Abstract
Mycoplasmatacea family comprises two genera: Mycoplasma and Ureaplasma. Ureaplasma parvum (previously known as U. urealyticum biovar 1) commonly colonises the urogenital tract in humans. Although Ureaplasma species have well-established pathogenicity in urogenital infections, its involvement in septic arthritis has been limited to prosthetic joint infections and immunocompromised individuals. We present a rare case of native right knee infection due to U. parvum identified using next-generation sequencing of microbial cell-free DNA testing and confirmed with PCR assays. This rare case of Ureaplasma septic arthritis was diagnosed using newer next-generation DNA sequencing diagnostic modalities and a literature review of prior cases, antibiotic coverage and antimicrobial resistance is incorporated as part of the discussion.
Keywords: bone and joint infections, drugs: infectious diseases, orthopaedics
Background
Ureaplasma parvum, a fastidious microorganism that exhibits urease activity, lacks a cell wall and along with U. urealyticum, comprises the two clinically significant strains of Ureaplasma species. In previous published reports, its role has been established as a rare causative agent for septic arthritis in immunocompromised individuals. We present a rare case of native right knee joint infection in an immunocompetent host. The case illustrates the importance of early use of next-generation DNA testing to facilitate diagnosis in culture-negative cases with a discussion of available antibiotic choices and growing resistance.
Case presentation
A 68-year-old woman with a history of total arthroplasty of the left knee and left hip, and severe tricompartmental degenerative osteoarthritis of right knee, initially presented with right knee pain and swelling. On inquiry, the patient reported having received a platelet-rich plasma injection to her right knee, 12 days prior to the presentation at a local clinic. Two days after the plasma injection, she started to endorse right knee pain and swelling. She described it as sharp, throbbing, intermittent and non-radiating. She was prescribed a tapering course of prednisone by the clinic without any improvement in her symptoms. Her pain continued to worsen with associated redness, to the point that she could not bear weight on her knee and ambulate. This prompted a diagnostic arthrocentesis at a walk-in clinic from where she was subsequently referred to our facility. She reported no recent trauma to her right knee and no history of gout or tick bites. She denied fever, chills, night sweats, nausea, vomiting, abdominal pain, cough, shortness of breath, diarrhoea or any urinary complaints. She did not smoke, drink alcohol or use any illicit intravenous drugs. She reported penicillin and sulfa drugs allergy causing a rash. Other medical history was significant for hypertension, asthma, lymphocytic colitis, mitral valve prolapse and left frontoparietal craniotomy for resection of a dural-based meningioma around 20 years ago. Family history was significant for Wegener’s granulomatosis in her mother. Examination was remarkable for a moderate intra-articular right knee effusion associated with tenderness to palpation, and range of motion significantly limited by severe pain.
Investigations
On admission, her white blood cell count (WBC) was 15×109 cells/L (reference range: 4.00–12.00×109 cells/L), erythrocyte sedimentation rate (ESR) was 23 mm/h (reference range: 0–20 mm/h) and C reactive protein (CRP) was 11.9 mg/dL (reference range:<0.50 mg/dL). X-ray of the right knee showed large effusion in the setting of tricompartmental degenerative osteoarthritis as shown in figure 1. The results from the diagnostic arthrocentesis prior to admission are shown in table 1. Gram stain did not reveal any organisms and aerobic, anaerobic and fungal cultures of blood, urine and synovial fluid were all negative. Next-generation sequencing (NGS) of microbial cell-free DNA (‘Karius Test’, Karius, Redwood City, California, USA) detected levels of U. Parvum at 90 molecules/mL of plasma. It detected no other organisms. Subsequent positive results on Ureaplasma-specific PCR testing of synovial fluid (Mayo Clinic Laboratories, Rochester, Minnesota, USA) confirmed the diagnosis. A urine Ureaplasma-specific PCR ruled out a urinary source of infection.
Figure 1.
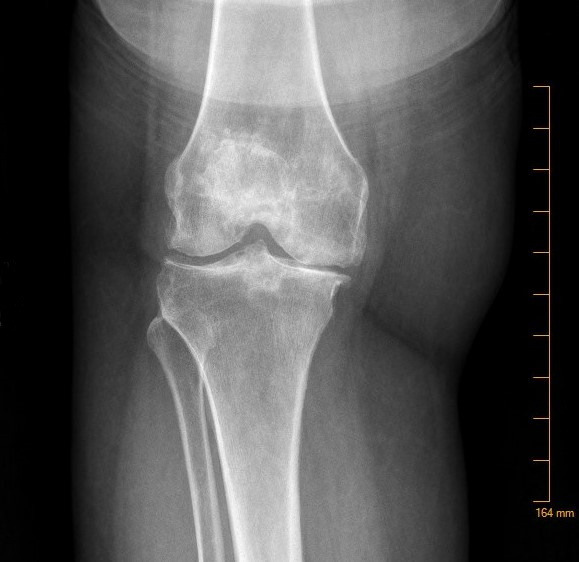
X-ray of the right knee showing joint effusion and severe tricompartmental degenerative changes.
Table 1.
Body fluid cell count with differential
| Site | Right knee |
| Clarity | Turbid |
| Colour | Yellow |
| Volume (mL) | 19 |
| Red cell count/mm3 | 16 000 |
| Total nucleated cell count/mm3 | 139 950 |
| Neutrophils, % | 87 |
| Lymphocyte, % | 5 |
| Monocyte, % | 8 |
| Eosinophil, % | 0 |
| Macrophage, % | 0 |
| Fluid lining cell, % | 0 |
Treatment
The patient underwent right knee arthroscopy with irrigation, debridement, extensive synovectomy and evacuation of grossly purulent fluid. He was then started empirically on vancomycin, cefepime and flagyl. The operative synovial fluid was also sent for Gram stain, aerobic, anaerobic and fungal cultures, which all came back negative. Unfortunately, the patient developed an allergic drug reaction after starting empiric therapy. Since it was unclear which antibiotic was responsible for it, all three empiric antibiotics were discontinued on postoperative day 3. Due to development of recurrent symptoms of right knee pain, swelling and tenderness, a repeat diagnostic and therapeutic arthrocentesis was performed which reported WBC of 61.6×109 cells/L (92% neutrophils). This prompted a second right knee arthroscopic irrigation, debridement with partial synovectomy and partial medial meniscectomy. Specimens for Karius testing were collected on day 2 of admission after the patient had already received a 1-day dose of vancomycin, cefepime and flagyl. Karius test results were reported within 3 days of collection. After confirming these results with Ureaplasma-specific PCR, antibiotic coverage was narrowed down to azithromycin 500 mg daily.
Outcome and follow-up
During the rest of her hospital stay, the patient’s clinical condition continued to improve with significant decrease in knee pain and swelling. She tolerated the antibiotic therapy well and reported no adverse effects. Serial CRP, WBC and ESR levels were evaluated frequently and showed a gradual decline. At the time of discharge, antibiotic coverage with azithromycin was initially planned for 6 weeks. She did well till 10 days after cessation of treatment which was resumed in light of relapse of right knee pain and persistent knee swelling with elevated CRP 2.15 mg/dL.
The patient sought a second opinion for refractory knee pain within 4 weeks of resumption of treatment with azithromycin and was switched to a 6-week course of oral levofloxacin and doxycycline. A right knee MRI scan 3 months post discharge showed no drainable fluid collection but could not exclude infectious aetiologies due to lack of T1-weighted sequences and history of prior surgery/debridement, as shown in figure 2.
Figure 2.
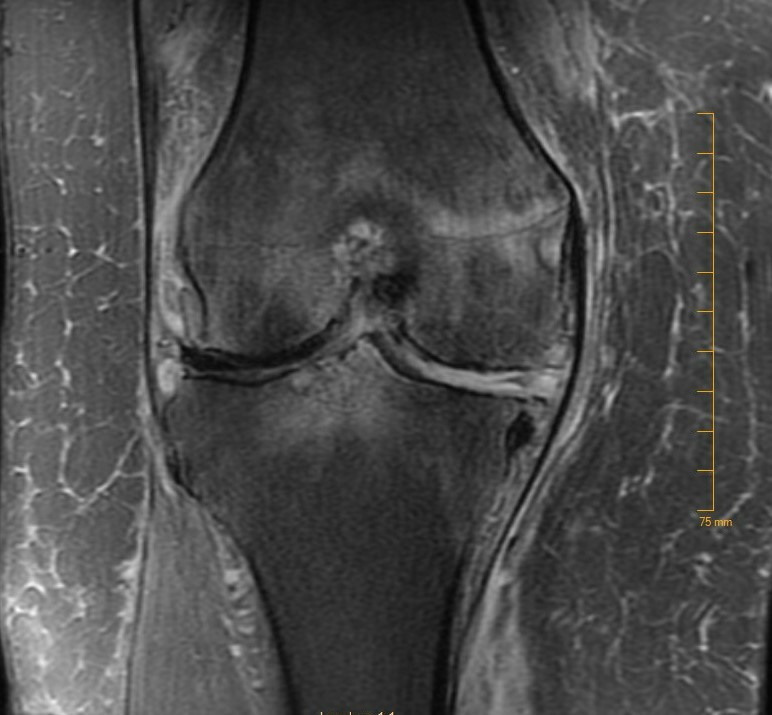
MRI of the right knee showing small right knee joint effusion with synovitis, prominent bone marrow oedema involving the distal femur, tibia, patella and postoperative changes and tricompartmental degenerative arthritis.
Discussion
Mycoplasmatacea family comprises two genera: Mycoplasma and Ureaplasma. U. parvum (previously known as U. urealyticum biovar 1) and U. urealyticum (previously known as U. urealyticum biovar 2) are two distinct Ureaplasma species of medical importance that colonise the urogenital tract in humans. Ureaplasma species are spherical or coccobacillary-shaped cells with a diameter of 0.1–1.0 µm. Absence of cell walls and ability to hydrolyze urea to generate ATP are some typical characteristics that help distinguish them from other microorganisms.1 2
The inability to isolate Ureaplasma species by Gram stain or detect their growth on the standard media represents a potential challenge in identifying them as a causative agent. Among the two species, U. urealyticum has had a greater role in causing septic polyarthritis, non-gonococcal urethritis and perinatal infections. However, over the past few years, U. parvum has increasingly been reported as a human pathogen.3 4
As per our literature review, there have been only four cases reported of septic arthritis due to U. parvum. Three of four reported cases were in immunocompromised patients or postoperative prosthetic joints.4–7 To date, one case of septic arthritis due to U. parvum in an immunocompetent patient, involving a native knee joint has been reported7 (table 2).
Table 2.
Reported cases of Ureaplasma parvum septic arthritis, risk factors for infection and antibiotics used
In our case, the patient did not have any history of receiving any immunosuppressants or history of immunocompromising conditions such as hypogammaglobulinaemia or haematologic malignancies. In our patient, the spread of pathogens was likely secondary to direct inoculation from the protein-rich plasma injection in the native right knee joint. Genitourinary infection, a possible primary source of septic arthritis reported in the medical literature, was ruled out in our case by urine culture and PCR. This is the second reported case of native knee joint septic arthritis in an immunocompetent patient.
Septic arthritis due to atypical microorganisms such as Ureaplasma species poses a diagnostic challenge as these species cannot be grown on standard culture media. The use of NGS can overcome this difficulty. The advantages of the test include detection of organisms that are difficult to grow on traditional culture, and further speciation of organisms. Though early collection of sample increases the sensitivity of the test, NGS can also help detect pathogens in serum after antibiotic administration.8
Concern for infection in spite of negative blood and synovial cultures in our patient and our previous experiences with alternate testing in culture-negative infections prompted the NGS of microbial cell-free DNA (Karius) testing that identified U. parvum as the causative agent. Karius test findings were also confirmed by Ureaplasma-specific PCR testing of synovial fluid aspirate. Our case also highlights the importance of using NGS testing early in the disease course, when diagnosis can potentially be delayed or confounded by negative cultures.
Up till now, only four classes of antibiotics; fluoroquinolones, tetracyclines, chloramphenicol and macrolides, have been recognised to combat ureaplasma infections. Lack of cell wall in Ureaplasma species renders resistance to all β-lactams and glycopeptide antibiotics. Inability to synthesise folic acid provides these organisms resistance against sulfonamides and trimethoprim.9 Macrolides remain the only safe option in pregnant women and neonates.
Most mycoplasma and ureaplasma infections are treated empirically. Antibiotic susceptibility testing comes of use especially in immunocompromised patients or patients who have failed prior antibiotic regimens. In an effort to allot quality control (QC) measures to ensure valid assay results and to standardise interlaboratory antibiotic susceptibility testing for Mycoplasma hominis, M. pneumoniae and U. urealyticum, an international multilaboratory collaborative study, by using methods and study designs approved by Clinical and Laboratory Standards Institute, established 3–4 dilution minimum inhibitory concentration (MIC) ranges (of specific strains with defined QC reference) for several antimicrobial classes, including tetracyclines, ketolides, lincosamides, macrolides and fluoroquinolones. This study also concluded that fluoroquinolones gave tighter QC ranges compared with other drugs.10
Fernández et al, in 2016, reported antimicrobial susceptibility of 250 clinical isolates of Ureaplasma species (202 U. parvum and 48 U. urealyticum). They determined the MIC of azithromycin, ciprofloxacin, doxycycline, erythromycin, tetracycline and levofloxacin, using broth microdilution. They concluded that 6.4% of U. parvum isolates were resistant to levofloxacin, primarily due to mutation in parC gene. Overall, 27.2% of the U. parvum isolates showed ciprofloxacin MICs of ≥4 µg/mL. Ten U. parvum isolates exhibited the presence of tet(M), which had low tetracycline and doxycycline MICs. In the past, tet(M) mutation has been associated with resistance to antimicrobials but there have been studies showing presence of the gene mutation in susceptible isolates as well.11 12 None of the 250 clinical isolates studies were found to be resistant to macrolides.13
Luo et al in an in vitro study evaluated the activities of tetracycline, erythromycin and levofloxacin, alone and in dual combination against Ureaplasma species. The study concluded that these drugs when used in combination prevented growth of organisms at lower antimicrobial concentration than when used alone.14 Due to the fastidious nature of Ureaplasma species and special growth medium requirements, antibiotic susceptibility testing is routinely not performed. In our patient, susceptibility testing could not be performed and she was started on treatment with macrolide. Her treatment had to be extended due to persistent pain and her antibiotic regimen was switched to combination therapy with levofloxacin and doxycycline based on the literature review. She responded well to the therapy and had not report problems on follow-up visits.
Learning points.
Ureaplasma species commonly colonise the urogenital tract. Septic arthritis due to U. parvum is rare.
Next-generation sequencing of microbial cell-free DNA testing is proving beneficial in isolating these organisms in culture-negative cases.
Standardised drug susceptibility testing is not available for this organism and so far only four classes of drugs including fluoroquinolones, tetracyclines, chloramphenicol and macrolides have shown to be effective against it.
Dual treatment using tetracycline, erythromycin or levofloxacin may be more effective in treating non-resolving infections.
Footnotes
Twitter: @sharjeel__ahmad
Contributors: AAA, MR and SA created the conception and design of the study. AA wrote in initial manuscript, performed data collection and analysis. MR performed subsequent article revising, data integration and image collection. SA obtained patient consent and performed final manuscript editing. All authors reviewed the final manuscript and approved the final version to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Robertson JA, Stemke GW, Davis JW, et al. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol 2002;52:587–97. 10.1099/00207713-52-2-587 [DOI] [PubMed] [Google Scholar]
- 2.Kong F, Ma Z, James G, et al. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol 2000;38:1175–9. 10.1128/JCM.38.3.1175-1179.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balsat M, Galicier L, Wargnier A, et al. Diagnosis of Ureaplasma urealyticum septic polyarthritis by PCR assay and electrospray ionization mass spectrometry in a patient with acute lymphoblastic leukemia. J Clin Microbiol 2014;52:3456–8. 10.1128/JCM.00963-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKenzie CR, Nischik N, Kram R, et al. Fatal outcome of a disseminated dual infection with drug-resistant Mycoplasma hominis and Ureaplasma parvum originating from a septic arthritis in an immunocompromised patient. Int J Infect Dis 2010;14 Suppl 3:e307–9. 10.1016/j.ijid.2010.02.2253 [DOI] [PubMed] [Google Scholar]
- 5.Korytny A, Nasser R, Geffen Y, et al. Ureaplasma parvum causing life-threatening disease in a susceptible patient. BMJ Case Rep 2017;53:bcr2017220383 10.1136/bcr-2017-220383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell JJ, Larson JA, Akeson JW, et al. Ureaplasma parvum prosthetic joint infection detected by PCR. J Clin Microbiol 2014;52:2248–50. 10.1128/JCM.00432-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suknuntha K, Lepak AJ, Rehrauer WM, et al. Identification of Ureaplasma parvum as a cause of culture-negative septic Monoarthitis using 16S rRNA gene sequencing. Infectious Diseases in Clinical Practice 2019;27:e12–14. 10.1097/IPC.0000000000000752 [DOI] [Google Scholar]
- 8.George MD, Cardenas AM, Birnbaum BK, et al. Ureaplasma septic arthritis in an immunosuppressed patient with juvenile idiopathic arthritis. JCR: Journal of Clinical Rheumatology 2015;21:221–4. 10.1097/RHU.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 9.Beeton ML, Spiller OB. Antibiotic resistance among Ureaplasma spp. isolates: cause for concern? J Antimicrob Chemother 2017;72:330–7. 10.1093/jac/dkw425 [DOI] [PubMed] [Google Scholar]
- 10.Waites KB, Duffy LB, Bébéar CM, et al. Standardized methods and quality control limits for agar and broth microdilution susceptibility testing of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum. J Clin Microbiol 2012;50:3542–7. 10.1128/JCM.01439-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeton ML, Chalker VJ, Maxwell NC, et al. Concurrent titration and determination of antibiotic resistance in Ureaplasma species with identification of novel point mutations in genes associated with resistance. Antimicrob Agents Chemother 2009;53:2020–7. 10.1128/AAC.01349-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beeton ML, Chalker VJ, Jones LC, et al. Antibiotic resistance among clinical Ureaplasma isolates recovered from neonates in England and Wales between 2007 and 2013. Antimicrob Agents Chemother 2016;60:52–6. 10.1128/AAC.00889-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández J, Karau MJ, Cunningham SA, et al. Antimicrobial susceptibility and clonality of clinical Ureaplasma isolates in the United States. Antimicrob Agents Chemother 2016;60:4793–8. 10.1128/AAC.00671-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo D-Q, Liu J-Y, Yang W, et al. In vitro activities of erythromycin, tetracycline and levofloxacin alone and in dual combinations against Ureaplasma spp. Chemotherapy 2011;57:128–33. 10.1159/000323629 [DOI] [PubMed] [Google Scholar]


