Abstract
Background:
Disparities in the control of hypertension and other cardiovascular disease risk factors are well-documented in the United States, even among patients seen regularly in the healthcare system. Few existing approaches explicitly address disparities in hypertension care and control. This paper describes the RICH LIFE Project (Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone) design.
Methods:
RICH LIFE is a two-arm, cluster-randomized trial, comparing the effectiveness of enhanced standard of care, “Standard of Care Plus” (SCP), to a multi-level intervention, “Collaborative Care/Stepped Care” (CC/SC), for improving blood pressure (BP) control and patient activation and reducing disparities in BP control among 1,890 adults with uncontrolled hypertension and at least one other cardiovascular disease risk factor treated at 30 primary care practices in Maryland and Pennsylvania. 15 practices randomized to the SCP arm receive standardized BP measurement training; race/ethnicity-specific audit and feedback of BP control rates; and quarterly webinars in management practices, quality improvement and disparities reduction. 15 practices in the CC/SC arm receive the SCP interventions plus implementation of the collaborative care model with stepped-care components (community health worker referrals and virtual specialist-panel consults). The primary clinical outcome is BP control (<140/90 mm Hg) at 12 months. The primary patient-reported outcome is change from baseline in self-reported patient activation at 12 months.
Discussion:
This study will provide knowledge about the feasibility of leveraging existing resources in routine primary care and potential benefits of adding supportive community-facing roles to improve hypertension care and reduce disparities.
Keywords: Hypertension, Health disparities, Pragmatic trial, Patient activation, Cardiovascular disease, Community-based participatory research, Study design, Federally Qualified Health Center
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of death and disability in the US -- despite the wide availability of effective therapy and prevention.1 Annually, the US spends about $231.1 billion treating cardiovascular disease with an additional $83.9 billion and $51.8 billion spent on the treatment of hypertension and hyperlipidemia, respectively.2 US racial and ethnic minorities are disproportionately burdened by CVD, with African Americans (24.5%), American Indians/Alaska Natives (18%), Asians/Pacific Islanders (23.2%), and Hispanics (20.8%) representing a greater percentage of deaths from heart disease than their overall representation in the US population (13.2%, 1.5%, 5.4%, and 17.4%, respectively).3, 4 Individuals with low income and those living in rural areas are also disproportionately affected by hypertension and other CVD risk factors, and experience less access and poorer quality of care.5–7 Numerous calls to action, including a recent Patient-Centered Outcomes Research Institute (PCORI) report,8 have highlighted the need to reduce the CVD burden by targeting the reduction of hypertension and other major CVD risk factors (e.g., diabetes, hyperlipidemia, and tobacco smoking) in underserved groups. Additionally, depressive symptoms among people with diabetes and hypertension are associated with higher rates of CVD, mortality, poorer adherence, and worse quality of life.9–11
Disparities in hypertension and other CVD risk factors are well-documented. For instance, the prevalence of hypertension in African Americans is among the highest in the world.12 African Americans have higher rates of Stage-3 hypertension, associated with an 80% higher stroke mortality rate, a 50% higher heart disease mortality rate, and a 320% greater incidence of end-stage renal disease than the general population.4,13–15 Other groups, such as Hispanics and Native Americans, also suffer disproportionately from hypertension in addition to other CVD-risk factors, such as diabetes.4,16 Disparities in control of hypertension are observed even among individuals seen regularly in the healthcare system.17 Barriers to reducing disparities in hypertension are complex and exist at multiple levels, including factors related to the individual;18–20 family and social support systems;21,22 healthcare providers, organizations and practice settings;23–26 the local community;27,28 and the policy environment.29
Effective approaches to treat and lower disparities in hypertension include practice-based quality improvement (QI) to improve control of hypertension and other CVD risk factors, with some tailored to racial and ethnic minority individuals.30 A recent systematic review suggests that audit and feedback interventions of clinical performance data are associated with modest (but variable) improvements in hypertension control and are most effective when they are delivered in written form, provide frequent (at least monthly) feedback, and suggest specific actions for improvement.31–33 The availability of reliable and valid blood pressure (BP) measurements can also enhance providers’ willingness to titrate anti-hypertensive medications.34 Results from our recent pragmatic trial suggest that rigorous BP measurement consistent with American Heart Association (AHA) guidelines can be successfully achieved, but other evidence suggests that this rigorous approach may be required only when patients’ initial clinic BP is ≥140/90 mm Hg.35–37 Care management interventions have been shown to be effective at improving hypertension control in vulnerable populations. Team-based care delivered by pharmacists, nurses, and community health workers, and self-management coaching and home BP monitoring for patients, when included as part of multicomponent strategies, are most effective for BP reduction.38 Specifically, CHW-delivered interventions have demonstrated effectiveness for improving adherence to antihypertensive medications, improving hypertension control, and reducing CVD-related hospitalizations and ED visits among African Americans and socially disadvantaged patient populations.39–42
However, few interventions were designed explicitly to reduce disparities in hypertension care and control. Additionally, ethnic minority groups (other than African Americans) and rural populations are understudied. The RICH LIFE (Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone) Project combines multi-level strategies into a pragmatic and sustainable approach to reduce multiple CVD-related risk factors (hypertension, diabetes, high cholesterol, smoking, and depressive symptoms) and improve patient-centered outcomes in persons from socially disadvantaged groups. A multi-level intervention is an intervention that targets two or more levels of contextual influence on health through interdependent interactions.
METHODS
Objective and study design
The project’s main objective is to implement a pragmatic clinical trial to test practical, scalable approaches to closing racial, ethnic, and geographic disparities in hypertension control. We used the Pragmatic-Explanatory Continuum Indicators (PRECIS) criteria for pragmatic trials43 in the development of the RICH LIFE Project. We have addressed the PRECIS criteria as follows: less stringent selection of trial participants; flexible interventions applied in routine practices; use of full range of existing staff to apply and monitor interventions; use of a best alternative comparison strategy; administrative routine follow up of patients; measurement of multiple relevant outcomes, and inclusive analysis (see Appendix for detail). The RICH LIFE Project was approved by the Johns Hopkins Institutional Review Board, protocol number 00085630.
The design of this study is a cluster-randomized trial comparing the effectiveness of two approaches in reducing hypertension disparities. The first is the “standard of care plus” (SCP) intervention, which includes race/ethnicity-specific audit and feedback; staff and provider training in BP measurement and management; and leader training in equity and management practices. The second is the “collaborative care/stepped care” (CC/SC) approach, which includes all of the SCP components and also employs a practice-based collaborative care team with options for escalating care. In the CC/SC approach, treatment for patients with prolonged uncontrolled hypertension is enhanced by adding a community health worker (CHW) to deliver community-based contextualized care, or consultation with a panel of sub-specialists, or both, as necessary, to improve patient-centered outcomes and reduce disparities in hypertension control. Some evidence exists for the effectiveness of both SCP and CC/SC intervention approaches; however, in many cases, these interventions have not been sustained or translated into practice in real-world settings caring for underserved populations. Further, comparison of these interventions has not been conducted within a pragmatic approach to address hypertension management to reduce disparities in patient outcomes. We hypothesize that the multi-level CC/SC intervention will be more effective than the SCP intervention in improving clinical outcomes and self-management behaviors among patients with hypertension and other common cardiovascular disease comorbidities. The CC/SC intervention is considered multi-level because it intervenes on different levels of the socio-ecological model; it targets factors related to individual patients, their social support systems, health care providers, practice settings, and health and community-based organizations that deliver care and other services to patients. Additionally, we hypothesize that the CC/SC intervention will reduce racial and ethnic disparities in hypertension control and patient self-management behaviors due to its focus on addressing social determinants of health that disproportionately affect African Americans and Hispanics.
Study setting and health system participants
We are implementing the RICH LIFE Project from 2015 to 2021 in 30 adult primary care practices across five health systems in Maryland and Pennsylvania (Table I, Table II). We invited health systems to participate if they were 1) located in Maryland, Washington, D.C., Delaware, or Pennsylvania, had two or more adult primary care practices in their systems, 3) were either a Federally Qualified Health Center (FQHC) or a practice that used nurse care managers (CM) and had at least 13% of their patient population from ethnic-minority groups or served a rural population.
Table I.
RICH LIFE Project Enrolled Health System Characteristics
| Characteristics | Health system practices randomized N=30 |
|---|---|
| Type of practice, n(%) | |
| FQHC | 17 (57) |
| Private | 13 (43) |
| Years in operation: | |
| N | 30 |
| Mean (SD) | 20.3 (13.5) |
| Range (Min – Max) | 2 – 45 |
| FTE – Providers: | |
| N | 30 |
| Mean (SD) | 4.1 (2.9) |
| Range (Min – Max) | 1 – 15.5 |
| FTE – Staff: | |
| N | 30 |
| Mean (SD) | 16.4 (10.1) |
| Range (Min – Max) | 3.25 – 44 |
Table II.
Description of Study Health Systems
| Health System A* | Health System B† | Health System C† | Health System D† | Health System E† | |
|---|---|---|---|---|---|
| Primary Care Practices, n | 25 | 3 | 8 | 5 | 3 |
| Patients, n | 266,890 | 6,799 | 33,783 | 28,302 | 11,658 |
| Adult Patients, % | 82 | 64 | 72 | 63 | 78 |
| African-American Patients, % | 30 | 97 | 91 | 26 | 14 |
| Hispanic/Latinx Patients, % | 7 | 9 | 6 | 15 | 77 |
| Hypertension, % | 30.0 | 35 | 39 | 40 | 36 |
| Uncontrolled hypertension (≥140/90 mm Hg), % | 45 | 46 | 27 | 37 | |
| Medicaid/CHIP, % | 12 | 67 | 64 | 33 | 53 |
| Uninsured, % | 2 | 12 | 18 | 17 | 17 |
2018 data reported by health system
2018 data from https://bphc.hrsa.gov/uds/datacenter.aspx
Health system recruitment and allocation
16 identified eligible health systems received an emailed letter of invitation to participate in the RICH LIFE Project and a two-page study overview. 12 health systems responded to the invitation and we invited leaders from these health systems to participate in individual meetings with key study team members where we reviewed the proposed project, addressed questions, and discussed resources available to support participation by study arm. Of the 12 responding health systems, six health systems agreed to participate in the study. One of the six health systems withdrew from the study after the initiation of clinic-level interventions but prior to the start of patient participant enrollment, resulting in a total of five participating health systems.
We shared practice eligibility criteria with health system organizational leadership to help inform the selection of sites from each health system. Practice randomization took place after eligible practices were identified at all of the health systems agreeing to participate in the trial. Randomization was stratified by health system to balance the intervention allocation within system. Health systems with an odd number of practices were grouped together and randomization was constrained so that it would be balanced overall. Given the pragmatic nature of the RICH LIFE Project, blinding of practice intervention assignment was not possible.
Patient participants
Participant inclusion and exclusion criteria are listed in Table III. We are enrolling an average of 63 adult patients from each of the 30 participating practices, for a total of 1890 patients. Given the pragmatic nature of the study and its emphasis on generalizability, we aim to maximize patient eligibility and are excluding patients only if they meet the limited exclusion criteria listed in Table III.
Table III:
Study Patient Participant Inclusion and Exclusion Criteria
| Inclusions (all criteria must be met) | Exclusions |
|---|---|
|
|
Certain conditions in ICD-10 have a coding convention that require the underlying conditions be sequenced first followed by manifestation. Wherever such a combination exists, coders are required to use additional code. For our purposes, any of the conditions that follow the initial code are acceptable. To save space, we are listing these codes with an “*” after the condition code.
Patient recruitment
Using eligibility criteria, the study biostatistician screens participating health systems’ electronic medical record (EMR) data to identify potentially eligible patients. Only patients seen within 6 months prior to the recruitment data pull are assessed for eligibility. In order to reduce the likelihood of unintentionally recruiting patients with well-controlled baseline BP into this study, only EMR measures obtained after the clinical staff are trained on proper BP measurement and use of automated BP measurement devices are used to determine eligibility. The BP readings recorded in participating practices’ EMR were either single BP readings that were less than 140/90 mm Hg, or obtained with automated office blood pressure (AOBP) devices as the average of three back-to-back readings. Potentially eligible patients then receive a mailed packet that includes a letter of invitation to participate, a study brochure, a copy of the oral consent, and a pre-posted “opt-out” postcard. After 10 days, trained recruiters call patients who have not opted out of the study and inquire about their interest in participating in the study. If the patient is interested, the recruiter screens for eligibility, obtains oral consent, and conducts the telephone baseline survey. In cases where a patient is unable to complete telephone baseline interview, a paper version of the baseline interview is mailed to the patient with a return envelope with prepaid postage. Upon completion of the baseline interview, recruiters inform the patient of the intervention assignment of their practice (Figure 1). We repeat this process of screening EMR data, sending mailed packets to potentially eligible patients, and calling patients who do not return a refusal postcard within 10 days of the mailing until the recruitment goal of an average of 63 participants per practice is met. Some practices have smaller patient populations and we may be unable to reach our target at those. We have been over-recruiting at other practices in the same intervention arm within the same health system when possible. If that is not possible, we are over-recruiting at practices in other health systems that have a similar demographic makeup to the small practice.
Figure 1.
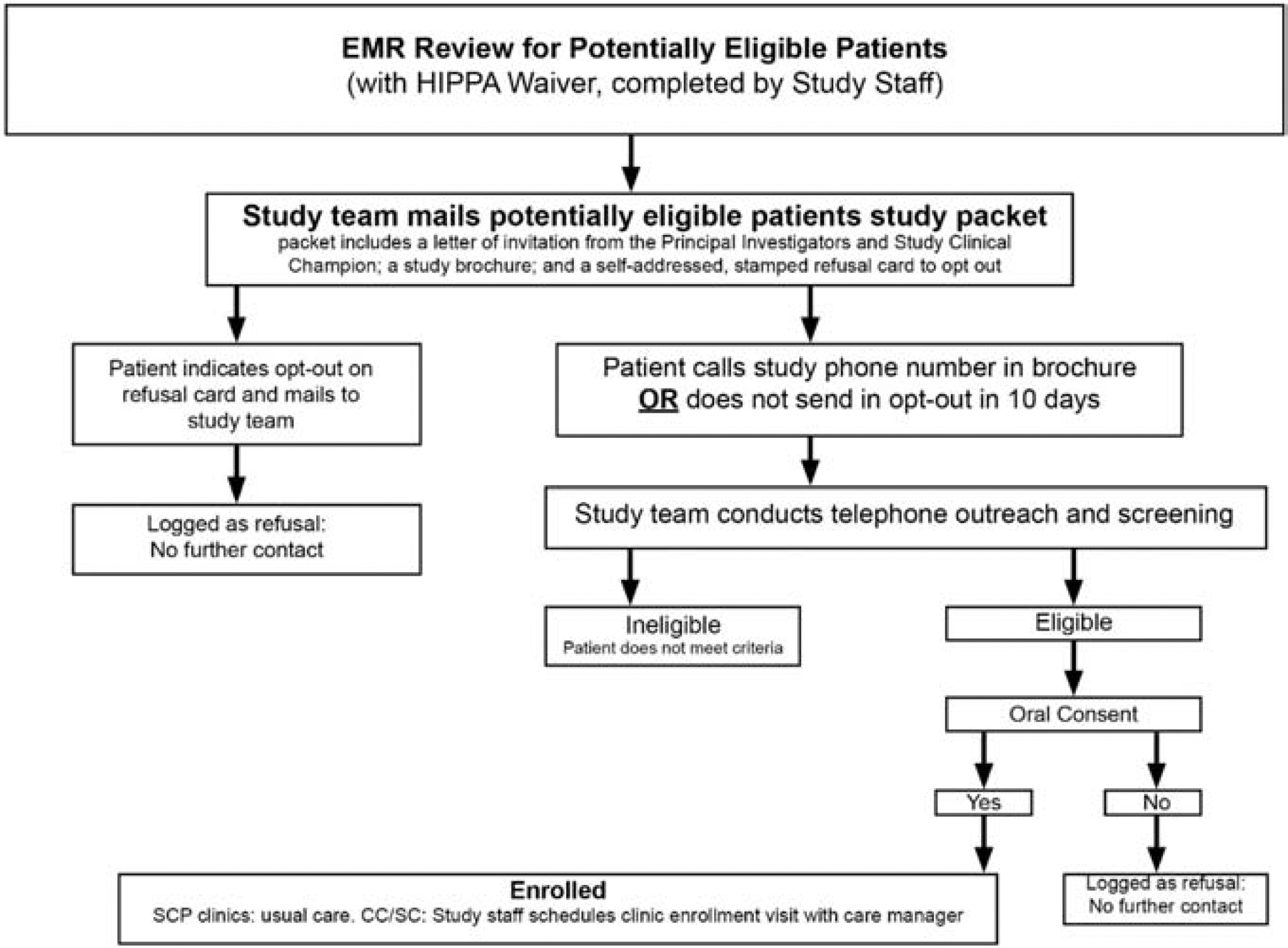
Participant Recruitment Flow
Patient withdrawal
Patients may choose to withdraw from the study at any time. The study team tries to collect as much data as possible from patients who withdraw – e.g., if patients move to another practice, we still try to collect survey data, and if they remain within the same health system, we are able to obtain EMR data.
Patient death
Patients who die before the primary outcome is measured will be treated the same as those lost to follow-up. We have a 6-month window around our 12-month follow-up (primary endpoint). If the patient has a measure during that window, the most recent value in that window will be used. If the patient dies prior to 6 months, then the patient will be omitted from the Generalized Estimating Equations (GEE) regression model for BP control, and only the baseline Patient Activation Measure will be included in the mixed effects regression. We will compare baseline demographic and clinical characteristics for those patients who die prior to follow-up to those who remain in the study.
Interventions
RICH LIFE Project champions
We asked each health system to have a project champion at each level of intervention – one health-system clinical champion, one health-system administrative champion, and at each practice, one clinical-practice champion. The health system clinical champion supports all research activities and promotes engagement of the participating practices and clinical staff while the administrative champion provides system-level administrative oversight and support. Practice-level clinical champions support the day-to-day implementation efforts of the RICH LIFE interventions at their practice.
Arm 1: Standard of care plus (SCP)
Practices in the comparator group receive interventions designed to reinforce and standardize evidence-based hypertension care best practices across both intervention and comparator practices. The “plus” in the standard care arm includes integration of proper BP measurement techniques, hypertension care best practices, and audit and feedback of hypertension control performance, as “usual care” at each practice. Additionally, health-system- and practice-level leaders at SCP practices participate in a system-level leadership engagement intervention consisting of quarterly, one hour calls.
Blood pressure measurement standardization
We aim to standardize BP measurement through the implementation of a “screen and confirm” BP measurement protocol, which incorporates use of automated office blood pressure measurement (AOBP).44
The screen and confirm protocol directs clinical practice staff to perform a single “screening” BP measurement on all patients at the beginning of the office visit. If this initial systolic BP (SBP) is ≥140 mm Hg or diastolic BP (DBP) is ≥ 90 mm Hg, the protocol then directs clinical staff to perform a “confirmatory” BP measurement, consisting of a five-minute rest period followed by three BP measurements separated by 30 seconds and to use the mean of the final three measurements as the patient’s BP reading for that visit.
All participating practices received one Omron HEM-907XL and key accessories for each full-time adult primary care provider (PCP) to facilitate adherence to the RICH LIFE protocol. The Omron HEM-907XL is one of four fully automated, commercially available BP measurement devices capable of performing AOBP.45 It has been validated against standards published by the Association for the Advancement of Medical Instrumentation and the British Hypertension Society,46 and it has been used in multiple clinical trials to measure study outcomes. The Omron HEM-907XL features a dial that allows users to easily toggle between a single “screening” BP measurement and a pre-programed “confirmatory” BP measurement that automatically performs and averages multiple BP readings after a timed rest period.
To standardize EMR BP measurements, we deliver the devices and train practice staff on their use at least three months before the start of patient recruitment at each practice. We employ a “train-the-trainer” model whereby study team members with expertise in clinical education and implementation of BP measurement processes conduct training sessions with each health system’s clinical education department (or equivalent) on the standardized BP measurement protocol and how to certify practice staff for BP measurement competency. Health system- and practice-level trainers receive a study-generated BP measurement guidebook detailing the importance of proper BP measurement, the standardized measurement protocol, staff competency checklists with corresponding questions, and troubleshooting resources. Clinical staff at each participating practice complete a study-developed online training course in proper BP measurement technique and demonstrate competency during assessments with the health system and practice identified trainers.
To monitor and support fidelity to the BP measurement protocol, trained research assistants conduct direct observations of medical assistants’ BP measurement process at all 30 participating practices, twice a year in the first year following implementation and then once a year until patient EMR data collection is complete. The research assistants use a standardized audit form for each visit and report their findings back to practice administrators and health system leaders through a standardized report and scorecard. The report includes recommended corrective actions to improve adherence. If requested by practice or health system leadership, members of the project team may return to practice sites to offer additional support, including providing staff re-training, and offering process improvement consultations to help integrate the protocol into clinical workflow.
Hypertension care and control best practices education
The hypertension care and control best practices education objectives are to enhance provider and staff awareness of evidence-based approaches to overcome practice inertia, improve follow-up, and engage patients in self-care. The intervention consists of online didactic training on evidence-based strategies to improve hypertension care and control. The training consists of four asynchronous, five-to-seven-minute online modules, with interactive knowledge assessment, which providers and staff can view at their convenience. Modules address critical aspects of improving hypertension care and control.
Hypertension dashboard: Audit and feedback
RICH LIFE provides guidance to participating health systems in developing and/or modifying existing reports and reporting tools on performance metrics, also known as “dashboards.” For this project, all health systems are required to display metrics that address hypertension differences in control rates in different patient groups and to display them in a way that allows viewers to monitor performance at both practice and provider levels. The practice dashboard provides a display of percentage of patients achieving BP control, defined as <140/90 mm Hg, for the overall practice, and the provider dashboard provides a display of the same percentage for each provider’s patient panel. Both the practice and provider dashboards break down hypertension control by race and ethnicity (White, non-Hispanic; Black, non-Hispanic; and All Hispanic). Data are uploaded to the clinical dashboard and new reports generated at least quarterly. Reports display data from the previous three months.
System-level leadership intervention
The system-level leadership intervention aims to create a learning network through an inter-organizational approach to promote health equity and reduce CVD disparities. The system-level leadership intervention for SCP practices include 16 quarterly one-hour training sessions presentations on leading for equity and discussion among system-level leaders, community organization leaders, and interested practice champions, conducted via conference call/webinar or in-person. Sessions provide management tools and define health disparities and health equity, describe the impact of health disparities on excess deaths, morbidity rates, costs, and lack of social justice in our society, and explain how the healthcare system contributes to health disparities and conversely, how it could help our society achieve health equity.
Arm 2: Collaborative care/stepped care (CC/SC) intervention
The intensive intervention includes all interventions in the SCP arm, plus the establishment of a collaborative care team with a stepped-care approach (Table IV); quarterly hypertension dashboard education and training; and twice quarterly “coaching calls” for system- and practice-level leaders, CMs, and CHWs in the CC/SC arm to discuss the interventions during their active intervention phase. We designed this intervention using an ecological intervention model developed by Fisher47 that we adapted to address domains relevant to hypertension identification, health behaviors, shared decision-making, guideline-concordant care, and disparate outcomes among racial and ethnic groups. This arm also aims to transform primary care delivery to address the needs of patients with hypertension and other chronic conditions; thus, we additionally selected the Expanded Chronic Care Model (ECCM)48 to guide our intervention implementation.
Table IV:
Components of Collaborative Care Intervention with Stepped-Care Approach
| Components of Practice-Based Collaborative Care Model | ||
|---|---|---|
| Collaborative Care Team Member | Collaborative Care Team Responsibilities | Description of Issues Addressed |
| Primary Care Physician or Advance practice clinician* | Development of Diagnosis and Treatment Plan | Diagnose and evaluate hypertension for secondary causes, additional risk factors, target organ damage |
| Medication Management | Modify medications; titrate and adjust dosages; address adherence issues | |
| Care manager | Care Management and Coordination | Conduct initial behavioral/psychosocial assessment that covers NCQA domains. Educate, activate, and counsel; provide ongoing case management |
| Medication Management | Recommend medication titration; address adherence issues and/or refer to PharmD/Pharmacist | |
| Patient Education and S elf-Management Support | Counsel about diet and weight loss strategies; promote medication adherence and/or refer to dietitian, health educator, or health coach | |
| Psychosocial/Behavioral/Mental Health Services | Provide counseling to address smoking, depression and other psychosocial stressors; assist patients in addressing health insurance, housing, employment, education, and other social or financial issues through referrals and advocacy and/or refer to Licensed clinical social worker, Psychologist, Psychiatrist, or Mental Health RN | |
| Components of Stepped Care Elements | ||
| Stepped Elements | Type of Clinician/Service Provider | Description of Issues Addressed |
| Subspecialist Consultation Services | Subspecialty trained physicians | Engage specialists in the areas of hypertension, diabetes, psychiatry, preventive cardiology, and smoking cessation to assist primary care team in providing consultation on patients with complex management issues. |
| Community-based Contextualization | Community health workers | Support patients in reaching self-management goals; help patients address social and environmental barriers through outreach and navigation services; engage, activate, and empower patients to participate in the care process |
Nurse practitioners, physician assistants
Collaborative care intervention
The collaborative care intervention seeks to establish a basic collaborative care team including a PCP and registered nurse (RN), licensed practical nurse (LPN), or licensed clinical social worker (LCSW) CM; however, depending on availability of other health professionals at a practice, the collaborative care teams may include additional team members such as pharmacists, nutritionists, health educators, and behavioral or mental health specialists. All CC/SC practices have access to the research team’s subspecialist consultation core to obtain care recommendations for patients enrolled in the RICH LIFE Project.
Within the RICH LIFE collaborative care intervention, the CM role is designed to: 1) develop the medical management plan in partnership with patients; 2) use care coordination to maximize interaction of the patients’ PCPs with other care providers addressing medication management, patient self-management, and psychosocial support on a regular, consistent basis; and 3) determine patient access to CHW support and make recommendations to the PCP for subspecialty group review of patient cases.
The CM uses the results of an assessment tool incorporating National Committee for Quality Assurance (NCQA) recommendations,49 administered at the first intervention visit, along with motivational interviewing (MI) to develop care plans with patients and allocate other care team resources in accordance with patients’ priorities and needs. The CM monitors all aspects of each patient’s progress toward improved cardiovascular health outcomes and disease self-management, with a focus on medical management of hypertension, coronary heart disease, diabetes, hyperlipidemia, depression, and on tobacco-use cessation. We designed the CM intervention protocols to address common comorbidities (diabetes, hyperlipidemia, depression and coronary heart disease), lifestyle factors (dietary intake, physical activity, and smoking), and medication adherence. CMs treat the “whole” patient and patient goals and priorities drive the care management encounters. CM and patient visits are not prescribed beyond the first encounter; rather CMs work with the patient to determine appropriate follow up based on the patient’s needs, goals, and preferences.
Regular CM and PCP communication about patients is a critical component of a functioning collaborative care team. CMs communicate with the PCP about patient encounters, including primary content covered, patient goals, and barriers to achieving health goals, through a variety of channels including EMR documentation, EMR messaging, and in-person conversations. CMs document each encounter with a study participant, including failed attempts at engagement and missed appointments, in the EMR and in the study database.
Stepped care interventions
Community health worker
The stepped care approach of the CHW intervention aims to strengthen support for patient self-management and address the community element of patient care. In the RICH LIFE Project, CHWs’ primary purpose is to support patients by: 1) educating participants on how to manage their own BP through self-monitoring and practicing healthy self-management behaviors; 2) reinforce participants’ positive BP self-management behaviors through follow-up encounters by telephone or in person; and 3) helping participants connect with existing clinical and administrative services. CHWs also support participants by making them aware of community resources they can access to support their chronic disease self-management and health-related social needs. CHWs engage with participants through home visits, telephone contacts, and in-person visits at their primary care practices during regular patient visits to CMs or PCPs. The form of CHW engagement is flexible and participant-centered.
The decision to refer a patient is based on CM-identified patient needs and patient and provider preferences. CMs deploy the CHW as a step-up intervention if the patient is willing to work with a CHW and if the patient’s blood pressure or other conditions remain uncontrolled or the patient continues to experience social or environmental stressors. The CHW initiates engagement with the patient upon notification from the CM that the patient requires CHW services. The CHW conducts the baseline visit at a mutually convenient location. The purpose of the CHW baseline visit is to support patient’s self-management of hypertension and to build rapport and trust with the patient and his/her family. The CHW may also begin to identify and address the array of proximal social/cultural/structural factors affecting patients’ self-management of hypertension during the baseline visit. During follow-up visits, the CHW monitors the patient’s progress with care plans; elicits and addresses patient’s concerns regarding treatment; coaches patients on communication with providers; reinforces health education provided by CMs; and facilitates patient and family empowerment. The CHW uses patient-centered motivational interviewing and models appropriate communication styles to elicit patient’s concerns in a compassionate manner. CHWs document each patient encounter, including failed contact attempts, in the EMR and study database.
Specialist core
The CC/SC arm’s specialist core intervention provides the 15 practice sites in the intensive arm with access to specialists for advice on the care of more complex patients. The specialist core includes physicians with clinical expertise in hypertension, preventive cardiology, endocrinology, psychiatry, addiction medicine, smoking cessation and nephrology. The specialist core team meets regularly to review cases and provide recommendations on referrals. The CM may recommend patients with complex medical needs for specialist consultation to their PCP; a PCP may request a consultation; or CMs and PCPs decide jointly to obtain a specialist core consultation based on the concerns they identify together.
CMs can submit a patient to the specialist core for consultation if the PCP agrees; the patient has poor disease control despite patient reports of adherence to several optimized regimens; there are conflicting treatment recommendations for different conditions; AND the CM believes the patient is unlikely to improve within three months of entering care management without specialist intervention.
To obtain a consultation, the CM, with input from the CHW, completes and submits a patient referral form to the specialist core. The specialist core then discusses the patient’s case and prepares recommendations based on the provided information using a standardized response form. The response form includes suggestions on the patient follow-up plan, recommended tests for the referring clinician to organize and communicate with the patient, and a statement on whether or not the specialist(s) believes the patient should be referred to a clinical specialist within the local referral network. The specialist response and recommendations are shared only with the CM and PCP and are suggested approaches to care, not clinical directives, and do not replace the patient’s pre-existing specialist relationships.
Timing of stepped-care
In most cases, CMs may refer patients to work with a CHW or to receive specialist core review after three months of care management sessions. However, an initial referral can occur at one month or sooner by special request from the patient and/or PCP, and for extenuating circumstances identified by the CM (e.g., no transportation, utility shut offs, food insecurity, domestic violence, poor/unstable housing, etc.), referral may occur immediately, or at any time prior to three months. If the patient’s BP or other CVD risk factors remain poorly controlled and/or the patient experiences psychosocial stressors, the CM may refer the patient again, to the remaining stepped care intervention. Thus, patients might receive: 1) CM only; 2) CM and CHW; 3) CM and specialist referral; or 4) CM, CHW, and specialist referral.
Protocol adherence
CMs and CHWs audio-record at least one patient encounter. We use the audio recordings to assess fidelity to motivational interviewing principles and other aspects of the intervention protocol. These evaluations support ongoing training and quality improvement efforts.
Timeframe
The study period is 6.5 years including a 1-year planning period and 5.5 years for intervention implementation and data collection. Health system recruitment occurred in the first year. Interventions were launched on a rolling basis across the different health systems. Patients participate in study data collection for two years. Enrolled patients from CC/SC practices receive 1 year of care manager intervention and, if needed, stepped-care intervention (Figure 2). Patients’ EMR and self-reported data are collected at baseline, 6- 12-, 18-, and 24-months (Figure 2a).
Figure 2.
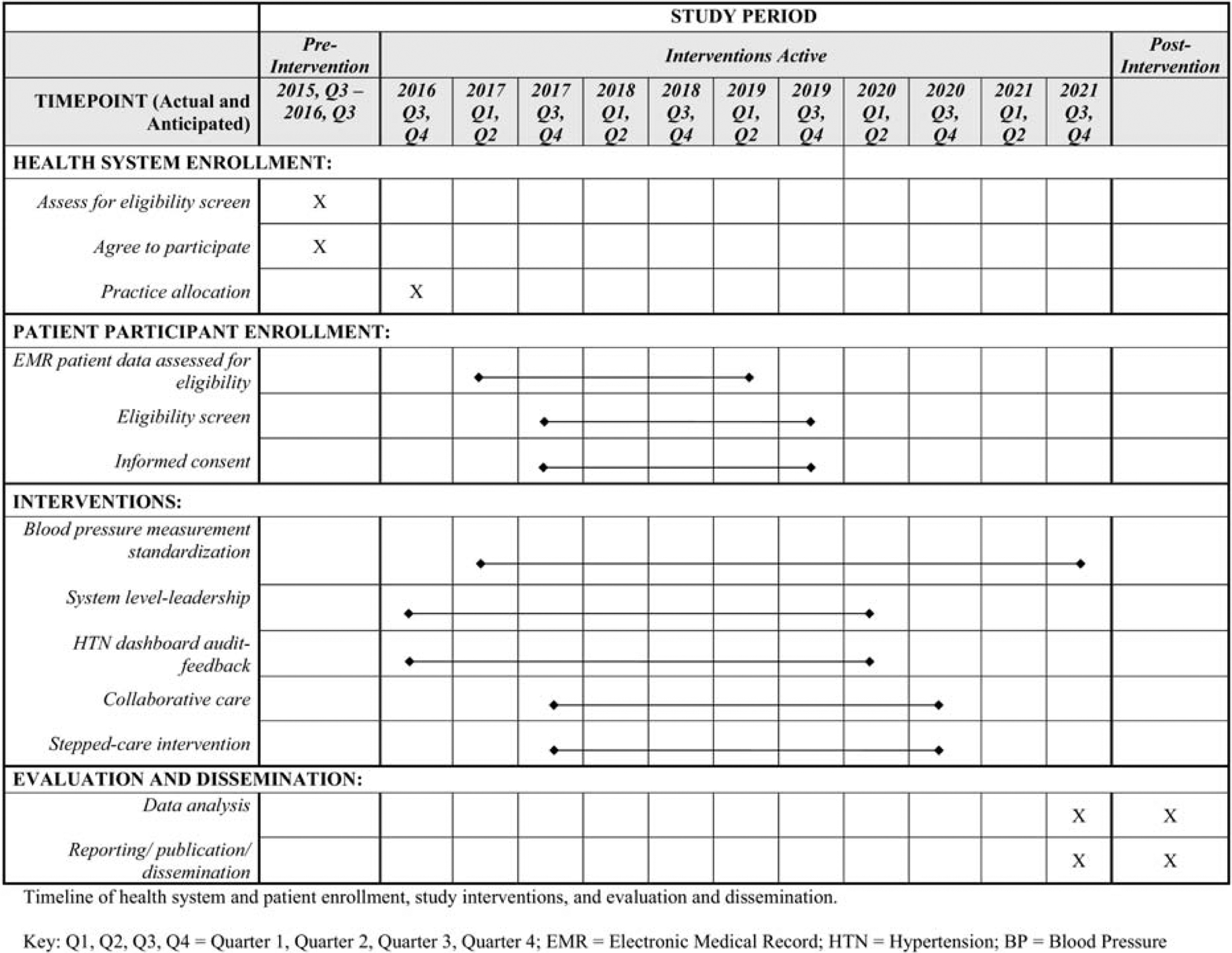
Study Timeline
Figure 2a.

Patient Data Collection Timeline
Outcomes
Primary endpoint
The primary endpoint is 12-month follow-up. Analysis on the following outcomes will take place after 12-month follow-up data collection has been completed.
Primary outcomes
The primary clinical outcome of the RICH LIFE Project is the percent of patients with BP <140/90 mm Hg at 12 months. The primary patient reported outcome is change from baseline in self-reported patient activation, measured by the Patient Activation Measure (PAM-13), at 12 months.
Secondary outcomes
The most important secondary clinical outcome is change from baseline in systolic BP at 12 months. Additional secondary clinical outcomes include: 1) change from baseline in diastolic BP at 12 months; 2) percent with BP<130/80 mm Hg and with BP<120/80 mm Hg at 12 months; 3) change from baseline in 10-year projected probability of a CVD event (global Framingham Risk Score) at 12 months; 4) mean change from baseline in total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein (HDL) and change from baseline in the percent with controlled total cholesterol, LDL, and HDL at 12 months for all patients and for the subgroup with hyperlipidemia; and 5) mean change from baseline in hemoglobin A1c and change from baseline in the percent with hemoglobin A1c< 7.0 at 12 months in patients with a diagnosis of diabetes. We have chosen to use the global Framingham Risk Score rather than the pooled atherosclerotic cardiovascular disease (ASCVD) risk score because the global Framingham Risk Score variables are available from the EMR, whereas the ASCVD variables may not be (in particular, aspirin therapy).
Patient-reported secondary outcomes include: 1) attainment of self-determined goals related to self-management behaviors (e.g., medication adherence, healthy diet, physical activity, and smoking cessation); 2) medication adherence; 3) health related quality of life; 4) depressive symptoms; 5) patient assessment of care for chronic conditions; 6) patient ratings of trust in their care teams; and 7) hypertension knowledge and attitudes. We will compare change from baseline to 12 months to determine the effect of the intervention.
Secondary endpoint and outcomes
We will assess the following outcomes after 24-month follow-up is completed to determine the durability or “late” intervention effect: 1) the percent of patients with BP <140/90 mm Hg at 24 months; 2) change from baseline in self-reported patient activation, measured by the Patient Activation Measure (PAM-13), at 24 months; 3) change from baseline in systolic and diastolic BP at 24 months. We will also compare the change from 12 months to 24 months.
Table V lists study measures, data sources, and collection time points.
Table V.
Study measures
| Baseline | Follow-Up | |
|---|---|---|
| Sources | ||
| Biomedical/Clinical Outcomes* | ||
| Systolic and diastolic BP, BP control (<140/90)25,50 | EMR | EMR |
| Hyperlipidemia control51 | ||
| Glycemic control52 | ||
| Global Framingham Risk Score53 | ||
| Chronic Kidney Disease (Estimated Glomerular Filtration Rate or eGFR) | ||
| Urine microalbumin (per National Kidney Foundation K/DOQI clinical practice guidelines 2000) | ||
| Patient-Reported Outcomes | ||
| PROMIS Global Scale | Telephone Interview | Telephone Interview |
| Patient activation (Patient Activation Measure, PAM-13)54,55 | ||
| Medication adherence56 | ||
| Physical Activity (The Framingham Heart Study (FHS). Physical Activity Questionnaire) | ||
| Fruit and Vegetable Intake (Diabetes Self-care Activities Measure) | ||
| Tobacco or cigarette use (National Health Interview Survey)57 | ||
| Stroke-free status (Questionnaire for Verifying Stroke-Free Status) | ||
| Hypertension Knowledge, Attitudes and Perceptions | ||
| Patient Health Questionnaire Depression (PHQ-8) | ||
| Perceived Stress Scale | ||
| Adverse Events (patient reported) | ||
| Patient attainment of self-defined goals (e.g., BP, weight, diet, exercise, medication adherence)+ | N/A | |
| Patient-Reported Experiences of Care | ||
| Resource Use (Chronic Illness Resources Survey)58 | Telephone Interview | Telephone Interview |
| Patient Assessment of Care for Chronic Conditions (PACIC-Plus) | ||
| AHRQ Care Coordination Quality Measure for Primary Care (CCQM-PC) | ||
| CAHPS Items from Health Literacy Subset | ||
| Satisfaction and Trust | ||
| Perceived usefulness of CHW (CHW Evaluation Questionnaire)59 | N/A | |
| Biomedical Covariates | ||
| Body mass index | EMR | EMR |
| Healthcare and Prescription Drug Utilization | ||
| Healthcare insurance status | Telephone Interview | Telephone Interview |
| Hospitalizations, emergency department use, 30-day Readmissions | Claims | Claims |
| Prescription refills and medication possession ratios for relevant medications | ||
| Social and Demographic Measures | ||
| Age, Gender, Ethnicity/Race, Primary Language, Employment, Insurance, Income, Wealth, Disability*57,60 | Telephone Interview | Telephone Interview |
| Emotional support (PROMIS Social Functioning Scale)61 | ||
| Informational support (PROMIS Social Functioning Scale)61 | ||
| Instrumental support (PROMIS Social Functioning Scale)61 | ||
| Health Literacy (CHEW) | N/A | |
| Subjective Numeracy (Three-Item Subjective Numeracy Scale) | ||
| Life Events | ||
| Everyday Discrimination | ||
| Community Stressors: Violence | ||
| Community Stressors: Total Victimization | ||
| Community Stressors: Disorder | ||
| Adverse Childhood Experience (ACE) Questionnaire | ||
| Provider and Staff Level Variables | ||
| Age, Gender, Race/Ethnicity, Education, Professional Role, Years of Experience | Online Survey | Online Survey |
| Cultural competence, Patient-centeredness (PPOS)62,63 | ||
| Perceptions of practice quality improvement capacity,64 patient-centeredness,65 cultural competency and barriers to addressing health disparities,66 teamwork67 | ||
| Provider and staff knowledge assessments | ||
| Practice Level Variables | ||
| Number and types of clinicians and staff, practice type (private, FQHC); resources | Survey EMR, Interview | EMR Telephone |
| Patient-level outcomes averaged across all patients from the site | ||
| System-Level Variables | ||
| System values, priorities, and capacity for change, leader attitudes | Survey, In-person interviews | Survey, In-person interviews |
Abbreviations: CHW, Community Health Worker; PHQ, Patient Health Questionnaire.
Collected at baseline only
Collected for patients in CC/Stepped Care only
Data collection
EMR data
This study will evaluate the primary outcome measure, BP control, using BP measurements that are collected by primary care providers and medical assistants using automated devices, and entered into practices’ EMR.
We will collect EMR clinical outcomes data from participating health systems on all enrolled patients, including patients at CC/SC practices who choose to withdraw from participation in the collaborative care interventions, unless patients submit a written letter of withdrawal from EMR data collection to the Johns Hopkins School of Medicine Institutional Review Board.
Surveys
Trained research staff complete a phone interview with each patient participant at baseline, 6, 12, 18, and 24 months. These data will be used to assess self-reported adverse events, patient-centered outcomes, including Patient Activation Measure (PAM-13), health-related quality of life, medication adherence, and patient satisfaction (Table V).
We are conducting brief surveys of providers and staff involved directly in the project to collect knowledge and attitudes toward the interventions. Additionally, we asked practice administrators and/or health system leaders to complete a practice characteristic data spreadsheet at the beginning of the study.
CMs and CHWs also complete a knowledge and attitudes survey prior to and immediately upon completion of their intensive two-week training at the start of their participation in the study, as well as demographic and professional characteristics surveys within 12 months of the first CM visit and 6 months of the first CHW visit. The overarching goal of the training is to build the capacity of RICH LIFE CMs and CHWs to support patients’ management of their hypertension and other commonly associated conditions, for reducing racial/ethnic, socioeconomic, and geographic disparities in blood pressure control. The training objectives include: 1) orienting CMs and CHWs to the RICH LIFE protocol, with an emphasis on their respective responsibilities and documentation; 2) providing didactic and group-based learning on the fundamentals of health disparities, health promotion/disease prevention, and patient-centered communication; and 3) strengthening patient engagement strategies utilizing motivational interviewing principles. CM training was centered on initiating and sustaining patients’ engagement and adherence to antihypertensive and other therapies through motivational interviewing, goal-setting, and patient activation approaches. Because CHWs play a supportive role in reinforcing the CM’s interventions and to address patients’ social determinants of health, there are specific modules on identifying and linking patients to community resources.
Finally, we are conducting exit interviews and focus groups with CMs and CHWs when they leave the project and/or at the end of the active patient intervention period. We hope to learn from these interviews about the implementation process of RICH LIFE within the clinics, as well as how RICH LIFE may influence clinic practice long term.
Directed interviews
Health system-level leadership participate in directed interviews, administered by a study team member, at baseline, to determine strategic priorities and readiness for change of all participating systems. In addition, we conduct exit interviews with senior leaders, CMs and CHWs departing from the study to learn more about their experiences executing the study protocol.
CM, CHW, and specialist records
RICH LIFE CMs, CHWs and specialists maintain consistent records on each patient participant that they work with during the study. The data collected includes both quantitative and qualitative data, which is entered into a study database. These data will be used to better understand the implementation process, potential mechanisms for intervention effectiveness, and targets for improvement of future interventions.
Statistical analysis plan
We will use descriptive statistics to characterize the organizations, providers, and patients, using means and standard deviations, medians and interquartile ranges, or frequencies and percentages where appropriate. Descriptive statistics of central tendency and variability will be generated for outcomes. The distributions of each continuous outcome variable will be assessed for normality, and appropriate transformations will be made if necessary. Two-sample t-tests, Wilcoxon rank-sum tests, and Fisher’s exact test will be used to compare baseline demographic and practice characteristics across intervention arms.
Primary statistical analyses testing the differences in outcome (or change in outcome over time) between groups will be conducted using the intention-to-treat principle. For our clinical outcomes at our primary endpoint of 12-month follow-up, we will select the EMR measure that is closest to 12 months following the baseline survey (enrollment date) for each patient as long as it is within the 6-month window.
General Approach
These analyses will utilize all available data through modeling approaches for correlated outcomes. All missing data will be assumed missing at random (MAR) conditioning on the observed data, and models will be adjusted for characteristics associated with missingness and key variables not balanced by the cluster randomization. In sensitivity analyses, missing data will be imputed using likelihood based longitudinal models developed under the MAR assumption. Missing data under plausible informative missing scenarios will be multiple-imputed using these likelihood based models with the mean models tuned according to the plausible informative missing scenarios. These sensitivity analyses will verify the robustness of the results derived under MAR assumption. All tests will be two-sided and significance will be set at alpha<0.05. Analyses will be conducted using SAS version 9.4 or higher (SAS Institute, Inc., Cary, NC) or Stata SE14 or higher (StataCorp, College Station, TX).
We will use mixed-effects regression models for continuous outcomes and Generalized Estimating Equations (GEE) analysis for dichotomous outcomes. For mixed-effects regression modeling, we will assume an unstructured correlation structure for longitudinal analyses of outcomes with repeated measurements over time, employ random effects to address clustering by practices, include appropriate covariate adjustment in the fixed effects, and use robust estimates for statistical inferences. The GEE approach is robust to outcome correlation misspecification, so we will just assume an exchangeable correlation structure for the outcomes, include appropriate covariates in the mean model, and use robust estimates for statistical inferences. We will use mixed-effects regression models under the MAR assumption as the basis to conduct multiple imputation for missing BP data or other outcomes as appropriate. In sensitivity analyses evaluating potential impacts under sensible informative missing (NMAR) scenarios, we will preserve the mixed-effects regression based variance-covariance estimates, and manipulate the mean model under each informative missing scenario to carry out multiple imputation. Missing binary outcomes (e.g., controlled BP or lipids) will be derived based on multiple-imputed continuous values using appropriate observed data likelihood models as described.
Statistical analysis plan for primary outcomes
For the primary clinical outcome of BP control at 12 months we will model the binary variable BP controlled (yes/no) at 12 months using GEE with a logit link on the intervention arm indicator (CC/SC arm vs. SCP arm). Additional covariates will be included as appropriate as described previously, including appropriate cluster level characteristics and variables associated with occurrence of missing data. With correctly specified mean model, the GEE is robust to misspecification of correlation structure so the statistical inferences will account for outcome correlation due to patients clustering within practice and be valid under MCAR. The regression coefficient for the intervention arm indicator estimates the log-odds ratio of BP control at 12 months for the CC/SC arm to the SCP arm. Multiple imputation of missing BP outcomes will be carried out to impute the missing BP control outcomes to produce proper inferences under MAR and to conduct sensitive analyses under NMAR scenarios.
For the primary patient-reported outcome of change from baseline in PAM-13 score at 12 months, we will model the baseline and 12-month PAM-13 measurements using a mixed-effects linear regression model, with the intervention arm indicator (CC/SC arm vs. SCP arm), 12-month time indicator, and the cross-product term of intervention by time interaction as the fixed mean effects, the practice indicator as a random effect, and additional covariates included as fixed effects as appropriate as described previously. The fixed effect regression coefficient for the cross-product term of intervention by time estimates the mean difference of 12-month change in PAM-13 between arms.
Statistical Analysis Plan for Secondary Outcomes
For the outcomes of systolic and diastolic BP measures at 12 months, we will select the blood pressure measure that is closest to 12 months of follow-up for each patient as long as it is within the 6-month window (either direction). We will model the baseline and 12-month BP measurements using a mixed-effects linear regression model, with the intervention arm indicator (CC/SC arm vs. SCP arm), 12-month time indicator, and the cross-product term of intervention by time interaction as the fixed mean effects, the practice indicator as a random effect, and adjusting for additional covariates as fixed effects as described previously. The random effect for the cluster ID will be used to capture the outcome correlation for patients nested within the cluster. The fixed effect regression coefficient for the cross-product term of intervention by time estimates the mean difference of 12-month change in BPs between arms.
For the other secondary measures, e.g., laboratory measures of hemoglobin A1c and lipids, we will use methods similar to the assessment of 12-month outcomes for blood pressure, i.e., select the within window measure in the EMR closest to 12 months after baseline and use mixed-effects linear regression or GEE models to assess the outcomes while controlling for outcome clustering within practice.
Subgroup and Exploratory Analyses
Durability of intervention at 24 months
For the important clinical outcome of BP control we will model the binary variable BP controlled (yes/no) at 12 and 24 months together using GEE with a logit link, and with the intervention arm indicator (CC/SC arm vs. SCP arm), the follow-up visit indicator (24 months vs. 12 months), and the interaction term of these two binary indicators as the main predictors for the mean model. Additional covariates will be included as appropriate as described previously. Multiple imputation of missing BP outcomes will be carried out to impute the missing BP control outcomes to produce proper inferences under MAR and to conduct sensitive analyses under NMAR scenarios.
For the durability outcomes of change in blood pressure and Patient Activation at 24 months, we will derive the statistical inference from mixed-effects models utilizing all outcome measures available from the three study time points. The regression coefficients of the arm by 24-month visit cross-product interaction term from the corresponding models estimate the difference in change in systolic BP as well as the difference in change in PAM-13 at 24 months from baseline between the intervention and the control arm, respectively. Estimates contrasting the 24-months change to the 12-months change from the modeling results will allow us to assess the durability or late effect of the intervention.
Subgroup Analyses
Patients, clinics, and health systems will be keen to know if the interventions have differential effects on different subpopulations. The pre-specified subgroup analyses include analyses by race and ethnicity. Specifically, we will compare outcomes among non-Hispanic African Americans and Hispanics to non-Hispanic whites. The analysis plan for testing heterogeneity of treatment effects is that using the full dataset, we will compare each disadvantaged subgroup (and the combined disadvantaged groups) to the presumed-advantaged group. We will test interactions of these with the intervention effect. We will target enrolling approximately 21 patients in each race/ethnicity category per clinic to maximize power; however, these analyses will be exploratory and are not powered to detect specific differences among the subgroups.
Exploratory analysis will also be conducted to test for differential intervention effects across clinics. For this analysis clinic-indicator dummy variables and their interaction with intervention arm will be included in the GEE and mixed effects models.
For the CC/SC arm, exploratory analysis will be conducted to determine which parts of the intervention, e.g., number of CM contacts, CHW, sub-specialist, were more successful in improving BP control and reducing systolic BP, or if there was an overall “dose” effect.
Power calculation
We aim to recruit a total of n=1,890 unique patients. We planned for 20% random attrition which would yield an analytic sample of 1,500 (effective size of 50 patients per practice). A review of cluster-randomized trials in primary care found a median intracluster correlation coefficient (ICC) of 0.04, with an interquartile range of −0.02 to 0.21.68 Assuming a 5% ICC, 15 clusters of 50 patients per intervention arm and a two-sided alpha of 0.05, we will have 80% power to detect a difference in proportion of patients with BP controlled of 11–13% (depending on whether the proportion controlled in the SCP arm is 50%−70%). With the same assumptions and sample size, using a two-sample t-test, we will be able to detect an effect size of 0.278. Operationalizing this for the primary and important secondary outcomes, we anticipate that for PAM-13, estimating a SD=10.0,69 we would be able to detect a mean difference of 2.78 between the CC/SC and SCP arms; for systolic BP, estimating a SD=19.5 mm Hg,63 we could detect a mean difference of 5.42 mm Hg between the CC/SC and SCP arms.
Data monitoring, data management, and patient safety
An independent Data and Safety Monitoring Board (DSMB) has responsibility to monitor all aspects of the study, including data and patient safety. Members have been appointed by the Patient-Centered Outcomes Research Institute (PCORI) and the National Heart, Lung, and Blood Institute (NHLBI) to provide oversight of the trial. The DSMB meets twice a year. Everyone on the study team is blinded to the interim results, except the study biostatistician, who presents them to the DSMB during semi-annual meetings. The DSMB has the responsibility to recommend to the NHLBI/PCORI whether the trial should continue, whether the protocol should be modified, or whether there should be early termination.
All data are stored on a secure file server on the Johns Hopkins Medical Institution (JHMI) network. The server is in a secure room with controlled access. We assigned study IDs to all participant patients. The study team stores all digital data, including audio files and transcripts, in a restricted, password-protected study shared drive, maintained by JHMI.
Surveillance for serious adverse events (SAE) and other relevant clinical events occurs by semi-annual questionnaire and by EMR review every 6 months. Physical measurements will not be done outside of the realm of routine clinical care, and all of the results will be available to the participant’s PCP through prompt EMR documentation.
The RICH LIFE Project is funded by the Patient-Centered Outcomes Research Institute (PCORI) and the National Heart, Lung, and Blood Institute (NHLBI). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
The RICH LIFE Project is registered at clinicaltrials.gov; registration number: NCT02674464.
DISCUSSION
Study rationale
This study aims to add significant value to current efforts to reduce hypertension disparities among underserved populations. In addition to benefiting patients’ self-management goals, activation levels, and BP control, identifying effective mechanisms to help patients achieve better overall CVD risk control is extremely beneficial to providers and insurers facing critical decisions about how best to provide supplemental behavioral approaches to enhance effectiveness of prescribed therapies among high-risk patients in real-world settings. The implementation of targeted and tailored approaches in these populations and settings is understudied and poorly understood. This study will also help to determine whether interventions guided by patients, providers, and other partners are more powerful and more sustainable than interventions developed without such input. Finally, rigorously evaluated interventions that demonstrate the feasibility of leveraging existing resources in routine primary care should provide health care providers and policy makers with the confidence to endorse the widespread use of similar interventions in a variety of healthcare practice settings that serve at-risk populations.
Early challenges and impact on study design
We anticipated modifications to the protocol given the pragmatic design of the RICH LIFE Project and its emphasis on meaningful stakeholder engagement. Table VI describes some of the early challenges we encountered in actualizing the original design and the resulting impact on the conduct of the study.
Table VI.
Effect of Implementation Challenges on Study Design
| Original Element of Study Design | Challenge | Impact on Study Design |
|---|---|---|
| Recruit 63 patients at each of the 30 participating practices | Smaller practices did not have enough eligible patients to reach recruitment target of 63 patients per practice. | Adjusted our recruitment target to an average of 63 patients per practice, over recruiting at other practices in the same intervention arm within the same health system when possible or, if not possible, over recruiting at practices in other health systems that have a similar demographic makeup to the small practice. |
| Recruit patients of White, Black and Latinx background in 1/3–1/3–1/3 proportions to examine racial and ethnic disparities among three racial groups | Participating practice sites had fewer eligible Latinx patients than would allow us to reach this goal. | Changed the analysis plan to examine racial and ethnic disparities among two racial groups (African-American and white), and changing the Latinx vs. white comparison to an exploratory one. |
| Embed one full-time CM and one full-time CHW at each of the 15 practices assigned to the Collaborative Care/Stepped Care study arm | There were limited health system and study resources to fully fund full-time CMs and CHWs at every practice site. | Health systems either identified an acceptable percentage of effort for CMs and CHWs to dedicate to the study or combined per practice resources to employ full-time CMs and CHWs responsible for more than one practice site. |
| CMs, CHWs, and PCPs would participate in at-least once weekly, in-person, “rounding” meetings as part of the collaborative care team | Several health system leaders expressed preferences for regular, within EMR, CM-CHW-PCP communication and ad hoc CM-CHW-PCP in-person or telephonic communication rather than regularly scheduled meetings. | Allowed for more flexibility in the communication among the collaborative care team to reflect health system leaders’ preferences. |
| CMs would “step-up” patients not achieving BP control or other health goals after 3 months to either a CHW or to the specialist core and initiate an additional step-up 3 months after the first step-up, if BP control/other health goals are not achieved | CM and CHW supervisors identified the phrase “step-up” was problematic as it implied a hierarchy between the CM and CHW. Health system leaders preferred the term “consultation” for specialists to avoid the perception that patients would actually need to see the specialist team. CMs and CHWs provided early feedback that patients were in need of additional resources, particularly resources related to social determinants of health, earlier than 3 months into working with the CM. |
Discontinued use of the phrase “step-up” and adopted “CHW referral and “specialist consultation.” Adapted the protocol to allow for earlier CHW referrals and specialist consultations. Identified specific criteria for CMs to follow to determine if a patient qualified for immediate, 1 month, or 3 month referral to a CHW. Identified specific criteria for CMs to follow to determine if a patient qualified for specialist consultation at 1 month of 3 months. |
| All participating practices would receive a hypertension dashboard, but only practices in the Collaborative Care/Stepped Care study arm would receive a race and ethnicity-stratified hypertension dashboard | Due to limitations of the population health management software at the time of the dashboard development and concerns about an inconsistent approach to quality improvement across the organization, the largest participating health system determined that they would prefer to release the race and ethnicity-stratified hypertension dashboard to all practices, regardless of study arm or study participation. | All participating practices receive a race and ethnicity-stratified hypertension dashboard. Leaders from the Collaborative Care/Stepped Care practices receive additional coaching on dashboard promotion and use through quarterly system level leadership coaching calls. |
| Each health system would identify a clinical and an administrative champion at the system-level and a clinical champion, preferably a provider, at the practice-level, for each participating practice | While all health systems identified clinical and administrative champions at the health system level, not all health systems identified practice-level clinical champions. | Allowed for flexibility in the practice-level champion role, including allowing medical assistants identified by health system leaders to serve as project champions. |
Strengths
This study has many strengths including its cluster-randomized design, extensive engagement with community-based organizations, patients, health system leaders, providers and staff, and payers; flexibility to adapt the protocol to respond to real-world implementation challenges; implementation at five different health systems including both FQHC and non-FQHC practices; and the use of existing clinical staff whenever possible to maximize study sustainability.
We selected a randomized design, which ensures balance between the two conditions (CC/SC and SCP) in all characteristics of practices so that in the aggregate, the only differences in outcomes between the two groups will be due to the intervention. This design will minimize confounding by variability in quality of care among practices. Cluster randomization sometimes results in imperfections in the balance between the two conditions at the patient level. Thus, we will examine trial-group characteristics for any imbalance so that we can adjust for that in quantitative analyses.
Extensive and on-going partner engagement is perhaps the RICH LIFE Project’s greatest strength. A community advisory board (CAB), whose membership reflects patient, advocate, community, governmental, payer, and health-system perspectives, has supported every stage of the RICH LIFE Project. CAB members contributed substantially to formulating the study’s research questions and identifying appropriate outcomes and continue to profoundly influence its implementation and the constellation of RICH LIFE interventions. The study responds directly to patient desires to feel more equipped to be involved in their care and manage multiple conditions that contribute to CVD. It also responds to provider and health system partner desires to address multiple CVD risk factors.
Given the pragmatic nature of the study and extensive health system and community engagement, flexibility to adapt the protocol to respond to practice-based concerns and partner suggestions is a major strength of this study. Implementing this study would not be possible without tailoring the protocol to meet the needs and resources of health systems, patients, CMs, and CHWs. On-going engagement with participating health systems and partners resulted in study design adaptations across all of the RICH LIFE interventions including team member training and staffing models of the collaborative care team, timing of stepped-care interventions, measures included on the hypertension dashboard, the frequency and content of the HELN webinars and coaching calls, and the process for training and certifying clinical staff in the BP standardization measurement protocol. Implementing the RICH LIFE Project across five unique health systems, including FQHCs and non-FQHCs, will result in greater generalizability of study findings. Significant engagement with the different health systems resulted in a flexible protocol, with permissible adaptations, that lends itself to wider dissemination.
Whenever possible, existing clinical staff at participating practices filled the roles of CM and CHW. The use of existing staff was a deliberate choice to increase the likelihood of CM and CHW integration into the practice and long-term sustainability of the interventions. Consistent with the tailored approach throughout RICH LIFE, staffing adaptions were made to respond to health systems’ needs. For example, two health systems expressed resource limitations to supervise the new CM and CHW positions created by RICH LIFE. As a result, the study team and health systems established partnerships with payers and community based organizations to support the hiring and staffing of these positions within the health systems. These new partnerships led to further collaboration and refinement of CM and CHW protocols, taking into consideration existing CM and CHW workflows and organizational cultures.
Potential limitations
The limitations of this study are worth noting. First, we cannot fully prevent cluster contamination between practices in the same health system. Health system leaders have a responsibility to oversee patient care at all practices and may inadvertently introduce new approaches as a result of exposure to the intensive CC/SC intervention. However, each leader is aware of the study design; to date we have not found significant expansions of hypertension or health equity-related activities in SCP practices. While not a common practice, some providers may see patients at both SCP and CC/SC practices; we will assess the extent to which this takes place and the potential impact on study outcomes through directed interviews with health system leaders.
SCP is an active comparator, which could drive our findings towards a null hypothesis. Any effects observed may have been greater if we had used usual care as our comparator.
Several practices in both the SCP and CC/SC arms have existing care management or patient wellness programs. Although patient participation in another care management program is an exclusion criterion, we must rely on patient self-report as EMR records do not consistently document care management participation. We cannot stop patients from participating in additional patient programs after their enrollment in the RICH LIFE Project. We will make every effort to monitor coincident programs at participating practices.
This study will evaluate the primary outcome measures of BP control based on PCP- and medical assistant-collected BP measurements using an automated device and entered into practices’ EMR rather than a more traditional approach used in clinical trials, where a small pool of highly trained research staff measure BP. It is a prerequisite of a pragmatic study to measure outcomes as they are measured in the real world. The measures used in this trial will resemble those used for quality improvement purposes as these metrics also are generated from BP measurements collected from clinical records. Outcomes from this study may offer a closer approximation of how studied interventions will affect measures as they are currently used for health system payment, quality reporting, and policymaking. Additionally, using measures from the EMR minimizes risk for intervention bias, as having study personnel obtain traditional research-quality BP measurements would require patients to interact with health care professionals outside of expected interactions.
Not all health systems require providers to participate in the BP standardization measurement best practices training. Some health-system leadership determined that provider training should be optional to reduce provider burden. It is mandatory for medical assistants to receive training and certification as medical assistants perform the vast majority of BP measurements, however, some providers may repeat measurements they find questionable rather than using the standardized approach. We will examine the rate of repeated BP measurements so that we can determine the extent to which differences exist in terminal digit preference in the measures done by providers versus medical assistants. If the rate of repeated measures is significant or differs by intervention group, we may conduct sensitivity analyses to determine the extent to which this phenomenon influences our study results. It is also optional for providers to utilize the hypertension dashboard which may dilute any potential effect of the audit and enhanced feedback intervention.
Our study protocol was written in 2016, and <140/90 mm Hg was the definition that we chose at the time. Although we are aware of the 2017 American College of Cardiology/American Heart Association hypertension guideline70 and we largely agree with the more aggressive goal of <130/80 mm Hg for control in most patients, we decided not to change the study goal for blood pressure control. Our rationale for keeping the study outcome is as follows: 1) we expect uptake of the new guidelines will be slow in the field and 2) we wish to maintain consistency in our data. We anticipate conducting a sub-analysis of study data at the close of the study period to examine what percentage of patients achieve lower targets (i.e. <130/80 mm Hg and <120/80 mm Hg) using the RICHLIFE interventions.
With respect to patient race and ethnicity balance across intervention arms, we are facing greater challenges reaching recruitment goals for Hispanic patients due to lower participation by healthcare organizations caring for this population, their clustering within a small number of practices, unbalanced allocation of practices to the interventions through randomization, and likely, the current social and political climate that has increased fear and institutional mistrust in many immigrant communities. To address this limitation, we have hired several Hispanic recruiters and attempted to recruit all eligible Hispanic patients across the 30 practices in the study.
This study includes patients who are regular attenders of primary care therefore the findings are likely only generalizable to those populations. Other approaches might be needed for populations not regularly engaged in primary care.
Human subject protections ensure that only patients who are willing to participate and provide consent will be included in this study. The patients who choose not to participate could be the patients who have poorer adherence or are less engaged in medical care. Due to human subjects research protections, we are unable to include them in the study. Nevertheless, our early experience of the data does suggest that we have enrolled both patients who are adherent to treatment and those who are not adherent.
Lastly, this study is funded by PCORI, administered through NHLBI, precluding us from conducting a cost-assessment or cost-effectiveness analysis. If this intervention is deemed to be effective, future research will incorporate such analyses.
CONCLUSION
The design of the RICH LIFE Project relies on a cluster-randomized controlled trial to assess effectiveness (relative to enhanced standard of care) of adding a CM to address the specific needs of patients with uncontrolled hypertension who may not be fully served by the currently available practice-based approaches. CMs have the ability to delve into the challenges that patients face in managing hypertension, which may well be different, and more difficult to manage, among historically disadvantaged racial and ethnic groups. RICH LIFE goes 1–2 steps further, to provide 1) a CHW who can assist the patient with social needs, and 2) access to a seasoned collaborative panel of specialists who can inform primary care clinicians and CMs on the best and most up-to-date evidence to assist their patients in meeting their disease control goals. Together, these interventions hold great promise to reduce disparities in BP control, and in CVD risk, between non-Hispanic whites and the underserved populations of non-Hispanic African Americans and Hispanics.
Trial status
The blood pressure intervention was introduced to all 30 practices between January 2017 and May 2018. Patient recruitment started at least three months after practice staff completed training and certification in the blood pressure measurement protocol. The launch of patient recruitment and the collaborative care intervention occurred on a rolling basis across the five health systems between August 1, 2017 and October 11, 2018. All participating health systems began participation in the HELN sessions beginning in September 2016. CC/SC practices participation in coaching calls coincided with the launch of the collaborative care intervention. Patient recruitment concluded on October 31, 2019; all 15 CC/SC practices have assigned care managers and community health workers. Providers at all 30 practices have access to the dashboard and the online best practices in hypertension control and care education videos.
Supplementary Material
Acknowledgements:
The investigators would like to acknowledge the following people and organizations for their contributions to patient recruitment, data collection, delivery of the study interventions, and operations of the study: Millie Aquino, Chara Bauer, Deven Brown, Tiffany Campbell, Lisa Carpenter, Maria Collazo, Stacye Cooper, Andria Coryatt, Cynthia Crandall, Keah Crosby, McKenzie Eakin, Dorethia Easley, Lia Escobar-Acosta, Sabrina Elias, Martha Franz, Kenya Ferguson, Minyun Fogg, Jennifer Halbert, Dairy Jennings, Jon Kawatachi, Mary Krob, Jolene Lambertis, Joseph Landavaso, Gee Eun Lee, Stella Marine, Erika McCannon, Krystle McConnell, Margaret Mejia, Theresa Messer, Nancy Molello, Kandice Oakley, Modupe Oduwole, Chibuzo Opara, Princess Osazuwa, Aidan Ottoni-Wilhelm, Helen Owhonda, Sherry Perkins, Jodi Peters, Lauren Phillips, Camila Montejo-Poll, Christine Richardson, Don Ritchie, Denise Saint-Jean, Abhay Singh, Sheryl Sprague, Anita Stokes, Tricia Taylor, Cleonda Thompson, Jodi Watkowski, Gloria Leonard-Witten, Randi Woods, Zehui Zhou, Sisters Together and Reaching, Inc., Johns Hopkins HealthCare, LLC, and i2i Population Health. The authors would also like to thank the members of the Johns Hopkins Center for Health Equity Community Advisory Board for guidance throughout the project and all of the staff, providers, organizational leaders, and patients at participating clinical sites at Berks Community Health Centers, Choptank Community Health System, Johns Hopkins Community Physicians, Park West Health System, and Total Health Care, Inc., for making the completion of this study possible. Lastly, the authors would like to thank the appointed DSMB and Hypertension Disparities Reduction Program Partnership membership for the oversight of this study.
The RICH LIFE Project is a National Heart, Lung, and Blood Institute and Patient-Centered Outcomes Research Institute supported trial; grant number UH3HL130688.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Clinicaltrials.gov NCT02674464
Competing interests:
The authors declare that they have no competing interests.
References
- 1.Murphy SL, Xu JQ, Kochanek KD. Deaths: Final data for 2010. Natl Vital Stat Rep. 2013;61(4). 2013; http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf, 2014. [PubMed] [Google Scholar]
- 2.Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996–2013. JAMA. 2016;316(24):2627–46. DOI: 10.1001/jama.2016.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Census Bureau. https://www.census.gov/. 2014. Retrieved on March 4, 2020.
- 4.Agency for Healthcare Research and Quality (AHRQ). National Healthcare Disparities Report. Rockville, MD: AHRQ Publication;2013. [Google Scholar]
- 5.Morenoff JD, House JS, Hansen BB, et al. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med. 2007. November;65(9):1853–66. DOI: 10.1016/j.socscimed.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics. Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 7.Melvin CL, Corbie-Smith G, Kumanyika SK, et al. Developing a research agenda for cardiovascular disease prevention in high-risk rural communities. Am J Public Health. 2013;e1–e11. DOI: 10.2105/AJPH.2012.300984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasgow R, Ashok M, Porterfield D, et al. Understanding Options to Reduce Disparities in Cardiovascular Disease through Comparative Effectiveness Research: Landscape Review. 2013. PCORI RFQ # PCO-CVDLDSR2013.
- 9.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003. October;26(10):2822–2828. DOI: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 10.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. DOI: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment non adherence: a meta-analysis. Diabetes Care. 2008. December;31(12):2398–2403. DOI: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper ES. Cardiovascular diseases and stroke in African Americans: a call for action. J Natl Med Assoc. 1993;85:97–100. [PMC free article] [PubMed] [Google Scholar]
- 13.Burt VL, Cutler JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to1991. Hypertension. 1995. July;26(1):60–69. DOI: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 14.Hall WD, Ferrario CM, Moore MA, et al. Hypertension-related morbidity and mortality in the southeastern United States. Am JMed Sci. 1997. April;313(4):195–209. [DOI] [PubMed] [Google Scholar]
- 15.Klag MJ, Whelton PK, Randall BL, et al. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997. April 23–30;277(16):1293–8. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. http://www.cdc.gov/diabetes/pubs/statsreport14.htm
- 17.Cooper LA, Marsteller JA, Noronha GJ, et al. A multi-level system quality improvement intervention to reduce racial disparities in hypertension care and control: study protocol. Implement Sci. 2013. June 4;8:60 DOI: 10.1186/1748-5908-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014. February 5;311(5):507–20. DOI: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 19.Ndumele CD, Shaykevich S, Williams D, et al. Disparities in adherence to hypertensive care in urban ambulatory settings. JHealth Care for the Poor & Underserved. 2010. February;21(1):132–143. DOI: 10.1353/hpu.0.0259. [DOI] [PubMed] [Google Scholar]
- 20.Sehgal AR. Overlap between whites and blacks in response to antihypertensive drugs. Hypertension. 2004. March;43(3):566–572. DOI: 10.1161/01.HYP.0000118019.28487.9c. [DOI] [PubMed] [Google Scholar]
- 21.Kressin NR, Orner MB, Manze M, et al. Understanding contributors to racial disparities in blood pressure control. Circulation. 2010. March;3(2):173–180. DOI: 10.1161/CIRCOUTCOMES.109.860841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis LM, Askie P, Randleman S, et al. Medication adherence beliefs of community-dwelling hypertensive African Americans. J Cardiovasc Nursing. 2010. May-Jun;25(3):199–206. DOI: 10.1097/JCN.0b013e3181c7ccde. [DOI] [PubMed] [Google Scholar]
- 23.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. New Engl JMed. 2004. August 5;351(6):575–84. DOI: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 24.Cene CW, Roter D, Carson KA, et al. The effect of patient race and blood pressure control on patient-physician communication. J Gen Intern Med. 2009. September;24(9):1057–1064. DOI: 10.1007/s11606-009-1051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper LA. Health disparities. Toward a better understanding of primary care patient-physician relationships. J Gen Intern Med. 2004. September;19(9):985–986. DOI: 10.1111/j.1525-1497.2004.46002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RL, Roter D, Powe NR, et al. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Pub Health. 2004. December;94(12):2084–90. DOI: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brondolo E, Love EE, Pencille M, et al. Racism and hypertension: a review of the empirical evidence and implications for clinical practice. Am J Hypertens. 2011. May;24(5):518–29. DOI: 10.1038/ajh.2011.9. [DOI] [PubMed] [Google Scholar]
- 28.Finkelstein EA, Khavjou OA, Mobley LR, et al. Racial/ethnic disparities in coronary heart disease risk factors among WISEWOMAN enrollees. J Women’s Health (2002). 2004. June;13(5):503–518. DOI: 10.1089/1540999041280963. [DOI] [PubMed] [Google Scholar]
- 29.Karve AM, Ou FS, Lytle BL, et al. Potential unintended financial consequences of pay-for-performance on the quality of care for minority patients. Am Heart J. 2008. March;155(3):571–6. doi: 10.1016/j.ahj.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Mills KT, Obst KM, Shen W, et al. Comparative Effectiveness of Implementation Strategies for Blood Pressure Control in Hypertensive Patients: A Systematic Review and Meta-analysis. Ann Intern Med. 2018. January 16;168(2):110–120. DOI: 10.7326/M17-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Medical Care. March 2009;47(3):356–363. DOI: 10.1097/MLR.0b013e3181893f6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Rev. 2012;6:CD000259 DOI: 10.1002/14651858.CD000259.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh J, McDonald KAM, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 3: Hypertension Care). Rockville MD: 2005. [PubMed] [Google Scholar]
- 34.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. May 20 2008;148(10):717–727. DOI: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- 35.Boonyasai RT, Carson KA, Marsteller JA, Dietz KB, Noronha GJ, Hsu YJ, Flynn SJ, Charleston JM, Prokopowicz GP, Miller ER 3rd, Cooper LA. A bundled quality improvement program to standardize clinical blood pressure measurement in primary care. J Clin Hypertens (Greenwich). 2018. February;20(2):324–333. DOI: 10.1111/jch.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handler J, Zhao Y, Egan BM. Impact of the number of blood pressure measurements onblood pressure classification in US adults: NHANES 1999–2008. J Clin Hypertension. November 2012;14(11):751–759. DOI: 10.1111/jch.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. February 8 2005;111(5):697–716. DOI: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 38.Hussain T, Franz W, Brown E, Kan A, Okoye M, Dietz K, Taylor K, Carson KA, Halbert J, Dalcin A, Anderson CA, Boonyasai RT, Albert M, Marsteller JA, Cooper LA. The Role of Care Management as a Population Health Intervention to Address Disparities and Control Hypertension: A Quasi-Experimental Observational Study. Ethn Dis. 2016. July 21;26(3):285–94. DOI: 10.18865/ed.26.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viswanathan M, Kraschnewski JL, Nishikawa B, et al. Outcomes and costs of community health worker interventions: a systematic review. Medical Care. September 2010;48(9):792–808. DOI: 10.1097/MLR.0b013e3181e35b51. [DOI] [PubMed] [Google Scholar]
- 40.Brownstein JN, Bone LR, Dennison CR, et al. Community health workers as interventionists in the prevention and control of heart disease and stroke. Am J Prev Med. 2005. December;29(5 Suppl 1):128–33. DOI: 10.1016/j.amepre.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007. May;32(5):435–47. DOI: 10.1016/j.amepre.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Boulware LE, Ephraim PL, Hill-Briggs F, et al. Hypertension Self-management in Socially Disadvantaged African Americans: the Achieving Blood Pressure Control Together (ACT) Randomized Comparative Effectiveness Trial. J Gen Intern Med. 2020. January;35(1):142–152. DOI: 10.1007/s11606-019-05396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ. 2009. May 12;180(10):E47–57. DOI: 10.1503/cmaj.090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension: a systematic review and meta-analysis. JAMA Intern Med. 2019. March 1;179(3):351–362. DOI: 10.1001/jamainternmed.2018.6551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boonyasai RT, McCannon EL, Landavaso JE. Automated Office-Based Blood Pressure Measurement: an Overview and Guidance for Implementation in Primary Care. Curr Hypertens Rep. 2019. April 4;21(4):29 DOI: 10.1007/s11906-019-0936-9. [DOI] [PubMed] [Google Scholar]
- 46.Coleman A, Steel S, Freeman P, et al. Validation of the Omron M7 (HEM-780-E) oscillometric blood pressure monitoring device according to the British Hypertension Society protocol. Blood Press Monit. 2008. February;13(1):49–54. DOI: 10.1097/MBP.0b013e3282cb57b6. [DOI] [PubMed] [Google Scholar]
- 47.Cooper LA, Boulware LE, Miller ER 3rd, et al. Creating a transdisciplinary research center to reduce cardiovascular health disparities in Baltimore, MD: Lessons learned. Am J Public Health. 2013. November;103(11):e26–38. DOI: 10.2105/AJPH.2013.301297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73–82. DOI: 10.12927/hcq.2003.16763. [DOI] [PubMed] [Google Scholar]
- 49.National Committee for Quality Assurance. (2013). Case management accreditation requirements. Retrieved from https://www.ncqa.org/programs/health-plans/case-management-cm/benefits-support/standards/.
- 50.Wright JT Jr, Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014. April 1;160(7):499–503. DOI: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 51.Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014March 4;160(5):339–43. DOI: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 52.American Diabetes Assoc. Clinical Practice Recommendations 2007. Diabetes Care. 2007; 30 (Suppl 1):S4–S41. 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 53.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014. June 24;129(25 Suppl 2):S49–73. DOI: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 54.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005. December;40(6 Pt 1):1918–30. DOI: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alegría M, Sribney W, Perez D, et al. The role of patient activation on patient-provider communication and quality of care for US and foreign born Latino patients. J Gen Intern Med. 2009. November;24 Suppl 3:534–41. DOI: 10.1007/s11606-009-1074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986. January;24(1):67–74. DOI: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. National Health Interview Survey. http://www.cdc.gov/nchs/nhis.htm. Accessed on August 6, 2014.
- 58.Glasgow RE, Toobert DJ, Barrera M Jr, et al. The Chronic Illness Resources Survey: cross-validation and sensitivity to intervention. Health Educ Res. 2005. August;20(4):402–9. DOI: 10.1093/her/cyg140. [DOI] [PubMed] [Google Scholar]
- 59.IOM (Institute of Medicine). Wallace RB, Ackermann RT, Basen-Engquist BA, et al. Living well with chronic illness: A call for public health action. Washington, DC: The National Academies Press; 2012. [Google Scholar]
- 60.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed on August 6, 2014.
- 61.PROMIS Network. Measures. http://www.nihpromis.org/measures/measureshome. Accessed August 6, 2014.
- 62.Saha S, Korthuis PT, Cohn JA, et al. Primary care provider cultural competence and racial disparities in HIV care and outcomes. J Gen Intern Med. 2013. May;28(5):622–9. DOI: 10.1007/s11606-012-2298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krupat E, Rosenkranz SL, Yeager CM, et al. The practice orientations of physicians and patients: the effect of doctor-patient congruence on satisfaction. Patient Educ Couns. 2000. January;39(1):49–59. DOI: 10.1016/s0738-3991(99)00090-7. [DOI] [PubMed] [Google Scholar]
- 64.Bobiak SN, Zyzanski SJ, Ruhe MC, et al. Measuring practice capacity for change: a tool for guiding quality improvement in primary care settings. Qual Manag Health Care. 2009. Oct-Dec;18(4):278–84. DOI: 10.1097/QMH.0b013e3181bee2f5. [DOI] [PubMed] [Google Scholar]
- 65.Shortell S, Marsteller JA, Lin M, et al. The Role of Team Effectiveness in Improving Chronic Illness Care. Med Care. November 2004;42(11):1040–1048. DOI: 10.1097/00005650-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 66.National Quality Forum Healthcare Disparities and Cultural Competency Measures. http://www.qualityforum.org/News_And_Resources/Press_Releases/2012/NQF_Endorses_Healthcare_Disparities_and_Cultural_Competency_Measures.aspx. Accessed on August 6, 2014.
- 67.Gittell JH, Seidner R, Wimbush J. A Relational Model of How High Performance Work Systems Work. Organization Science. 2010;21(2): 490–506. 10.1287/orsc.1090.0446. [DOI] [Google Scholar]
- 68.Mosen DM, Schmittdiel S, Hibbard S, et al. Is patient activation associated with outcome of care for adults with chronic conditions? J Ambul Care Manage. 2007;30(1):21–29. DOI: 10.1097/00004479-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991April;10(4):585–98. DOI: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 70.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018. June;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


