Highlights
-
•
Subsolid nodules, which may be divided into pure ground glass and part-solid nodules, are increasingly identified at CT.
-
•
Subsolid nodules have higher risk of malignancy than solid nodules and represent lesions along the adenocarcinoma spectrum.
-
•
Subsolid adenocarcinomas are more indolent than solid adenocarcinomas.
-
•
Emerging evidence suggests longer intervals and shorter duration of follow-up may be used for stable subsolid nodules.
-
•
Definitive therapy may be less aggressive, such as sublobar resection rather than lobectomy.
Keywords: Subsolid nodule, Ground glass nodule, Part-solid nodule
Abstract
Ground glass and part-solid nodules, collectively referred to as subsolid nodules, present a challenge in management, with a high risk of malignancy but, when malignant, demonstrating indolent behavior. Emerging data suggest longer follow-up intervals and shorter duration of follow-up is likely appropriate in these nodules. Additionally, definitive therapy is shifting to less aggressive approaches such as sub-lobar resection. Patients may benefit from individualized approaches, incorporating both patient and imaging features to determine whether treatment is necessary.
1. Introduction
Subsolid pulmonary nodules, which comprise pure ground glass and part-solid nodules, are commonly identified incidentally or as part of lung cancer screening. Our detection of subsolid nodules has grown dramatically over the last decade as thin section CT has become widely available. Additionally, our understanding of these nodules has progressed recently with the updated classification of lung adenocarcinoma in 2011 [1]. Subsolid nodules present a particular dilemma for radiologists and clinicians, as they have a higher rate of malignancy than solid nodules but, when malignant, demonstrate indolent behavior compared to solid lung cancers. In this review, we will discuss controversies in existing management guidelines, genetics and epidemiology, emerging evidence, and future directions of research in subsolid nodules.
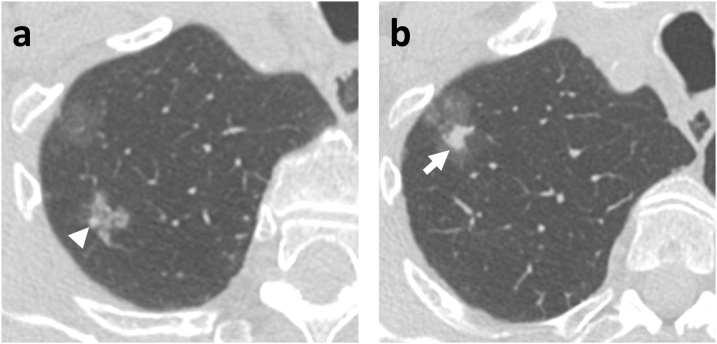
Fig. 1.
88-year-old man with two part-solid nodules in the right upper lobe; the more superior nodule (a) is 16 mm with 3 mm solid component (arrowhead), and the inferior nodule (b) is 29 mm with 9 mm solid component (arrow). Pathology of the lobectomy specimen demonstrated that the smaller nodule was a minimally-invasive adenocarcinoma and the larger nodule was an invasive adenocarcinoma.
2. Behavior of subsolid nodules
Subsolid nodules are common, identified in approximately 9% of lung cancer screening patients [2,3]. We define a pure ground glass nodule (GGN) as a well-circumscribed nodular lesion in the lung parenchyma with attenuation less than adjacent pulmonary vessels. A part-solid nodule is a nodule that has both a ground glass component and one or more solid components, defined by having density visually equal to that of pulmonary vessels. Identification and measurement of solid components should be done on thin section images (1 mm or thinner) and lung windows [4]. Subsolid nodules may contain internal cystic change, sometimes referred to as pseudocavitation or bubbly cysts; this is often seen with early adenocarcinomas [5].
Approximately one quarter of these nodules will resolve, representing an inflammatory process [2,3,6]. Those that persist have a high risk of malignancy, representing a spectrum of neoplastic growth ranging from atypical adenomatous hyperplasia (AAH) to adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and fully invasive adenocarcinoma [1]. Typically, AAH presents as a small round ground-glass opacity, more frequently seen in the upper lobes [7]. AIS lesions are typically associated with a more irregular shape and small solid components. The solid components and overall lesion size are generally larger in MIA lesions and still larger in invasive adenocarcinomas. In pathology, an invasive adenocarcinoma must have at least a 5 mm invasive component to distinguish it from MIA; this size threshold is often applied on CT as well, although as we note below, the solid component on CT does not exactly represent the invasive component. Size and density are the two principal features that correspond to increasing invasiveness pathologically [1], although other features such as internal heterogeneity and irregular lesion margins are also associated with lesions on the more invasive part of the spectrum. However, in the real world, the relationship between pathologic classification and radiologic appearance is more complicated, as seen in the following examples (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
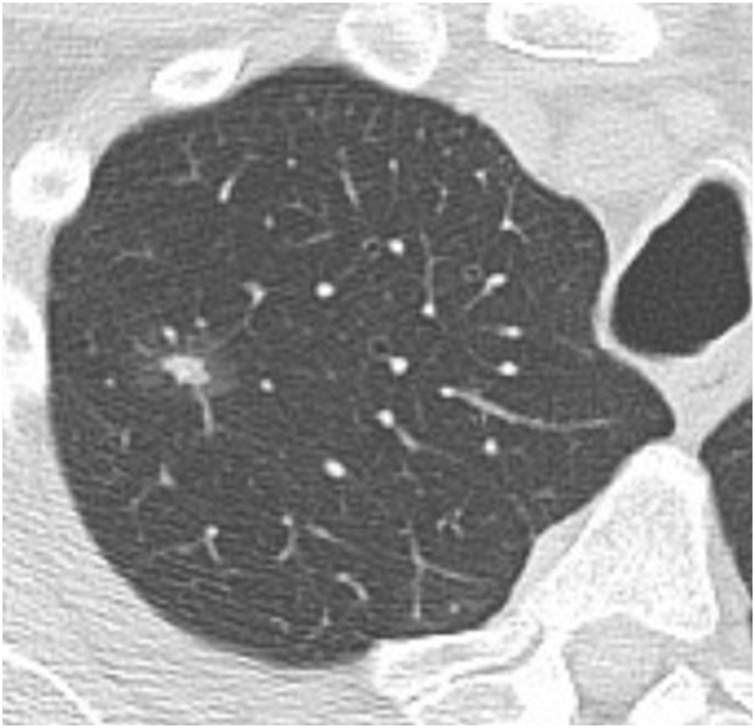
Fig. 2.
71-year-old man with a part-solid nodule in the right upper lobe (15 mm total diameter with 6 mm solid component). This was resected, with pathology showing adenocarcinoma in situ.
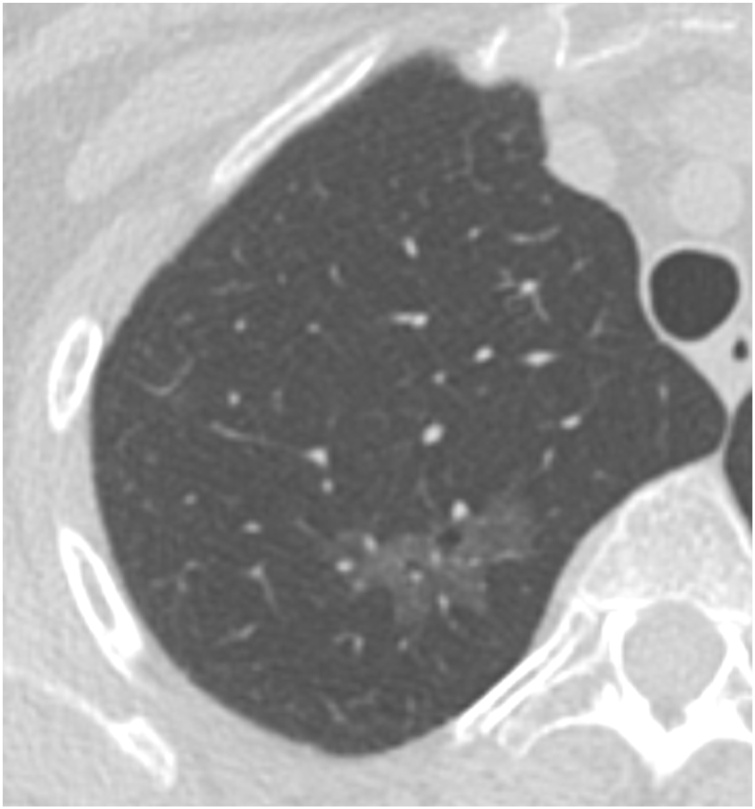
Fig. 3.
56-year-old woman with a pure ground glass nodule in the right upper lobe measuring 30 × 15 mm. This was resected, with pathology showing lepidic-predominant invasive adenocarcinoma. Genomic analysis showed a mutation in the KRAS proto-oncogene.
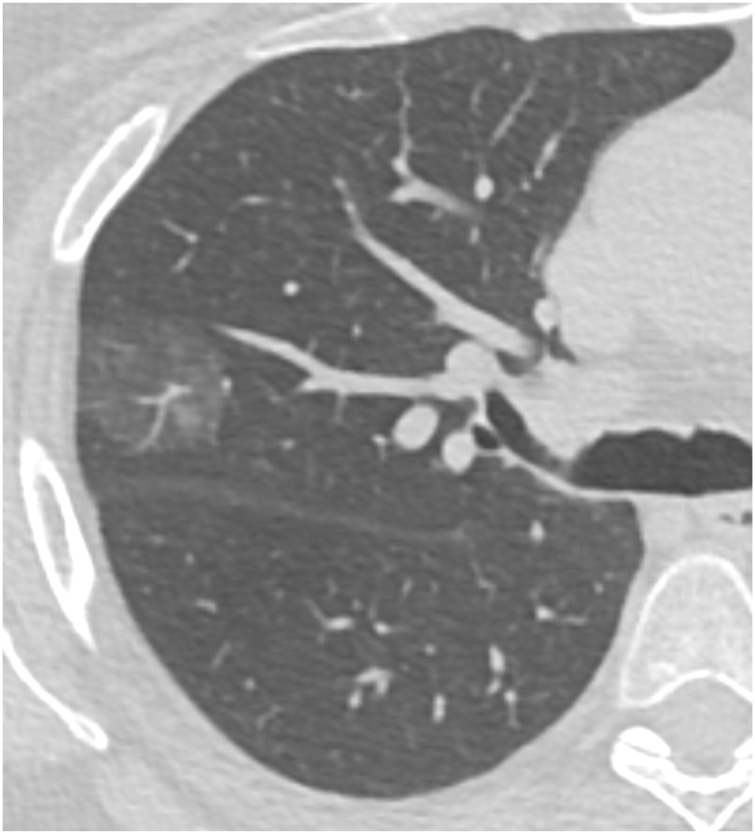
Fig. 4.
65-year-old woman with a pure ground glass nodule (29 mm in diameter) with multiple small areas of increased density in the right upper lobe. This was resected, with pathology showing lepidic-predominant invasive adenocarcinoma. Genomic analysis showed a mutation in the MET proto-oncogene.
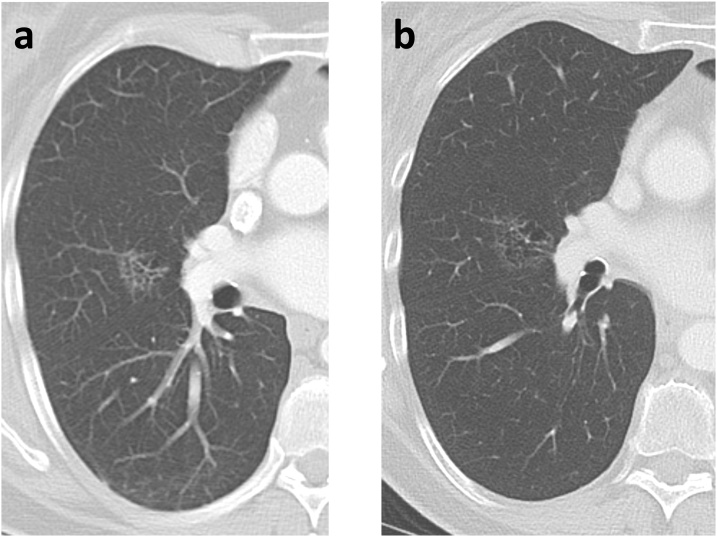
Fig. 5.
66-year-old woman with a ground glass nodule in the right middle lobe. (a) Baseline CT and (b) CT 4 years later show slow growth over time, with the lesion measuring 26 mm on the later CT. Note extensive internal ‘bubbly’ cystic change within the lesion. Surgical resection after the CT in (b) showed invasive adenocarcinoma.
Overall, subsolid nodules, particularly part-solid nodules, have a higher risk of malignancy than solid nodules [8]. The risk of malignancy increases by nodule size as well as presence and size of a solid component: pure ground glass nodules < 1 cm have a malignancy rate of approximately 1%, whereas part-solid nodules with a solid component ≥ 6 mm have a malignancy rate of around 20 % (Fig. 1) [6]. Many radiologists associate the solid components in part-solid nodules with the pathologic invasive component. However, the solid component on imaging often over-estimates the size of the invasive component, since it contains atelectatic lung as well as malignant cells (Fig. 2) [9]. Radiologists should also note that pure ground glass nodules may be malignant (Fig. 3, Fig. 4) [5].
However, while subsolid nodules have a higher rate of malignancy than solid nodules, those that are malignant exhibit more indolent behavior than purely solid malignancies. Subsolid cancers grow more slowly than solid cancers (Fig. 5) [10]. In addition, they are less likely to develop lymph node and distant metastases [3] and do not appear to benefit from routine lymph node dissection at surgery [11]. Recurrence-free survival for T1 adenocarcinomas is worst for those presenting as solid nodules and is much better for part-solid nodules and even better for pure ground glass nodules, the latter of which had a 100 % 5-year recurrence-free survival in one series [12].
3. Genetics and epidemiology
Subsolid adenocarcinomas have traditionally been associated with female nonsmokers, particularly in Asian populations, but recent data show smoking status and male sex being risk factors for growth of such nodules [9]. Subsolid nodules are frequently multiple, complicating treatment decisions [13]. Subsolid nodules frequently contain mutations in the epidermal growth factor receptor (EGFR) gene, seen in 64–74 % of such nodules in several series [14,15]. There may be an association between development of a solid component within a ground glass nodule and acquisition of EGFR mutation [16]. Notably, EGFR mutations are also associated with female sex, Asian populations, and non-smokers, supporting the association between these demographic factors and subsolid adenocarcinomas [17]. Other mutations such as KRAS, ALK and HER2 were found in 3–4 % of resected GGNs, respectively, in one Japanese study [18]. The exact role of mutations in subsolid nodules remains to be elucidated.
4. Follow-up guidelines
A number of guidelines exist to assist radiologists and clinicians in the management of pulmonary nodules (Table 1). These include guidelines from the Fleischner Society for incidental nodules and the Lung-RADS guidelines from the American College of Radiology for nodules identified at lung cancer screening [19,20]. To briefly summarize, the Fleischner Society guidelines recommend no follow-up for subsolid nodules < 6 mm; and, after an initial short follow-up to confirm persistence, annual follow-up for part-solid nodules and biennial follow-up for ground glass nodules for 5 years. The Lung-RADS guidelines are to be used within lung cancer screening, in which patients undergo annual follow-up (screening) by default. For the most part, pure ground glass nodules are followed annually under this scheme; part-solid nodules are managed by the size of their solid component but if stable are then followed annually as well. Unfortunately, while these guidelines are widely used, they are predominantly based on expert opinion. No data from prospective trials exists at the moment to support specific nodule management guidelines.
Table 1.
Guidelines and Recommendations for Follow-up of Stable Subsolid Nodules.
| Recommendation | Patient Population | Nodule Characteristic | Follow-up Interval | Follow-up Duration |
|---|---|---|---|---|
| Fleischner Society(19) | Incidental nodule | GGN or PSN < 6 mm GGN ≥ 6 mm PSN ≥ 6 mm |
No follow-up CT at 6−12 months, then every 2 years CT at 3−6 months, then every 1 year |
No follow-up 5 years 5 years |
| Lung-RADS(20) | Lung cancer screening | PSN < 6 mm GGN < 30 mm Any PSN or GGN stable for ≥ 3 months |
Every 1 year | Indefinite (screening) |
| Hammer, et al.(26) | High-risk patients | PSN < 6 mm GGN < 30 mm Any PSN or GGN stable for ≥ 3 months |
Every 2 years | For GGN, 2 years For PSN, 5 years |
GGN, ground glass nodule; PSN, part solid nodule.
These nodule guidelines are based upon malignancy risk and, to some extent, tumor aggressiveness. The Lung-RADS guidelines, in fact, specify malignancy risk for each category (<1% for category 2, 1–2 % for category 3, 5–15 % for category 4A, and >15 % for category 4B). However, these numbers substantially underestimate risk for the subsolid nodules that are assigned to the Lung-RADS categories [21,22]. Because subsolid nodules behave more indolently than their solid counterparts, assigning subsolid nodules to lower risk categories may be justified, but this requires further study.
Because subsolid nodules typically have very indolent growth, they may appear stable for years before growing. In some cases, a more aggressive malignancy may develop years after initial detection of a subsolid nodule. For these reasons, it remains unclear how frequently and for how long subsolid nodules should be surveilled. If a subsolid nodule is stable for several years, does such a patient need perpetual follow-up? Fleischner guidelines recommend annual follow-up for part-solid nodules and biennial follow-up for ground glass nodules, both for 5 years duration, but again this is based in large part on expert opinion.
A number of recent articles have studied the growth of subsolid nodules that are initially stable for a 3−5 year period [[23], [24], [25]]. The rate of subsequent growth of subsolid nodules after an initial period of stability is low but variable, found to be 2 %, 3 %, and 13 % in those studies. However, even if such a nodule grows, the clinical relevance (i.e. potential for impacting the patient’s life expectancy) is likely low. A simulation-based cost-effectiveness analysis that evaluated several follow-up durations found that the most cost-effective were 2 years for ground glass nodules and 5 years for part-solid nodules (Table 1) [26].
Of note, the Fleischner Society guidelines do not apply in patients with known malignancy or immunocompromised state. As noted earlier, approximately one quarter of subsolid nodules are inflammatory. These nodules are frequently seen in immunocompromised patients as signs of viral or (if the ground glass component is actually a halo) fungal pneumonias. In that setting, the nodules are typically multiple and resolve in the short term. A more complicated question is how to address subsolid nodules in patients with known malignancy. Subsolid nodules are a very uncommon presentation of metastatic disease from non-lung cancer. Thus, in a patient in whom a subsolid nodule is detected as part of a staging exam, that nodule should be regarded as incidental and may be managed using the same guidelines, e.g. Fleischner, depending upon the prognosis of the patient’s primary malignancy.
Subsolid nodules in patients with lung cancer present a more complicated clinical problem. These may occur as multiple synchronous primary lesions. Typically, the appearance in that case is of multiple ground glass or part-solid nodules of varying sizes with a mild upper lung predominance. These are generally slowly growing, though one lesion may start behaving more aggressively at any time. Surgical management of such patients is difficult because the surgeon must spare lung parenchyma, envisioning the likelihood that additional nodules may develop aggressive features and require treatment as well.
Less commonly, multiple subsolid nodules, particularly multiple ground glass nodules, may be the presentation of pulmonary metastatic disease from lung cancer. These are generally mucinous or lepidic adenocarcinomas, and the primary tumor typically will have a ground glass component. Such lesions tend to grow far more rapidly than in the synchronous primary case, and they tend to have a dependent distribution since they spread through endogenous aspiration of tumor cells.
5. Therapeutic options
In contrast to stable, low risk subsolid nodules, those that are large or exhibit progressive growth are typically taken to definitive therapy. There are a number of options for definitive therapy, including anatomic surgical resection (lobectomy), limited (sub-lobar or wedge) surgical resection, stereotactic beam radiation therapy (SBRT), and percutaneous ablation.
The standard of care treatment for patients with non-small cell lung cancer who are surgical candidates is lobectomy [27], although guidelines do allow sublobar resection for certain subsolid nodules. As noted earlier, the excellent post-resection prognosis of subsolid nodules seems to support the idea of using less aggressive surgical therapy, namely sublobar resection [12]. A number of retrospective studies support good outcomes for treating subsolid nodules with sub-lobar resection [28,29], and there are ongoing randomized trials in the US and Japan that aim to answer this question definitively [30,31].
Use of non-surgical treatment, i.e. radiation or ablation, is generally reserved for patients who are not surgical candidates because of limited lung function or comorbidities [27]. Overall, retrospective analyses of elderly patients found better outcomes with surgical resection compared to non-surgical treatment, although one study found comparable outcomes [[32], [33], [34]]. However, these studies included all patients with non-small cell lung cancer and did not specifically focus on subsolid nodules. Given the indolent behavior and low metastatic potential for subsolid adenocarcinomas, the benefits of surgery are likely reduced in these cancers. One simulation-based analysis suggested that radiation therapy may even provide improved outcomes in these patients because of less treatment-related morbidity and mortality [35]. Future research is needed in this arena to better define the benefits of surgery versus non-surgical management of subsolid malignancies.
More broadly, the question of which patients should even undergo definitive therapy is the subject of some debate. Guidelines for management of patients within lung cancer screening programs, such as Lung-RADS or the NELSON trial guidelines, have high thresholds for treatment of subsolid nodules [20,36]. Lung-RADS advises against treatment of most pure ground glass nodules; and NELSON uses volume doubling time to determine which nodules need intervention. However, we note that in clinical practice, many patients with persistent subsolid nodules may go to definitive treatment based on local practice patterns and/or patient preference.
6. Risk stratification and future directions
As noted, currently the most commonly used nodule management guidelines are those from the Fleischner Society for incidental nodules and Lung-RADS for lung cancer screening cases. These guidelines rely principally on nodule size measurements and for the most part do not take into account other patient or nodule factors in assigning risk categories. However, there are more sophisticated nodule risk triage tools, the most well-known of which is the Brock University nodule risk calculator [8]. This calculator includes patient risk factors such as age, sex, and emphysema, as well as nodule factors such as upper lobe location and size. It has been shown to outperform Lung-RADS in nodules from the National Lung Screening Trial [21,37].
Besides risk calculators based on patient characteristics and nodule size, there is emerging literature about use of more complex CT features and more advanced image processing tools to triage nodules. Several articles have shown promise of certain CT features for predicting invasive adenocarcinomas from ground glass nodules, including attenuation and iodine content derived from dual energy CT [5,[38], [39], [40]]. More advanced image processing tools include radiomics, which evaluates the texture characteristics of nodules and surrounding lung parenchyma, and deep learning (artificial intelligence) with convolutional neural networks. Radiomics has been shown to be helpful in evaluating malignancy risk in pulmonary nodules, including subsolid nodules [[41], [42], [43], [44]]. Deep learning, which relies on convolutional neural networks to classify whole images, has also shown initial promise in estimating malignancy risk in pulmonary nodules [45].
7. Conclusion
Subsolid nodules present a paradox in clinical management, with a high risk of malignancy but, when malignant, demonstrating indolent behavior. This leads to conundrums in clinical management – how often and how long should such nodules be surveilled? And when should patients undergo treatment? Emerging data suggest less aggressive management of these nodules is appropriate. Additionally, patients will likely benefit from a personalized approach, incorporating both patient and imaging features in a risk calculator to determine whether treatment is necessary. Future research is needed to evaluate follow-up and management strategies in prospective trials, as well as to identify novel imaging techniques such as deep learning that may provide better nodule triage.
References
- 1.Travis W.D., Brambilla E., Noguchi M. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International multidisciplinary classification of lung adenocarcinoma. J. Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yankelevitz D.F., Yip R., Smith J.P. CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology. 2015;277(2):555–564. doi: 10.1148/radiol.2015142554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henschke C.I., Yip R., Smith J.P. CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am. J. Roentgenol. 2016;207(6):1176–1184. doi: 10.2214/AJR.16.16043. [DOI] [PubMed] [Google Scholar]
- 4.Bankier A.A., MacMahon H., Goo J.M., Rubin G.D., Schaefer-Prokop C.M., Naidich D.P. Recommendations for measuring pulmonary nodules at CT: a statement from the fleischner society. Radiology. 2017;285(2):584–600. doi: 10.1148/radiol.2017162894. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Li K., Sun D. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as pure ground-glass nodules: differentiation using enhanced dual-source dual-energy CT. Am. J. Roentgenol. Am. Roentgen Ray Soc. 2019;213(3):W114–W122. doi: 10.2214/AJR.19.21245. [DOI] [PubMed] [Google Scholar]
- 6.Hammer M.M., Palazzo L.L., Kong C.Y., Hunsaker A.R. Cancer risk in subsolid nodules in the national lung screening trial. Radiology. 2019:190905. doi: 10.1148/radiol.2019190905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oda S., Awai K., Liu D. Ground-glass opacities on thin-section helical CT: differentiation between bronchioloalveolar carcinoma and atypical adenomatous hyperplasia. Am. J. Roentgenol. 2008;190(5):1363–1368. doi: 10.2214/AJR.07.3101. [DOI] [PubMed] [Google Scholar]
- 8.McWilliams A., Tammemagi M.C., Mayo J.R. Probability of cancer in pulmonary nodules detected on first screening CT. N. Engl. J. Med. 2013;369(10):910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakinuma R., Noguchi M., Ashizawa K. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J. Thoracic Oncol. 2016;11(7):1012–1028. doi: 10.1016/j.jtho.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Obayashi K., Shimizu K., Nakazawa S. The impact of histology and ground-glass opacity component on volume doubling time in primary lung cancer. J. Thorac Dis. 2018;10(9):5428–5434. doi: 10.21037/jtd.2018.08.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon Y., Sung Sw, Namkoong M., Park Jk. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J. Thorac Dis. 2016;8(9):2617–2625. doi: 10.21037/jtd.2016.08.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattori A., Matsunaga T., Hayashi T., Takamochi K., Oh S., Suzuki K. Prognostic impact of the findings on thin-section computed tomography in patients with subcentimeter non-small cell lung cancer. J. Thoracic Oncol. 2017;12(6):954–962. doi: 10.1016/j.jtho.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y., Fujimoto D., Morimoto T. Natural history and clinical characteristics of multiple pulmonary nodules with ground glass opacity. Respirology. 2017;22(8):1615–1621. doi: 10.1111/resp.13089. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi Y., Mitsudomi T., Sakao Y., Yatabe Y. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol. 2015;26(1):156–161. doi: 10.1093/annonc/mdu505. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida Y., Kokubu A., Suzuki K. Molecular markers and changes of computed tomography appearance in lung adenocarcinoma with ground-glass opacity. Jpn. J. Clin. Oncol. Oxford Acad. 2007;37(12):907–912. doi: 10.1093/jjco/hym139. [DOI] [PubMed] [Google Scholar]
- 16.Hsu K.-H., Chen K.-C., Yang T.-Y. Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J. Thoracic Oncol. 2011;6(6):1066–1072. doi: 10.1097/JTO.0b013e31821667b0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y.-L., Yuan J.-Q., Wang K.-F. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi Y., Ambrogio C., Mitsudomi T. Ground-glass nodules of the lung in never-smokers and smokers: clinical and genetic insights. Transl. Lung Cancer Res. 2018;7(4):487–497. doi: 10.21037/tlcr.2018.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMahon H., Naidich D.P., Goo J.M. Guidelines for management of incidental pulmonary nodules detected on CT images: from the fleischner society. Radiology. 2017;284(1):228–243. doi: 10.1148/radiol.2017161659. 2017. [DOI] [PubMed] [Google Scholar]
- 20.American College of Radiology . 2019. Lung‐RADS® Version 1.1.https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf?la=en Accessed June 21, 2019. [Google Scholar]
- 21.Hammer M.M., Palazzo L.L., Kong C.Y., Hunsaker A.R. Cancer risk in subsolid nodules in the national lung screening trial. Radiology. 2019;293(2):441–448. doi: 10.1148/radiol.2019190905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins H.A., Katki H.A., Cheung L.C., Landy R., Berg C.D. Insights for management of ground-glass opacities from the national lung screening trial. J. Thoracic Oncol. Elsevier. 2019;14(9):1662–1665. doi: 10.1016/j.jtho.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho J., Kim E.S., Kim S.J. Long-term follow-up of small pulmonary ground-glass nodules stable for 3 years: implications of the proper follow-up period and risk factors for subsequent growth. J. Thoracic Oncol. 2016;11(9):1453–1459. doi: 10.1016/j.jtho.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.W., Jin K.-N., Lee J.-K. Long-term follow-up of ground-glass nodules after 5 years of stability. J. Thoracic Oncol. Elsevier. 2019;14(8):1370–1377. doi: 10.1016/j.jtho.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.H., Lim W.H., Hong J.H. Growth and clinical impact of 6-mm or larger subsolid nodules after 5 years of stability at chest CT. Radiology. 2020;295(2):448–455. doi: 10.1148/radiol.2020191921. [DOI] [PubMed] [Google Scholar]
- 26.Hammer M.M., Palazzo L.L., Paquette A. Cost-effectiveness of follow-up for subsolid pulmonary nodules in High-risk patients. J. Thoracic Oncol. Elsevier. 2020 doi: 10.1016/j.jtho.2020.03.001. https://www.jto.org/article/S1556-0864(20)30193-3/abstract Accessed May 17, 2020. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network . 2020. Non-Small Cell Lung Cancer (Version 4.2020)https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf Accessed May 17, 2020. [Google Scholar]
- 28.Tsutani Y., Miyata Y., Nakayama H. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. 2014;145(1):66–71. doi: 10.1378/chest.13-1094. [DOI] [PubMed] [Google Scholar]
- 29.Cho J.H., Choi Y.S., Kim J., Kim H.K., Zo J.I., Shim Y.M. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann. Thorac Surg. 2015;99(1):218–222. doi: 10.1016/j.athoracsur.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 30.Altorki N.K., Wang X., Wigle D. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503) Lancet Respir. Med. Elsevier. 2018;6(12):915–924. doi: 10.1016/S2213-2600(18)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki K., Saji H., Aokage K. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J. Thoracic Cardiovasc. Surg. Elsevier. 2019;158(3):895–907. doi: 10.1016/j.jtcvs.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 32.Paul S., Lee P.C., Mao J., Isaacs A.J., Sedrakyan A. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ. 2016;354:i3570. doi: 10.1136/bmj.i3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirvani S.M., Jiang J., Chang J.Y. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 2014;149(12):1244–1253. doi: 10.1001/jamasurg.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwan S.W., Mortell K.E., Talenfeld A.D., Brunner M.C. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J. Vasc. Interv. Radiol. 2014;25(1):1–9. doi: 10.1016/j.jvir.2013.10.018. e1. [DOI] [PubMed] [Google Scholar]
- 35.Hammer M.M., Palazzo L.L., Eckel A.L., Barbosa E.M., Kong C.Y. A decision analysis of follow-up and treatment algorithms for nonsolid pulmonary nodules. Radiology. 2018;290(2):506–513. doi: 10.1148/radiol.2018180867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Koning H.J., van der Aalst C.M., de Jong P.A. Reduced lung-cancer mortality with volume CT screening in a randomized trial. New England J. Med. Massachusetts Med. Soc. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 37.White C.S., Dharaiya E., Dalal S., Chen R., Haramati L.B. Vancouver risk calculator compared with ACR lung-RADS in predicting malignancy: analysis of the national lung screening trial. Radiology. 2019;291(1):205–211. doi: 10.1148/radiol.2018181050. [DOI] [PubMed] [Google Scholar]
- 38.Zhan Y., Peng X., Shan F. Attenuation and morphologic characteristics distinguishing a ground-glass nodule measuring 5–10 mm in diameter as invasive lung adenocarcinoma on thin-slice CT. Am. J. Roentgenol. 2019;213(4):W162–W170. doi: 10.2214/AJR.18.21008. [DOI] [PubMed] [Google Scholar]
- 39.Chu Z., Li W., Fu B., Lv F. CT characteristics for predicting invasiveness in pulmonary pure ground-glass nodules. Am. J. Roentgenol. 2020:1–8. doi: 10.2214/AJR.19.22381. [DOI] [PubMed] [Google Scholar]
- 40.Ko J.P., Suh J., Ibidapo O. Lung adenocarcinoma: correlation of quantitative CT findings with pathologic findings. Radiology. 2016;280(3):931–939. doi: 10.1148/radiol.2016142975. [DOI] [PubMed] [Google Scholar]
- 41.Lee G., Lee H.Y., Park H. Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: State of the art. Eur. J. Radiol. 2017;86:297–307. doi: 10.1016/j.ejrad.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y., Lu L., E L Application of radiomics in predicting the malignancy of pulmonary nodules in different sizes. Am. J. Roentgenol. 2019;213(6):1213–1220. doi: 10.2214/AJR.19.21490. [DOI] [PubMed] [Google Scholar]
- 43.Chae H.-D., Park C.M., Park S.J., Lee S.M., Kim K.G., Goo J.M. Computerized texture analysis of persistent part-solid Ground-glass nodules: differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology. 2014;273(1):285–293. doi: 10.1148/radiol.14132187. [DOI] [PubMed] [Google Scholar]
- 44.Li M., Narayan V., Gill R.R. Computer-aided diagnosis of ground-glass opacity nodules using open-source software for quantifying tumor heterogeneity. Am. J. Roentgenol. 2017;209(6):1216–1227. doi: 10.2214/AJR.17.17857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Causey J.L., Zhang J., Ma S. Highly accurate model for prediction of lung nodule malignancy with CT scans. Scientific Rep. 2018;8(1):9286. doi: 10.1038/s41598-018-27569-w. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]