Abstract
Objectives
To demonstrate the application of the Large-scale Evidence Generation and Evaluation across a Network of Databases (LEGEND) principles described in our companion article to hypertension treatments and assess internal and external validity of the generated evidence.
Materials and Methods
LEGEND defines a process for high-quality observational research based on 10 guiding principles. We demonstrate how this process, here implemented through large-scale propensity score modeling, negative and positive control questions, empirical calibration, and full transparency, can be applied to compare antihypertensive drug therapies. We assess internal validity through covariate balance, confidence-interval coverage, between-database heterogeneity, and transitivity of results. We assess external validity through comparison to direct meta-analyses of randomized controlled trials (RCTs).
Results
From 21.6 million unique antihypertensive new users, we generate 6 076 775 effect size estimates for 699 872 research questions on 12 946 treatment comparisons. Through propensity score matching, we achieve balance on all baseline patient characteristics for 75% of estimates, observe 95.7% coverage in our effect-estimate 95% confidence intervals, find high between-database consistency, and achieve transitivity in 84.8% of triplet hypotheses. Compared with meta-analyses of RCTs, our results are consistent with 28 of 30 comparisons while providing narrower confidence intervals.
Conclusion
We find that these LEGEND results show high internal validity and are congruent with meta-analyses of RCTs. For these reasons we believe that evidence generated by LEGEND is of high quality and can inform medical decision-making where evidence is currently lacking. Subsequent publications will explore the clinical interpretations of this evidence.
Keywords: hypertension, treatment effects, observational studies, open science, empirical calibration
INTRODUCTION
The Observational Health Data Sciences and Informatics (OHDSI) Large-Scale Evidence Generation and Evaluation across a Network of Databases (LEGEND)1–3 strives to produce reproducible evidence based on existing observational healthcare data, such as electronic health records (EHRs) and administrative claims data, and thus aims to fill in evidence gaps in medicine. Many studies document the limitations of much of the current observational research.4,5 There are challenges related to selective reporting, nonreproducibility, confounding, imprecision, and lack of robust validation. As such, these studies are relegated to lower levels of evidence, and confidence in their ability to make causal inference is low. Meanwhile, evidence gaps in medicine are profound and the vast majority of recommendations in guidelines, even in the most evidence-based fields such as cardiology, are not supported by randomized trials. Clearly, we need more randomized trials, but, in parallel, there is a great need for credible observational evidence to support clinical decision-making.
The LEGEND design and execution is based on its 10 guiding principles,2 aimed at addressing the current concerns about observational research. In brief, these principles prescribe the generation and dissemination of evidence on many research questions at once, for example, comparing all treatments for a disease for a wide range of outcomes, thus increasing the comprehensiveness of evidence and preventing publication bias.6 These questions should be answered using a prespecified and systematic approach, preventing p-hacking (selective reporting). Best-practice statistical methods address measured confounding,7 and control questions (research questions where the answer is known) quantify potential residual bias, expressed in calibrated confidence intervals (CIs)8 and P values.9 Finally, the evidence is generated in a network of databases to assess consistency, by sharing open source analytics code to enhance transparency and reproducibility but without sharing patient-level information, and ensuring patient confidentiality.
To demonstrate and evaluate LEGEND, in this article we apply the LEGEND principles to treatments for hypertension. Antihypertensive therapies carry well-established benefits in reducing blood pressure and the risk of major cardiovascular events. There remain large gaps in evidence about antihypertensive therapy—the health benefits and drug safety concerns of any 1 antihypertensive drug relative to other drugs as first-line therapy remain debatable—but among clinical areas, hypertension is less sparse than others. Reboussin et al10 perform a systematic review of the current evidence from RCTs, and this review constitutes the basis of the recent 2017 American College of Cardiology / American Heart Association Guidelines11 and the 2018 European Society of Cardiology and European Society of Hypertension Guidelines for the management of arterial hypertension.12 However, the study by Reboussin et al presents data on only 40 head-to-head treatment comparisons and is based largely on studies completed before 2000.
We use the LEGEND methods to compare antihypertensive drugs and drug classes on effectiveness and safety outcomes. We start by illustrating our evidence generation process for a single research question. We then assess internal validity of the generated results for all the hundreds of thousands of research questions, based on LEGEND diagnostics defined in the Materials and Methods, and we use the meta-analysis of the Reboussin review as a benchmark to assess external validity. Discussion of the evidence on hypertension treatment itself, and the implications of this evidence for medicine, are beyond the scope of this paper and are instead presented in hypothesis-specific papers.13
MATERIALS AND METHODS
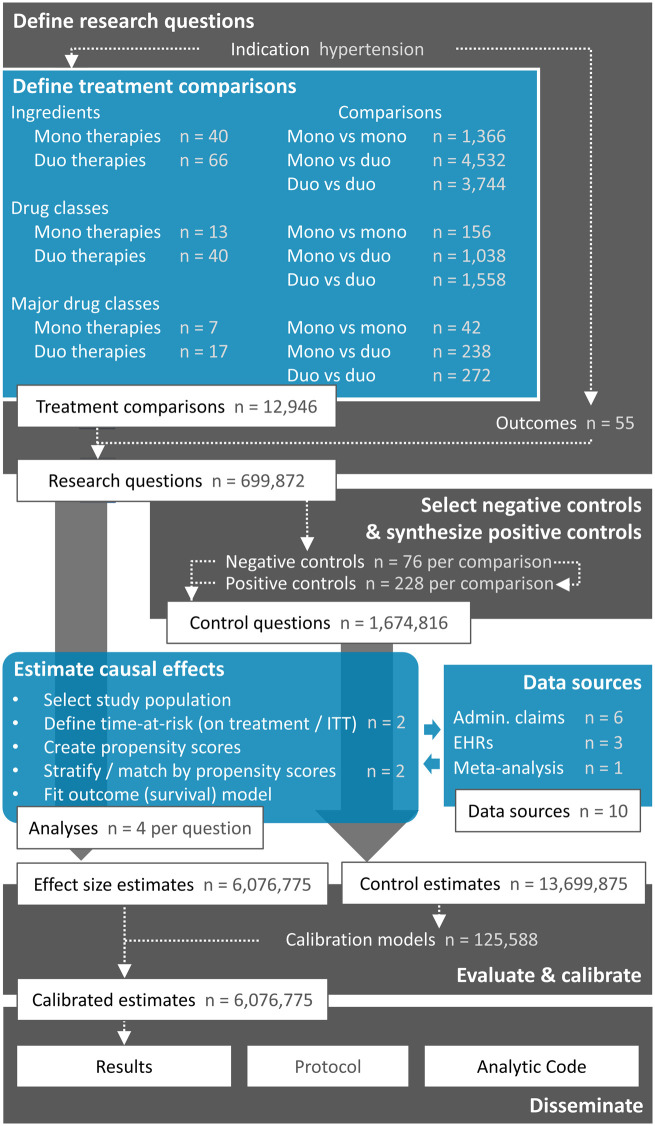
Figure 1 summarizes our approach based on the principles of LEGEND.2 We define a large set of research questions and additionally define a set of control questions where the answer is known. We use effect estimates for the control questions to estimate systematic error distributions (eg, due to confounding, measurement error, and selection bias) and subsequent empirical calibration. We apply a systematic causal effect estimation procedure reflecting current best practices to generate estimates for all questions in an international network of healthcare databases. Each site runs the analysis locally and only shares aggregated statistics. The full result set is made available in an online database, accessible through various web applications. The protocol has been prespecified and made available online, alongside the open source code for executing the entire study.
Figure 1.
Overview of the Large-scale Evidence Generation and Evaluation across a Network of Databases (LEGEND) Hypertension study. The numbers reported here only include those data elements that were actually observed in the data. For exposures, a minimum of 2500 new users in at least 1 database was required. Outcomes had to be observed at least once. Admin. claims = administrative claims, EHRs = electronic health records, ITT = intent-to-treat.
Define a large set of research questions
Treatment comparisons
We analyze all pharmaceutical therapies indicated for hypertension treatment, as listed in the 2017 American Heart Association Guidelines,11 categorized at 3 levels: drug ingredient, class, and major class (Table 1). Because medications are often prescribed in combinations, we also include all possible combinations of 2 treatments (either coprescribed individual drugs or combination products containing both ingredients). Similar to the guidelines, we focus on first-line therapies; we only evaluate the first hypertension treatment a patient receives and disregard all subsequent treatments. Only observed (at least 2500 new users in at least 1 database) treatments are considered and enumerated in Figure 1. For example, even though Table 1 lists 58 ingredients, we observe only 40 in the data and include those in the analysis. Similarly, even though we could hypothetically study 58 * (58 − 1) = 3306 duo-ingredient therapies, only 66 are observed. We define our set of treatment comparisons as all possible (ordered) pairs of treatments.
Table 1.
Hypertension treatments included in this study
| Ingredient | Class | Major class | |
|---|---|---|---|
| Benazepril | Moexipril | ACE inhibitors | Angiotensin converting enzyme (ACE) inhibitors |
| Captopril | Perindopril | ||
| Enalapril | Quinapril | ||
| Fosinopril | Ramipril | ||
| Lisinopril | Trandolapril | ||
| Doxazosin | Terazosin | Alpha-1 blockers | Alpha-1 blockers |
| Prazosin | |||
| Azilsartan | Losartan | Angiotensin receptor blockers | Angiotensin receptor blockers |
| Candesartan | Olmesartan | ||
| Eprosartan | Telmisartan | ||
| Irbesartan | Valsartan | ||
| Atenolol | Bisoprolol | BB cardioselective | Beta-blockers (BB) |
| Betaxolol | Metoprolol | ||
| Nebivolol | BB cardioselective and vasodilatory | ||
| Carvedilol | Labetalol | BB combined alpha and beta receptor | |
| Acebutolol | BB intrinsic sympathomimetic activity | ||
| Penbutolol | Pindolol | ||
| Nadolol | Propranolol | BB non-cardioselective | |
| Amlodipine | Nicardipine | Dihydropyridine CCB (dCCB) | Calcium Channel Blockers (CCB) |
| Felodipine | Nifedipine | ||
| Isradipine | Nisoldipine | ||
| Diltiazem | Verapamil | Nondihydropyridine CCB (ndCCB) | |
| Hydralazine | Minoxidil | Direct vasodilators | Direct vasodilators |
| Eplerenone | Spironolactone | Aldosterone antagonist diuretics | Diuretics |
| Bumetanide | Torsemide | Loop diuretics | |
| Furosemide | |||
| Amiloride | Triamterene | Potassium sparing diuretics | |
| Chlorthalidone | Indapamide | Thiazide or thiazide-like diuretics (THZ) | |
| Hydrochlorothiazide | Metolazone | ||
| Aliskiren | Guanfacine | ||
| Clonidine | Methyldopa | ||
Abbreviations: ACE, angiotensin converting enzyme; BB, beta blocker; CCB, Calcium Channel Blockers; dCCB, Dihydropyridine CCB; ndCCB, Nondihydropyridine CCB; THZ, thiazide.
Outcomes
Table 2 lists the 55 outcomes we include in our study. The primary effectiveness outcomes are acute myocardial infarction (AMI), hospitalization with heart failure, ischemic or hemorrhagic stroke, and a composite cardiovascular event outcome including the first 3 outcomes and sudden cardiac death. Additionally, we define a large set of safety outcomes based on known and suspected side effects of hypertension treatments that are listed on the product labels. The large number of treatment comparisons and outcomes not only allows us to answer more questions, but it also allows us to assess the operating characteristics of our design, answering questions such as whether we have an unexpected number of statistically significant results or whether there is an unusual pattern to the significance (eg, publication bias cuts at P = .05).6
Table 2.
Health outcomes of interest
| Abdominal pain | Dementia | Ischemic stroke |
| Abnormal weight gain | Depression | Malignant neoplasm |
| Abnormal weight loss | Diarrhea | Measured renal dysfunction |
| Acute myocardial infarction | End stage renal disease | Nausea |
| Acute pancreatitis | Fall | Neutropenia or agranulocytosis |
| Acute renal failure | Gastrointestinal bleeding | Rash |
| All-cause mortality | Gout | Rhabdomyolysis |
| Anaphylactoid reaction | Headache | Stroke |
| Anemia | Heart failure | Sudden cardiac death |
| Angioedema | Hemorrhagic stroke | Syncope |
| Anxiety | Hepatic failure | Thrombocytopenia |
| Bradycardia | Hospitalization with heart failure | Transient ischemic attack |
| Cardiac arrhythmia | Hospitalization with preinfarction syndrome | Type 2 diabetes mellitus |
| Cardiovascular event | Hyperkalemia | Vasculitis |
| Cardiovascular-related mortality | Hypokalemia | Venous thromboembolic events |
| Chest pain or angina | Hypomagnesemia | Vertigo |
| Chronic kidney disease | Hyponatremia | Vomiting |
| Cough | Hypotension | |
| Decreased libido | Impotence |
We detect each outcome in the healthcare databases using carefully designed logic combining observed diagnosis, procedure, and other codes (see the protocol in the Supplementary Materials). For example, AMI is detected as the occurrence of an AMI diagnosis code in an emergency room or inpatient setting, with no AMI diagnosis in a similar setting in the prior 180 days. The AMI diagnosis codes are specified using OHDSI’s Observational Medical Outcomes Partnership vocabulary standard concepts that map to the coding systems used in each of the databases (eg, 410.* in ICD-9).
Generate the evidence using best practices
For causal effect estimation, we compare a target treatment (T) to a comparator treatment (C) for the risk of an outcome (O). We define T and C as the first exposure to the treatments defined in Table 1, while requiring at least 1 year of prior observation, no prior hypertension treatment, and no other hypertension treatment starting within 7 days of treatment initiation. We further require a diagnosis of hypertension recorded on or before the day of treatment initiation and no prior outcome O. We use 2 time-at-risk definitions: The on-treatment definition considers risk to start on the day after treatment initiation and end at treatment end, allowing for a maximum gap of 30 days between prescriptions. The intent-to-treat definition starts on the day after treatment initiation and stops at the end of observation. For each comparison, the study is restricted to the calendar time period when both treatments are observed in the database so that we compare drugs only during times when both are on the market.
We conduct our cohort study using the open-source OHDSI CohortMethod R package,14 whose large-scale analytics are achieved through the Cyclops R package.15 To account for the fact that treatment assignment is not random, resulting in imbalance between the target and comparator cohorts, we employ large-scale regularized regression to fit propensity models16 using tens of thousands of baseline covariates.7 These covariates include demographics, all prior drugs, conditions, procedures, etc. Hazard ratios are computed using Cox proportional hazards models conditioned on the propensity score-matched or stratified sets and are combined using meta-analysis for random effects. In addition, diagnostics on patient characteristic balance (ie, is every covariate in the propensity model balanced between the cohorts) and empirical clinical equipoise between exposure cohorts (is there sufficient overlap between the cohorts that an adjustment is possible, quantified using a preference score derived from the propensity score16,17) are generated.
Empirically evaluate through the use of control research questions
To diagnose and correct for residual confounding, we use control questions. Control questions are questions where the answer is known. We distinguish between negative controls, where the true hazard ratio is assumed to be 1, and positive controls with a known effect size greater than 1. We identify 76 negative controls—outcomes that are not believed to be caused by any hypertension treatment—through a data-rich algorithm18 based on the literature, drug product labels, spontaneous reports, drug knowledge bases, and manual review (see the protocol in the Supplementary Materials). We use these to additionally generate 3*76 = 228 synthetic positive controls with effect sizes 1.5, 2, and 4 by inserting simulated events into real data based on a prognostic model.8 We estimate effect sizes for these control outcomes in each treatment comparison using the same evidence generation process as used for the research questions of interest. This allows us to evaluate the operating characteristics of our process (eg, how often the 95% CI contains the true effect size), and these characteristics are used to subsequently calibrate our CIs8 and P values.9
Generate the evidence for all questions across a network of databases
We executed the LEGEND Hypertension study across the OHDSI research network1 and included 9 databases covering 4 countries. Six databases contain administrative claims: IBM MarketScan Commercial Claims and Encounters, IBM MarketScan Medicare Supplemental Beneficiaries, IBM MarketScan Multi-state Medicaid, Optum ClinFormatics, Japan Medical Data Center, and the Korea National Health Insurance Service National Sample Cohort. Three databases contain electronic health records (EHRs): Optum deidentified Electronic Health Record Dataset (PanTher), Columbia University Medical Center, and QuintilesIMS Disease Analyzer Germany. In addition to the per-database effect size estimates, we also report summary estimates across databases using meta-analysis for random effects.19 We compute the I2 heterogeneity metric to assess between-database consistency.20 An I2 of zero means no between-database heterogeneity is observed.
Disseminate the generated evidence
We support open dissemination of generated evidence. Access to the database server containing the full results is available from the authors upon request. We have developed 2 web applications that connect to the database for exploring the results: The LEGEND Basic Viewer and LEGENDMed Central. The protocol and analytic code are posted publicly (https://github.com/OHDSI/Legend).
Internal validity
To assess internal validity, we examine the following properties.
Balance of the covariates demonstrates whether propensity score adjustment successfully creates comparison groups with similar characteristics. Our very strict goal is to achieve a standardized difference of the mean of 0.1 or less for every 1 of the thousands of measured covariates. Negative and positive controls allow us to assess residual confounding and calibrate CIs so that calibrated 95% CIs actually contains the true effect size 95% of the time. We report measured coverage of the calibrated CIs,21 aiming for 95%.
The quantity I2 is often used to test whether there is consistency among studies in a meta-analysis.20 It is the percentage of total variation across studies that is due to heterogeneity rather than chance. We report between-database consistency as the proportion of hypotheses, after calibration, for which I2 is below 0.25. We also graph the studies so that readers can inspect the overlap among estimates and among CIs.
We report transitivity as the proportion of studies for which superiority of 1 drug over a second, and that second drug over a third is accompanied by the finding that the first is superior to the third.
We assess whether balancing on a large number of covariates can improve balance on an unmeasured confounder using baseline blood pressure as an example. Blood pressure is not captured adequately in most of the databases included in LEGEND, except for the PanTher database. Using this database, we report covariate balance and the difference in effect size estimates with and without baseline blood pressure in the propensity model.
External validity
We compare the results of a meta-analysis across our databases to the direct meta-analysis of all RCTs included in the recent systematic review.10 For each comparison, we compute the P value against the null hypothesis of no difference using:
Where and denote the hazard ratios of the RCT and LEGEND meta-analyses, respectively. and denote the standard errors of the RCT and LEGEND meta-analyses, respectively, and denotes the cumulative distribution function of the standard normal distribution. We report concordance between our results and those of the RCT meta-analysis as the number of comparisons where . We also inspect the statistical significance and direction of the results.
RESULTS
In total, we analyze data from 21.6 million unique antihypertensive new users (the union of all unique patients entered in at least 1 Cox regression) to generate 6 076 775 effect size estimates answering 699 872 research questions, each including full diagnostics and all additional validity checks.
Exemplar research question: The effect of lisinopril compared to amlodipine on the risk of angioedema
For illustration, we highlight just 1 of the 699 872 research questions: the effect of lisinopril compared to amlodipine on the risk of angioedema. Table 3 reports the numbers of patients initiating treatment of 1 of these drugs (monotherapy) in each database and includes the number of angioedema events observed during the on-treatment time-at-risk. We furthermore report the number of covariates constructed in each database for these patients, and the maximum absolute standardized difference of the mean across covariates before and after propensity score matching. As shown, all of the thousands of covariates had an absolute standardized difference of the mean smaller than 0.1, indicating good balance. Note that some databases do not have sufficient exposure to both drugs and are therefore omitted.
Table 3.
Counts and maximum standardized difference per database. T = lisinopril, C = amlodipine, Outcome = angioedema, Max std. diff. = Maximum absolute standardized difference of means between T and C
| Subjects |
Patient years |
Outcomes |
Covariate count | Max std. diff. |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Source | T | C | T | C | T | C | Before | After | |
| CCAE | 647 212 | 183 776 | 465 516 | 126 173 | 671 | 97 | 9765 | 0.407 | 0.029 |
| MDCD | 59 897 | 26 525 | 29 464 | 11 187 | 150 | 26 | 12 703 | 0.526 | 0.048 |
| MDCR | 73 821 | 32 375 | 61 864 | 28 107 | 99 | 18 | 11 217 | 0.363 | 0.029 |
| Optum | 447 905 | 143 079 | 340 148 | 107 631 | 475 | 88 | 11 903 | 0.432 | 0.028 |
| PanTher | 651 707 | 201 527 | 243 121 | 71 438 | 236 | 44 | 10 548 | 0.425 | 0.022 |
| Total | 1 884 874 | 590 945 | 1 146 421 | 348 158 | 1646 | 279 | |||
Abbreviations: CCAE, Commercial Claims and Encounters; MDCD, Multi-state Medicaid; MDCR, Medicare Supplemental Beneficiaries; Optum, Optum ClinFormatics; PanTher, Optum deidentified Electronic Health Record Dataset.
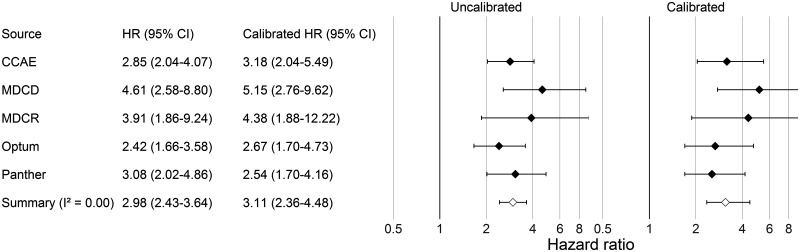
Figure 2 reports the estimated hazard ratios for the risk of angioedema with lisinopril compared to amlodipine, for each database separately as well as a summary estimate. This same procedure is used not only for angioedema, but also for the 304 control outcomes. Using the estimates for these control questions, we fit a systematic error model and use it to compute the empirically calibrated estimates reported in Figure 2.
Figure 2.
Hazard ratio (HR) estimates (and 95% CIs) before and after empirical calibration for lisinopril compared to amlodipine for the risk of angioedema, when using propensity score matching and an on-treatment time-at-risk window.
The results above use the on-treatment time-at-risk window and propensity score matching, producing a calibrated hazard ratio of 3.11 (2.36–4.48). When using an intent-to-treat window instead, the calibrated summary hazard ratio is 1.92 (1.57–2.40). Using propensity score stratification, the calibrated summary hazard ratio is 2.52 (1.95–3.32) and 1.59 (1.31–1.95) for the on-treatment and intent-to-treat window, respectively.
Internal validity across all research questions
We now report the internal and external validity checks across all research questions examined in this LEGEND hypertension study.
Balance
When using propensity score matching, we achieve balance (standardized difference of the means < 0.1 on every 1 of the thousands of included covariates) for 75% of the 1 665 176 effect size estimates. When using propensity score stratification this is 19% of 2 280 73 estimates. Note that we can compute fewer estimates when using matching compared to stratification because matching may lead to removal of subjects for which no match could be found.
Empirical evaluation and calibration
Of the 13 699 875 control estimates, 79.9% contain the true effect size within the 95% CI before calibration. That is, according to our negative and positive controls, when we calculate a 95% CI, that interval includes the true value only 79.9% of the time, implying more than an expected number of false-positive results would be reported. After our empirical calibration the coverage becomes 95.7% (nominal = 95%).
Between-database consistency
We identify 37 953 target-comparator-outcome triplets having sufficient data in at least 4 databases to compute an estimate for the analysis specifying an on-treatment time-at-risk using propensity score matching. Across databases, 78% of calibrated estimates have an I2 below 0.25, corresponding to low heterogeneity.20 That is, in 78% of our eligible comparisons, the databases were consistent with each other under the most conservative threshold (ie, 0.25). In contrast, only 64% of the estimates have an I2 below 0.25 when no calibration is applied. The I2 score is computed and available for all meta-analyses in the results database. In this manuscript, we report all meta-analysis estimates irrespective of I2.
Transitivity
If treatment A has a statistically significant higher risk than treatment B for a particular outcome, and treatment B has a statistically significant higher risk than C for that same outcome, we expect A to have a statistically significant higher risk than C. In total, we identify 653 595 such A-B-C combinations in the calibrated meta-analyses results, of which for 554 268 triplets (84.8%) the transitivity property holds.
Effect of not explicitly adjusting for baseline blood pressure
Supplementary Table S1 evaluates the balance on blood pressure before and after propensity score matching for a select number of treatment comparisons in the PanTher database. Before matching, there is an absolute standardized difference of means greater than 0.1 for almost all comparisons, suggesting the treatment groups have different baseline blood pressure. After matching on propensity scores using the original set of covariates—which excludes blood pressure—we see a reduced imbalance in blood pressure, but a few comparisons cross our predefined threshold of 0.1. To evaluate whether these residual imbalances should cause bias, we repeat our analysis including blood pressure in the propensity model. Supplementary Table S1 shows that after matching on these propensity scores, baseline blood pressure is nearly perfectly balanced. Supplementary Figure S1 shows that use of the 2 different propensity scores has little to no impact on the hazard ratio estimates produced.
External validity
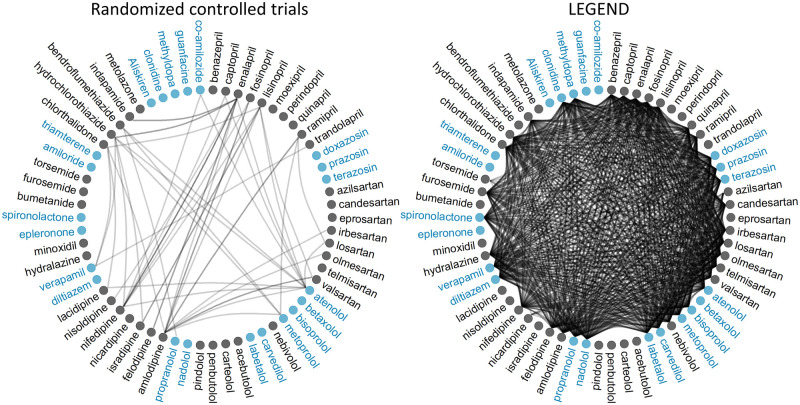
We evaluate external validity by comparing the LEGEND results to meta-analyses of randomized controlled trials (RCTs). All RCTs included in the recently published systematic review cover the 40 head-to-head comparisons of drugs shown in the left of Figure 3.10 In contrast, LEGEND analyzes 12 946 treatment comparisons for each outcome. The mono-ingredient versus mono-ingredient comparisons performed are shown in the right of Figure 3. The sample size (subjects) for comparisons in published RCTs varied from 102 to 33 000,10 with a median of 1148. In contrast, the sample size for comparisons in LEGEND (excluding meta-analyses) varied from 692 to 1.2 million, with a median of 33 771.
Figure 3.
Comparisons of single-drug hypertension treatments in randomized controlled trials (left) and in LEGEND (right). Each circle represents an ingredient. Color groupings indicate drug classes. A line between circles indicates the 2 drugs are compared in at least 1 study.
Concordance
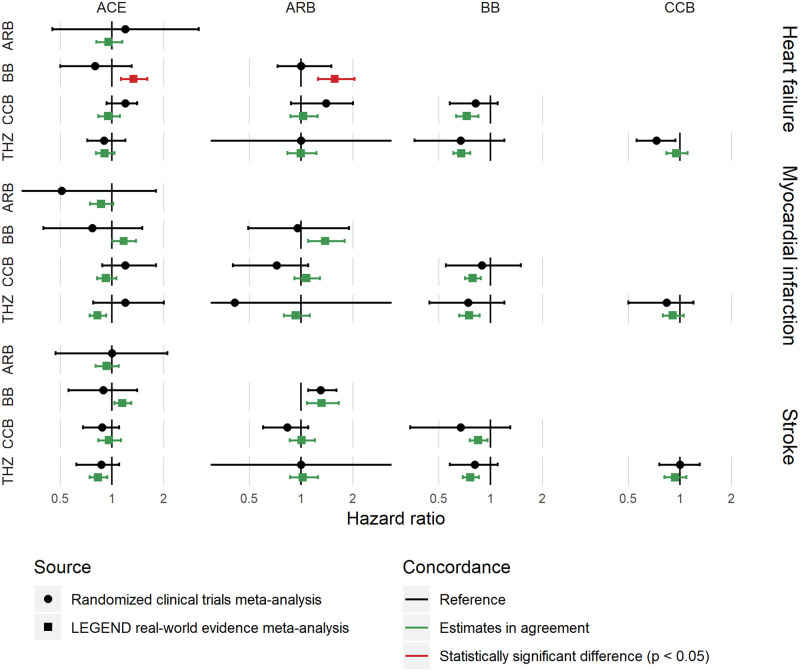
Of the 40 comparisons reported in the systematic review, 30 overlapped with research questions addressed in LEGEND. Of these 30 comparisons, 28 (93%) show no statistical difference in estimates, and 2 (7%) have a nominal P value < .05 indicating different estimates. Note that these numbers do not correct for multiple testing, so 5% are expected to have P < 0.05 when no real differences exist. Figure 4 shows, for 3 effectiveness outcomes, the concordance between results from LEGEND and direct meta-analyses of RCTs.10 Of the 30 comparisons, 16 agreed on the significance and direction of the result (both not significant or both significant in the same direction), 1 was significant in the systematic review but not significant in LEGEND, and 13 were significant in LEGEND but not in the systematic review. The interpretation of this is in the Discussion.
Figure 4.
Concordance between LEGEND meta-analysis results (using propensity score matching with an on-treatment risk window as well as empirical calibration) and the results from meta-analyses of randomized controlled trials. ACE = ACE inhibitors, ARB = Angiotensin receptor blockers, BB = Beta-blockers, CCB = Calcium channel blockers, THZ = Thiazide or thiazide-like diuretics. Hazard ratios greater than 1 indicate greater risk for the drug class at the left.
DISCUSSION
In this study we apply the LEGEND principles to systematically compare all pharmaceutical therapies indicated for hypertension treatment, considering a wide range of effectiveness and safety outcomes. We find that these LEGEND results have high internal validity and are statistically congruent with available meta-analyses of RCTs, although with its greater sample size, LEGEND often found statistical significance where the meta-analyses did not.
For internal validity, we find that propensity score matching achieves balance on every 1 of the thousands of covariates for 75% of effect size estimates, despite our very stringent criterion (absolute standardized difference < 0.1), which is normally used in studies with only a handful of covariates.22 Even without empirical calibration, coverage of the 95% CIs remained relatively high at 79.9% compared to the 6%–88% observed in prior research.8 After calibration, this coverage became almost identical to the nominal 95%. High consistency between databases, as expressed in low I2 scores, suggests the results are robust and not due to database idiosyncrasies. This is even more remarkable when considering the heterogeneity of the populations across the OHDSI research network, which included EHR and administrative claims data from 4 countries. We observe a transitivity of 84.8%, but this number is hard to interpret. To our knowledge, no one has ever attempted to quantify transitivity in scientific results before. We would not expect a transitivity of 100% because of varying standard errors in our meta-analytic estimates (which assume random effects), and because for each comparison we restrict to the calendar time when both drugs are on the market.
For external validity, we compare the LEGEND results to a set of direct meta-analyses of RCTs, which were used to generate recent hypertension treatment guidelines. LEGEND results showed high concordance with the meta-analysis (Figure 4), showing a statistically significant difference from the meta-analysis at about the rate one would expect based on random error alone, if no real differences exist (7% observed, 5% expected). Because of the larger sample size, LEGEND results tend to have narrower CIs. In some cases, the LEGEND point estimate can be on the opposite side of 1 from the RCTs point estimate, and still be concordant if their CIs overlap sufficiently. We emphasize that when a meta-analysis CI includes 1, the formal conclusion is not “no effect” but instead insufficient evidence to conclude an effect. Therefore, even if LEGEND shows a statistically significant effect where the RCT meta-analysis demonstrates no effect (eg, beta blockers compared to thiazides for all 3 outcomes), they may still be consistent. In fact, this is 1 of the goals of LEGEND, to use its larger sample size to more precisely measure effects and to uncover effects masked by the RCTs’ small sample sizes. Therefore, while we do not know the underlying true effect sizes and cannot credit LEGEND for getting a better estimate than the meta-analysis, similarly we cannot count it against LEGEND that it had a narrower interval and apparently uncovered an effect. The best we can say is that the meta-analysis and LEGEND overlapped sufficiently (ie, sufficiently that they could have been drawn from the same theoretical population of studies). Of the 2 hypotheses that were statistically significant in the meta-analysis, LEGEND was statistically consistent with both (ie, the CIs were overlapping enough) but in 1 of the 2, LEGEND was not statistically significant. Here again, in theory, the meta-analysis and LEGEND could have been drawn from the same theoretical population of studies, just as RCTs for the same comparison sometimes differ in statistical significance. We recognize that merely guessing no effect with a wide CI would have captured all the RCT meta-analysis results, producing even better concordance, but LEGEND did not in fact do that and instead produced narrower intervals that overlapped the meta-analysis results.
The reason for the discordance between LEGEND and the meta-analysis on beta-blockers versus angiotensin-converting enzyme inhibitors and versus angiotensin receptor blockers remains unclear. It could be chance, residual bias in the observational study, or a difference in populations (RCTs tend to be run on sicker patients with a history of hypertension treatment, sometimes on several drugs, whereas this LEGEND study was focused on first-time use of an antihypertensive drug).
The LEGEND meta-analyses provide estimates for 699 872 unique research questions and can thus help inform clinical decision-making when other information is not available. Reviewing all these other results goes beyond what can be discussed in this article. We are in the process of writing several clinical papers on specific questions likely to be of high interest, and have already published a paper based on these LEGEND results comparing first-line hypertension treatments at the class level.13 This article demonstrates that even though the current guidelines11 do not distinguish between a wide set of drugs as the recommended choice for first-line treatment, there are differences in the effectiveness and safety of these drugs that warrant consideration. Clinical researchers and appropriately trained clinicians can consult the web apps themselves to seek answers for specific questions they have. Each LEGEND result comes with full diagnostics, including a description of the study population, key characteristics, propensity score distribution, covariate balance, bias distribution as estimated using negative and positive controls, and Kaplan–Meier plots. We invite others to develop other apps or to help interpret these results in other ways.
LEGEND answers each question using best practices, including advanced methods for adjusting for confounding, producing study diagnostics, and replication across a network of databases. For these reasons, we believe the evidence LEGEND generates, in this case for treatments of hypertension, is of high quality, and can inform medical decision-making where evidence is currently lacking.
LEGEND uses large-scale propensity scores as the primary means to adjust for confounding. The technique has shown promise7 and, in this experiment, demonstrated that it adjusted for an important potential confounder, baseline blood pressure, without including it in the model. Other methods are also possible, such as use of a more traditional (ie, not large-scale) propensity score, but incorporating a prognostic score23 to produce an approach that may be doubly robust. Comparing these techniques is out of scope for this article, but further research in this area is warranted.
Limitations
Like all observational research, LEGEND is still vulnerable to residual bias due to confounding and measurement error. However, in contrast to the vast majority of published observational studies, LEGEND provides a rich set of diagnostics to evaluate whether we can trust the results, including covariate balance and estimates for control questions. Any systematic error observed through the control questions is incorporated in calibrated CIs and P values, thus conveying the limits of what can be learned from these data.
One limitation of using existing healthcare data for research purposes is that some variables of interest may not be recorded systematically. In this study, the most prominent example is blood pressure, which ideally would have been included in the propensity score, but is not available in all databases. Our sensitivity analysis in PanTher indicates that access to these data does not meaningfully change the effect size estimates, suggesting that baseline blood pressure is either not an important confounder or, more likely, is already sufficiently adjusted for by the large-scale propensity scores through observed proxy variables. Similarly, postintervention blood pressure could have been included as an outcome, but, because of its unavailability in the databases, we did not use it.
We purposely do not correct for multiple hypotheses in our results because that can only be done once researchers choose a specific hypothesis or set of hypotheses. As when using results from the literature, it is important to consider false positives when faced with multiple testing, and our results readily allow for adjustment for multiple testing because we disseminate all results.
CONCLUSION
We find that the LEGEND results have high internal validity and are congruent with direct meta-analyses of RCTs. Even though many RCTs inform on the effects of hypertension treatments, much uncertainty remains. By following the LEGEND guiding principles that address study bias, p-hacking, and publication bias, LEGEND seeks to augment existing knowledge by generating reliable evidence from existing healthcare data, answering hundreds of thousands of research questions simultaneously using a transparent, reproducible, and systematic approach.
FUNDING
This work was supported in part by National Science Foundation grant IIS 1251151, National Institutes of Health grant R01 LM006910, and Australian National Health and Medical Research Council grant GNT1157506.
AUTHOR CONTRIBUTIONS
Each author made substantial contributions to the conception or design of the work; was involved in drafting the work or revising it critically for important intellectual content; gave final approval of the version to be published; and has agreed to be accountable for all aspects of the work.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1.Hripcsak. G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform 2015; 216: 574–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Schuemie MJ, Ryan PB, Pratt N, et al. Principles of Large-Scale Evidence Generation and Evaluation across a Network of Databases (LEGEND). J Am Med Inform Association. 27(8):1331--1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioannidis JPA. Why Most Published Research Findings Are False. CHANCE 2019; 32: 4–13. doi:10.1080/09332480.2019.1579573 [Google Scholar]
- 4.Rush CJ, Campbell RT, Jhund PS, et al. Association is not causation: treatment effects cannot be estimated from observational data in heart failure. Eur Heart J 2018; 39: 3417–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein HC, McMurray J, Holman RR. Real-world studies no substitute for RCTs in establishing efficacy. Lancet 2019; 393: 210–1. [DOI] [PubMed] [Google Scholar]
- 6.Schuemie MJ, Ryan PB, Hripcsak G, et al. Improving reproducibility by using high-throughput observational studies with empirical calibration. Philos Trans A Math Phys Eng Sci 2018; 376. doi: 10.1098/rsta.2017.0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol 2018; 47 (6): 2005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuemie MJ, Hripcsak G, Ryan PB, et al. Empirical CI calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci USA 2018; 115 (11): 2571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuemie MJ, Ryan PB, DuMouchel W, et al. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med 2014; 33 (2): 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138: e595–616. [DOI] [PubMed] [Google Scholar]
- 11.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71 (6): 1269–324. [DOI] [PubMed] [Google Scholar]
- 12.Williams B, Mancia G, Spiering W, et al. ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36 (10): 1953–2041. [DOI] [PubMed] [Google Scholar]
- 13.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019; 394 (10211): 1816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuemie MJ, Suchard MA, Ryan PB. CohortMethod: New-user cohort method with large scale propensity and outcome models. 2018. https://ohdsi.github.io/CohortMethod/
- 15.Suchard MA, Simpson SE, Zorych I, et al. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul 2013; 23 (1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects.Biometrika 1983;70:41–55.; doi: 10.21236/ada114514. [Google Scholar]
- 17.Walker A, Lauer Patrick, et al. A tool for assessing the feasibility of comparative effectiveness research. Comparative Effect Res 2013; 3: 11–20. [Google Scholar]
- 18.Voss EA, Boyce RD, Ryan PB, et al. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform 2017; 66: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7 (3): 177–88. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327 (7414): 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuemie MJ, Soledad Cepede M, Suchard MA, et al. How Confident Are We about Observational Findings in Health Care: A Benchmark Study. 2.1. 2020; doi: 10.1162/99608f92.147cc28e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28 (25): 3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen BB. The prognostic analogue of the propensity score. Biometrika 2008; 95 (2): 481–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.