive diffusion. Mechanisms of NP uptake may vary depending on the activation state of microglia. Lipopolysaccharide (LPS)-activated microglia show higher in vitro uptake of dendrimers approximately 4–10 nm in size compared to resting microglia, which can be attributed to increased endocytosis in activated microglia.139–141 The physicochemical properties of NPs such as the size, surface chemistry, surface charge, and shape are important design criteria that influence the binding to and uptake by microglia, which can affect intracellular delivery of therapeutic cargo and the subsequent biological response.142–145 After in vivo administration, serum proteins adsorb on the surface of NPs, forming a layer known as the protein corona.146 The surface characteristics of NPs affect the identity, thickness, and orientation of the protein corona, which, in turn, also significantly affects cell interactions and uptake.146–148 The morphology of NPs may also affect their uptake by microglia.142 Independent of surface coating, spiky “urchin-shaped” gold NPs (which have numerous “bumps” and “thorns” covering the surface) showed a significantly greater extent of microglial internalization compared to spherical or rod-shaped gold NPs.143 Further, rod and urchin NPs caused transient microglial activation, with studies indicating that the mechanism of activation was also shape-dependent.143
NP design criteria for microglial targeting
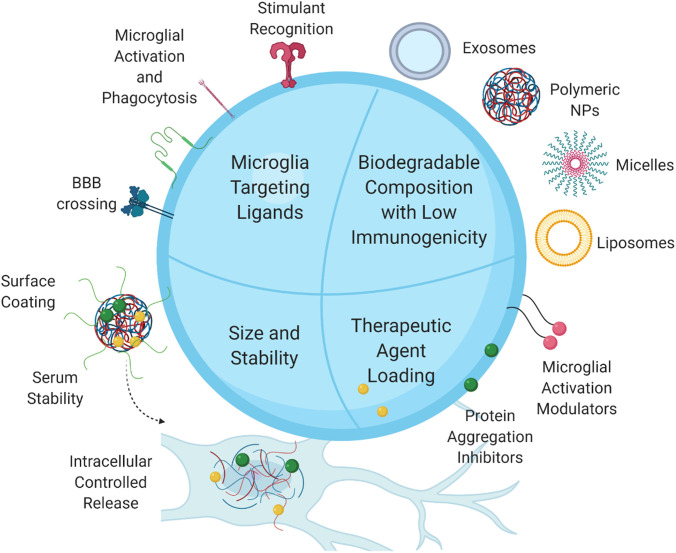
As described above, advances in nanotechnology have enabled the design of nanotherapeutic platforms with the ability to cross or bypass the BBB, and the tunability of NPs built on these platforms ensures the delivery of therapeutics via the BBB through a variety of mechanisms.149 Here, we will expand the following discussion on NP design criteria unique to microglial targeting. These criteria are meant to be taken into consideration along with BBB penetration benchmarks (Fig. 4).
FIG. 4.
Microglia-targeting nanoparticle design criteria. To achieve modulation of neurodegeneration associated with microglia-induced neuroinflammation, nanoparticles should be designed incorporating1 microglial receptor-targeting ligands taking advantage of microglia's phagocytic nature,2 therapeutic agents for inhibition of microglial activation and toxic protein aggregation, and3 biodegradable materials with low immunogenicity,4 without compromising the nanoparticle size and serum stability.
Microglia possess a wide range of receptors that sense exogenous or endogenous CNS disturbance and initiate a tailored immune response.150 By incorporating targeting ligands or peptides specific for microglial receptors involved in neurodegeneration onto or within the NP structure, the targeting capabilities of nanotherapeutics would be greatly improved and, thus, more efficiently deliver therapeutic agents with minimal side effects. PRRs expressed on microglia are a key group of receptors that not only facilitate the membrane-association and aggregation of different species of Aβ and ASYN proteins but also have elevated expression levels upon cellular disturbance.151,152 In addition to the TLR receptors mentioned previously, receptors for advanced glycation endproducts (RAGE) and scavenger receptors are also considered as PRRs.151 A previous study investigated the use of TLR4 receptor-targeting liposomes to improve the delivery of minocycline treatment in a mouse model of ALS with spinal neuron degeneration.153 The surface of the liposomes was modified with LPS, a TLR4 agonist, to facilitate delivery to microglia. Results showed that this targeting ligand significantly increased the uptake of encapsulated drug compared to non-targeted liposomes and that the microglia-targeted liposomes significantly delayed disease progression.153 Scavenger receptors, such as SR-A1 and CD36, are also PRRs that are involved in modulating aggregated protein interaction with microglia and subsequent activation. Previous research has demonstrated the uptake of different NPs through scavenger receptors, such as polystyrene-based NPs and silver NPs.154 NPs can also be designed as scavenger receptor ligand mimetics, such as lipoproteins and long chain fatty acids to achieve specific targeting.154,155 Other microglial receptors have also been investigated as potential avenues to deliver nanotherapeutics to microglia specifically. For example, Lee et al. studied the use of ceria-zirconia NPs (CZ NPs) for inhibiting microglial activation in a neuropathic pain mouse model.156 The NP surface was decorated with CD11b antibody via NHS-ester conjugation, and in vitro results showed that these NPs had much higher microglial internalization than NPs with isotype control antibody conjugates.156 Brain slides obtained after intrathecal administration of CZ NPs into mice showed that microglia had significantly higher uptake of NPs with antibody conjugates than any other CNS cell types.156
In addition to its first-responder role during the CNS inflammatory development, microglia are also highly functional as phagocytes.157 Previous research has shown that expression of phagocytic receptors on microglia is elevated under neuroinflammatory conditions and this microglial property is particularly beneficial for targeted delivery of nanotherapeutics to microglia.157,158 While the studies on NP interactions specifically with microglia are limited, a few studies have highlighted the selective uptake of NPs by microglia and macrophages considering their origin and functional similarities.159–161 Veglianese et al. demonstrated the use of poly-ε-caprolactone and PEG-based NPs (PCL-based NPs) for targeted delivery of minocycline to reduce microglia/macrophage activation.160 In vitro results showed that only activated microglia or microglia with an ameboid shape were able to internalize a significant amount of these PCL-based NPs, while resting microglia did not.160 Most importantly, local injections of NPs in mice with spinal cord injury (SCI) were able to shift the microglial population at the lesion site from phagocytic to a more arborized resting phenotype, suggesting a reduction in the overall level of microglial activation.160 Similarly, another study conducted in the following year explored the use of an alternative polymeric NP formulation composed of polymethylmethacrylate (PMMA) for the treatment of SCI and both studies took the microglia/macrophage activation and its heightened phagocytic ability involved in the disease development as an opportunity for targeted drug delivery.160,161
NPs can also be designed to incorporate inhibitor molecules for enzymes involved in microglial inflammatory pathways during the neuroinflammatory response in PD and AD.162 For example, DNL747, a small-molecular inhibitor of receptor-interacting serine/threonine-protein kinase 1 (RIPK1)—a kinase enzyme involved in the downstream signaling of the TNF-α receptor, is currently under phase I clinical trial investigation for its efficacy in modulating microglial activation and related neuroinflammation in patients with AD.163 Preliminary research has demonstrated that RIPK1 mediates the occurrence of disease-associated microglial phenotypes, reduction in microglial phagocytic activity, and the etiology of AD.164 Anti-aggregation small molecules could also be incorporated within the NP formulation to improve the bioavailability of hydrophobic molecules and further valorize the NP as a drug delivery vehicle. Zheng et al. have compared the Aβ aggregation and ROS-inhibiting properties of resveratrol and selenium NPs functionalized with resveratrol.165 In vitro evaluation showed that functionalization with resveratrol significantly strengthened the inhibition of copper ion-induced Aβ aggregation, ROS production, and toxic protein aggregates associated with neuron death.165 More recently, Ren et al. have demonstrated the use of a mitochondria-targeted polymeric material-coated quantum dot nanoparticle to scavenge free radicals released from microglia induced by Aβ aggregation, modulating microglial activation and ameliorating neuron death in vivo.166 The incorporation of lipophilic (3-carboxypropyl)triphenyl-phosphonium bromide (TPP) molecule within the polymer shell not only enabled the mitochondria targeting ability of NPs but also strengthened the free radical scavenging ability of these NPs.166
In addition, NP design should also ensure reasonable colloidal stability of the NP structure and encapsulated agents in serum, which will ensure their activity once it has reached the target site and can potentially reduce side effects compared to untargeted therapeutics. This is particularly challenging for NPs decorated with targeting ligands on the surface since non-specific interactions will likely occur between targeting ligands and other proteins or receptors in serum or other cells in the CNS and modify its structure before reaching the site of pathology. One of the modifications made to improve NP stability is with PEG, a long chain hydrophilic polyether compound, which improves the NP's structural and chemical stability and performance in physiological environments.167,168 The PEG shields the active agent within the NP core from enzymatic degradation and reduces nonspecific protein binding of NPs, and the hydrophilic nature of PEG increases the circulation time of the therapeutic.167,169 With these criteria taken into account, NPs could be designed to have high bioavailability to microglia and improved pharmacodynamic profiles when used via systemic administration.
Addressing NP-mediated toxicity
The unique physicochemical properties of NPs, which can be markedly different from bulk materials, can lead to unpredictable interactions with cells and tissues and subsequent neurotoxic effects.170,171 Neurotoxicity can be induced through direct effects on the morphology or function of cells in the CNS or by triggering glial activation and affecting the interactions between microglia and neurons, leading to neurological damage and cognitive or behavioral impairments.170,172 Most of these effects are due to oxidative stress, with other mechanisms of neurotoxicity including inflammation, apoptosis, alterations in gene expression and signaling pathways, and epigenetic modifications.173 Various physicochemical properties of NPs can contribute to toxic effects, including the size, shape, chemical composition, surface chemistry, and aggregation. Cationic NPs have been widely shown to have toxic properties, through binding to serum proteins and disrupting the structure and function of cell membranes, including forming holes and eroding/thinning the membrane.174 Many commonly used metallic NPs, such as gold, silver, silica, iron oxide, and titanium dioxide, have been reported to have neurotoxic effects.175 Some studies have found that pre-treatment or co-treatment with antioxidants can inhibit the inflammatory response to metallic NPs, reducing apoptosis and protecting against NP-induced neurotoxicity.175–177 Antioxidants can also be incorporated within NPs for controlled release. Polymeric NPs have several advantages including controlled release, biodegradability, specific cell targeting, and the ability to protect encapsulated drugs, which makes them attractive for use as drug delivery vehicles to the CNS.178,179 Their disadvantages include aggregation and potential neurotoxicity, which could be caused by the degradation process and residual byproducts.178
Strategies to attenuate neurotoxicity include controlling the NP size and shape, coating the NPs to modify the surface chemistry, and removing toxic components from the fabrication process.178 Surface coating with biocompatible polymers, such as PEG, can create a protective hydrophilic layer around the NPs, shielding positive surface charges, which will reduce toxicity, and also extending the circulation time of systemically administered NPs by delaying clearance via the reticuloendothelial system.180,181 Adding targeting ligands on the surface of NPs is another approach to reduce toxicity since targeted NPs have improved efficacy at much lower concentrations compared to untargeted NPs.180,182,183 Targeting ligands specific for microglia will also improve the binding and uptake into these cells, enhancing the desired biological response. Extensive characterization and in vitro evaluation can and should be conducted prior to selecting a particular NP formulation for use in vivo in order to minimize or avoid potential neurotoxicity.
There are various methods to assess nanotoxicity in vitro, including evaluation of the effects on cell viability and proliferation, apoptosis and necrosis, oxidative stress, and genotoxicity.184 NP toxicity can be assessed using various neural cell culture models in order to determine the potential adverse effects of NPs in the brain, including monolayer cell cultures, co-cultures, and tissue slices. Cultures including primary brain cells are more likely to accurately recapitulate the morphology and function of the respective cells in vivo compared to immortalized cell lines.185 NPs can also induce toxicity outside of the brain, particularly when they are administered systemically through intravenous injection. In vivo NP toxicity should be assessed through evaluation of organ distribution and clearance, NP degradation, immunotoxicity, histopathology, and single dose/repeated dose toxicity measurements.184
Using nanotherapeutics to modulate microglial activation
As the primary endogenous immune cell in the CNS, microglia constantly sense endogenous or exogenous stimuli and respond to cues by shifting its phenotype while clearing undesirable debris. Considering the sensitive nature of microglia, certain types of NPs may actually stimulate microglial activation rather than ameliorating it.113 Previous research has shown that silver NPs administered via the intranasal route can reach the CNS and cause subsequent NP size-dependent microglial activation in rats.186 From this perspective, biocompatible materials such as biodegradable polymeric NPs would be preferred since the structure is less likely to cause hyperactivity of the immune system, and its rapid degradation via physiological enzymes will reduce any toxicity cause by residual material accumulated in the CNS.187 More recently, researchers have also investigated the effect of human mesenchymal stem cell (MSC)-derived exosomes on microglia-mediated neuroinflammation. Not only did MSC-derived exosomes reduce pro-inflammatory cytokine production from activated microglia in vitro, but also intranasally administrated exosomes reached the CNS and reduced microglial activation in rats with perinatal brain injury.188
Considering the complex and multifaceted disease mechanisms of neurodegeneration, it would be beneficial to utilize combinatorial therapeutics targeting more than one aspect of pathology. One way to achieve this goal is to design nanotherapeutics that incorporate multiple therapeutic agents and targeting peptides or small molecule ligands to interfere with more than one inflammatory pathway in microglia. One example is a scavenger receptor-targeting amphiphilic macromolecule-based NP (AM NP) design, carrying ferulic acid-derived polymer as a therapeutic agent.189 These AM NPs were designed to have strong affinity to scavenger receptors, specifically CD36 and SR-A1 on microglia, and can weaken the receptor binding of ASYN, thus reducing ASYN aggregation-induced microglial activation. In vivo studies in a PD mouse model showed that the AM NPs can reduce ASYN deposition in the CNS as well as reduce microglial activation and recruitment, which were achieved by the combinatorial effect of the scavenger receptor-targeting AM shell and anti-inflammatory ferulic acid polymer components.189 This result highlights the use of NP formulations as a unique drug delivery platform, which not only supports but also strengthens the activity of the bioactive agent and enables targeted delivery.
An alternative approach to tackle the complex mechanism of neurodegeneration can be directed toward screening molecules that have multiple targeting enzymes in different pathways involved in microglial activation and also toward designing nanotherapeutics wisely to achieve simultaneous microglial targeting, toxic protein aggregate clearance, and neuroinflammation modulation. A bioinspired nanostructure, apolipoprotein E3-reconstituted high density lipoprotein (ApoE3-rHDL), is one example with a simple structural design, which not only binds to Aβ and facilitates protein clearance but also reduces microglial activation and neuron damage in an AD mouse model.190
While genetic manipulation of microglia in animal models of neurodegeneration has greatly improved our understanding of disease mechanisms, gene therapy approaches to modulate microglial activation for patients are still under investigation.191 For example, extracellular vesicles loaded with long intergenic non-coding RNA-cyclooxygenase-2 (LincRNA-Cox2) were shown to control LPS-induced microglial proliferation in mice, which is an early event in the development of neurodegeneration.192 However, similar genetic manipulations also need to take into consideration that activated microglia also contribute to clearing the CNS environment and protecting neuronal damage. In this case, while early stage microglial proliferation is controlled, in the long run, the silenced gene expression may interfere with microglia's normal function as immune cells and leave other CNS cells vulnerable to insults. Rather than silencing gene expression or deleting specific microglial population, nanotherapeutics should be designed to guide a shift in the spectrum of microglial phenotypes toward a more neuroprotective state via targeted delivery and controlled release of therapeutics to counteract microglial activation. With this taken under consideration, nanotherapeutics designed, either for early stages of neurodegeneration where microglial activity needs to be promoted or for late stages where microglial reactivity requires suppression, will not interfere with the microglial population's diversity and natural plasticity and have long-term effectiveness for patients.193
CONCLUSION
Microglia are an essential cell type involved in the neuroinflammation process. The challenge in designing microglia-targeting nanotherapeutics for neurodegeneration is to identify microglia-targeting peptides or small molecule ligands, utilize biocompatible and biodegradable nanomaterials with low immunoreactivity, and incorporate agents to modulate microglial activation while maintaining population diversity. The identification of unique microglial markers and NP interaction with microglia will greatly guide the design of successful nanomedicine platforms that enable the targeted delivery of agents while minimizing off-target effects and system level toxicity.
ACKNOWLEDGMENTS
This work was supported by Nos. NIH-NINDS NS095082, NSF 1803675, and NIH-NIA AG060024 and Rutgers Brain Health Institute. Figures 1–4 were created with Biorender.com.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M. F., Conway S. J., Ng L. G., and Stanley E. R., “ Fate mapping analysis reveals that adult microglia derive from primitive macrophages,” Science 330(6005), 841–845 (2010). 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Szepesi Z., Manouchehrian O., Bachiller S., and Deierborg T., “ Bidirectional microglia–neuron communication in health and disease,” Front. Cellular Neurosci. 12, 323 (2018). 10.3389/fncel.2018.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferreira S. A. and Romero-Ramos M., “ Microglia response during Parkinson's disease: Alpha-synuclein intervention,” Front. Cellular Neurosci. 12, 247 (2018). 10.3389/fncel.2018.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Subramaniam S. R. and Federoff H. J., “ Targeting microglial activation states as a therapeutic avenue in Parkinson's disease,” Front. Aging Neurosci. 9, 176 (2017). 10.3389/fnagi.2017.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angelova D. M. and Brown D. R., “ Microglia and the aging brain: Are senescent microglia the key to neurodegeneration?,” J. Neurochem. 151(6), 676–688 (2019). 10.1111/jnc.14860 [DOI] [PubMed] [Google Scholar]
- 6. Graeber M. B., Li W., and Rodriguez M. L., “ Role of microglia in CNS inflammation,” FEBS Lett. 585(23), 3798–3805 (2011). 10.1016/j.febslet.2011.08.033 [DOI] [PubMed] [Google Scholar]
- 7.“ Microglia and macrophages of the Central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration,” Seminars in Immunopathology, edited by Perry V. H. and Teeling J. ( Springer, 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soto M. S. and Sibson N. R., “ The multifarious roles of microglia in brain metastasis,” Front. Cellular Neurosci. 12, 414 (2018). 10.3389/fncel.2018.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T. K., David E., Baruch K., Lara-Astaiso D., and Toth B., “ A unique microglia type associated with restricting development of Alzheimer's disease,” Cell 169(7), 1276–1290 (2017). 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 10. Deczkowska A., Keren-Shaul H., Weiner A., Colonna M., Schwartz M., and Amit I., “ Disease-associated microglia: A universal immune sensor of neurodegeneration,” Cell 173(5), 1073–1081 (2018). 10.1016/j.cell.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 11. Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O'Loughlin E., Xu Y., and Fanek Z., “ The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases,” Immunity 47(3), 566–581 (2017). 10.1016/j.immuni.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathys H., Adaikkan C., Gao F., Young J. Z., Manet E., Hemberg M., De Jager P. L., Ransohoff R. M., Regev A., and Tsai L.-H., “ Temporal tracking of microglia activation in neurodegeneration at single-cell resolution,” Cell Rep. 21(2), 366–380 (2017). 10.1016/j.celrep.2017.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sevenich L., “ Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer,” Front. Immunol. 9, 697 (2018). 10.3389/fimmu.2018.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Block M. L. and Hong J.-S., “ Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism,” Prog. Neurobiol. 76(2), 77–98 (2005). 10.1016/j.pneurobio.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 15. Hoenen C., Gustin A., Birck C., Kirchmeyer M., Beaume N., Felten P., Grandbarbe L., Heuschling P., and Heurtaux T., “ Alpha-synuclein proteins promote pro-inflammatory cascades in microglia: Stronger effects of the A53T mutant,” PLoS One 11(9), e0162717 (2016). 10.1371/journal.pone.0162717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown G. C. and Vilalta A., “ How microglia kill neurons,” Brain Res. 1628, 288–297 (2015). 10.1016/j.brainres.2015.08.031 [DOI] [PubMed] [Google Scholar]
- 17. Bal-Price A. and Brown G. C., “ Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity,” J. Neurosci. 21(17), 6480–6491 (2001). 10.1523/JNEUROSCI.21-17-06480.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown G. C. and Neher J. J., “ Inflammatory neurodegeneration and mechanisms of microglial killing of neurons,” Mol. Neurobiol. 41(2–3), 242–247 (2010). 10.1007/s12035-010-8105-9 [DOI] [PubMed] [Google Scholar]
- 19. Spittau B., “ Aging microglia—phenotypes, functions and implications for age-related neurodegenerative diseases,” Front. Aging Neurosci. 9, 194 (2017). 10.3389/fnagi.2017.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mecca C., Giambanco I., Donato R., and Arcuri C., “ Microglia and aging: The role of the TREM2–DAP12 and CX3CL1-CX3CR1 axes,” Int. J. Mol. Sci. 19(1), 318 (2018). 10.3390/ijms19010318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang Y. and Le W., “ Differential roles of M1 and M2 microglia in neurodegenerative diseases,” Mol. Neurobiol. 53(2), 1181–1194 (2016). 10.1007/s12035-014-9070-5 [DOI] [PubMed] [Google Scholar]
- 22. Heneka M. T., Kummer M. P., and Latz E., “ Innate immune activation in neurodegenerative disease,” Nat. Rev. Immunol. 14(7), 463–477 (2014). 10.1038/nri3705 [DOI] [PubMed] [Google Scholar]
- 23. Poupot R., Bergozza D., and Fruchon S., “ Nanoparticle-based strategies to treat neuro-inflammation,” Mater. 11(2), 270 (2018). 10.3390/ma11020270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kigerl K. A., de Rivero Vaccari J. P., Dietrich W. D., Popovich P. G., and Keane R. W., “ Pattern recognition receptors and central nervous system repair,” Exp. Neurol. 258, 5–16 (2014). 10.1016/j.expneurol.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stuart L. M., Bell S. A., Stewart C. R., Silver J. M., Richard J., Goss J. L., Tseng A. A., Zhang A., El Khoury J. B., and Moore K. J., “ CD36 signals to the actin cytoskeleton and regulates microglial migration via a p130Cas complex,” J. Biol. Chem. 282, 27392 (2007). 10.1074/jbc.M702887200 [DOI] [PubMed] [Google Scholar]
- 26. El Khoury J. B., Moore K. J., Means T. K., Leung J., Terada K., Toft M., Freeman M. W., and Luster A. D., “ CD36 mediates the innate host response to β-amyloid,” J. Exp. Med. 197(12), 1657–1666 (2003). 10.1084/jem.20021546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coraci I. S., Husemann J., Berman J. W., Hulette C., Dufour J. H., Campanella G. K., Luster A. D., Silverstein S. C., and El Khoury J. B., “ CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to β-amyloid fibrils,” Am. J. Pathol. 160(1), 101–112 (2002). 10.1016/S0002-9440(10)64354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart C. R., Stuart L. M., Wilkinson K., Van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., and Frazier W. A., “ CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer,” Nat. Immunol. 11(2), 155 (2010). 10.1038/ni.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ransohoff R. M., “ How neuroinflammation contributes to neurodegeneration,” Science 353(6301), 777–783 (2016). 10.1126/science.aag2590 [DOI] [PubMed] [Google Scholar]
- 30.Alzheimer's Association, “ Alzheimer's disease facts and figures,” Alzheimer's Dementia 14(3), 367–429 (2018). 10.1016/j.jalz.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 31. Murphy M. P. and LeVine H. III “ Alzheimer's disease and the amyloid-β peptide,” J. Alzheimer's Dis. 19(1), 311–323 (2010). 10.3233/JAD-2010-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erdo F., Denes L., and de Lange E., “ Age-associated physiological and pathological changes at the blood-brain barrier: A review,” J. Cereb. Blood Flow Metab. 37(1), 4–24 (2017). 10.1177/0271678X16679420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bamberger M. E., Harris M. E., McDonald D. R., Husemann J., and Landreth G. E., “ A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation,” J. Neurosci. 23(7), 2665–2674 (2003). 10.1523/JNEUROSCI.23-07-02665.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., Jacobs A. H., Wyss-Coray T., Vitorica J., and Ransohoff R. M., “ Neuroinflammation in Alzheimer's disease,” Lancet Neurol. 14(4), 388–405 (2015). 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mawuenyega K. G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J. C., Yarasheski K. E., and Bateman R. J., “ Decreased clearance of CNS β-amyloid in Alzheimer's disease,” Science 330(6012), 1774 (2010). 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakanishi H. and Wu Z., “ Microglia-aging: Roles of microglial lysosome-and mitochondria-derived reactive oxygen species in brain aging,” Behav. Brain Res. 201(1), 1–7 (2009). 10.1016/j.bbr.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 37. Hickman S. E., Allison E. K., and El Khoury J., “ Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer's disease mice,” J. Neurosci. 28(33), 8354–8360 (2008). 10.1523/JNEUROSCI.0616-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hickman S., Izzy S., Sen P., Morsett L., and El Khoury J., “ Microglia in neurodegeneration,” Nat. Neurosci. 21(10), 1359–1369 (2018). 10.1038/s41593-018-0242-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T., Wolozin B., Butovsky O., Kügler S., and Ikezu T., “ Depletion of microglia and inhibition of exosome synthesis halt tau propagation,” Nat. Neurosci. 18(11), 1584 (2015). 10.1038/nn.4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dani M., Wood M., Mizoguchi R., Fan Z., Walker Z., Morgan R., Hinz R., Biju M., Kuruvilla T., and Brooks D. J., “ Microglial activation correlates in vivo with both tau and amyloid in Alzheimer's disease,” Brain 141(9), 2740–2754 (2018). 10.1093/brain/awy188 [DOI] [PubMed] [Google Scholar]
- 41. Lee H. J., Suk J. E., Bae E. J., and Lee S. J., “ Clearance and deposition of extracellular alpha-synuclein aggregates in microglia,” Biochem. Biophys. Res. Commun. 372(3), 423–428 (2008). 10.1016/j.bbrc.2008.05.045 [DOI] [PubMed] [Google Scholar]
- 42. Tansey M. G. and Goldberg M. S., “ Neuroinflammation in Parkinson's disease: Its role in neuronal death and implications for therapeutic intervention,” Neurobiol Dis 37(3), 510–518 (2010). 10.1016/j.nbd.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gerhard A., Pavese N., Hotton G., Turkheimer F., Es M., Hammers A., Eggert K., Oertel W., Banati R. B., and Brooks D. J., “ In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease,” Neurobiol. Dis. 21(2), 404–412 (2006). 10.1016/j.nbd.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 44. McGeer P. L., Itagaki S., Boyes B. E., and McGeer E. G., “ Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains,” Neurology 38(8), 1285–1291 (1988). 10.1212/WNL.38.8.1285 [DOI] [PubMed] [Google Scholar]
- 45. Brooks D. J., “ Imaging amyloid in Parkinson's disease dementia and dementia with Lewy bodies with positron emission tomography,” Mov. Disord. 24(S2), S742–S747 (2009). 10.1002/mds.22581 [DOI] [PubMed] [Google Scholar]
- 46. Sanchez-Guajardo V., Barnum C. J., Tansey M. G., and Romero-Ramos M., “ Neuroimmunological processes in Parkinson's disease and their relation to alpha-synuclein: Microglia as the referee between neuronal processes and peripheral immunity,” ASN Neuro 5(2), AN20120066 (2013). 10.1042/AN20120066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang W., Wang T., Pei Z., Miller D. S., Wu X., Block M. L., Wilson B., Zhou Y., Hong J. S., and Zhang J., “ Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson's disease,” FASEB J. 19(6), 533–542 (2005). 10.1096/fj.04-2751com [DOI] [PubMed] [Google Scholar]
- 48. Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., Bennett M. L., Münch A. E., Chung W.-S., and Peterson T. C., “ Neurotoxic reactive astrocytes are induced by activated microglia,” Nature 541(7638), 481 (2017). 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loeffler D. A., Demaggio A. J., Juneau P. L., Havaich M., and Lewitt P. A., “ Levodopa administration to normal rats: Influence on striatal oxidized glutathione concentration,” Ann. New York Acad. Sci. 738, 421–426 (2006). 10.1111/j.1749-6632.1994.tb21832.x [DOI] [PubMed] [Google Scholar]
- 50. Loeffler D. A., DeMaggio A. J., Juneau P. L., Havaich M. K., and LeWitt P. A., “ Effects of enhanced striatal dopamine turnover in vivo on glutathione oxidation,” Clin. Neuropharmacol. 17(4), 370–379 (1994). 10.1097/00002826-199408000-00009 [DOI] [PubMed] [Google Scholar]
- 51. George S. and Brundin P., “ Immunotherapy in Parkinson's Disease: Micromanaging alpha-synuclein aggregation,” J. Parkinson's Dis. 5(3), 413–424 (2015). 10.3233/JPD-150630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boillée S., Yamanaka K., Lobsiger C. S., Copeland N. G., Jenkins N. A., Kassiotis G., Kollias G., and Cleveland D. W., “ Onset and progression in inherited ALS determined by motor neurons and microglia,” Science 312(5778), 1389–1392 (2006). 10.1126/science.1123511 [DOI] [PubMed] [Google Scholar]
- 53. Henkel J. S., Engelhardt J. I., Siklós L., Simpson E. P., Kim S. H., Pan T., Goodman J. C., Siddique T., Beers D. R., and Appel S. H., “ Presence of dendritic cells, MCP‐1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue,” Ann. Neurol. 55(2), 221–235 (2004). 10.1002/ana.10805 [DOI] [PubMed] [Google Scholar]
- 54. Liao B., Zhao W., Beers D. R., Henkel J. S., and Appel S. H., “ Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS,” Exp. Neurol. 237(1), 147–152 (2012). 10.1016/j.expneurol.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haukedal H. and Freude K., “ Implications of microglia in amyotrophic lateral sclerosis and frontotemporal dementia,” J. Mol. Biol. 431, 1818 (2019). 10.1016/j.jmb.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 56. Daneman R. and Prat A., “ The blood–brain barrier,” Cold Spring Harbor Perspect. Biol. 7(1), a020412 (2015). 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bors L. A. and Erdo F., “ Overcoming the blood-brain barrier. Challenges and tricks for CNS drug delivery,” Sci Pharm 87(1), 6 (2019). 10.3390/scipharm87010006 [DOI] [Google Scholar]
- 58. Vlieghe P. and Khrestchatisky M., “ Peptide-based vectors for blood-brain barrier targeting and delivery of drugs to the central nervous system,” Ther. Delivery 1(4), 489–494 (2010). 10.4155/tde.10.44 [DOI] [PubMed] [Google Scholar]
- 59. Mikitsh J. L. and Chacko A. M., “ Pathways for small molecule delivery to the central nervous system across the blood-brain barrier,” Perspect Med. Chem. 6, 11–24 (2014). 10.4137/PMC.S13384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Banks W. A. and Greig N. H., “ Small molecules as central nervous system therapeutics: Old challenges, new directions, and a philosophic divide,” Future Med. Chem. 11(6), 489–493 (2019). 10.4155/fmc-2018-0436 [DOI] [PubMed] [Google Scholar]
- 61. Pardridge W. M., “ Blood-brain barrier and delivery of protein and gene therapeutics to brain,” Front. Aging Neurosci. 11, 373 (2020). 10.3389/fnagi.2019.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pardridge W. M., “ Delivery of biologics across the blood-brain barrier with molecular Trojan horse technology,” BioDrugs 31(6), 503–519 (2017). 10.1007/s40259-017-0248-z [DOI] [PubMed] [Google Scholar]
- 63. Pardridge W. M., “ CSF, blood-brain barrier, and brain drug delivery,” Expert Opin. Drug Delivery 13(7), 963–975 (2016). 10.1517/17425247.2016.1171315 [DOI] [PubMed] [Google Scholar]
- 64. Kalia L. V. and Lang A. E., “ Parkinson's disease,” Lancet 386(9996), 896–912 (2015). 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 65. Scheltens P., Blennow K., Breteler M. M. B., de Strooper B., Frisoni G. B., Salloway S., and Van der Flier W. M., “ Alzheimer's disease,” Lancet 388(10043), 505–517 (2016). 10.1016/S0140-6736(15)01124-1 [DOI] [PubMed] [Google Scholar]
- 66. Charvin D., Medori R., Hauser R. A., and Rascol O., “ Therapeutic strategies for Parkinson disease: Beyond dopaminergic drugs,” Nat. Rev. Drug Discovery 17(11), 844 (2018). 10.1038/nrd.2018.184 [DOI] [PubMed] [Google Scholar]
- 67. Aldape K., Brindle K. M., Chesler L., Chopra R., Gajjar A., Gilbert M. R., Gottardo N., Gutmann D. H., Hargrave D., Holland E. C., Jones D. T. W., Joyce J. A., Kearns P., Kieran M. W., Mellinghoff I. K., Merchant M., Pfister S. M., Pollard S. M., Ramaswamy V., Rich J. N., Robinson G. W., Rowitch D. H., Sampson J. H., Taylor M. D., Workman P., and Gilbertson R. J., “ Challenges to curing primary brain tumours,” Nat. Rev. Clin. Oncol. 16(8), 509–520 (2019). 10.1038/s41571-019-0177-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Siegal T., “ Which drug or drug delivery system can change clinical practice for brain tumor therapy?,” Neuro-Oncol. 15(6), 656–669 (2013). 10.1093/neuonc/not016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rankovic Z., “ CNS drug design: Balancing physicochemical properties for optimal brain exposure,” J. Med. Chem. 58(6), 2584–2608 (2015). 10.1021/jm501535r [DOI] [PubMed] [Google Scholar]
- 70. Penalver P., Belmonte-Reche E., Adan N., Caro M., Mateos-Martin M. L., Delgado M., Gonzalez-Rey E., and Morales J. C., “ Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases,” Eur. J. Med. Chem. 146, 123–138 (2018). 10.1016/j.ejmech.2018.01.037 [DOI] [PubMed] [Google Scholar]
- 71. Peauger L., Azzouz R., Gembus V., Tintas M. L., Sopkova-de Oliveira Santos J., Bohn P., Papamicael C., and Levacher V., “ Donepezil-based central acetylcholinesterase inhibitors by means of a “bio-oxidizable” prodrug strategy: Design, synthesis, and in vitro biological evaluation,” J. Med. Chem. 60(13), 5909–5926 (2017). 10.1021/acs.jmedchem.7b00702 [DOI] [PubMed] [Google Scholar]
- 72. Petros R. A. and DeSimone J. M., “ Strategies in the design of nanoparticles for therapeutic applications,” Nat. Rev. Drug Discovery 9(8), 615–627 (2010). 10.1038/nrd2591 [DOI] [PubMed] [Google Scholar]
- 73. Gao H., “ Progress and perspectives on targeting nanoparticles for brain drug delivery,” Acta Pharm. Sin. B 6(4), 268–286 (2016). 10.1016/j.apsb.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gonda A., Zhao N., Shah J. V., Calvelli H. R., Kantamneni H., Francis N. L., and Ganapathy V., “ Engineering tumor-targeting nanoparticles as vehicles for precision nanomedicine,” Med One 4, e190021 (2019). 10.20900/mo.20190021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Soares S., Sousa J., Pais A., and Vitorino C., “ Nanomedicine: Principles, properties, and regulatory issues,” Front. Chem. 6, 360 (2018). 10.3389/fchem.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rafiyath S. M., Lamba G., Singh P., Rasul M., and Liu D., “ Comparison of liposomal doxorubicin versus conventional anthracyclines: A meta-analysis,” J. Clin. Oncol. 28(15), e13128 (2010). 10.1200/jco.2010.28.15_suppl.e13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang M., Gu X., and Gao X., Nanotherapeutic Strategies for the Treatment of Neurodegenerative Diseases. Brain Targeted Drug Delivery System ( Academic Press, 2019), p. 321–356. [Google Scholar]
- 78. Chang D., Lim M., Goos J., Qiao R., Ng Y. Y., Mansfeld F. M., Jackson M., Davis T. P., and Kavallaris M., “ Biologically targeted magnetic hyperthermia: Potential and limitations,” Front. Pharmacol. 9, 831 (2018). 10.3389/fphar.2018.00831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Luan X., Sansanaphongpricha K., Myers I., Chen H. W., Yuan H. B., and Sun D. X., “ Engineering exosomes as refined biological nanoplatforms for drug delivery,” Acta Pharmacol. Sin. 38(6), 754–763 (2017). 10.1038/aps.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang M. J., Zang X. L., Wang M. Y., Li Z., Qiao M. X., Hu H. Y., and Chen D. W., “ Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: Recent advances and challenges,” J. Mater. Chem. B 7(15), 2421–2433 (2019). 10.1039/C9TB00170K [DOI] [PubMed] [Google Scholar]
- 81. Chen I.-C., Hsiao I.-L., Lin H.-C., Wu C.-H., Chuang C.-Y., and Huang Y.-J., “ Influence of silver and titanium dioxide nanoparticles on in vitro blood-brain barrier permeability,” Environ. Toxicol. Pharmacol. 47, 108–118 (2016). 10.1016/j.etap.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 82. Sela H., Cohen H., Elia P., Zach R., Karpas Z., and Zeiri Y., “ Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB),” J. Nanobiotechnol. 13(1), 71 (2015). 10.1186/s12951-015-0133-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baghirov H., Snipstad S., Sulheim E., Berg S., Hansen R., Thorsen F., Mørch Y., de Lange Davies C., and Åslund A. K., “ Ultrasound-mediated delivery and distribution of polymeric nanoparticles in the normal brain parenchyma of a metastatic brain tumour model,” PLoS One 13(1), e0191102 (2018). 10.1371/journal.pone.0191102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Peviani M., Palmiero U. C., Cecere F., Milazzo R., Moscatelli D., and Biffi A., “ Biodegradable polymeric nanoparticles administered in the cerebrospinal fluid: Brain biodistribution, preferential internalization in microglia and implications for cell-selective drug release,” Biomaterials 209, 25–40 (2019). 10.1016/j.biomaterials.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 85. Wang X., Chi N., and Tang X., “ Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting,” Eur. J. Pharm. Biopharm. 70(3), 735–740 (2008). 10.1016/j.ejpb.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 86. Helmschrodt C., Höbel S., Schöniger S., Bauer A., Bonicelli J., Gringmuth M., Fietz S. A., Aigner A., Richter A., and Richter F., “ Polyethylenimine nanoparticle-mediated siRNA delivery to reduce α-synuclein expression in a model of Parkinson's disease,” Mol. Ther.--Nucleic Acids 9, 57–68 (2017). 10.1016/j.omtn.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rodriguez M., Lapierre J., Ojha C. R., Kaushik A., Batrakova E., Kashanchi F., Dever S. M., Nair M., and El-Hage N., “ Intranasal drug delivery of small interfering RNA targeting Beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation,” Sci. Rep. 7(1), 1862 (2017). 10.1038/s41598-017-01819-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nagpal K., Singh S. K., and Mishra D. N., “ Chitosan nanoparticles: A promising system in novel drug delivery,” Chem. Pharm. Bull. 58(11), 1423–1430 (2010). 10.1248/cpb.58.1423 [DOI] [PubMed] [Google Scholar]
- 89. Rao K. S., Reddy M. K., Horning J. L., and Labhasetwar V., “ TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs,” Biomaterials 29(33), 4429–4438 (2008). 10.1016/j.biomaterials.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tong W. Y., Alnakhli M., Bhardwaj R., Apostolou S., Sinha S., Fraser C., Kuchel T., Kuss B., and Voelcker N. H., “ Delivery of siRNA in vitro and in vivo using PEI-capped porous silicon nanoparticles to silence MRP1 and inhibit proliferation in glioblastoma,” J. Nanobiotechnol. 16(1), 38 (2018). 10.1186/s12951-018-0365-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pardridge W. M., “ Molecular Trojan horses for blood-brain barrier drug delivery,” Curr. Opin. Pharmacol. 6(5), 494–500 (2006). 10.1016/j.coph.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 92. Gabathuler R., “ Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases,” Neurobiol Dis. 37(1), 48–57 (2010). 10.1016/j.nbd.2009.07.028 [DOI] [PubMed] [Google Scholar]
- 93. Kreuter J., “ Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain,” J. Nanosci. Nanotechnol. 4(5), 484–488 (2004). 10.1166/jnn.2003.077 [DOI] [PubMed] [Google Scholar]
- 94. Kreuter J., Shamenkov D., Petrov V., Ramge P., Cychutek K., Koch-Brandt C., and Alyautdin R., “ Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier,” J. Drug Targeting 10(4), 317–325 (2002). 10.1080/10611860290031877 [DOI] [PubMed] [Google Scholar]
- 95. Zensi A., Begley D., Pontikis C., Legros C., Mihoreanu L., Wagner S., Büchel C., and Kreuter J., “ Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones,” J. Controlled Release 137(1), 78–86 (2009). 10.1016/j.jconrel.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 96. Ulbrich K., Knobloch T., and Kreuter J., “ Targeting the insulin receptor: Nanoparticles for drug delivery across the blood–brain barrier (BBB),” J. Drug Targeting 19(2), 125–132 (2011). 10.3109/10611861003734001 [DOI] [PubMed] [Google Scholar]
- 97. Lalani J., Raichandani Y., Mathur R., Lalan M., Chutani K., Mishra A. K., and Misra A., “ Comparative receptor based brain delivery of tramadol-loaded poly (lactic-co-glycolic acid) nanoparticles,” J. Biomed. Nanotechnol. 8(6), 918–927 (2012). 10.1166/jbn.2012.1462 [DOI] [PubMed] [Google Scholar]
- 98. Clark A. J. and Davis M. E., “ Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core,” Proc. Nat. Acad. Sci. U.S.A 112(40), 12486–12491 (2015). 10.1073/pnas.1517048112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fornaguera C., Dols-Perez A., Caldero G., Garcia-Celma M., Camarasa J., and Solans C., “ PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier,” J. Controlled Release 211, 134–143 (2015). 10.1016/j.jconrel.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 100. Ulbrich K., Hekmatara T., Herbert E., and Kreuter J., “ Transferrin-and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB),” Eur. J. Pharm. Biopharm. 71(2), 251–256 (2009). 10.1016/j.ejpb.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 101. Ding H., Sagar V., Agudelo M., Pilakka-Kanthikeel S., Atluri V. S. R., Raymond A., Samikkannu T., and Nair M. P., “ Enhanced blood–brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation,” Nanotechnology 25(5), 055101 (2014). 10.1088/0957-4484/25/5/055101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gu J., Al-Bayati K., and Ho E. A., “ Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes,” Drug Delivery Trans. Res. 7(4), 497–506 (2017). 10.1007/s13346-017-0368-5 [DOI] [PubMed] [Google Scholar]
- 103. Mcgowan J., Shao Q., Vig P., and Bidwell G. III, “ Intranasal administration of elastin-like polypeptide for therapeutic delivery to the central nervous system,” Drug Des., Dev. Ther. 10, 2803 (2016). 10.2147/DDDT.S106216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Elnaggar Y. S., Etman S. M., Abdelmonsif D. A., and Abdallah O. Y., “ Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer's disease: Optimization, biological efficacy, and potential toxicity,” J. Pharm. Sci. 104(10), 3544–3556 (2015). 10.1002/jps.24557 [DOI] [PubMed] [Google Scholar]
- 105. Battaglia L., Panciani P. P., Muntoni E., Capucchio M. T., Biasibetti E., De Bonis P., Mioletti S., Fontanella M., and Swaminathan S., “ Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery,” Expert Opin. Drug Delivery 15(4), 369–378 (2018). 10.1080/17425247.2018.1429401 [DOI] [PubMed] [Google Scholar]
- 106. Erdo F., Bors L. A., Farkas D., Bajza A., and Gizurarson S., “ Evaluation of intranasal delivery route of drug administration for brain targeting,” Brain Res. Bull. 143, 155–170 (2018). 10.1016/j.brainresbull.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 107. Sukumar U. K., Bose R. J., Malhotra M., Babikir H. A., Afjei R., Robinson E., Zeng Y., Chang E., Habte F., and Sinclair R., “ Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide,” Biomaterials 218, 119342 (2019). 10.1016/j.biomaterials.2019.119342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Illum L., “ Nasal drug delivery—possibilities, problems and solutions,” J. Controlled Release 87(1–3), 187–198 (2003). 10.1016/S0168-3659(02)00363-2 [DOI] [PubMed] [Google Scholar]
- 109. Xu W., Ling P., and Zhang T., “ Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs,” J. Drug Delivery 2013, 1. 10.1155/2013/340315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chen T., Liu W., Xiong S., Li D., Fang S., Wu Z., Wang Q., and Chen X., “ Nanoparticles mediating the sustained puerarin release facilitate improved brain delivery to treat Parkinson's disease,” ACS Appl. Mater. Interfaces 11(48), 45276–45289 (2019). 10.1021/acsami.9b16047 [DOI] [PubMed] [Google Scholar]
- 111. Augustine R., Kalva N., Kim H. A., Zhang Y., and Kim I., “ pH-responsive polypeptide-based smart nano-carriers for theranostic applications,” Molecules 24(16), 2961 (2019). 10.3390/molecules24162961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Olson J. K. and Miller S. D., “ Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs,” J. Immunol. 173(6), 3916–3924 (2004). 10.4049/jimmunol.173.6.3916 [DOI] [PubMed] [Google Scholar]
- 113. Duffy C. M., Ahmed S., Yuan C., Mavanji V., Nixon J. P., and Butterick T., “ Microglia as a surrogate biosensor to determine nanoparticle neurotoxicity,” J. Visualized Exp. 116, e54662 (2016). 10.3791/54662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang Z., Liu Z. W., Allaker R. P., Reip P., Oxford J., Ahmad Z., and Ren G., “ A review of nanoparticle functionality and toxicity on the central nervous system,” J. R. Soc., Interface 7, S411–S422 (2010). 10.1098/rsif.2010.0158.focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Arkin M. R. and Wells J. A., “ Small-molecule inhibitors of protein-protein interactions: Progressing towards the dream,” Nat. Rev. Drug Discovery 3(4), 301–317 (2004). 10.1038/nrd1343 [DOI] [PubMed] [Google Scholar]
- 116. Young L. M., Ashcroft A. E., and Radford S. E., “ Small molecule probes of protein aggregation,” Curr. Opin. Chem. Biol. 39, 90–99 (2017). 10.1016/j.cbpa.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 117. Giorgetti S., Greco C., Tortora P., and Aprile F. A., “ Targeting amyloid aggregation: An overview of strategies and mechanisms,” Int. J. Mol. Sci. 19(9), 2677 (2018). 10.3390/ijms19092677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Freyssin A., Page G., Fauconneau B., and Bilan A. R., “ Natural polyphenols effects on protein aggregates in Alzheimer's and Parkinson's prion-like diseases,” Neural Regener. Res. 13(6), 955–961 (2018). 10.4103/1673-5374.233432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Caruana M., Hogen T., Levin J., Hillmer A., Giese A., and Vassallo N., “ Inhibition and disaggregation of alpha-synuclein oligomers by natural polyphenolic compounds,” FEBS Lett. 585(8), 1113–1120 (2011). 10.1016/j.febslet.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 120. Renaud J. and Martinoli M. G., “ Considerations for the use of polyphenols as therapies in neurodegenerative diseases,” Int. J. Mol. Sci. 20(8), 1883 (2019). 10.3390/ijms20081883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Javed H., Nagoor Meeran M. F., Azimullah S., Adem A., Sadek B., and Ojha S. K., “ Plant extracts and phytochemicals targeting alpha-synuclein aggregation in Parkinson's disease models,” Front. Pharmacol. 9, 1555 (2018). 10.3389/fphar.2018.01555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yang J. E., Rhoo K. Y., Lee S., Lee J. T., Park J. H., Bhak G., and Paik S. R., “ EGCG-mediated protection of the membrane disruption and cytotoxicity caused by the 'active oligomer' of alpha-synuclein,” Sci. Rep. 7, 17945 (2017). 10.1038/s41598-017-18349-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lu J. M., Lin P. H., Yao Q., and Chen C., “ Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems,” J. Cell Mol. Med. 14(4), 840–860 (2010). 10.1111/j.1582-4934.2009.00897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhao N., Yang X., Calvelli H. R., Cao Y., Francis N. L., Chmielowski R. A., Joseph L. B., Pang Z. P., Uhrich K. E., Baum J., and Moghe P. V., “ Antioxidant-nanoparticles for concerted inhibition of alpha-synuclein fibrillization, and attenuation of microglial intracellular aggregation and activation,” Front. Bioeng. Biotechnol. 8, 112 (2020). 10.3389/fbioe.2020.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fu Y., Yang J. M., Wang X. Y., Yang P., Zhao Y., Li K., Chen Y. J., and Xu Y., “ Herbal compounds play a role in neuroprotection through the inhibition of microglial activation,” J. Immunol. Res. 2018, 1. 10.1155/2018/9348046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Maraldi T., “ Natural compounds as modulators of NADPH oxidases,” Oxid. Med. Cell Longevity 2013, 1. 10.1155/2013/271602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ajmone-Cat M. A., Bernardo A., Greco A., and Minghetti L., “ Non-steroidal anti-inflammatory drugs and brain inflammation: Effects on microglial functions,” Pharmaceuticals 3(6), 1949–1965 (2010). 10.3390/ph3061949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Imbimbo B. P., “ An update on the efficacy of non-steroidal anti-inflammatory drugs in Alzheimer's disease,” Expert Opin. Invest. Drugs 18(8), 1147–1168 (2009). 10.1517/13543780903066780 [DOI] [PubMed] [Google Scholar]
- 129. Masters C., Wyss-Coray T., and Pasinetti G. M., “ Anti-inflammatory drugs fall short in Alzheimer's disease,” Nat. Med. 14(9), 916 (2008). 10.1038/nm0908-916 [DOI] [PubMed] [Google Scholar]
- 130. Rees K., Stowe R., Patel S., Ives N., Breen K., Clarke C. E., and Ben-Shlomo Y., “ Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson's disease: Evidence from observational studies,” Cochrane Database Syst. Rev. 11, CD008454 (2011). 10.1002/14651858.CD008454.pub2 [DOI] [PubMed] [Google Scholar]
- 131. Hemonnot A. L., Hua J., Ulmann L., and Hirbec H., “ Microglia in Alzheimer disease: Well-known targets and new opportunities,” Front. Aging Neurosci. 11, 233 (2019). 10.3389/fnagi.2019.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Janda E., Boi L., and Carta A. R., “ Microglial phagocytosis and its regulation: A therapeutic target in Parkinson's disease?,” Front. Mol. Neurosci. 11, 144 (2018). 10.3389/fnmol.2018.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhong L., Xu Y., Zhuo R. G., Wang T. T., Wang K., Huang R. Z., Wang D. X., Gao Y., Zhu Y. F., Sheng X., Chen K., Wang N., Zhu L., Can D., Marten Y., Shinohara M., Liu C. C., Du D., Sun H., Wen L., Xu H. X., Bu G. J., and Chen X. F., “ Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer's disease model,” Nat. Commun. 10, 1365 (2019). 10.1038/s41467-019-09118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhong L. and Chen X. F., “ The emerging roles and therapeutic potential of soluble TREM2 in Alzheimer's disease,” Front. Aging Neurosci. 11, 328 (2019). 10.3389/fnagi.2019.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fiebich B. L., Batista C. R. A., Saliba S. W., Yousif N. M., and de Oliveira A. C. P., “ Role of microglia TLRs in neurodegeneration,” Front. Cell Neurosci. 12, 329 (2018). 10.3389/fncel.2018.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. De Paola M., Mariani A., Bigini P., Peviani M., Ferrara G., Molteni M., Gemma S., Veglianese P., Castellaneta V., Boldrin V., Rossetti C., Chiabrando C., Forloni G., Mennini T., and Fanelli R., “ Neuroprotective effects of toll-like receptor 4 antagonism in spinal cord cultures and in a mouse model of motor neuron degeneration,” Mol. Med. 18, 971–981 (2012). 10.2119/molmed.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Behzadi S., Serpooshan V., Tao W., Hamaly M. A., Alkawareek M. Y., Dreaden E. C., Brown D., Alkilany A. M., Farokhzad O. C., and Mahmoudi M., “ Cellular uptake of nanoparticles: Journey inside the cell,” Chem. Soc. Rev. 46(14), 4218–4244 (2017). 10.1039/C6CS00636A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Foroozandeh P. and Aziz A. A., “ Insight into cellular uptake and intracellular trafficking of nanoparticles,” Nanoscale Res. Lett. 13(1), 339 (2018). 10.1186/s11671-018-2728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Alnasser Y., Kambhampati S. P., Nance E., Rajbhandari L., Shrestha S., Venkatesan A., Kannan R. M., and Kannan S., “ Preferential and increased uptake of hydroxyl-terminated PAMAM dendrimers by activated microglia in rabbit brain mixed glial culture,” Molecules 23(5), 1025 (2018). 10.3390/molecules23051025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pickford F., Marcus J., Camargo L. M., Xiao Q., Graham D., Mo J.-R., Burkhardt M., Kulkarni V., Crispino J., and Hering H., “ Progranulin is a chemoattractant for microglia and stimulates their endocytic activity,” Am. J. Pathol. 178(1), 284–295 (2011). 10.1016/j.ajpath.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zabala A., Vazquez‐Villoldo N., Rissiek B., Gejo J., Martin A., Palomino A., Perez‐Samartín A., Pulagam K. R., Lukowiak M., and Capetillo‐Zarate E., “ P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis,” EMBO Mol. Med. 10(8), e8743 (2018). 10.15252/emmm.201708743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Champion J. A. and Mitragotri S., “ Role of target geometry in phagocytosis,” Proc. Nat. Acad. Sci. U. S. A. 103(13), 4930–4934 (2006). 10.1073/pnas.0600997103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hutter E., Boridy S., Labrecque S., Lalancette-Hébert M., Kriz J., Winnik F. M., and Maysinger D., “ Microglial response to gold nanoparticles,” ACS Nano 4(5), 2595–2606 (2010). 10.1021/nn901869f [DOI] [PubMed] [Google Scholar]
- 144. Jo D. H., Kim J. H., Lee T. G., and Kim J. H., “ Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases,” Nanomedicine 11(7), 1603–1611 (2015). 10.1016/j.nano.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 145. Zhang F., Lin Y. A., Kannan S., and Kannan R. M., “ Targeting specific cells in the brain with nanomedicines for CNS therapies,” J. Control Release 240, 212–226 (2016). 10.1016/j.jconrel.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lu X., Xu P., Ding H. M., Yu Y. S., Huo D., and Ma Y. Q., “ Tailoring the component of protein corona via simple chemistry,” Nat. Commun. 10(1), 4520 (2019). 10.1038/s41467-019-12470-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Saha K., Rahimi M., Yazdani M., Kim S. T., Moyano D. F., Hou S., Das R., Mout R., Rezaee F., and Mahmoudi M., “ Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona,” ACS Nano 10(4), 4421–4430 (2016). 10.1021/acsnano.6b00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang Z., Guan J., Jiang Z., Yang Y., Liu J., Hua W., Mao Y., Li C., Lu W., Qian J., and Zhan C., “ Brain-targeted drug delivery by manipulating protein corona functions,” Nat. Commun. 10(1), 3561 (2019). 10.1038/s41467-019-11593-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hersh D. S., Wadajkar A. S., Roberts N., Perez J. G., Connolly N. P., Frenkel V., Winkles J. A., Woodworth G. F., and Kim A. J., “ Evolving drug delivery strategies to overcome the blood brain barrier,” Curr. Pharm. Des. 22(9), 1177–1193 (2016). 10.2174/1381612822666151221150733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kofler J. and Wiley C. A., “ Microglia: Key innate immune cells of the brain,” Toxicol. Pathol. 39(1), 103–114 (2011). 10.1177/0192623310387619 [DOI] [PubMed] [Google Scholar]
- 151. Sarlus H. and Heneka M. T., “ Microglia in Alzheimer's disease,” J. Clin. Invest. 127(9), 3240–3249 (2017). 10.1172/JCI90606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wiley N. J., Madhankumar A. B., Mitchell R. M., Neely E. B., Rizk E., Douds G. L., Simmons Z., and Connor J. R., “ Lipopolysaccharide modified liposomes for amyotropic lateral sclerosis therapy: Efficacy in SOD1 mouse model,” Adv. Nanopart. 1(3), 44 (2012). 10.4236/anp.2012.13007 [DOI] [Google Scholar]
- 153. Doens D. and Fernandez P. L., “ Microglia receptors and their implications in the response to amyloid beta for Alzheimer's disease pathogenesis,” J. Neuroinflammation 11, 48 (2014). 10.1186/1742-2094-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shannahan J. H., Bai W., and Brown J. M., “ Implications of scavenger receptors in the safe development of nanotherapeutics,” Recept. Clin. Invest. 2(3), e811 (2015). 10.14800/rci.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Silverstein R. L. and Febbraio M., “ CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior,” Sci. Signal 2(72), re3 (2009). 10.1126/scisignal.272re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Choi B., Soh M., Manandhar Y., Kim D., Han S. I., Baik S., Shin K., Koo S., Kwon H. J., Ko G., Oh J., Hwang H., Hyeon T., and Lee S. J., “ Highly selective microglial uptake of ceria-zirconia nanoparticles for enhanced analgesic treatment of neuropathic pain,” Nanoscale 11(41), 19437–19447 (2019). 10.1039/C9NR02648G [DOI] [PubMed] [Google Scholar]
- 157. Sierra A., Abiega O., Shahraz A., and Neumann H., “ Janus-faced microglia: Beneficial and detrimental consequences of microglial phagocytosis,” Front. Cell Neurosci. 7, 6 (2013). 10.3389/fncel.2013.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. David S. and Kroner A., “ Repertoire of microglial and macrophage responses after spinal cord injury,” Nat. Rev. Neurosci. 12(7), 388–399 (2011). 10.1038/nrn3053 [DOI] [PubMed] [Google Scholar]
- 159. Wang J., Wang J., Wang J., Yang B., Weng Q., and He Q., “ Targeting microglia and macrophages: A potential treatment strategy for multiple sclerosis,” Front. Pharmacol. 10, 286 (2019). 10.3389/fphar.2019.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Papa S., Rossi F., Ferrari R., Mariani A., De Paola M., Caron I., Fiordaliso F., Bisighini C., Sammali E., Colombo C., Gobbi M., Canovi M., Lucchetti J., Peviani M., Morbidelli M., Forloni G., Perale G., Moscatelli D., and Veglianese P., “ Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury,” ACS Nano 7(11), 9881–9895 (2013). 10.1021/nn4036014 [DOI] [PubMed] [Google Scholar]
- 161. Papa S., Ferrari R., De Paola M., Rossi F., Mariani A., Caron I., Sammali E., Peviani M., Dell'Oro V., Colombo C., Morbidelli M., Forloni G., Perale G., Moscatelli D., and Veglianese P., “ Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury,” J. Control Release 174, 15–26 (2014). 10.1016/j.jconrel.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 162. Bachiller S., Jimenez-Ferrer I., Paulus A., Yang Y., Swanberg M., Deierborg T., and Boza-Serrano A., “ Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response,” Front. Cell Neurosci. 12, 488 (2018). 10.3389/fncel.2018.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mullard A., “ Microglia-targeted candidates push the Alzheimer drug envelope,” Nat. Rev. Drug Discovery 17(5), 303–305 (2018). 10.1038/nrd.2018.65 [DOI] [PubMed] [Google Scholar]
- 164. Ofengeim D., Mazzitelli S., Ito Y., DeWitt J. P., Mifflin L., Zou C. Y., Das S., Adiconis X., Chen H. B., Zhu H., Kelliher M. A., Levin J. Z., and Yuan J. Y., “ RIPK1 mediates a disease-associated microglial response in Alzheimer's disease,” Proc. Natl. Acad. Sci. U. S. A. 114(41), E8788–E97 (2017). 10.1073/pnas.1714175114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yang L., Wang W., Chen J., Wang N., and Zheng G., “ A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid beta aggregation and reactive oxygen species formation properties,” J. Biomed. Mater. Res. A 106(12), 3034–3041 (2018). 10.1002/jbm.a.36493 [DOI] [PubMed] [Google Scholar]
- 166. Ren C., Li D., Zhou Q., and Hu X., “ Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer's disease model,” Biomaterials 232, 119752 (2020). 10.1016/j.biomaterials.2019.119752 [DOI] [PubMed] [Google Scholar]
- 167. Vissers C., Ming G. L., and Song H., “ Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders,” Adv. Drug Delivery Rev. 148, 239–251 (2019). 10.1016/j.addr.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jokerst J. V., Lobovkina T., Zare R. N., and Gambhir S. S., “ Nanoparticle PEGylation for imaging and therapy,” Nanomedicine 6(4), 715–728 (2011). 10.2217/nnm.11.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Gref R., Luck M., Quellec P., Marchand M., Dellacherie E., Harnisch S., Blunk T., and Muller R. H., “ Stealth corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption,” Colloids Surf., B 18(3–4), 301–313 (2000). 10.1016/S0927-7765(99)00156-3 [DOI] [PubMed] [Google Scholar]
- 170. Teleanu D. M., Chircov C., Grumezescu A. M., Volceanov A., and Teleanu R. I., “ Impact of nanoparticles on brain health: An up to date overview,” J. Clin. Med. 7(12), 490 (2018). 10.3390/jcm7120490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Aillon K. L., Xie Y., El-Gendy N., Berkland C. J., and Forrest M. L., “ Effects of nanomaterial physicochemical properties on in vivo toxicity,” Adv. Drug Delivery Rev. 61(6), 457–466 (2009). 10.1016/j.addr.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wang Y., Xiong L., and Tang M., “ Toxicity of inhaled particulate matter on the central nervous system: Neuroinflammation, neuropsychological effects and neurodegenerative disease,” J. Appl. Toxicol. 37(6), 644–667 (2017). 10.1002/jat.3451 [DOI] [PubMed] [Google Scholar]
- 173. Lovisolo D., Dionisi M., Ruffinatti F. A., and Distasi C., “ Nanoparticles and potential neurotoxicity: Focus on molecular mechanisms,” AIMS Mol. Sci. 5, 1–13 (2018). 10.3934/molsci.2018.1.1 [DOI] [Google Scholar]
- 174. Leroueil P. R., Berry S. A., Duthie K., Han G., Rotello V. M., McNerny D. Q., J. R. Baker, Jr. , Orr B. G., and Banaszak Holl M. M., “ Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers,” Nano Lett. 8(2), 420–424 (2008). 10.1021/nl0722929 [DOI] [PubMed] [Google Scholar]
- 175. Song B., Zhang Y., Liu J., Feng X., Zhou T., and Shao L., “ Is neurotoxicity of metallic nanoparticles the cascades of oxidative stress?,” Nanoscale Res. Lett. 11(1), 291 (2016). 10.1186/s11671-016-1508-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. El-Ghor A., Noshy M. M.., Galal A., and Mohamed H. R. H., “ Normalization of nano-sized TiO2-induced clastogenicity, genotoxicity and mutagenicity by chlorophyllin administration in mice brain, liver, and bone marrow cells,” Toxicol. Sci. 142(1), 21–32 (2014). 10.1093/toxsci/kfu157 [DOI] [PubMed] [Google Scholar]
- 177. Niska K., Santos-Martinez M. J., Radomski M. W., and Inkielewicz-Stepniak I., “ CuO nanoparticles induce apoptosis by impairing the antioxidant defense and detoxification systems in the mouse hippocampal HT22 cell line: Protective effect of crocetin,” Toxicol. In Vitro 29(4), 663–671 (2015). 10.1016/j.tiv.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 178. Teleanu D. M., Chircov C., Grumezescu A. M., and Teleanu R. I., “ Neurotoxicity of nanomaterials: An up-to-date overview,” Nanomaterials 9(1), 96 (2019). 10.3390/nano9010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Crucho C. I. and Barros M. T., “ Polymeric nanoparticles: A study on the preparation variables and characterization methods,” Mater. Sci. Eng., C 80, 771–784 (2017). 10.1016/j.msec.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 180. Sharma A., Madhunapantula S. V., and Robertson G. P., “ Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems,” Expert Opin. Drug Metab. Toxicol. 8(1), 47–69 (2012). 10.1517/17425255.2012.637916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Yang Q. and Lai S. K., Engineering Well-Characterized PEG-Coated Nanoparticles for Elucidating Biological Barriers to Drug Delivery ( Springer, 2017), p. 125–137. [DOI] [PubMed] [Google Scholar]
- 182. Wyatt E. A. and Davis M. E., “ Nanoparticles containing a combination of a drug and an antibody for the treatment of breast cancer brain metastases,” Mol. Pharm. 17, 717–721 (2020). 10.1021/acs.molpharmaceut.9b01167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Martin-Rapun R., De Matteis L., Ambrosone A., Garcia-Embid S., Gutierrez L. M., and de la Fuente J., “ Targeted nanoparticles for the treatment of Alzheimer's disease,” Curr. Pharm. Des. 23(13), 1927–1952 (2017). 10.2174/1381612822666161226151011 [DOI] [PubMed] [Google Scholar]
- 184. Kumar V., Sharma N., and Maitra S., “ In vitro and in vivo toxicity assessment of nanoparticles,” Int. Nano Lett. 7(4), 243–256 (2017). 10.1007/s40089-017-0221-3 [DOI] [Google Scholar]
- 185. Timmerman R., Burm S. M., and Bajramovic J. J., “ An overview of in vitro methods to study microglia,” Front. Cellular Neurosci. 12, 242 (2018). 10.3389/fncel.2018.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Patchin E. S., Anderson D. S., Silva R. M., Uyeminami D. L., Scott G. M., Guo T., Van Winkle L. S., and Pinkerton K. E., “ Size dependent deposition, translocation, and microglial activation of inhaled silver nanoparticles in the rodent nose and brain,” Environ. Health Perspect. 124(12), 1870–1875 (2016). 10.1289/EHP234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Calzoni E., Cesaretti A., Polchi A., Di Michele A., Tancini B., and Emiliani C., “ Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies,” J. Funct. Biomater. 10(1), 4 (2019). 10.3390/jfb10010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Thomi G., Surbek D., Haesler V., Joerger-Messerli M., and Schoeberlein A., “ Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury,” Stem Cell Res. Ther. 10(1), 105 (2019). 10.1186/s13287-019-1207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bennett N. K., Chmielowski R., Abdelhamid D. S., Faig J. J., Francis N., Baum J., Pang Z. P., Uhrich K. E., and Moghe P. V., “ Polymer brain-nanotherapeutics for multipronged inhibition of microglial alpha-synuclein aggregation, activation, and neurotoxicity,” Biomaterials 111, 179–189 (2016). 10.1016/j.biomaterials.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Song Q. X., Huang M., Yao L., Wang X. L., Gu X., Chen J., Chen J., Huang J. L., Hu Q. Y., Kang T., Rong Z. X., Qi H., Zheng G., Chen H. Z., and Gao X. L., “ Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer's disease by accelerating the clearance of amyloid-beta,” ACS Nano 8(3), 2345–2359 (2014). 10.1021/nn4058215 [DOI] [PubMed] [Google Scholar]
- 191. Chen W., Hu Y., and Ju D., “ Gene therapy for neurodegenerative disorders: Advances, insights and prospects,” Acta Pharm. Sin. B (to be published). 10.1016/j.apsb.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Liao K., Niu F., Dagur R. S., He M., Tian C., and Hu G., “ Intranasal delivery of lincRNA-Cox2 siRNA loaded extracellular vesicles decreases lipopolysaccharide-induced microglial proliferation in mice,” J. Neuroimmune Pharmacol. (published online 2019). 10.1007/s11481-019-09864-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Moore Z., Taylor J. M., and Crack P. J., “ The involvement of microglia in Alzheimer's disease: A new dog in the fight,” Br. J. Pharmacol. 176(18), 3533–3543 (2019). 10.1111/bph.14546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.