Abstract
Background
Assessing adherence to growth hormone (GH) is challenging. The Easypod™ connect device delivers pre-set doses of recombinant human GH (r-hGH) and stores a digital record of adherence that can be shared with healthcare provider. We assessed adherence to r-hGH delivered with Easypod™ according to the approved pediatric indications for r-hGH: growth hormone deficiency (GHD), born small for gestational age (SGA) who failed to show catch-up growth and Turner syndrome (TS).
Methods
ECOS (NCT01555528) was a multicenter (24 countries), 5-year, longitudinal, observational study, which aimed to evaluate country-specific adherence to r-hGH therapy prescribed via the Easypod™ electronic injection device. The primary endpoint was yearly adherence. Secondary endpoints were height velocity, height velocity standard deviation scores (SDS), height, height SDS and IGF-1 concentrations. Clinical and auxological data were obtained from medical records and adherence from Easypod™ logs.
Results
This study included 147 Easypod™-naïve Mexican children assessed during 3 years (mean age: 9.96 ± 3.41 years, 56.8% boys, mean height SDS at baseline: − 2.17 ± 0.97): 118 with GHD, 24 SGA and 5 with TS. A total of 105 (71.4%) patients were GH naïve. Overall median adherence was > 90% over the first year of treatment and > 80% at 3 years. Adherence was not different by r-hGH indication or between GH-naïve or experienced patients. At 1-year follow-up, mean change in height SDS was 0.57 ± 0.34, whereas mean height velocity SDS was 2.85 ± 2.51. In all, 84.7% patients had normal IGF-1 concentrations at 1-year follow-up. Adherence was associated with change in height SDS (r = 0.239, p = 0.005) and height velocity SDS (r = 0.194, p = 0.027).
Conclusion
Adherence rates with the Easypod™ device are high and maintained over time in GHD, SGA and TS Easypod™-naïve Mexican patients. High adherence is associated with better outcomes. Easypod™ assists physicians in monitoring adherence to r-hGH.
Keywords: Adherence, Auto-injector, Device, Growth hormone, Somatotropin
Introduction
The human recombinant growth hormone (h-rGH) is indicated to treat clinical conditions that imply short stature, such as GH deficiency (GHD), children born small for gestational age (SGA) who failed to show catch-up growth and Turner syndrome (TS), among other medical conditions [1–6]. Adherence to h-rGH is one of the most important factors that determine achievement of clinical targets [7−10]. Unfortunately, adherence to GH therapy has been reported suboptimal in the majority of patients [11−13]. But an additional barrier is actual recognition of adherence by healthcare providers due to patients and/or family under reporting [6]. Hence, detection of poor adherence can be challenging in real-life settings.
The EasypodTM auto-injector connected digital device (approved in Mexico in June 2016) is designed to make daily administration of recombinant human growth hormone (r-hGH) comfortable and easier to patients. EasypodTM device delivers pre-set doses of r-hGH (Saizen®) and stores a digital record of adherence to therapy that can be electronically shared with healthcare providers for evaluation [5, 6, 13−21]. Furthermore, EasypodTM is integrated into an e-Health ecosystem for management of growth disorders treated with Saizen (r-hGH) available to healthcare professionals through a personal-data secure web solution.
The aim of the present report was to assess adherence to r-hGH therapy delivered via the EasypodTM device according to the approved pediatric indications for Saizen® in Mexico: GHD, SGA and TS. A secondary objective of the present analysis is to evaluate the potential association of adherence with growth outcomes.
Methods
The EasypodTM connect observational study (ECOS, NCT01555528) was multicenter (24 countries), 5-year (November 2010–February 2016), phase IV, prospective, longitudinal and observational study, to assess country-specific adherence to therapy among children receiving r-hGH via the EasypodTM electromechanical auto-injector device. Herein, we present the subanalysis for the Mexican population included in ECOS. The study was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice (ICH-GCP E6) guidelines and applicable local regulatory requirements. A central Institutional Review Board and Ethics Committee in Mexico approved this part of the study. All patients agreed for participating in the study and their parents or legal proxies provided signed informed consent.
Methods of ECOS have already been reported elsewhere [6]. To be eligible, patients should be EasypodTM naïve but could be experienced with r-hGH delivered by other methods. Briefly, EasypodTM-naïve children included in the study were aged 2–18 years or >18 years without fusion of growth plates and were receiving r-hGH via the EasypodTM electromechanical device (Saizen®, Merck KGaA, Darmstadt, Germany). Eligible patients from each participating country were enrolled and attended one baseline visit followed by 1–4 visits each year, at healthcare provider discretion and according to local clinical practice. Also, all diagnoses and treatment decisions were at the discretion of the researcher physician.
The primary endpoint was the recorded adherence as assessed at yearly intervals. Secondary endpoints were height velocity, height velocity standard deviation scores (SDS), height, height SDS, as well as IGF-1 concentrations after each year of treatment. Demographic, auxological and diagnostic data were obtained from medical records, with adherence data obtained directly from the patients’ EasypodTM electronic records.
Relative frequencies are expressed as percentages. Parametric continuous variables are expressed as means and standard deviations (SD). Non-parametric continuous variables will be expressed as medians with minimum and maximum or interquartile range. Pearson Chi-square or Fisher exact tests are used to assess proportions in categorical variables. To compare quantitative variables between two groups, Student t test and Mann–Whitney U test were performed in distributions of parametric and non-parametric variables, respectively. Adherence was determined as the percentage adherence over time (number of days with injections received divided by the number of days with injections planned). Correlations between adherence and growth outcomes were calculated using Spearman’s product–moment correlation. Alpha errors (p values) reported are two sided and considered significant when p < 0.05.
Results
ECOS included 147 Mexican patients (mean age: 9.96 ± 3.41 years, 56.8% boys, mean height SDS at baseline: − 2.17 ± 0.97): 118 with GHD, 24 SGA patients who failed to show catch-up growth and 5 with TS (Table 1). A total of 105 (71.4%) patients were GH naïve. Mean age was not significantly different among groups, however, with a trend for patients with TS being younger (Table 2).
Table 1.
Baseline characteristics of the cohort (n = 147)
| GHD (n = 118) | SGA (n = 24) | TS (n = 5) | Overall (n = 147) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 9.95 (3.52) | 10.13 (2.40) | 8.60 (4.88) | 9.96 (3.41) |
| Median | 10.5 | 10 | 10 | 10 |
| Min; max | 1; 18 | 5; 15 | 3; 14 | 1; 18 |
| Sex, n (%) | ||||
| Female | 50 (42.4) | 9 (37.5) | 5 (100) | 64 (43.2) |
| Male | 68 (57.6) | 15 (62.5) | 0 | 84 (56.8) |
| Ethnicity, n (%) | ||||
| Caucasian | 5 (4.2) | 1 (4.2) | 0 | 6 (4.1) |
| Other | 113 (95.8) | 23 (95.8) | 5 (100) | 142 (95.9) |
GHD growth hormone deficiency, SGA small for gestational age patient who failed to show catch-up growth, TS Turner syndrome
Table 2.
Growth outcomes at 1-year follow-up (n = 147)
| GHD (n = 118) | SGA (n = 24) | TS (n = 5) | Overall (n = 147) | |
|---|---|---|---|---|
| Height SDS at baseline | ||||
| Mean (SD) | − 2.17 (0.98) | − 1.93 (0.76) | − 3.36 (1.06) | − 2.17 (0.97) |
| Median (IQR) | − 2.06 (−2.61 to − 1.72) | − 1.89 (−2.43 to −1.55) | − 3.10 (−3.79 to −2.58) | − 2.08 (−2.61 to −1.72) |
| Change in height SDS | ||||
| Mean (SD) | 0.58 (0.35) | 0.51 (0.30) | 0.63 (0.42) | 0.57 (0.34) |
| Median (IQR) | 0.56 (0.36–0.76) | 0.44 (0.32–0.76) | 0.80 (0.40–0.88) | 0.54 (0.36–0.77) |
| Height velocity (cm per year) SDS | ||||
| Mean (SD) | 2.97 (2.62) | 2.64 (1.97) | 0.64 (1.65) | 2.85 (2.51) |
| Median (IQR) | 3.04 (1.58–4.17) | 2.65 (1.54–3.71) | 1.05 (0.60–1.88) | 2.91 (1.49–4.07) |
| 1-year IGF-1 standard score (%) | ||||
| Abnormal low (< 84 µ/L) | 9.6 | 16.7 | 0 | 10.2 |
| Normal (84–100 µ/L) | 86.5 | 66.7 | 100 | 84.7 |
| Abnormal high (> 100 µ/L) | 3.8 | 16.7 | 0 | 5.1 |
GHD growth hormone deficiency, SDS standard deviation scores, SGA small for gestational age patient who failed to show catch-up growth, TS Turner syndrome
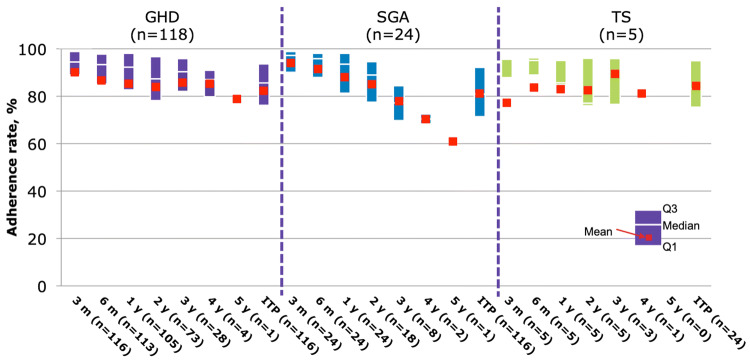
Overall median adherence was >90% over the first year of treatment and >80% over 3 years (Fig. 1); however, as this was an observational study in Mexico and most patients were treated privately significant study dropouts where observed after the first year of therapy on patients enrolled the study. Adherence was not different by r-hGH indication of between GH-naïve or experienced patients (Fig. 2). At 1-year follow-up, mean change in height SDS was 0.57 ± 0.34, whereas mean height velocity SDS was 2.85 ± 2.51.
Fig. 1.
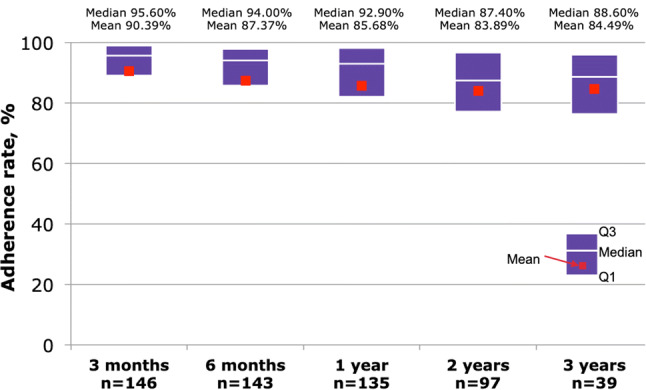
Adherence to r-hGH therapy delivered via the Easypod™ device from baseline through year 3 follow-up
Fig. 2.
Adherence to r-hGH therapy delivered via the Easypod™ device, according to diagnosis. GHD Growth hormone deficiency, SGA small for gestational age patient who failed to show catch-up growth, TS Turner syndrome
At 1-year follow-up, 84.7% patients had normal IGF-1 concentrations (84–100 µ/L), 10.2% had abnormal low (<84 µ/L) and 5.1% abnormal high IGF-1 concentrations (>100 µ/L). Overall, statistically significant correlations were observed with therapy adherence and change in height SDS (p = 0.005), as well as with height velocity SDS (p = 0.027) (Table 3). Nonetheless, correlation of treatment adherence with change in height SDS was lower and not significant in the GH-naïve subgroup (Spearman’s product–moment correlation = 0.147, p = 0.134).
Table 3.
Spearman’s product–moment correlation of therapy adherence with growth outcomes at 1-year follow-up
| GHD (n = 118) | SGA (n = 24) | TS (n = 5) | Overall (n = 147) | |
|---|---|---|---|---|
| Change in height SDS | ||||
| Spearman's rho | 0.215 | 0.376 | − 0.500 | 0.239 |
| p value | 0.028 | 0.070 | 0.391 | 0.005 |
| Height velocity SDS | ||||
| Spearman's rho | 0.187 | 0.184 | 0.200 | 0.194 |
| p value | 0.061 | 0.390 | 0.800 | 0.027 |
GHD growth hormone deficiency, SDS standard deviation scores, SGA small for gestational age patient who failed to show catch-up growth, TS Turner syndrome
Discussion
The present analysis shows that adherence to r-hGH delivered by the Easypod™ electromechanical auto-injection device in Mexican children with different causes of short stature remains high after 3 years of treatment. Furthermore, the magnitude of adherence was directly associated with anthropometric and auxological milestones during r-hGH therapy. Although we cannot discard the Hawthorne effect (i.e., the reactivity in which individuals modify an aspect of their behavior in response to their awareness of being observed), our data and those of the global ECOS report [6] suggest that children under r-hGH therapy delivered by the Easypod™ device are prone to present a high rate of adherence in the mid- and long terms.
In the present study, the observed adherence rate was above 80% up to 3 years of clinical follow-up, which contrasts with the expected adherence rate of 55–65% observed in other studies using different r-hGH delivery technologies [22, 23] and confirms the findings of other reports with the same methodology [5, 6, 13, 14, 24]. Additionally, this study suggests that adherence to therapy is predictor of clinical response, a factor that has been described in other scenarios where electromechanical auto-injectors are used to deliver drugs and to assess compliance to therapy [25−30]. As can be inferred, poor adherence is usually associated with failure to reaching clinical outcomes [6, 13]. The global ECOS study found a positive correlation between adherence and attainment of growth milestones [6]. Contrary to previous reports [5, 13], here, we found a significant correlation between adherence and growth outcomes at 1-year after initiating r-hGH therapy with Easypod™. This difference among ECOS subpopulations may be due to sample size differences as well as the proportions of r-hGH therapy indications, which may lead to potential differences in the magnitude of clinical response.
A limitation of this study, however, is the low sample of patients that remained after 3 years of follow-up, which make difficult assumptions regarding very long-term adherence, associated factors and attainment of clinical objectives of the r-hGH therapy in the long run. Moreover, determinants of adherence such as socioeconomical status, parental education status, achievement of therapeutic goals and patient’s perspective about therapy were not taken into account for the present analysis. Side effects associated with therapy were not analyzed since in this cohort as global ECOS data did not show any new safety findings [6] and the main objective of this study was to assess adherence to an electronic auto-injector device in real-world settings in Mexican patients. Although efficacy was not an objective of this study, we observed attainment of clinical milestones of r-hGH therapy as can be expected by clinical indication. Nevertheless, this ECOS subanalysis on Mexican patients demonstrates that adherence to therapy is high with Easypod™ device and that adherence relates with clinical response.
Conclusion
In conclusion, ECOS has produced robust, real-world adherence data in patients receiving Saizen® via Easypod™ and provided useful insights into growth response to Saizen® treatment. Adherence rates with the EasypodTM device are high and maintained over time in GHD and SGA Mexican patients. The positive correlations between adherence and growth outcomes suggest an influence of adherence on treatment outcomes. Nonetheless, this may also be influenced by the fact that in Mexico, most patients were treated privately, have regular consultations and received a slightly higher mean dose of r-hGH, as compared with other countries of the ECOS study.
Acknowledgements
The authors would like to thank the patients and their families, the investigators, co-investigators and study teams at each of the participating centers and at Merck Biopharma Distribution S.A. de C.V., Mexico and Merck Healthcare KGaA, Darmstadt, Germany. The company, however, was not involved in patient’s data management, analysis, manuscript preparation or the decision to submit for publication. This manuscript was written and edited by the authors, who take full responsibility for its content.
Author contributions
All authors have contributed to the conception and design of the work and the analysis of the data in a manner substantial enough to take public responsibility for it; each believes the manuscript represents valid work; and each has reviewed the final version of the manuscript and approves it for publication. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
Merck Healthcare KGaA, Darmstadt, Germany and Merck S.A. de C.V., Mexico sponsored this study.
Compliance with ethical standards
Conflict of interest
Carmen Celeste Rosas-Guerra and Ekaterina Koledova are Employees of Merck Serono. Armando Blanco-López, Raúl Calzada-León and Arturo Ayala-Estrada have received research support and lecture fees from Merck S.A. de C.V., Mexico. Authors of this paper declare that the paper is original and has not been published or submitted for publication elsewhere, and that there is no any affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript that may affect the reporting of the work submitted.
Ethical approval
The local ethical committees of each participating center in Mexico approved the study.
Informed consent
Written informed consent was obtained from the patients and/or from their legal guardians prior to enrollment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
E. Chiquete, Email: erwinchiquete@hotmail.com
A. Ayala-Estrada, Email: dr_arturo78@hotmail.com
References
- 1.Richmond E, Rogol AD. Current indications for growth hormone therapy for children and adolescents. Endocr Dev. 2010;18:92–108. doi: 10.1159/000316130. [DOI] [PubMed] [Google Scholar]
- 2.Johannsson G, Nespithal K, Plöckinger U, Alam V, McLean M. Multi-centre phase IV trial to investigate the immunogenicity of a new liquid formulation of recombinant human growth hormone in adults with growth hormone deficiency. J Endocrinol Invest. 2018;41:919–927. doi: 10.1007/s40618-017-0818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatrics. 2016;86:361–397. doi: 10.1159/000452150. [DOI] [PubMed] [Google Scholar]
- 4.Mancini A, Vergani E, Bruno C, Palladino A, Brunetti A. Relevance of adherence monitoring in adult patients with growth hormone deficiency under replacement therapy: preliminary monocentric data with Easypod(TM) connect. Front Endocrinol (Lausanne) 2019;10:416. doi: 10.3389/fendo.2019.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dommelen P, Koledova E, Wit JM. Effect of adherence to growth hormone treatment on 0–2 year catch-up growth in children with growth hormone deficiency. PLoS ONE. 2018;13:e0206009. doi: 10.1371/journal.pone.0206009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koledova E, Stoyanov G, Ovbude L, Davies PSW. Adherence and long-term growth outcomes: results from the easypod(™) connect observational study (ECOS) in paediatric patients with growth disorders. Endocr Connect. 2018;7:914–923. doi: 10.1530/EC-18-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapoor R, Burke S, Sparrow S, Hughes I, Dunger D, Ong K, Acerini C. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93:147–148. doi: 10.1136/adc.2006.114249. [DOI] [PubMed] [Google Scholar]
- 8.Cutfield WS, Derraik JG, Gunn AJ, Reid K, Delany T, Robinson E, Hofman PL. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS ONE. 2011;6:e16223. doi: 10.1371/journal.pone.0016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79:189–196. doi: 10.1159/000350251. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers P, O’Brien F, Blethen S. Patient outcomes in the GH monitor: the effect of delivery device on compliance and growth. Pediatr Endocrinol Rev. 2005;2:327–331. [PubMed] [Google Scholar]
- 11.Smith S, Hindmarsh P, Brook C. Compliance with growth hormone treatment—are they getting it? Arch Dis Child. 1993;68:91–93. doi: 10.1136/adc.68.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyarzabal M, Aliaga M, Chueca M, Echarte G, Ulied A. Multicentre survey on compliance with growth hormone therapy: what can be improved? Acta Paediatr. 1998;87:387–391. doi: 10.1080/08035259850156959. [DOI] [PubMed] [Google Scholar]
- 13.Centonze C, Guzzetti C, Orlando G, Loche S, Investigators IECOS. Adherence to growth hormone (GH) therapy in naïve to treatment GH-deficient children: data of the Italian Cohort from the Easypod Connect Observational Study (ECOS) J Endocrinol Invest. 2019;42:1241–1244. doi: 10.1007/s40618-019-01046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrabal Vela MA, García Gijón CP, Pascual Martin M, Benet Giménez I, Áreas Del Águila V, Muñoz-Rodríguez JR, Palomo Atance E. Adherence to somatotropin treatment administered with an electronic device. Endocrinol Diabetes Nutr. 2018;65:314–318. doi: 10.1016/j.endinu.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Loche S, Salerno M, Garofalo P, Cardinale GM, Licenziati MR, Citro G, Caruso Nicoletti M, Cappa M, Longobardi S, Maghnie M, Perrone R. Adherence in children with growth hormone deficiency treated with r-hGH and the easypod™ device. J Endocrinol Invest. 2016;39:1419–1424. doi: 10.1007/s40618-016-0510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann K, Ittner J, Müller-Rossberg E, Schönau E, Stephan R, Ullrich KP, Hoppe B, Ramseger R, Brämswig J. Growth hormone treatment adherence in prepubertal and pubertal children with different growth disorders. Horm Res Paediatr. 2013;80(1):1–5. doi: 10.1159/000351800. [DOI] [PubMed] [Google Scholar]
- 17.Pfützner A, Hartmann K, Winter F, Fuchs GS, Kappelgaard AM, Rohrer TR. Intuitiveness, ease of use, and preference of a prefilled growth hormone injection pen: a noninterventional, randomized, open-label, crossover, comparative usability study of three delivery devices in growth hormone-treated pediatric patients. Clin Ther. 2010;32:1918–1934. doi: 10.1016/j.clinthera.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Kirk J. Improving adherence to GH therapy with an electronic device: first experience with easypod. Pediatr Endocrinol Rev. 2006;6(Suppl 4):549–552. [PubMed] [Google Scholar]
- 19.Tauber M, Payen C, Cartault A, Jouret B, Edouard T, Roger D. User trial of Easypod, an electronic autoinjector for growth hormone. Ann Endocrinol (Paris) 2008;69:511–516. doi: 10.1016/j.ando.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Dahlgren J. Easypod: a new electronic injection device for growth hormone. Expert Rev Med Devices. 2008;5:297–304. doi: 10.1586/17434440.5.3.297. [DOI] [PubMed] [Google Scholar]
- 21.Dahlgren J, Veimo D, Johansson L, Bech I. Patient acceptance of a novel electronic auto-injector device to administer recombinant human growth hormone: results from an open-label, user survey of everyday use. Curr Med Res Opin. 2007;23:1649–1655. doi: 10.1185/030079907x210589. [DOI] [PubMed] [Google Scholar]
- 22.Mohseni S, Heydari Z, Qorbani M, Radfar M. Adherence to growth hormone therapy in children and its potential barriers. J Pediatr Endocrinol Metab. 2018;31:13–20. doi: 10.1515/jpem-2017-0157. [DOI] [PubMed] [Google Scholar]
- 23.Michaelidou M, Whitten S, Bajaj P, Knight A, Spoudeas HA. Improved adherence and growth outcomes with jet-delivered growth hormone. J Pediatr Endocrinol Metab. 2019;32:207–213. doi: 10.1515/jpem-2018-0067. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez Arnao MD, Rodríguez Sánchez A, Díez López I, Ramírez Fernández J, Bermúdez de la Vega JA, Yeste Fernández D, Chueca Guindulain M, Corripio Collado R, Pérez Sánchez J, Fernández González A. Adherence and long-term outcomes of growth hormone therapy with easypod™ in pediatric subjects: Spanish ECOS study. Endocr Connect. 2019;8:1240–1249. doi: 10.1530/EC-19-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayas A, Ouallet JC, Kallmann B, Hupperts R, Fulda U, Marhardt K, for the SMART study (2015) Adherence to, and effectiveness of, subcutaneous interferon b-1a administered by RebiSmart in patients with relapsing multiple. Expert Opin Drug Deliv 12:1239–1250 [DOI] [PubMed]
- 26.Pedersen ED, Stenager E, Vadgaard JL, Jensen MB, Schmid R, Meland N, Magnussen G, Frederiksen JL. Adherence to subcutaneous interferon beta-1a treatment using an electronic injection device: a prospective open-label Scandinavian noninterventional study (the ScanSmart study) Patient Prefer Adherence. 2018;12:569–575. doi: 10.2147/PPA.S154417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edo Solsona MD, Monte Boquet E, Casanova Estruch B, Poveda Andrés JL. Impact of adherence on subcutaneous interferon beta-1a effectiveness administered by Rebismart(®) in patients with multiple sclerosis. Patient Prefer Adherence. 2017;11:415–421. doi: 10.2147/PPA.S127508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaminetsky JC, McCullough A, Hwang K, Jaffe JS, Wang C, Swerdloff RS. A 52-week study of dose adjusted subcutaneous testosterone enanthate in oil self-administered via disposable auto-injector. J Urol. 2019;201:587–594. doi: 10.1016/j.juro.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 29.Xiao X, Li W, Clawson C, Karvani D, Sondag P, Hahn JK. Evaluation of performance, acceptance, and compliance of an auto-injector in healthy and rheumatoid arthritic subjects measured by a motion capture system. Patient Prefer Adherence. 2018;12:515–526. doi: 10.2147/PPA.S160394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pricci F, Villa M, Maccari F, Agazio E, Rotondi D, Panei P, Roazzi P. The Italian Registry of GH Treatment: electronic Clinical Report Form (e-CRF) and web-based platform for the national database of GH prescriptions. J Endocrinol Invest. 2019;42:769–777. doi: 10.1007/s40618-018-0980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]