Abstract
INTRODUCTION
End-stage renal disease is associated with elevations in circulating prolactin concentrations, but the association of prolactin concentrations with intermediate health outcomes and the effects of hemodialysis frequency on changes in serum prolactin have not been examined.
METHODS
The FHN Daily and Nocturnal Dialysis Trials compared the effects of conventional thrice weekly hemodialysis with in-center daily hemodialysis (6 days/week) and nocturnal home hemodialysis (6 nights/week) over 12 months and obtained measures of health-related quality of life, self-reported physical function, mental health and cognition. Serum prolactin concentrations were measured at baseline and 12-month follow-up in 70% of the FHN Trial cohort to examine the associations among serum prolactin concentrations and physical, mental and cognitive function and the effects of hemodialysis frequency on serum prolactin.
FINDINGS
Among 177 Daily Trial and 60 Nocturnal Trial participants with baseline serum prolactin measurements, the median serum prolactin concentration was 65 ng/mL (25th–75th percentile 48–195 ng/mL) and 81% had serum prolactin concentrations >30 ng/mL. While serum prolactin was associated with sex (higher in women), we observed no association between baseline serum prolactin and age, dialysis vintage, and baseline measures of physical, mental and cognitive function. Furthermore, there was no significant effect of hemodialysis frequency on serum prolactin in either of the two trials.
DISCUSSION
Serum prolactin concentrations were elevated in the large majority of patients with ESRD, but were not associated with several measures of health status. Circulating prolactin levels also do not appear to decrease in response to more frequent hemodialysis over a one-year period.
Keywords: prolactin, pituitary, daily dialysis, nocturnal dialysis, end stage renal disease
INTRODUCTION
Higher serum prolactin concentrations are common in patients with advanced chronic kidney disease and end stage renal disease (ESRD), secondary to increased secretion and, to a lesser extent, reduced metabolic clearance.1–4 It has been reported that serum prolactin concentrations may be higher in patients with advanced, non-dialysis-requiring chronic kidney disease when compared to patients receiving maintenance hemodialysis, suggesting that factors responsible for uremic hyperprolactinemia may only be partially removed by hemodialysis.5 Stimulation and suppression of prolactin secretion also appears to be impaired in ESRD but not in transplant recipients, with circulating prolactin concentrations reported to decline and/or normalize after successful kidney transplantation.5–8 The elevated level of circulating prolactin in ESRD represents biologically active hormone9 and is thought to contribute to the high prevalence of hypogonadism, anovulation, and sexual dysfunction in patients on dialysis (due to prolactin inhibition of gonadotropin secretion).10 It is unknown whether higher serum prolactin concentrations observed in patients with ESRD are associated with symptoms other than those directly related to relative gonadal deficiency. Several observational studies conducted in women with idiopathic hyperprolactinemia or a prolactin-secreting adenoma provide provocative data that hyperprolactinemia may be directly associated with measures of psychological distress such as anxiety and depression.11,12
Few studies have examined the association of serum prolactin with health status in patients receiving dialysis and/or the effects of hemodialysis intensity or frequency on changes in serum prolactin. Data from a small observational cohort of patients receiving home hemodialysis who converted to nocturnal home hemodialysis were notable for significant reductions in serum prolactin in men and spontaneous return of menses in two of three women of reproductive age.13 The Frequent Hemodialysis Network (FHN) Daily and Nocturnal Trials are the largest randomized studies to date comparing conventional thrice weekly hemodialysis to either daily in-center hemodialysis or nocturnal home hemodialysis, aiming to determine the effects of hemodialysis frequency on multiple intermediate outcomes, including cardiac structure and function, physical health and performance, mental health, cognitive function, hypertension, nutritional status, anemia, bone and mineral metabolism, and general health-related quality of life.14 Based on findings from the Daily Trial, more frequent hemodialysis was associated with improvement in self-reported physical health, based on the physical health composite score of the RAND SF-36 item health survey.15 This ancillary study utilized data from the FHN randomized trials to examine hyperprolactinemia in ESRD, the associations of serum prolactin concentrations with multiple intermediate outcomes, and the effects of hemodialysis frequency on circulating serum prolactin concentrations. If there were changes in serum prolactin in response to frequent hemodialysis, we aimed to explore whether changes in serum prolactin were associated with changes in multiple parameters of health status.
MATERIALS AND METHODS
The FHN trials compared the effects of conventional thrice weekly hemodialysis with in-center daily hemodialysis (6 days per week) and nocturnal home hemodialysis (6 nights per week) over a 12-month treatment interval, with examination of two co-primary outcomes: death or change in left ventricular mass by cardiac magnetic resonance imaging and death or change in self-reported physical health (the Physical Health Composite score from the RAND SF-36 health survey). Secondary outcomes were examined in multiple domains at baseline, month 4 and month 12. For the Daily Trial, a total of 245 patients were randomly assigned to 3 (N=120) or 6 (N = 125) times per week hemodialysis; for the Nocturnal Dialysis Trial, a total of 87 patients were randomly assigned to 3 (N=42) or 6 (N = 45) times per week hemodialysis, both with follow-up to 12 months. The FHN Daily and Nocturnal Dialysis Trials were approved by the Institutional Review Board at each participating site, with biosamples stored at the NIDDK Repository obtained by informed consent for future biomarker analyses. This ancillary study, using stored biosamples and clinical data from the FHN Trials was also approved by the Institutional Review Board at Kaiser Permanente Northern California.
Measurements
Age, sex, race/ethnicity, dialysis vintage (years since first ESRD treatment), diabetes status,16 and a modified Charlson Comorbidity Index adapted for patients with ESRD16,17 were obtained from the FHN trials as previously described. Prolactin laboratory measurements were conducted at the University of California Davis (Biomedical Sciences Laboratory) using stored serum frozen at −70C obtained from the NIDDK Biorepository. Samples were taken at baseline and month 12 (or if not available then, used month closest to month12, ranging from 10–15 months). Serum prolactin (ng/dL) was measured in duplicate using a magnetic microsphere Luminex bead panel system (Millipore, Chicago IL). All samples were initially diluted 1:10, with subsequent dilutions at 1/40 and higher as needed for out-of-range high values. Samples with a coefficient of variation greater than 20% were repeated. The intra- and inter-assay coefficients of variation (CV) were 7.0% and 8.2%, respectively.
The following parameters of self-reported health status and physical and cognitive performance were examined in the FHN Trials at baseline, 4 and 12 months of follow-up. The RAND SF-36 item health survey18 was used to derive physical and mental health composite (PHC and MHC) scores, where higher scores reflect better physical or mental health status, along with a general health scale (0 to 100, where 100 indicates perfect health); the Beck Depression Inventory,19,20 a 21-item survey designed to measure the presence and severity of depressive symptoms; the Health Utilities Index (HUI-3), scored with a final result from 0 to 1 with a function derived from community preferences for various health attributes;21 the Feeling Thermometer, a visual analog scale (0 to 100, where 100 indicates perfect health); and the time to recovery (in minutes) after a dialysis session. The Short Physical Performance Battery (SPPB) included three mobility tests pertaining to standing balance, timed chair stand and timed walk with an overall physical function score (0–12). The Modified Mini Mental Status (3MS) test and the Trail Making Test B (Trails B) were used to test general cognitive function and executive function, respectively.
Statistical Analyses
Standard descriptive statistics were used to compare dialysis frequency groups, including the Student t-test for continuous variables and chi-square or Fisher’s exact test for categorical variables. The association between prolactin concentrations and measures of health status were examined using Pearson correlations, and partial correlations were used to adjust for differences by sex and other clinical factors. To examine the effect of dialysis frequency on change in serum prolactin, and to include all observations, even in those subjects with no 12-month measurements, we employed a mixed-effects model with an unstructured covariance matrix, with adjustment for clinical center. Since prolactin measurements were highly positively skewed in each trial, a log-transformation was used so that treatment effects were expressed as percent differences in geometric means. The interactions of dialysis frequency and sex, dialysis vintage or residual kidney function were examined using mixed-effects models. Regression analyses were conducted to examine the interaction of dialysis frequency with baseline prolactin (log-transformed). Stratified analyses were conducted when a significant interaction was observed. We conducted all analyses using SAS statistical software version 9.4 (SAS Institute, Cary NC) with a p-value criterion of <0.05 for statistical significance.
RESULTS
Baseline characteristics and distribution of serum prolactin concentrations
There were a total of 237 FHN Trial participants (38% female) with stored biosamples and measures of serum prolactin: 177 (72% of those randomized) participants in the Daily Trial and 60 (69% of those randomized) participants in the Nocturnal Trial. Table 1 shows the baseline demographic and clinical characteristics among participants included in these analyses. For the Daily Trial, the average (± SD) age was 50.6 ± 13.8 years old and 71% received hemodialysis ≥2 years before study entry. For the Nocturnal Trial, the average age was 54.5 ± 13.3 years old and 33% had received hemodialysis for ≥2 years before study entry. Compared to patients with measured serum prolactin, FHN Trial participants without prolactin data for analysis did not differ by age, sex, race/ethnicity, vintage or comorbidity status in the Daily Trial, but did differ by race (48% white versus 38%) and were less likely (26% versus 50%) to have diabetes mellitus in the Nocturnal Trial. Over both trials, 81% had a serum prolactin concentrations >30 ng/mL; the median serum prolactin concentration was 65.2 ng/mL (25th–75th percentile range, 35.9–108.7 ng/mL) and more than one-fourth (27%) had serum prolactin concentrations >100 ng/mL. Figure 1 shows the distribution of serum prolactin by sex, with lower prolactin concentrations among men (median 52.6 ng/mL, 25th–75th percentile range 32.0–85.7 ng/mL) compared to women (median 89.8 ng/mL, 25th–75th percentile range 47.8–194.5, p=0.01). However, there were no associations between baseline serum prolactin and age (r=0.027, p=0.68) or vintage (r=0.012, p=0.85).
Table 1.
Baseline characteristics among FHN trial participants with prolactin concentration
| FHN Daily Dialysis Trial | FHN Nocturnal Dialysis Trial | |||
|---|---|---|---|---|
| 3x/week (N=91) |
6x/week (N=86) |
3x/week (N=29) |
6x/week (N=31) |
|
| Age (mean ± SD), years | 52.1 ± 14.0 | 49.1 ± 13.5 | 56.4 ± 12.0 | 52.8 ± 14.4 |
| Female sex, N (%) | 40 (44%) | 28 (33%) | 10 (34%) | 13 (42%) |
| Race/Ethnicity, N (%) | ||||
| Non-Hispanic white | 18 (20%) | 16 (19%) | 16 (55%) | 19 (61%) |
| Black | 42 (46%) | 35 (41%) | 11 (38%) | 11 (36%) |
| All others | 31 (34%) | 35 (41%) | 2 (7%) | 1 (3%) |
| Years since ESRD (Vintage), N (%) | ||||
| < 2 years | 26 (29%) | 25 (29%) | 22 (76%) | 18 (58%) |
| ≥ 2 years | 65 (71%) | 61 (71%) | 7 (24%) | 13 (42%) |
| Charlson Comorbidity Index, N (%) | ||||
| 0 | 37 (41%) | 30 (35%) | 9 (31%) | 11 (36%) |
| 1–2 | 23 (25%) | 32 (37%) | 10 (35%) | 11 (36%) |
| ≥ 3 | 31 (34%) | 24 (28%) | 10 (35%) | 9 (29%) |
| Diabetes mellitus, N (%) | 38 (42%) | 35 (41%) | 14 (48%) | 16 (52%) |
| Prolactin (ng/mL), Median | 64.3 | 59.7 | 66.2 | 72.6 |
| Interquartile range (IQR) | (34.9, 108.7) | (40.5, 99.1) | (42.2, 100.9) | (33.4, 151) |
| Prolactin (ng/mL), N (%) | ||||
| < 30 | 19 (21%) | 16 (19%) | 4 (14%) | 6 (19%) |
| 30 – 49 | 19 (21%) | 17 (20%) | 7 (24%) | 5 (16%) |
| 50 – 99 | 27 (30%) | 32 (37%) | 10 (35%) | 10 (32%) |
| ≥ 100 | 26 (29%) | 21 (24%) | 8 (28%) | 10 (32%) |
No significant differences were seen by treatment arm at baseline for the Daily and Nocturnal Trial
Figure 1.
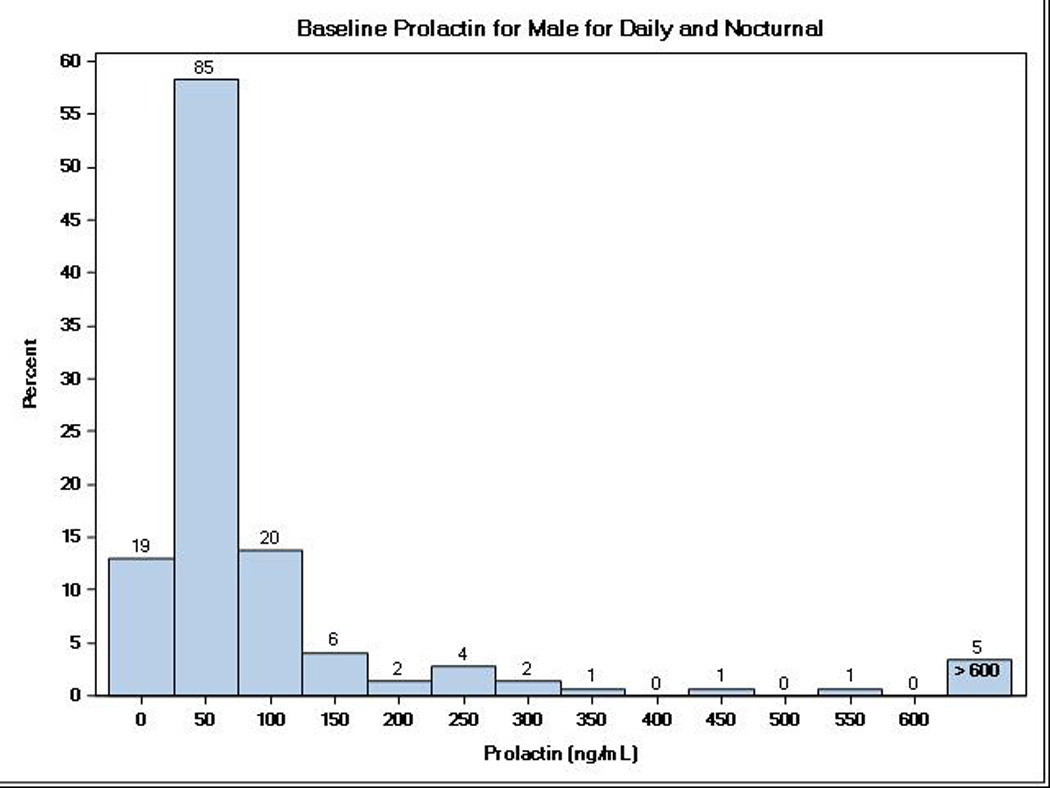
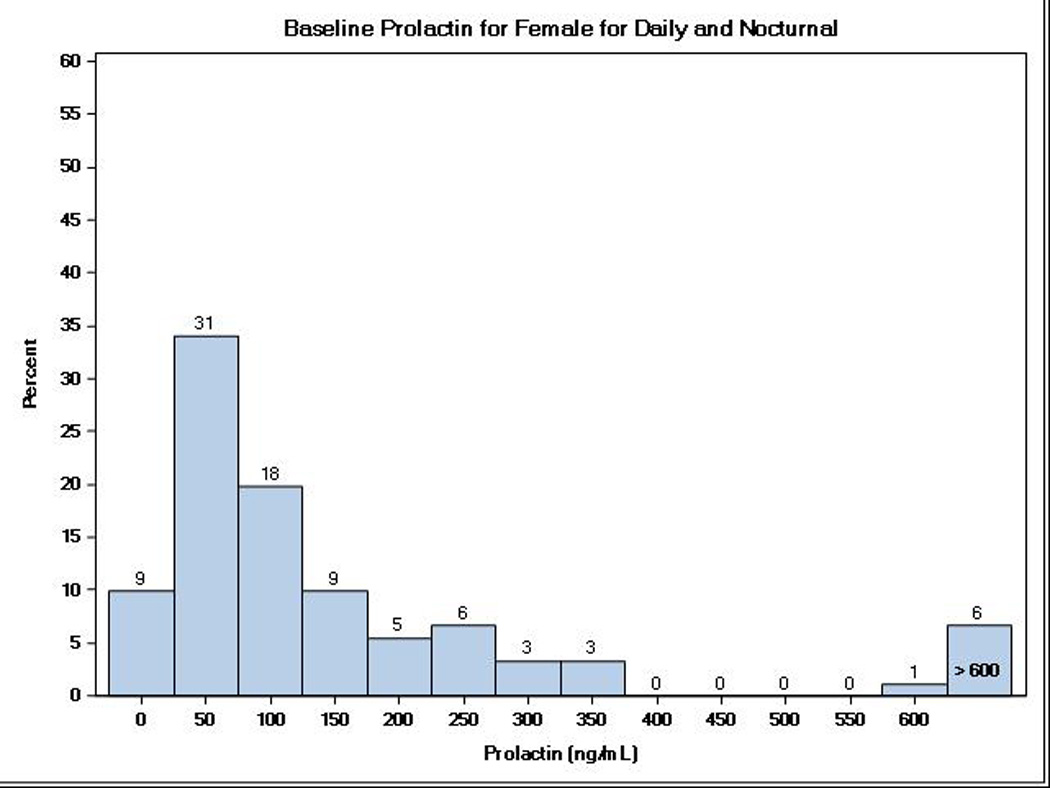
Distribution of baseline serum prolactin levels among ESRD patients enrolled in the Daily and Nocturnal FHN Trials by sex.
Baseline serum prolactin and physical, mental and cognitive function
We next examined the association of baseline serum prolactin with measures of self-reported physical and mental health and physical and cognitive performance and found no significant associations between baseline serum prolactin and the PHC or MHC (from the RAND SF-36 survey), Beck Depression Index, Health Utilities Index, Feeling Thermometer visual scale, time to recovery following a dialysis session, SPPB, 3MS, and Trails B (all |r| ≤0.11, p>0.1) overall. These results were largely unchanged after adjustment for age, sex, vintage, diabetes status and level of comorbidity burden.
Effect of dialysis frequency on prolactin level
Overall, the median change from baseline to 12 months in prolactin concentration was −6.7 (interquartile range −30.5 to +15.6) in the Daily Trial and −9.1 (interquartile range −48.8 to +12.5) in the Nocturnal Trial. Figure 2 shows the change in prolactin concentration by treatment arm (3 times versus 6 times weekly hemodialysis) in those with both baseline and follow-up measurements. Although more participants in the six times weekly arm of the Nocturnal Trial experienced a decline in circulating prolactin level, the change in prolactin did not differ significantly from those receiving thrice weekly hemodialysis. We also examined the effect of hemodialysis frequency (3 versus 6 times weekly) on serum prolactin concentration for the Daily and Nocturnal Trial respectively, using mixed models analyses to account for missing prolactin data at follow-up (61 in the Daily Trial and 14 in the Nocturnal Trial). As shown in Table 2, there were no significant changes in serum prolactin level between conventional and daily hemodialysis treatment arms in both trials. A positive mean change indicates that the month 12 prolactin increased from baseline. The treatment effect compares the change from baseline to month 12 between the dialysis frequency arms. A treatment effect with a positive sign indicates a net increase comparing daily to conventional hemodialysis; a negative sign indicates a net decrease comparing daily to conventional hemodialysis. No significant interaction of dialysis frequency and sex, dialysis vintage or residual renal function were observed. Effect modification of baseline prolactin on the effect of dialysis frequency on Month 12 prolactin (both log-transformed) was also not seen in the Daily Trial, but a significant effect was observed in the Nocturnal Trial (p=0.02). Further stratification of the mixed model analyses by baseline prolactin above or below the median level of 65 ng/dL, showed a significant treatment effect only in the subset of 27 Nocturnal Trial participants with baseline prolactin level ≤65 ng/dL, largely due to an increase in prolactin for the thrice weekly treatment arm (Table 2 footnote). These stratified findings should be interpreted cautiously given the small number of Nocturnal Trial participants in our study.
Figure 2.
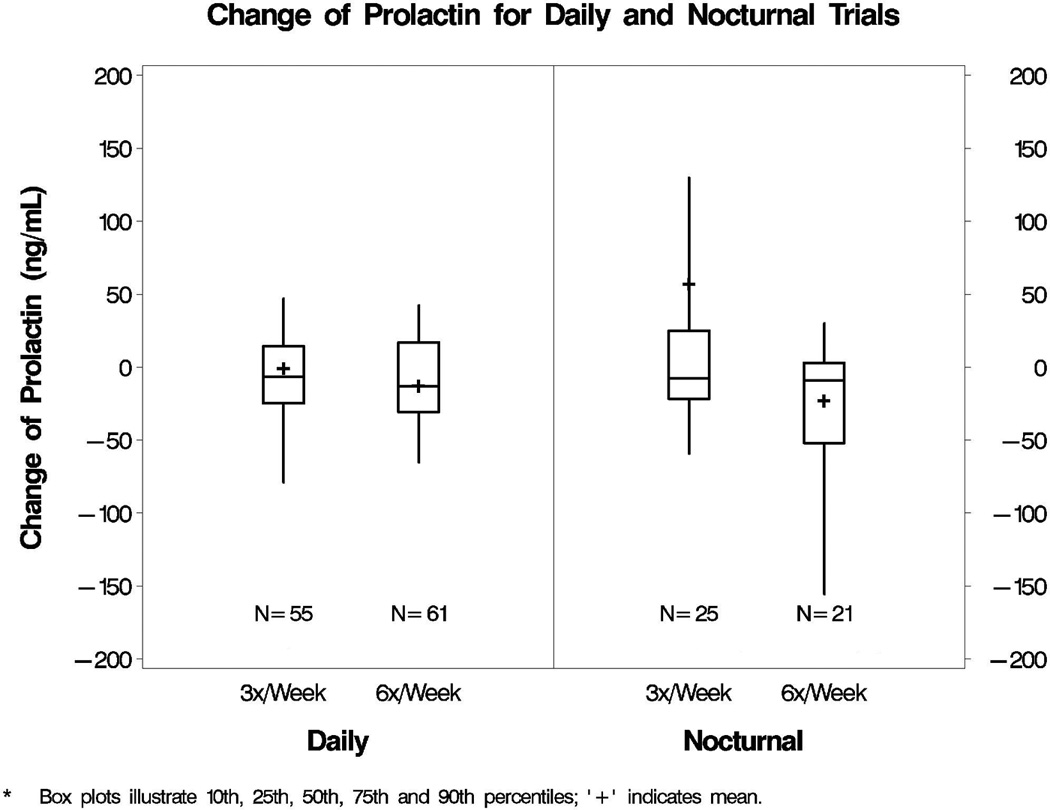
Change in serum prolactin concentration in the Daily and Nocturnal FHN Trials participants with paired measurements. [LEGEND] Box plots represent median and interquartile range prolactin (ng/mL) values (box) and 10th and 90th percentile range (bars), with plus symbols representing mean values. These results are based on 116 and 46 participants in the Daily and Nocturnal Trials, respectively, with both baseline and 12-month prolactin measurements. No significant differences were seen between treatment arms (p=0.70 for Daily and p=0.28 for Nocturnal Trials).
Table 2.
The effect of dialysis frequency on change in serum prolactin* in the Frequent Hemodialysis Trials
| Daily Trial | Nocturnal Trial | |||
|---|---|---|---|---|
| 3x/week | 6x/week | 3x/week | 6x/week | |
| Baseline median (n) prolactin (interquartile range, ng/mL) | 64.3 (91) (34.9, 108.7) |
59.7 (86) (40.5, 99.1) |
66.2 (29) (42.2, 100.9) |
72.6 (31) (33.4, 151.0) |
| Month 12 median (n) prolactin (interquartile range, ng/mL) | 45.8 (55) (29.0, 90.4) |
58.4 (61) (36.0, 110.1) |
65.4 (25) (46.1, 97.8) |
58.1 (21) (25.8, 206.1) |
| Adjusted mean change* in prolactin from baseline (95% confidence interval) | −14.6% (−33.1%, 8.9%) |
−15.5% (−34.6%, 9.1%) |
11.4% (−21.4%, 57.8%) |
−8.1% (−37.1%, 34.4%) |
| Treatment effect* for prolactin (95% confidence interval) | −1.0% (−26.7%, 33.5%) p=0.95 |
−17.4% (−50.4%, 37.4%) p = 0.46 |
||
Adjusted for clinical center (Daily Trial only) and expressed as percent difference based on log-transformed analyses. There was no significant interaction of dialysis frequency and sex, dialysis vintage or residual renal function in either trial (p at least 0.28). Regression analyses showed no effect modification of baseline log-prolactin on the effect of dialysis frequency on log-prolactin at 12 months in the Daily Trial (p=0.88) but a significant effect in the Nocturnal Trial (p=0.02). When the mixed model analyses were stratified by baseline prolactin above or below the median level for the cohort (65 ng/mL), no significant treatment effect was seen for those with baseline prolactin >65 (p≥0.28 for Daily and Nocturnal Trials) or baseline prolactin ≤65 ng/mL for the Daily Trial (p=0.78), but a significant treatment effect was seen among the 27 participants with baseline prolactin ≤65 ng/mL in the Nocturnal Trial (p=0.04) that was largely due to increased prolactin in the 3x/week arm.
These data are based on the 177 Daily and 60 Nocturnal Trial participants with serum prolactin at baseline or baseline and follow-up.
DISCUSSION
These data, derived from two randomized trials of conventional versus more frequent hemodialysis, demonstrate that circulating prolactin concentrations are high in the majority of patients receiving maintenance hemodialysis, including levels exceeding 100 ng/mL in about one-fourth of the cohort examined. However, serum prolactin was unassociated with dialysis vintage, and no associations were seen among circulating prolactin concentrations and multiple health measures relating to physical function, mental health and cognitive function. We also found that compared to conventional dialysis administered 3 times per week, serum prolactin did not decline with more frequent hemodialysis (6 times per week) over a one-year time period.
Historical data demonstrate that hyperprolactinemia occurs in 25–57% of men and 73–91% of women receiving hemodialysis.8,10,22 Others have observed that up to two-thirds of men receiving maintenance hemodialysis have an elevated prolactin level.7 Our results, conducted in more than 200 adults with ESRD receiving hemodialysis, confirm that hyperprolactinemia is prevalent within the hemodialysis population. However, our findings are in contrast to data from a study of 37 patients (30 men) receiving maintenance home hemodialysis (3–5 hours, 3.5–5 sessions per week) converted to nocturnal home hemodialysis (6–9 hours, 3.5–5 sessions weekly), where a significant reduction in serum prolactin was seen in 25 men (median 281 to 243 mU/L or, represented in conventional units, 13.2 to 11.5 ng/mL) after six months.13 In our study, the median serum prolactin concentration was somewhat higher than that published in other studies, although assay methodology differed and included dilution steps that may have optimized detection of extremely high serum concentrations. In the Nocturnal Trial, we did find a significant treatment effect among those with lower baseline prolactin, but this was driven by an increase in prolactin in the conventional treatment arm. Collectively, these data point to the need to further study the spectrum of hyperprolactinemia in patients with ESRD, associated clinical sequelae, and the short- versus long-term effects of dialysis frequency on elevated prolactin concentration.
The strengths of our study include examination of baseline and longitudinal change in serum prolactin in a multicenter randomized trial of hemodialysis frequency in patients with ESRD, with standardized collection of data for patient-centered outcomes. However, our study has important limitations, including modest sample size, incomplete serum prolactin data and, perhaps most importantly, measures of prolactin obtained only at baseline and 12 months, which may not reflect early effects of hemodialysis frequency on circulating prolactin. Furthermore, without available serum testosterone concentrations in men, we were unable to directly address gonadal-specific outcomes secondary to hyperprolactinemia. Finally, these data were derived from two randomized clinical trials; while participants were diverse by age, sex, and race/ethnicity, patients who agree to a major time-consuming intervention may be different in important ways from other patients with ESRD.
In summary, serum prolactin concentrations were high in the large majority of patients with ESRD, but were not associated with a variety of measures of health status. Circulating prolactin levels also do not appear to decrease in response to more frequent hemodialysis when compared to conventional thrice weekly hemodialysis regimens, although further studies may be warranted in larger populations, including those receiving nocturnal hemodialysis.
Acknowledgments
The Frequent Hemodialysis Network Trials (registered at Clinical Trials.gov NCT00264758 and NCT00271999) were supported by grants from the National Institutes of Health (U01 DK066579, U01 DK066597, U01 DK066480, U01 DK066481) and the Centers for Medicare and Medicaid Services and the National Institutes of Health Research Foundation (with contributions from Amgen, Baxter, DaVita, Dialysis Clinics, Inc., Fresenius Medical Care North America, Renal Research Institute and Satellite Healthcare). This Frequent Hemodialysis Network Trial ancillary study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (R01 DK076165).
Footnotes
CONFLICT OF INTEREST: None
REFERENCES
- 1.Sievertsen GD, Lim VS, Nakawatase C, Frohman LA. Metabolic clearance and secretion rates of human prolactin in normal subjects and in patients with chronic renal failure. J Clin Endocrinol Metab. 1980;50:846–852. doi: 10.1210/jcem-50-5-846. [DOI] [PubMed] [Google Scholar]
- 2.Handelsman DJ. Hypothalamic-pituitary gonadal dysfunction in renal failure, dialysis and renal transplantation. Endocr Rev. 1985;6:151–182. doi: 10.1210/edrv-6-2-151. [DOI] [PubMed] [Google Scholar]
- 3.Cowden EA, Ratcliffe WA, Ratcliffe JG, Dobbie JW, Kennedy AC. Hyperprolactinaemia in renal disease. Clin Endocrinol (Oxf) 1978;9:241–248. doi: 10.1111/j.1365-2265.1978.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 4.McKenna TM, Woolf PD. Prolactin metabolic clearance and resistance to dopaminergic suppression in acute uremia. Endocrinol. 1985;116:2003–2007. doi: 10.1210/endo-116-5-2003. [DOI] [PubMed] [Google Scholar]
- 5.Peces R, Horcajada C, Lopez-Novoa JM, Frutos MA, Casado S, Hernando L. Hyperprolactinemia in chronic renal failure: impaired responsiveness to stimulation and suppression. Normalization after transplantation. Nephron. 1981;28:11–16. doi: 10.1159/000182087. [DOI] [PubMed] [Google Scholar]
- 6.Ghahramani N, Habili H, Karimi M, Ghalambor MA, Jan-Ghorban P. Renal transplantation: levels of prolactin, leutinizing hormone, follicle-stimulating hormone and testosterone. Transplant Proc. 1999;31:3145. doi: 10.1016/s0041-1345(99)00758-7. [DOI] [PubMed] [Google Scholar]
- 7.Yadav R, Mehta SN, Kumar A, Guleria S, Seenu V, Tiwari SC. A prospective analysis of testicular androgenic function in recipients of a renal allograft. Int Urol Nephrol. 2008;40:397–403. doi: 10.1007/s11255-007-9277-8. [DOI] [PubMed] [Google Scholar]
- 8.Lim VS, Kathpalia SC, Frohman LA. Hyperprolactinemia and impaired pituitary response to suppression and stimulation in chronic renal failure: reversal after transplantation. J Clin Endocrinol Metab. 1979;48:101–107. doi: 10.1210/jcem-48-1-101. [DOI] [PubMed] [Google Scholar]
- 9.Smith CR, Butler J, Iggo N, Norman MR. Serum prolactin in uraemia: correlations between bioactivity and activity in two immunoassays. Acta Endocrinol (Copenh) 1989;120:295–300. doi: 10.1530/acta.0.1200295. [DOI] [PubMed] [Google Scholar]
- 10.Gomez F, de la Cueva R, Wauters JP, Lemarchand-Beraud T. Endocrine abnormalities in patients undergoing long-term hemodialysis. The role of prolactin. Am J Med. 1980;68:522–530. doi: 10.1016/0002-9343(80)90296-x. [DOI] [PubMed] [Google Scholar]
- 11.Reavley A, Fisher AD, Owen D, Creed FH, Davis JR. Psychological distress in patients with hyperprolactinaemia. Clin Endocrinol (Oxf) 1997;47:343–348. doi: 10.1046/j.1365-2265.1997.2701073.x. [DOI] [PubMed] [Google Scholar]
- 12.Fava GA, Fava M, Kellner R, Serafini E, Mastrogiacomo I. Depression hostility and anxiety in hyperprolactinemic amenorrhea. Psychother Psychosom. 1981;36:122–128. doi: 10.1159/000287535. [DOI] [PubMed] [Google Scholar]
- 13.van Eps C, Hawley C, Jeffries J, et al. Changes in serum prolactin, sex hormones and thyroid function with alternate nightly nocturnal home haemodialysis. Nephrology (Carlton) 2012;17:42–47. doi: 10.1111/j.1440-1797.2011.01520.x. [DOI] [PubMed] [Google Scholar]
- 14.Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 15.Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocco MV, Larive B, Eggers PW, et al. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. Am J Kidney Dis. 2011;57:90–100. doi: 10.1053/j.ajkd.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42:125–132. doi: 10.1016/s0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 18.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Craven JL, Rodin GM, Johnson L, Kennedy SH. The diagnosis of major depression in renal dialysis patients. Psychosom Med. 1987;49:482–492. doi: 10.1097/00006842-198709000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33:375–384. doi: 10.3109/07853890109002092. [DOI] [PubMed] [Google Scholar]
- 22.Molitch ME, Hou SH. Neuroendocrine alterations in systemic disease. Clin Endocrinol Metab. 1983;12:825–851. doi: 10.1016/s0300-595x(83)80066-8. [DOI] [PubMed] [Google Scholar]


