Abstract
Background:
It is well-documented that African Americans have elevated risk for cognitive impairment and dementia in late life, but reasons for the racial disparities remain unknown. Stress processes have been linked to premature age-related morbidity, including Alzheimer’s and related dementias (ADRD), and plausibly contribute to social disparities in cognitive aging.
Objective:
We examined the relationship between stressful life events and cognitive decline among African American and White participants enrolled in the Wisconsin Registry for Alzheimer’s Prevention (WRAP).
Methods:
Linear mixed models including demographic, literacy, and health-related covariates were used to estimate (1) relationships between a life event index score and decline in cognitive test performance in two domains of executive function (Speed & Flexibility, Working Memory) and one domain of episodic memory (Verbal Learning & Memory) among 1,241 WRAP enrollees, stratified by race, and (2) contributions of stressful life events to racial differences in cognition within the full sample.
Results:
African Americans (N = 50) reported more stressful life events than Whites (N = 1,191). Higher stress scores associated with poorer Speed & Flexibility performance in both groups, though not with declines across time, and partially explained racial differentials in this domain. Among African Americans only, stressor exposure also associated with age-related decline in Verbal Learning & Memory. Stressor-cognition relationships were independent of literacy and health-related variables.
Conclusions:
Greater lifetime stress predicted poorer later-life cognition, and, in a small sample of African Americans, faster declines in a key domain of episodic memory. These preliminary findings suggest that future work in large minority aging cohorts should explore stress as an important source of modifiable, socially-rooted risk for impairment and ADRD in African Americans, who are disproportionately exposed to adverse experiences across the life course.
Keywords: African Americans, Alzheimer’s disease, life course, stress
INTRODUCTION
The prevalence of Alzheimer’s disease and related dementias (ADRD) and age-related cognitive impairment are increasing across sociodemographic strata as the U.S. population ages, but the burden is not equally distributed [1]. Though African Americans continue to be underrepresented in cognitive aging research, there is substantial evidence that when compared with age-matched Whites, they are at increased risk for dementia [2-4]. Cognitive function in midlife and early old age may be an important determinant of ADRD onset in racial minority populations [5], and studies in diverse combined [6] and population-based [7] cohorts demonstrate that African Americans also experience more subclinical impairment in both episodic memory and executive function. Cognitive aging disparities remain underexplained: genetic factors such as the apolipoprotein E (APOE) ε4 allele that contribute to ADRD-related changes in predominantly White cohorts [8, 9] appear more prevalent but less relevant in African Americans [10], and substantial efforts dedicated to identifying cardiovascular mechanisms that might operate differentially by race have also failed to fully account for the disproportionate risk [3, 11].
In this paper, we propose that stress-related mechanisms empirically shown to contribute to cognitive aging processes are likely to be an important determinant of cognitive outcomes among older African Americans, and to play a role in racial disparities in cognitive health. Exploration of socioenvironmental contexts across the life course is likely to clarify disproportionate risk for dementia and age-related cognitive impairment in racial and ethnic minorities including African Americans. Health disparities frameworks posit African American race as a fundamental determinant of health [12] operating through pervasive structural and institutionalized inequalities that shape more proximal risk exposures and systematically disadvantage members of minority racial groups across the life course. As such, at a population level African American race is often a risk marker for a number of independent ADRD risk factors including neighborhood disadvantage, reduced healthcare access, undertreated comorbidities, and socioeconomic deprivations including poorer quality of early life schooling [13, 14]. Given these associations, it is unsurprising that a substantial body of evidence suggests African Americans of all ages also report experiencing more acute and chronic stressors than their White counterparts [15-17].
Disproportionate exposure to stressors plausibly contributes to racial disparities in age-related cognitive impairment and ADRD risk. In cross-sectional and longitudinal studies, measures of both objective and perceived stress have been associated with poorer cognitive function [18, 19] and increased risk for Alzheimer’s disease [20, 21]. Stress may impact memory and executive function processes directly through structural and functional changes in key brain regions: chronic stress associates with lower hippocampal volume in animal [22, 23] and human [24, 25] models, and cumulative exposure to adverse life events has been linked to reduced volume in the hippocampus [26] and the medial prefrontal cortex [27]. Additionally, stress exposures have been associated with longer-term metabolic and vascular disorders such as diabetes [28] and hypertension [29] that represent strong independent risk factors for cognitive decline and dementia in later life [30, 31], and to acute risk factors for impairment such as depression [32, 33] and sleep disturbance [34, 35].
Despite well-established dual associations between African American race and stress, and between stress and cognitive aging, the contribution of stress to cognitive aging processes among African Americans and to racial disparities in cognitive health has only rarely been explored [15, 36, 37]. In this paper, we describe an analysis designed to preliminarily assess the role of cumulative stressful life events. We utilize a middle-aged and older cognitive aging study cohort that includes a small but well-characterized sample of African American participants. In primary analyses, we stratify by race to explore within-group relationships [38] between a life event index score and rate of cognitive decline over time in three cognitive domains vulnerable to early age-related changes [39]. In secondary analyses, we explore the contribution of stressful life event exposure to race differentials in cognitive function among the sample as a whole.
METHODS
Participants
Participants were selected from the Wisconsin Registry for Alzheimer’s Prevention (WRAP). The WRAP study is a longitudinal study of cognitive aging in a cohort of middle-aged and older adults enriched for a parental history of Alzheimer’s disease [40]. Participants in the parental history-positive group (73% of all WRAP enrollees) have at least one biological parent with dementia attributed to Alzheimer’s disease as determined by a review of parental medical records (and autopsy records, when available) or by administering a Dementia Questionnaire [41] to the adult child. Most WRAP participants volunteered for the study after learning of it via statewide educational presentations or word of mouth. A portion of the parental history-positive participants were recruited during a clinical evaluation of their parent in a University of Wisconsin or satellite Memory Assessment Clinic. WRAP participants were between the ages of 40 and 65 at enrollment, were cognitively intact at enrollment as determined by neuropsychological test performance, and speak English as their primary language. Enrollment began in 2001 and has been conducted on a rolling basis with recent efforts prioritizing racial diversification of the cohort [42].
The WRAP protocol includes a baseline visit (Wave 1), a second visit after approximately four years (Wave 2), and subsequent visits approximately every two years (Waves 3–6). Visits are conducted at one of three study sites: a large urban center, a mid-sized city that is home to a state university, and a small city. A majority (90%) of African American participants attend the site based in the large urban center. The Wave 1 protocol does not include collection of social data; a stressful life events questionnaire was included in the Wave 2 protocol between 2006 and 2015. WRAP participants were only included in the current analytic sample if they attended their Wave 2 visit within that time frame and provided complete lifetime experience data. Seven participants who were determined by clinical consensus to meet diagnostic criteria for mild cognitive impairment, Alzheimer’s disease, or another dementia at any follow-up WRAP study visit were excluded. Finally, 35 participants who identified their primary race as Hispanic/Latino, American Indian, Asian, or Other were excluded from analyses because their numbers were not large enough to create independent racial categories. The analytic sample ultimately included 1,191 White participants and 50 African American participants. Of these 1,241 individuals, the number of participants who most recently completed a Wave 2, 3, 4, 5, or 6 visit was 92, 161, 319, 469, or 200, respectively. 91% of White participants and 92% of African American participants in the analytic sample remained actively enrolled at the time of analysis. This study was conducted with the approval of the University of Wisconsin Health Sciences Institutional Review Board and all participants provided signed informed consent prior to enrollment.
Measures
Each WRAP visit is approximately five hours in duration, and participants provide self-reported data on health history, psychosocial factors, and lifestyle. A nurse collects blood samples and clinical data. A trained psychometrist administers a battery of neuropsychological tests. A detailed description of the WRAP study protocol and overall sample characteristics is available in a recent publication [40].
Cognitive outcomes
Key cognitive outcome variables included three cognitive factor scores from each WRAP visit, determined via a previously published factor analysis [43]. These composite factor scores represent domains of episodic memory and executive function, and were used to reduce the potential for measurement error and Type I error associated with multiple comparison testing. The cognitive factors in this analysis, Speed & Flexibility, Working Memory, and Verbal Learning & Memory were selected for their sensitivity to age-related change. Speed & Flexibility is derived from time to completion on Trailmaking Tests A & B [44] and the number of items completed on the Stroop Test Color-Word Interference condition [45]. A second measure of executive function, Working Memory, is derived from the number of correct items on the Digit Span Forward, Digit Span Backward, and Letter-Number Sequencing subtests of the Wechsler Adult Intelligence Scale-III [46]. Verbal Learning & Memory, representing episodic memory, was derived from the Rey Auditory Verbal Learning Test (RAVLT), specifically RAVLT Learning Trials 3–5 and the RAVLT Delayed Recall Trial [47]. Factor scores have been standardized [~N (0,1)] into z-scores, using means and SDs obtained from the WRAP Wave 1 baseline sample.
Lifetime stressors
Data were drawn from a Stressful Life Events Inventory adapted from a Midlife in the United States study [48] measure [49] that was itself based on standard life event instrumentation [50]. The inventory, administered at Wave 2 visits for every participant, included 27 different potentially stressful events (e.g., flunking out of school, parental alcohol abuse, involuntary unemployment, the death of a child) spanning the life course. Lifetime stressor exposure was operationalized as a count of self-reported events. Every event a participant reported experiencing was scored as a “1” on a binary 0-1 scale and these were summed to create a cumulative index of stressful life events.
Self-identified race and covariates
WRAP participants are asked to specify their race/ethnicity at their baseline visit and questionnaire formatting requires participants to select a single response. The current study included those participants who identified as “White/Caucasian” or as “Black/African American”.
Covariates were chosen a priori based on a review of cognition and disparities literature. Demographic covariates included age at visit, gender, and educational attainment. Age was calculated for each visit based on date of birth and analyzed as a continuous variable. To maximize model parsimony and minimize collinearity, baseline age was not included, and it should be noted that with this approach, age-at-visit estimates represent a weighted average of the within-person (time) effects and between-person (cohort) age effects [51]. Gender was self-reported as “Male” or “Female” and analyzed as a binary variable. Educational attainment was reported and analyzed as a continuous variable but was winsorized at a maximum of 20 years. In addition to base demographics, covariates included APOE ε4 carrier status and a control for practice effects, operationalized as the number of visits completed following Wave 1. Late-life literacy, as measured by the WRAT 3 Reading Score [52], was included as a proxy for cumulative educational quality in early life and intellectually enriching activities in adulthood [13]. Two stress-correlated health variables, smoking status and body mass index (BMI), were also included in final models. Participants were coded as never, ever, or current smokers. Height and weight were measured at the time of visit and BMI was calculated as kg/m2.
Data analyses
Analyses were performed using SAS version 9.4 and R version 3.5.1. Characteristics of African American and White participants were compared using Mann-Whitney U tests and Chi-squared tests within the analytic sample. In our primary stratified analyses, we utilized linear mixed effects regression within White and African American samples. We modeled each of the three cognitive factor scores as a function of the life event index score while adjusting for additional covariates. Time was operationalized as the age at each WRAP visit. Covariates were sequentially added to three nested models to assess the contributions of each set to stress-cognition relationships. Ultimately, the three cognitive factor scores were regressed on the life event index in (1) a base model controlling for time [age at visit, centered at sample mean across all visits], gender, years of education, APOE ε4 carrier status, practice effects, the interaction of stress*age, and subject specific random effects for intercept and age-related slope; (2) a model that added a measure of literacy to assess any confounding of stress-cognition relationships by poor educational quality; and (3) a model, including literacy, that added two health-related factors, smoking and BMI, that potentially confound relationships between stress and cognitive health.
In order to directly assess the contribution of stressful life event exposure to racial disparities in cognitive function, we utilized the combined African American and White sample. In order to assess potential disparity in level of cognitive test performance and in rate of decline, we first explored the main effect for African American race and a race*age interaction term in mixed models similarly structured to Model 3 above. Where significant race effects were observed, we then quantified the attenuation of race estimates when life event index score and the life event*age interaction term were included in models. Naïve non-parametric bootstrapping was used to construct 95% confidence intervals around each attenuation estimate. 2,000 replicates were performed for each attenuation analysis and the bootstrap quantile method was used to construct the confidence intervals.
In unconditional growth models used to assess inter-individual variability for all cognitive domains, null hypotheses were rejected, indicating that intercepts and slopes should be included in conditional models. Regression diagnostics were performed on the model with the most parameters for each race/ethnicity and cognitive factor pairs (6 model total). Each of these models was checked for residual outliers, heteroscedasticity, trends, distribution, and random effects’ distribution and correlation with residuals. No concerning violations were noted for these models.
RESULTS
Sample characteristics
A total of 1,241 participants had completed at least two WRAP visits and had complete data available. Participant characteristics from their baseline WRAP visit are presented in Table 1. Only a small percentage of the analytic sample (4%) identified as African American or Black. African Americans had completed fewer WRAP visits on average than White participants, reflecting the implementation of a priority outreach program beginning in 2008 [42]. There were no significant differences by race in age at enrollment, proportion identifying as female, or proportion of APOE ε4 carriers. African American participants were more likely to currently smoke and on average had a higher BMI. African Americans also reported marginally fewer years of educational attainment, and showed worse performance on a measure of literacy and on the outcome measures of Speed & Flexibility, Working Memory, and Verbal Learning & Memory.
Table 1.
Descriptive statistics by race for study participants (N== 1,241) at Visit 1
| Variable | African American (n = 50) |
White (n = 1,191) |
Group comparison (p) |
|---|---|---|---|
| Age in years, M (SD) | 54.84 (6.55) | 53.91 (6.70) | 0.19 |
| Female gender, N (%) | 36 (72) | 834 (70) | 0.74 |
| Years of education, M (SD) | 15.36 (2.51) | 16.09 (2.45) | 0.02 |
| APOE ε4 carriers, N (%) | 25 (50) | 453 (38) | 0.13 |
| WRAP visits completed, M (SD)* | 3.4 (1.1) | 4.5 (1.1) | <0.001 |
| Body mass index, M (SD) | 33.43 (7.34) | 28.77 (6.16) | <0.001 |
| Smoking status, N (%) | |||
| Never | 19 (38) | 707 (59) | <0.001 |
| Past | 15 (30) | 397 (33) | |
| Current | 16 (32) | 87 (8) | |
| Life Event Index Score, M (SD) | 5.12 (3.51) | 2.79 (2.01) | <0.001 |
| Literacy Score, M (SD) | 46.28 (6.48) | 51.02 (4.35) | <0.001 |
| Speed & Flexibility, M (SD) | −0.96 (1.03) | 0.10 (0.95) | <0.001 |
| Working Memory, M (SD) | −0.70 (0.98) | 0.06 (0.98) | <0.001 |
| Verbal Learning & Memory, M (SD) | −0.55 (1.11) | 0.07 (0.97) | <0.001 |
Number of WRAP visits completed was based on most recent (last) visit rather than Visit 1.
African Americans reported experiencing significantly more stressful life events than their White counterparts. The mean number of life events among African Americans was nearly 84% higher, and as seen in Fig. 1, the upper tail of the index score distribution was longer.
Fig. 1.
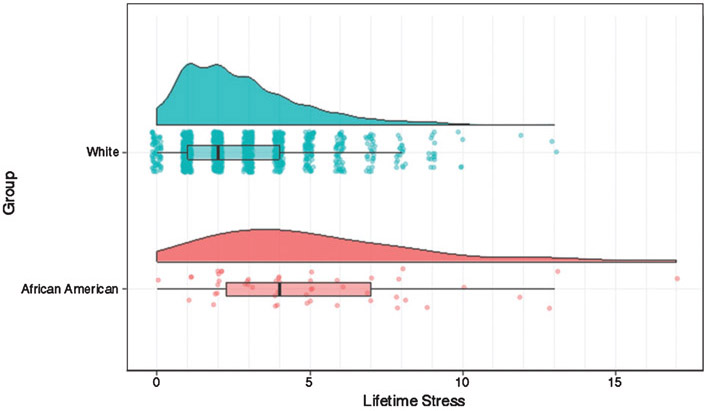
Distribution of lifetime stressful experiences by race.
Stressful life events and cognitive outcomes in African Americans
Associations between the life event index score and cognitive outcomes in nested models can be seen in Table 2. Within the small African American stratum and utilizing Model 1, greater exposure to stressful life events marginally associated with poorer overall level of performance within the domain of Speed & Flexibility (β = −0.11, p = 0.06), but not with rate of decline. Though literacy was strongly and positively associated with level of function in this domain (β = 0.07, p < 0.001), its inclusion in the analytic model does not appear to change the association between lifetime stressor index and level of performance (β = −0.12, p = 0.02), nor does the inclusion of BMI and smoking status. No associations were seen between stressful life events and performance on tests of another domain of executive function, Working Memory.
Table 2.
Regression coefficients (95% confidence intervals) for stressful life events and change in cognitive function by domain, race, and model
| Speed & flexibility | African American (N = 50) |
White (N = 1191) |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Stress | −0.11 (−0.23, 0.006) | −0.12 (−0.23, −0.02)* | −0.13 (−0.24, −0.03)* | −0.03 (−0.05, −0.01)* | −0.03 (−0.05, −0.01)* | −0.03 (−0.05, −0.01)* |
| Age | −0.07 (−0.12, −0.02)* | −0.08 (−0.13, −0.03)† | −0.08 (−0.14, −0.04)† | −0.05 (−0.06, −0.05)† | −0.06 (−0.06, −0.05)† | −0.06 (−0.07, −0.05)† |
| Stress* Age | 0.003 (−0.007, 0.013) | 0.001 (−0.008, 0.011) | 0.001 (−0.009, 0.010) | −0.000 (−0.001, 0.002) | −0.000 (−0.001, 0.002) | −0.000 (−0.002, 0.002) |
| Literacy | – | 0.07 (0.03, 0.11)† | 0.07 (0.03, 0.11)† | – | 0.03 (0.02, 0.05)† | 0.03 (0.02, 0.05)† |
| BMI | – | – | 0.01 (−0.02, 0.04) | – | – | −.001 (.−0.007, 0.005) |
| Past smoking | – | – | 0.10 (−0.49, 0.69) | – | – | −0.03 (−0.12, 0.07) |
| Current smoking | – | – | −0.14 (−0.77, 0.50) | – | – | −0.13 (−0.26, 0.01) |
| Working memory | African American (N = 50) |
White (N = 1191) |
||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Stress | −0.06 (−0.15, 0.03) | −0.05 (−0.13, 0.02) | −0.06 (−0.13, 0.02) | 0.01 (−0.02, 0.03) | 0.01 (−0.01, 0.03) | 0.01 (−0.02, 0.03) |
| Age | −0.03 (−0.07, 0.02) | −0.04 (−0.08, −0.001)* | −0.04 (−0.08, −0.005)* | −0.01 (−0.02, −0.01)† | −0.01 (−0.02, −0.01)† | −0.01 (−0.02, −0.01)† |
| Stress* Age | 0.00 (−0.01, 0.01) | −0.001 (−0.006, 0.005) | −0.002 (−0.007, 0.004) | −0.000 (−0.001, 0.001) | 0.000 (−0.001, 0.001) | 0.000 (−0.001, 0.002) |
| Literacy | – | 0.10 (0.06, 0.13)† | 0.10 (0.07, 0.14)† | – | 0.09 (0.08, 0.10)† | 0.09 0.08, 0.10)† |
| BMI | – | – | 0.02 (−0.01, 0.04) | – | – | −0.002 (−0.007, 0.004) |
| Past smoking | – | – | 0.12 (−0.41, 0.65) | – | – | 0.13 (0.04, 0.23)* |
| Current smoking | – | – | 0.06 (−0.49, 0.60) | – | – | 0.21 (0.09, 0.34)* |
| Verbal learning & memory | African American (N = 50) |
White (N = 1191) |
||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Stress | 0.00 (−0.08, 0.08) | −0.001 (−0.08, 0.08) | −0.01 (−0.09, 0.08) | −0.002 (−0.03, 0.02) | −0.002 (−0.03, 0.02) | −0.002 (−0.03, 0.02) |
| Age | 0.01 (−0.03, 0.04) | 0.01 (−0.04, 0.04) | −0.01 (−0.05, 0.04) | –0.03 (−0.04, −0.02)† | −0.03 (−0.04, −0.03)† | −0.03 (−0.04, −0.03)† |
| Stress*Age | −0.01 (−0.02, 0.00) | −0.01 (−0.02, −0.001)* | −0.01 (−0.02, −0.002)* | 0.001 (−0.001, 0.003) | 0.001 (−0.001, 0.003) | 0.001 (−0.001, 0.003) |
| Literacy | – | 0.02 (−0.01, 0.06) | 0.02 (−0.01, 0.06) | – | 0.05 (0.04, 0.06)† | 0.05 (0.04, 0.06)† |
| BMI | – | – | −0.01 (−0.04, 0.01) | – | – | 0.003 (−0.003, 0.009) |
| Past smoking | – | – | −0.07 (−0.58, 0.44) | – | – | −0.04 (−0.13, 0.06) |
| Current smoking | – | – | −0.45 (−1.06, 0.16) | – | – | −0.11 (−0.26, 0.03) |
All models additionally adjust for gender, years of education, APOE ε4 carrier status, and practice effects.
Significant at the p < 0.05 level.
Significant at the p < 0.001 level.
Within this African American sample, stressful life events were associated with rate of decline in the Verbal Learning & Memory domain (β = −0.01, p = 0.05) in Model 1, and again, this negative relationship did not change after controlling for literacy (β = −0.01, p = 0.04) and health factors (β = −0.01, p = 0.02).
Lifetime stressful events and cognitive outcomes in Whites
Within the much larger White stratum, greater exposure to stressful life events significantly associated with poorer level of performance on tests of Speed & Flexibility (β = −0.03, p = 0.008) in the Model 1 and, as in African Americans, this association does not appear to be attenuated by the inclusion of literacy and health factors in regression models. Also, similarly to the African American cohort, there were no associations between life events and rate of decline in Speed & Flexibility skills over time, or with any cognitive outcomes in the Working Memory or Verbal Learning & Memory domains.
Racial disparities across domain and attenuation by stress and stress related decline
African American race was significantly associated with poorer level of test performance across all three cognitive domains, but was not associated with rate of decline in Speed & Flexibility (β = −0.01, p = 0.37), Working Memory (β = 0.001, p = 0.86), or Verbal Learning & Memory (β = −0.01, p = 0.40) domains. Results from secondary analyses conducted to directly assess contributions of stressful life event exposure to racial disparities in level of cognitive test performance can be seen in Table 3. In otherwise full models excluding the life event index score and its interaction with age, African American race was associated with poorer overall performance in all three domains of cognition. Inclusion of life event index terms (stress and stress*time) led to attenuation of the association between African American race and Speed & Flexibility by 6.9% (95% CI: 4.4, 8.8). However, no significant stress-related attenuation of associations between African American race and level of Working Memory [0.02% (95% CI: −1.8, 6.0)] or Verbal Learning & Memory [1.5% (95% CI: −1.8, 13.1)] was observed.
Table 3.
Attenuation of race differences in level of cognitive function by lifetime stress in each cognitive domain
| Speed & flexibility |
Working memory |
Verbal learning & memory |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| African American race | −0.93 (−1.18, −0.69)† | −0.87 (−1.12, −0.62)† | −0.39 (−0.63, −0.15)* | −0.39 (−0.63, −0.15)* | −0.33 (−0.58, −0.09)* | −0.33 (−0.58, −0.08)* |
| Stress | – | −0.04 (−0.06, −0.01)† | – | 0.00 (−0.02, 0.02) | – | –0.003 (−0.03, 0.02) |
| Stress* Age | – | 0.00 (0.00, 0.00) | – | 0.00 (−0.001, 0.001) | – | 0.00 (−0.002, 0.002) |
| Attenuation (%, CI) | – | 6.91 (4.43, 8.78)* | – | 0.02 (−1.77, 5.97) | – | 1.51 (−1.83, 13.06) |
All models additionally adjust for age at visit, gender, years of education, APOE ε4 carrier status, and practice effects.
Significant at the p < 0.05 level.
Significant at the p < 0.001 level.
DISCUSSION
In a sample of cognitively normal middle-aged and older adults, we explored associations between stressful life events and cognitive decline across time. Although there were only a small number of African American participants with full stressor and cognitive data available, we disaggregated the sample by race for assessment of within-group stress-cognition relationships. This approach is advantageous because the lifetime social environment and experiences of White and African American participants are likely to differ in significant ways, with unique implications for adversity, resilience, and health [38]. Data were aggregated only to preliminarily quantify the contribution of lifetime stressful events to racial disparities in cognition seen among the whole sample. Racial disparity was observed only in level of performance: African American participants performed more poorly in each cognitive domain but no differences by race were observed for rate of decline. Our analysis ultimately showed that exposure to self-reported lifetime stressful events associated negatively with cognitive test performance within both White and African American participants, though the domains affected varied by race. African Americans also reported significantly greater exposure to stressors. Cumulative stressful life events, as measured here, partially accounted for observable racial gaps in Speed & Flexibility. The attenuation of <10% observed in this study was quite modest compared to results reported for the most well-studied social determinants of cognitive health disparity, educational quality and literacy, which are robust predictors of cognitive aging trajectories in racial and ethnic minority populations [53, 54] and have been shown to attenuate racial disparities in both executive function and episodic memory by as much as 32% [13] and to the point of non-significance [55]. Here, we provide evidence that another social determinant, lifetime stress, contributes to later-life cognitive outcomes, including memory decline, for African American communities. In our study, this relationship appears robust to controls for literacy and for BMI and smoking, two additional cognitive aging risk factors that are more prevalent in individuals with a history of stressful life events [56, 57].
Our findings regarding stress and executive function are generally consistent with the current body of literature. A relationship between stressful life events and overall level of performance on tests of Speed & Flexibility, observed in both subsamples, echoes previous findings on stress and cognition in WRAP. Our group has reported cross-sectional relationships between recent stressful events and poorer performance in the same domain [19]. That earlier study was conducted in a small subset of the current study’s White sample, and when paired with our current findings suggests robustness in the associations of both past and present stressful experiences with this measure of executive function within our cohort. Past studies in both White and African American cohorts have reported consistently negative associations of stress constructs including negative life events [58] and perceived stress [36] with overall level but not decline in processing speed. However, given strong experimental evidence suggesting that induced stress is associated with cognitive detriment in both domains of executive function across all ages [59], the lack of observable relationships between stressful life events and tests of working memory in the current data is somewhat surprising. In the small sample of African Americans, this may be due primarily to limited estimate precision. And among White participants in the WRAP cohort, Working Memory performance has previously been shown to have a strong genetic component [60]. Additionally, the timing of events could be important: some prior work has suggested that recency of stress exposure may be important in working memory, with recent stressors negatively impacting working memory through cognitive interference [61]. We did not distinguish recent from distal experiences in this analysis, though we also reported a lack of association in the previously mentioned study on recent stress [19].
A relationship between stressful life events and episodic memory was seen only in the African American sample, where greater number of events associated with faster rate of decline. Though research on stress and cognition in African Americans is still rare, this association is consistent with findings from a recent study that showed perceived stress to be associated with faster rate of decline in performance on word list recall tasks in a much larger cohort of older African Americans [36]. Racial differences in stressor-episodic memory relationships may be consistent with “weathering,” a model commonly used to account for health disparities [62], which posits that accumulating disadvantage across the life course provokes systemic dysregulation, accelerated biological aging [63], and premature onset of age-related morbidities including cognitive aging [64]. Executive function has been shown to mediate the association between age and episodic memory [65] and between vascular pathology and episodic memory [66]. As such, early age-related declines in episodic memory are likely to be most observable in a sample of African American participants disproportionately experiencing both stress-related weathering and poorer executive function. Interestingly, our findings differ from those reported in a seminal study [10] assessing early life adversity and later-life cognitive change in a large, population-based African American and White sample. Barnes and colleagues also observed a lack of association among Whites, but found that African Americans who self-reported two markers of childhood adversity, being thinner than average and not having enough food to eat during childhood, exhibited slower rates of cognitive decline than their peers who did not experience these conditions [15]. The Barnes et al. sample included much older adults than the current sample, and the adversity measure differed substantially from the life events inventory utilized in this analysis. The contrasting findings highlight the need for additional research that parses both nature and timing of adverse events.
Our reported findings on stress and cognition within a small sample of African Americans provide suggestive preliminary data that should inform replicating or expanded research questions to be answered within larger and population-based cohorts. Both African American and White samples were drawn from a cohort enriched for AD risk, and sampling on family history of AD may limit generalizability based on issues of ancestry, selection into the cohort, and prevalence of dementia caregiving. Only WRAP participants who were interested enough and healthy enough to attend the first WRAP follow-up visit and provide the stressful life events data that were collected for the Wave 2 protocol were included in the analytic sample. The high educational attainment in both samples similarly limits generalizability. Further, information on recruitment source (from a memory clinic during the clinical evaluation of a parent versus community-based) was not available for WRAP participants, but Gleason and colleagues [67] recently used a national cognitive aging database to illustrate how restricting the use of strong participatory research-based recruitment programming [42] to underrepresented racial minority communities while sampling White participants from memory clinic settings can result in selection bias and threats to internal validity when race is a predictor of interest.
In addition to sample limitations, there are measurement issues that bear mention. First, it must be noted that while cognitive trajectories began at each participant’s baseline visit, they did not provide information on their experiences of stressful life events until a follow-up visit Wave 2 visit four years later. Exposure over that four-year period is reflected in the index score despite it having occurred after the first outcome measure timepoint, and exposure that occurred after Wave 2 was not reflected. Additionally, as mentioned earlier, stress operationalization in a given study is likely to influence findings. Our measure, a count of binary 0/1 responses indicating the absence or presence of a given experience on a finite checklist of possible life events, is commonly used and is appropriate for studies incorporating accumulation-of-risk models [48]. However, such models do not account for potentially synergistic detriment arising from repeated stressor exposures, nor do they incorporate appraisal [16], and in fact they have been shown to underestimate stressor exposure in African Americans and other underrepresented groups [17].
Finally, our small African American sample did not allow for disambiguation of age, cohort, and period effects on the relationships between stressful life events and cognitive outcomes in older age. Recent epidemiological work has suggested that consideration of cohort effects in dementia and impairment, and how they differ by race, is critical [4] given that risk exposures and their influence are likely to be influenced by the year an individual was born, schooled, entered the labor force, encountered health problems, and so on. As de jure and de facto environments that shape key modifiable cognitive risk factors such as education quality and access to cardiovascular health care shift, the relationships between exposures and cognitive outcomes are also likely to change. Period effects may also differ between the predominantly White adults enrolled at the beginning of the study and similarly-aged African American adults enrolled ten years later. Future studies in larger middle-aged and older cohorts should explore potentially distinct age, cohort, and period effects in relationships between stress and health [68].
The current study nonetheless contributes additional evidence to a growing body of literature that suggests that life course socio-environmental factors are important determinants of later-life cognitive trajectories and dementia risk, and that this may be particularly true for racial minority and other historically disadvantaged populations. Further, cumulative disadvantage as measured by a life event index may influence cognitive trajectories independent of other strong, well-established social determinants including education quality and literacy. Observed racial differences in stress-cognition relationships highlight the need for prioritization of cohort diversity, and delineation of AD-specific biomarker and other relevant brain changes in non-White populations. Social-biological processes represent a modifiable source of ADRD and impairment risk, one that with further study could clarify underexplained disparity and illuminate strategic points for targeted intervention.
ACKNOWLEDGMENTS
This work was supported by the Alzheimer’s Association [AARF-18-562958]; by the National Institute on Aging-National Institutes of Health [R01 AG054059, R01 AG027161, K23 AG045957-S1]; and by the Helen Bader Foundation. The authors gratefully acknowledge the contributions of Gina Green-Harris, Nia Norris, and the WRAP participants who make this research possible.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0439r2).
REFERENCES
- [1].Glymour MM, Manly JJ (2008) Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev 18, 223–254. [DOI] [PubMed] [Google Scholar]
- [2].Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM (2017) Contribution of socioeconomic status at 3 lifecourse periods to late-life memory function and decline: Early and late predictors of dementia risk. Am J Epidemiol 186, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA (2016) Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 12, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weuve J, Rajan KB, Barnes LL, Wilson RS, Evans DA (2018) Secular trends in cognitive performance in older Black and White U.S. adults, 1993-2012: Findings from the Chicago Health and Aging Project. J Gerontol B Psychol Sci Soc Sci 73, S73–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J (2009) Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. Am J Epidemiol 170, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gross AL, Mungas DM, Crane PK, Gibbons LE, MacKay-Brandt A, Manly JJ, Mukherjee S, Romero H, Sachs B, Thomas M, Potter GG, Jones RN (2015) Effects of education and race on cognitive decline: An integrative study of generalizability versus study-specific results. Psychol Aging 30, 863–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, Hebert LE, Bennett DA, Wilson RS, Evans DA (2018) Cognitive aging in Black and White Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology 29, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tsuang DW, Wilson RK, Lopez OL, Luedecking-Zimmer EK, Leverenz JB, DeKosky ST, Kamboh MI, Hamilton RL (2005) Genetic association between the APOE*4 allele and Lewy bodies in Alzheimer disease. Neurology 64, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang R, Fratiglioni L, Laukka EJ, Lovden M, Kalpouzos G, Keller L, Graff C, Salami A, Backman L, Qiu C (2015) Effects of vascular risk factors and APOE epsilon4 on white matter integrity and cognitive decline. Neurology 84, 1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, Hebert LE, Aggarwal N, Beckett LA, Joglekar R, Berry-Kravis E, Schneider J (2003) Incidence of Alzheimer disease in a biracial urban community: Relation to apolipoprotein E allele status. Arch Neurol 60, 185–189. [DOI] [PubMed] [Google Scholar]
- [11].Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R (2001) Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 56, 49–56. [DOI] [PubMed] [Google Scholar]
- [12].Hill CV, Perez-Stable EJ, Anderson NA, Bernard MA (2015) The National Institute on Aging Health Disparities Research Framework. Ethn Dis 25, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sisco S, Gross AL, Shih RA, Sachs BC, Glymour MM, Bangen KJ, Benitez A, Skinner J, Schneider BC, Manly JJ (2015) The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci 70, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Williams DR, Jackson PB (2005) Social sources of racial disparities in health. Health Aff (Millwood) 24, 325–334. [DOI] [PubMed] [Google Scholar]
- [15].Barnes LL, Wilson RS, Everson-Rose SA, Hayward MD, Evans DA, Mendes de Leon CF (2012) Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology 79, 2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brown LL, Mitchell UA, Ailshire J (2018) Disentangling the stress process: Race/ethnic differences in the exposure and appraisal of chronic stressors among older adults. J Gerontol B Psychol Sci Soc Sci, doi: 10.1093/geronb/gby072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Turner RJ, Avison WR (2003) Status variations in stress exposure: Implications for the interpretation of research on race, socioeconomic status, and gender. J Health Soc Behav 44, 488–505. [PubMed] [Google Scholar]
- [18].Aggarwal NT, Wilson RS, Beck TL, Rajan KB, Mendes de Leon CF, Evans DA, Everson-Rose SA (2014) Perceived stress and change in cognitive function among adults 65 years and older. Psychosom Med 76, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zuelsdorff ML, Engelman CD, Friedman EM, Koscik RL, Jonaitis EM, Rue AL, Sager MA (2013) Stressful events, social support, and cognitive function in middle-aged adults with a family history of Alzheimer’s disease. J Aging Health 25, 944–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johansson L, Guo X, Hallstrom T, Norton MC, Waern M, Ostling S, Bengtsson C, Skoog I (2013) Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: A38-year longitudinal population study. BMJ Open 3, e003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peavy GM, Jacobson MW, Salmon DP, Gamst AC, Patterson TL, Goldman S, Mills PJ, Khandrika S, Galasko D (2012) The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Dis Assoc Disord 26, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee T, Jarome T, Li SJ, Kim JJ, Helmstetter FJ (2009) Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport 20, 1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA (2017) Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatry 82, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA (2007) Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage 35, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Piccolo LR, Noble KG, Pediatric Imaging N, Genetics S (2018) Perceived stress is associated with smaller hippocampal volume in adolescence. Psychophysiology 55, e13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A (2011) Effects of stressful life events on human brain structure: A longitudinal voxel-based morphometry study. Stress 14, 227–232. [DOI] [PubMed] [Google Scholar]
- [27].Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R (2012) Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry 72, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, Parks SE (2015) Childhood adversity and adult chronic disease: An update from ten states and the District of Columbia, 2010. Am J Prev Med 48, 345–349. [DOI] [PubMed] [Google Scholar]
- [29].Din-Dzietham R, Nembhard WN, Collins R, Davis SK (2004) Perceived stress following race-based discrimination at work is associated with hypertension in African-Americans. The Metro Atlanta Heart Disease Study, 1999-2001. Soc Sci Med 58, 449–461. [DOI] [PubMed] [Google Scholar]
- [30].Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH (2014) Midlife hypertension and 20-year cognitive change: The Atherosclerosis Risk in Communities neurocognitive study. JAMA Neurol 71, 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rajan KB, Arvanitakis Z, Lynch EB, McAninch EA, Wilson RS, Weuve J, Barnes LL, Bianco AC, Evans DA (2016) Cognitive decline following incident and preexisting diabetes mellitus in a population sample. Neurology 87, 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA (2012) Midlife vs late-life depressive symptoms and risk of dementia: Differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 69, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hamilton JL, Brickman AM, Lang R, Byrd GS, Haines JL, Pericak-Vance MA, Manly JJ (2014) Relationship between depressive symptoms and cognition in older, non-demented African Americans. J Int Neuropsychol Soc 20, 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pase MP, Himali JJ, Grima NA, Beiser AS, Satizabal CL, Aparicio HJ, Thomas RJ, Gottlieb DJ, Auerbach SH, Seshadri S (2017) Sleep architecture and the risk of incident dementia in the community. Neurology 89, 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Virta JJ, Heikkila K, Perola M, Koskenvuo M, Raiha I, Rinne JO, Kaprio J (2013) Midlife sleep characteristics associated with late life cognitive function. Sleep 36, 1533–1541, 1541A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Turner AD, James BD, Capuano AW, Aggarwal NT, Barnes LL (2017) Perceived stress and cognitive decline in different cognitive domains in a cohort of older African Americans. Am J Geriatr Psychiatry 25, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zahodne LB, Manly JJ, Smith J, Seeman T, Lachman ME (2017) Socioeconomic, health, and psychosocial mediators of racial disparities in cognition in early, middle, and late adulthood. Psychol Aging 32, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Whitfield KE, Allaire JC, Belue R, Edwards CL (2008) Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? J Gerontol B Psychol Sci Soc Sci 63, P301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Backman L, Jones S, Berger AK, Laukka EJ, Small BJ (2005) Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology 19, 520–531. [DOI] [PubMed] [Google Scholar]
- [40].Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, Bendlin BB, Engelman CD, Okonkwo OC, Hogan KJ, Asthana S, Carlsson CM, Hermann BP, Sager MA (2018) The Wisconsin Registry for Alzheimer’s Prevention: A review of findings and current directions. Alzheimers Dement (Amst) 10, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ (1994) A validation study of the Dementia Questionnaire. Arch Neurol 51, 901–906. [DOI] [PubMed] [Google Scholar]
- [42].Green-Harris G, Coley SL, Koscik RL, Norris NC, Houston SL, Sager MA, Johnson SC, Edwards DF (2019) Addressing disparities in Alzheimer’s disease and African-American participation in research: An asset-based community development approach. Front Aging Neurosci 11, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Koscik RL, La Rue A, Jonaitis EM, Okonkwo OC, Johnson SC, Bendlin BB, Hermann BP, Sager MA(2014)Emergence of mild cognitive impairment in late middle-aged adults in the Wisconsin Registry for Alzheimer’s Prevention. Dement Geriatr Cogn Disord 38, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Heaton RK, Miller SW, Taylor MJ, Grant I (2004) Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults, Psychological Assessment Resources Inc., Lutz, FL. [Google Scholar]
- [45].Trenerry M, Crosson B, Deboe J, Leber L (1989) Stroop Neuropsychological Screening Test, Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- [46].Weschler D (1997) Weschler Adult Intelligence Scale, 3rd ed., Psychological Corporation, San Antonio. [Google Scholar]
- [47].Schmidt M (1996) Rey Auditory Verbal Learning Test: A Handbook, Western Psychological Services, Los Angeles, CA. [Google Scholar]
- [48].Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, Miller GE (2015) Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Dev Psychol 51, 1630–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ryff CD, Almeida DM, Ayanian JS, Carr DS, Cleary PD, Coe C, Davidson R, Krueger RF, Lachman ME, Marks NF, Mroczek DK, Seeman TE, Seltzer MM, Singer BH, Sloan RP, Tun PA, Weinstein M, Williams D, Midlife in the United States (MIDUS 2), 2004 - 2006: SAQ Quesionnaires 1 and 2, E11 (ISCPR Version), http://midus.wisc.edu/midus2/project1/, Accessed 16 October 2019.
- [50].Turner RJ, Wheaton B (1995) Checklist measurement of stressful life events In Measuring stress: A guide for health and social scientists., Cohen S, Kessler R, Underwood GL, eds. Oxford University Press, New York, pp. 29–58. [Google Scholar]
- [51].Morrell CH, Brant LJ, Ferrucci L (2009) Model choice can obscure results in longitudinal studies. J Gerontol A Biol Sci Med Sci 64, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wilkinson G (1993) WRAT3 Administrative Manual, Wide Range, Wilmington, Del. [Google Scholar]
- [53].Manly JJ, Schupf N, Tang MX, Stern Y (2005) Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol 18, 213–217. [DOI] [PubMed] [Google Scholar]
- [54].Manly JJ, Touradji P, Tang MX, Stern Y (2003) Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol 25, 680–690. [DOI] [PubMed] [Google Scholar]
- [55].Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y (2002) Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc 8, 341–348. [DOI] [PubMed] [Google Scholar]
- [56].Rogers CJ, Forster M, Unger JB (2018) Ethnic variations in the relationship between multiple stress domains and use of several types of tobacco/nicotine products among a diverse sample of adults. Addict Behav Rep 7, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Palmisano GL, Innamorati M, Vanderlinden J (2016) Life adverse experiences in relation with obesity and binge eating disorder: A systematic review. J Behav Addict 5, 11–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Comijs HC, van den Kommer TN, Minnaar RW, Penninx BW, Deeg DJ (2011) Accumulated and differential effects of life events on cognitive decline in older persons: Depending on depression, baseline cognition, or ApoE epsilon4 status? J Gerontol B Psychol Sci Soc Sci 66 Suppl 1, i111–120. [DOI] [PubMed] [Google Scholar]
- [59].Shields GS, Sazma MA, Yonelinas AP (2016) The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci Biobehav Rev 68, 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Darst BF, Koscik RL, Hermann BP, La Rue A, Sager MA, Johnson SC, Engelman CD (2015) Heritability of cognitive traits among siblings with a parental history of Alzheimer’s disease. J Alzheimers Dis 45, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Korten NC, Sliwinski MJ, Comijs HC, Smyth JM (2014) Mediators of the relationship between life events and memory functioning in a community sample of adults. Appl Cogn Psychol 28, 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Geronimus AT, Hicken M, Keene D, Bound J (2006) “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 96, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Simons RL, Lei MK, Beach SR, Philibert RA, Cutrona CE, Gibbons FX, Barr A (2016) Economic hardship and biological weathering: The epigenetics of aging in a U.S. sample of black women. Soc Sci Med 150, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hatton SN, Franz CE, Elman JA, Panizzon MS, Hagler DJ Jr., Fennema-Notestine C, Eyler LT, McEvoy LK, Lyons MJ, Dale AM, Kremen WS (2018) Negative fateful life events in midlife and advanced predicted brain aging. Neurobiol Aging 67, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Troyer AK, Graves RE, Cullum CM (1994) Executive functioning as a mediator of the relationship between age and episodic memory in healthy aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 1, 45–53. [Google Scholar]
- [66].Parks CM, Iosif AM, Farias S, Reed B, Mungas D, DeCarli C (2011) Executive function mediates effects of white matter hyperintensities on episodic memory. Neuropsychologia 49, 2817–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gleason CE, Norton D, Zuelsdorff M, Benton SF, Wyman MF, Nystrom N, Lambrou N, Salazar H, Koscik RL, Jonaitis E, Carter F, Harris B, Gee A, Chin N, Ketchum F, Johnson SC, Edwards DF, Carlsson CM, Kukull W, Asthana S (2019) Association between enrollment factors and incident cognitive impairment in Blacks and Whites: Data from the Alzheimer’s Disease Center. Alzheimers Dement, doi: 10.1016/j.jalz.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lewis TT, Van Dyke ME (2018) Discrimination and the health of African Americans: The potential importance of intersectionalities. Curr Dir Psychol Sci 27, 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]


