Abstract
This case aims to remind all providers to scrutinise for atypical presentations of multisystem inflammatory syndrome in children (MIS-C) which may mimic a more routine diagnosis. In the absence of mucocutaneous symptoms, the diagnosis of MIS-C can be missed. Given the potential for rapid deterioration of patients with MIS-C, early treatment and inpatient interventions are necessary.
Keywords: tropical medicine (infectious disease), paediatric intensive care, medical management
Background
The novel SARS-CoV-2 (COVID-19) pandemic has presented a wide range of clinical manifestations, varying from asymptomatic to severe acute respiratory distress syndrome and death.1 2 Paediatric patients are believed to exhibit a milder course than adults.3–6 While the complications of COVID-19 in adult patients are better recognised, the morbidities in paediatric patients have only recently become apparent.5 6
A new syndrome affecting paediatric patients with COVID-19, multisystem inflammatory syndrome in children (MIS-C), has been defined by the Centers for Disease Control and Prevention (CDC) and the WHO.7 8 The spectrum of MIS-C is characterised by an unopposed inflammatory state that may rapidly progress to multiorgan failure.9–11 Children with MIS-C present with persistent fever (100%), conjunctivitis (68%), rash (75%), elevated inflammatory markers (100%), coagulopathy (100%), gastrointestinal complaints (85%) and cardiac abnormalities (75%).12 The overlap between MIS-C, Kawasaki disease (KD) and macrophage activation syndrome suggests that MIS-C represents a spectrum of diseases.9 11 The presence of positive SARS-CoV-2 antigen by PCR, serological testing for antibodies or report of close contact with a person diagnosed with COVID-19 helps differentiate MIS-C from other illnesses.11 Unlike traditional KD, affected patients with MIS-C often require intensive care and benefit from additional targeted therapies against the inflammatory response.13
Providers are learning more about the spectrum of MIS-C as the incidence rises, which has led to an emphasis on worldwide collaboration to define this syndrome and its risk factors for severe morbidity. In this case report, we describe a novel presentation of MIS-C with rapid deterioration which was initially overlooked during three emergency room visits in an effort to educate providers to remain vigilant and more quickly diagnose this illness.
Case presentation
A previously healthy 8-year-old African-American twin boy, presented to an outside emergency department (OED) with a mild periumbilical abdominal pain and 2 days of fever unresponsive to antipyretics. No other symptoms were identified during the initial encounter. There was a history of recent in-state travel, and the patient’s mother had screened positive for COVID-19 by nasopharyngeal PCR 28 days prior to presentation. The mother was never symptomatic, and she subsequently screened negative on repeat testing per work policy as a geriatric nurse. No other family members were tested, and all remained asymptomatic. Physical examination in the OED was significant for mild abdominal tenderness in the periumbilical area without guarding or peritoneal signs. An abdominal X-ray showed significant stool burden, and the patient was discharged with a diagnosis of mild gastritis, early gastroenteritis and constipation.
The patient returned to the OED the following day due to persistently high fever, continued abdominal pain and decreased appetite. He was febrile and tachycardic, and his examination revealed mild periumbilical pain. An abdominal ultrasound was performed due to suspicion of appendicitis, but proved inconclusive. Laboratory tests revealed mild thrombocytopenia and an elevated C reactive protein (CRP) of 15.8 mg/dL. The patient was then transferred to our paediatric emergency department (PED) for further evaluation with a working diagnosis of appendicitis.
In our PED, the patient tested negative for COVID-19 by nasopharyngeal swab PCR, ordered per hospital policy. A repeat abdominal ultrasound remained inconclusive for appendicitis, but the consulting paediatric surgery team recommended admission for serial abdominal examinations overnight. The patient was admitted to the general paediatrics floor in the late afternoon.
Investigations
On admission, the patient remained febrile and tachycardic but was otherwise well appearing. He was given a normal saline bolus for tachycardia and antipyretics for fever control. A chest radiograph did not reveal cardiopulmonary anomalies. Given the elevated CRP and a 4-day history of fever, clinical suspicion was high for atypical KD, macrophage activation syndrome (MAS), or viral myocarditis, so additional laboratory studies were performed. Urinalysis revealed pyuria and haematuria. Blood and urine cultures and a nasopharyngeal PCR swab for common respiratory viruses, otherwise known as a respiratory viral panel (RVP), were collected, and the patient was empirically started on ceftriaxone. The patient had elevations in CRP, D-dimer, pro-brain natriuretic peptide (pro-BNP), lactate dehydrogenase and coagulation studies, but a normal troponin level (table 1).
Table 1.
Summary of pertinent laboratory values with institutional normals
| Laboratory | Admission to the floor | 12 hours post-admission | 36 hours post-admission | Days 3–4 of admission, post-anakinra and post-remdesevir |
| CRP (<0.9 mg/dL) |
24.7 | 30.9 | 29.5 | 26.1 |
| ESR (<10 mm/hour) |
61 | 118 | ||
| Troponin (<0.034 ng/mL) |
<0.012 | <0.012 | 0.551 | 0.668 |
| Pro-BNP (<125 pg/mL) |
264 | 1420 | 7850 | 9500 |
| LDH (313–618 U/L) |
687 | 622 | 804 | 939 |
| Fibrinogen (195–515 mg/dL) |
623 | 534 | 769 | |
| Procalcitonin (<0.08 ng/mL) |
14.5 | 7.3 | ||
| Ferritin (<400 ng/mL) |
362 | 426 | 904 | |
| D-dimer (<0.49 µg/mL) |
2.7 | 3.1 | 5.3 | 4.4 |
By 24 hours of admission, when the patient clinically deteriorated, troponin and pro-BNP levels were significantly elevated.
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; pro-BNP, pro-brain natriuretic peptide.
The following morning, approximately 12 hours after admission and 5 days since symptom onset, the patient continued to spike high-grade fevers with persistent tachycardia despite antipyretics and intravenous fluids (figure 1). His generalised abdominal pain remained unchanged without guarding or peritoneal signs. An abdominal MRI was completed to definitively evaluate for appendicitis, showing diffuse thickening of bowel walls throughout the ileum and large intestine and a retrocecal appendix. Paediatric surgery determined that no surgical intervention was indicated. A 12-lead ECG demonstrated sinus tachycardia, and repeated inflammatory, coagulation and cardiac markers from that morning had worsened (table 1). The RVP, blood culture and urine culture were negative. Given our clinical suspicion for viral myocarditis, paediatric infectious disease and paediatric cardiology were consulted.
Figure 1.
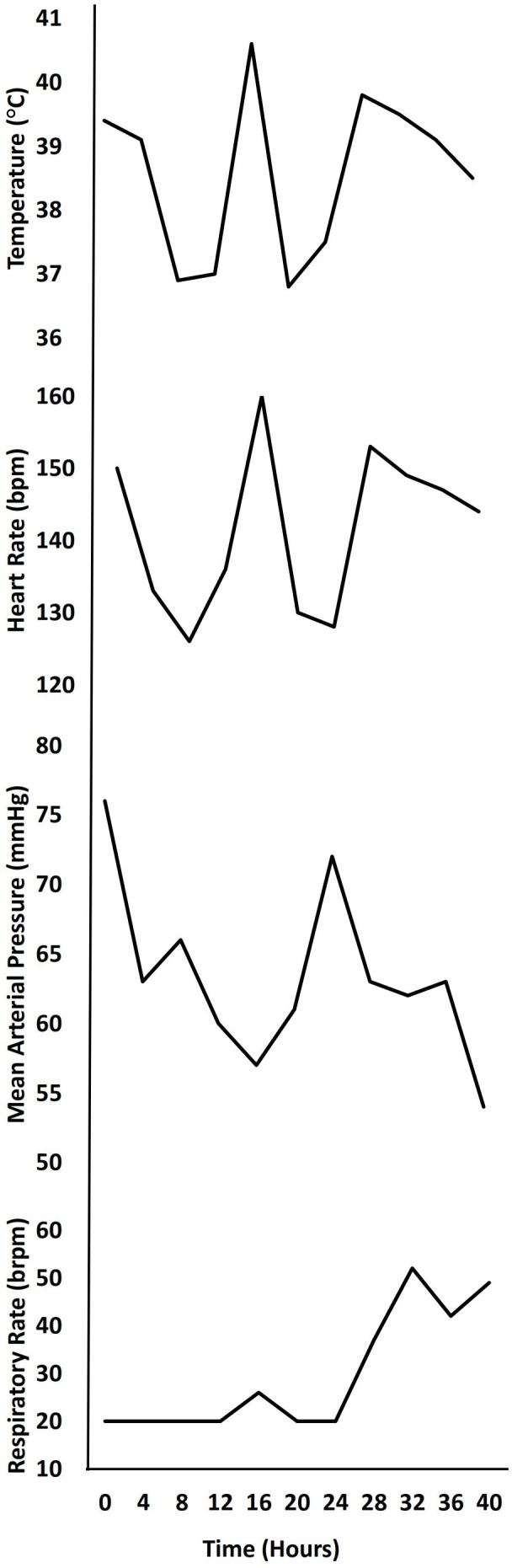
Trends in vital signs. Heart rate remained elevated from normal for age (70–110 beats/min) even when patient was afebrile. Blood pressure and respiratory rate deteriorated quickly at 24 hours.
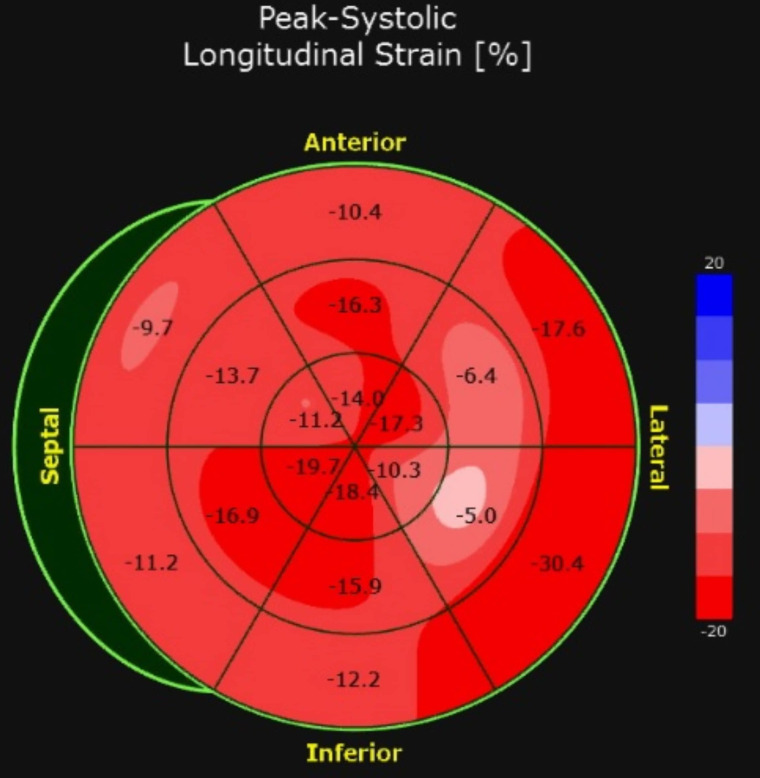
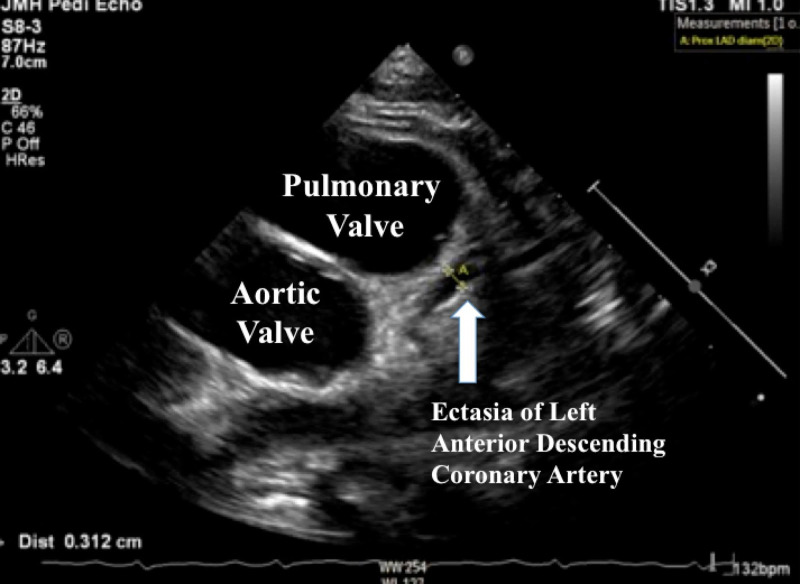
Work-ups including traditional viral causes of myocarditis were ordered, and serologies for SARS-CoV-2 were sent given the maternal exposure. An echocardiogram showed reduced peak global longitudinal strain, a sensitive marker of cardiac dysfunction (figure 2), significant ectasia of the left anterior descending (LAD) coronary artery (Z =+2.5), no aneurysms, normal biventricular systolic function (ejection fraction 64%) and no pericardial effusion (figure 3).
Figure 2.
Myocardial strain imaging before treatment demonstrated abnormalities in left ventricular function at the base and lateral wall. Global longitudinal strain of −13.9% (reference normal value for our centre and equipment −19.6% ± 1.9%).
Figure 3.
Echocardiogram demonstrating ectasia of left anterior descending coronary artery which measured 3.12 mm (Z-score = +2.5).
Differential diagnosis
At 18 hours after admission, our differential diagnoses included atypical KD, viral myocarditis, and COVID-19-related Kawasaki-like inflammatory syndrome that was later known as MIS-C. The echocardiogram findings were consistent with KD, but our patient did not demonstrate mucocutaneous symptoms at any point, contrary to the definition of KD. Although our patient initially tested negative for COVID-19, the presenting symptoms and laboratory markers were similar to early reports of what is now known as MIS-C.9–11 The elevated pro-BNP and persistent tachycardia were indicative of myocarditis, and with concurrent abdominal symptoms, viral aetiologies were most likely. MAS was preliminarily ruled-out based on the lack of cytopenias and a ferritin below 500 ng/mL.
Due to the possibility of rapid deterioration, the patient was placed on telemetry and treated with high-dose aspirin and intravenous immunoglobulin (IVIG) for treatment of presumed atypical KD based on echocardiogram findings. Shortly after initiating treatment, and 24 hours after admission, the patient developed hypotension, cool extremities with diminished pulses and tachypnea (figure 1). The patient was transferred to the paediatric intensive care unit (PICU) for closer monitoring. Post-transfer, viral antibodies for SARS-CoV-2 returned positive for mixed IgM and IgG, and he was formally diagnosed with post-viral MIS-C, and coincidentally, this diagnosis was made on the same day the CDC announced their case definition for MIS-C. Additional viral myocarditis work-ups for adenovirus, Epstein-Barr virus, cytomegalovirus, hepatitis C, herpes simplex virus, and HIV returned negative, and he started on low molecular weight heparin, received a second dose of IVIG, and continued on aspirin.
Treatment
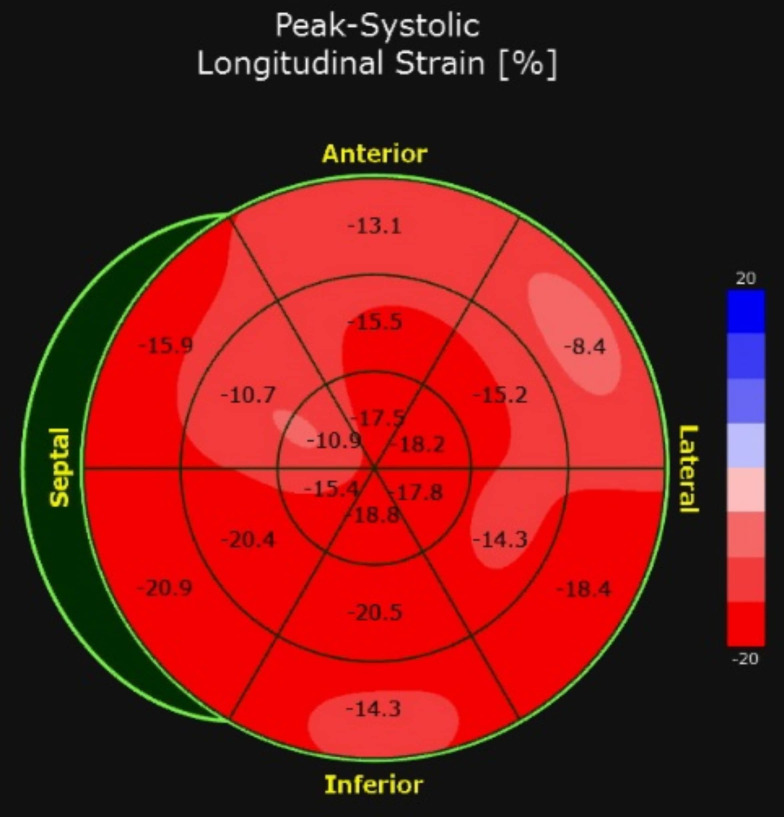
In the PICU, he rapidly decompensated due to cardiogenic shock (ejection fraction 30%), leading to acute hypoxic respiratory failure with pulmonary oedema and acute kidney injury. He was intubated and started on inotropic support. His treatment was dictated by the New York Presbyterian Children’s Hospital clinical protocol for moderately severe MIS-C; therefore he received methylprednisolone.14 A repeat nasal swab for SARS-CoV-2 PCR was ordered per protocol and subsequently resulted as positive.14 There was suspicion for cytokine storming with declining cardiac function, and based on several case reports of improving coronary ectasia, anakinra was initiated.15 Remdesivir was also started given the positive SARS-CoV-2 PCR finding. A repeat echocardiogram performed approximately 20 hours after administration of anakinra and remdesivir showed improvement in the patient’s ejection fraction from 30% to 60% and improvement in myocardial strain (figure 4).
Figure 4.
Myocardial strain imaging after treatment with anakinra and remdesivir demonstrating improvement in left ventricular dysfunction at the base. Global longitudinal strain of −15.3% (reference normal value for our centre and equipment −19.6% ± 1.9%).
Outcome and follow-up
Thirty-six hours post-anakinra and post-remdesivir, the patient’s fever curve had decreased, his laboratory tests demonstrated improved gas exchange and he was weaned to minimal ventilator settings. The patient was successfully extubated to room air 66 hours after administration of the two agents. He was discharged without additional cardiopulmonary complications or laboratory anomalies after completing 10 days of remdesivir and 14 days of anakinra. An echocardiogram at the time of discharge demonstrated diffuse ectasia in the LAD, along with normal biventricular size, wall thickness and systolic function. Discharge medications included famotidine, aspirin, enalapril, enoxaparin, and prednisolone. Prednisolone was tapered over 2 weeks and famotidine was discontinued at its conclusion. Aspirin and enalapril will be continued for a total of 90 days of treatment. Enoxaparin is to be continued pending follow-up with the haematology service.
Discussion
MIS-C associated with SARS-CoV-2 is thought to occur secondary to a cytokine storm that damages numerous organ systems. The inflammatory response results in blood vessel dilation, leading to hypotension, fluid accumulation and shock. Despite initially presenting with fever and abdominal pain only, our patient quickly deteriorated due to cardiogenic shock, consistent with other reported cases of MIS-C. It is important for providers to be aware of the diagnosis of MIS-C so that antiviral and targeted immunotherapy can be initiated quickly.
Prior to the COVID-19 pandemic, a group of autoinflammatory disorders similar to MIS-C was defined. They are collectively known as cryopyrin-associated periodic syndromes (CAPS), with gene mutations that affect interleukin-1 (IL-1) activity. These diseases present similarly to MIS-C, with episodic fevers, rash, conjunctivitis and progressive multisystem damage.16 MIS-C is also thought to affect IL-1 activity levels, so anakinra has been used in its treatment. While the pathogenesis of CAPS and MIS-C remains largely unknown, there are suspicions that affected patients may have some underlying genetic susceptibility. It is remarkable that our patient has an identical twin brother with similar exposure, who did not manifest with MIS-C, which we found to be notable from an epidemiological perspective.
Interestingly, procalcitonin had long been used as a marker of a serious bacterial infection, but a recent meta-analysis of four studies found that patients with COVID-19 with increased procalcitonin levels had a fivefold higher risk for severe SARS-CoV-2 infections.17 Our patient had significantly elevated procalcitonin that was consistent with his clinical deterioration. Further studies in critically ill paediatric patients with COVID-19 are needed to fully understand how to interpret procalcitonin levels during this pandemic.
As we expand our understanding of MIS-C secondary to COVID-19, we recognise that there is likely a broader spectrum of signs and symptoms beyond those of classical CAPS. A recent case series from the UK described eight previously healthy children who developed MIS-C with an initial presentation of fevers >39°C for at least 4 days, diarrhoea, conjunctivitis, peripheral oedema and rash.18 In addition to MIS-C, there have also been numerous case series of typical and atypical KD presenting in the setting of COVID-19. In an Italian case series, all children who presented with COVID-19-related atypical KD fulfilled at least two clinical criteria for KD.11
Our case is unique in that the presenting symptoms of our patient were only fever and abdominal pain. Even after 5 days of fever, our patient did not exhibit peripheral oedema, mucocutaneous changes, signs of conjunctivitis, diarrhoea, vomiting or chest pain, findings inconsistent with the current MIS-C literature.9–11 15 We suspect the lack of initial multi-organ involvement led to misdiagnosis in the PED as a more routine entity. In reviewing this clinical case, our hospital developed and implemented a new screening protocol for MIS-C to promote earlier diagnosis. Given the risk of rapid and severe deterioration, we advocate for a high level of vigilance in all providers during this current pandemic and recommend that patients with significant fevers and abdominal pain be evaluated for MIS-C.
Patient’s perspective.
My son was admitted to Jackson Memorial Hospital on 13 May 2020 with unusual and puzzling symptoms. I have never seen a team so dedicated to figuring out what was wrong with him. Days had passed and I saw my son getting sicker. They were there with encouraging words. No words can express the love and gratitude I have for this awesome medical, cardiac and intensive care unit team of nurses and doctors. Thank you with all my heart. (N’s mom)
Learning points.
Multisystem inflammatory syndrome in children (MIS-C) can present with a broad range of clinical symptoms including just fever and abdominal pain.
Early recognition of MIS-C is critical as patients can deteriorate rapidly.
Given the worldwide COVID-19 pandemic, it is paramount for all providers to be familiar with MIS-C and to keep this entity in their differential diagnosis, even in cases where classic diagnostic criteria are not initially fulfilled.
Footnotes
Contributors: NM, HTC and RL drafted the initial manuscript, collected pertinent data, and reviewed and revised the manuscript. RM provided key data and figures, as well as, reviewed and revised the manuscript. WRG designed the table and revised the figures, analysed the data, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R, Votto M, Licari A, et al. . Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020. 10.1001/jamapediatrics.2020.1467. [Epub ahead of print: 22 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 4.Mehta NS, Mytton OT, Mullins EWS, et al. . SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis 2020. 10.1093/cid/ciaa556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sankar J, Dhochak N, Kabra SK, et al. . COVID-19 in children: clinical approach and management. Indian J Pediatr 2020;87:433–42. 10.1007/s12098-020-03292-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J 2020;39:469–77. 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center for Disease Control and Prevention, US Department of Health & Human Services . Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19), 2020. Available: https://emergency.cdc.gov/han/2020/han00432.asp [Accessed 20 May 2020].
- 8.Freedman SG-C S, Gorman R, Lodha R, et al. . Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19, 2020. Available: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 [Accessed 20 May 2020].
- 9.Mahase E. Covid-19: cases of inflammatory syndrome in children surge after urgent alert. BMJ 2020;369:m1990. 10.1136/bmj.m1990 [DOI] [PubMed] [Google Scholar]
- 10.Mahase E. Covid-19: concerns grow over inflammatory syndrome emerging in children. BMJ 2020;369:m1710. 10.1136/bmj.m1710 [DOI] [PubMed] [Google Scholar]
- 11.Verdoni L, Mazza A, Gervasoni A, et al. . An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771–8. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung E. NYC experience with COVID-19: from ICU adaptations to multi-system inflammatory syndrome in children New York Presbyterian/Columbia University Children’s Medicine; 2020. [Google Scholar]
- 13.Royal College of Paediatrics and Child Health Health RCoPaC. Guidance - Paediatric multisystem inflammatory syndrome temporally associated with COVID-19, 2020. Available: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19 [Accessed 20 May 2020].
- 14.Jona B, Cheung E. Pediatric guidelines for COVID-19 multi-system inflammatory syndrome New York Presbyterian Kids; 2020. [Google Scholar]
- 15.Belhadjer Z, Méot M, Bajolle F, et al. . Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 16.Yu JR, Leslie KS. Cryopyrin-Associated periodic syndrome: an update on diagnosis and treatment response. Curr Allergy Asthma Rep 2011;11:12–20. 10.1007/s11882-010-0160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. . Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020;505:190–1. 10.1016/j.cca.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]